Measurement of retinol-binding protein 4 (RBP4) in urine plays an important role as a sensitive functional biomarker of proximal renal tubular disease, a diagnostic feature of renal Fanconi syndrome.1, 2 The syndrome may be hereditary, as in Dent 1 or Dent 2 diseases and many other syndromes, or acquired, as in several drug toxicities targeting the proximal tubule.2, 3 The “free” form of RBP4 in plasma (molecular weight: 21 kDa) comprises about 14% of total RBP4, the remainder being complexed to the carrier protein, transthyretin, as RBP4–transthyretin, with a molecular weight of 76 kDa.4 The literature generally refers to the “free” form of plasma RBP4 as being freely filtered by the renal glomerulus, in much the same way as β2-microglobulin, with a molecular weight of 11.6 kDa.2, 5 β2-microglobulin has an estimated glomerular-sieving coefficient (GSC) of 0.94 ± 0.1 and 0.97, as measured directly in animal studies,6, 7 and 0.91 ± 0.14 from indirect measurement in humans.8 Curiously, the GSC of RBP4 has not been measured in animals, and there is only one indirect measurement in humans.8
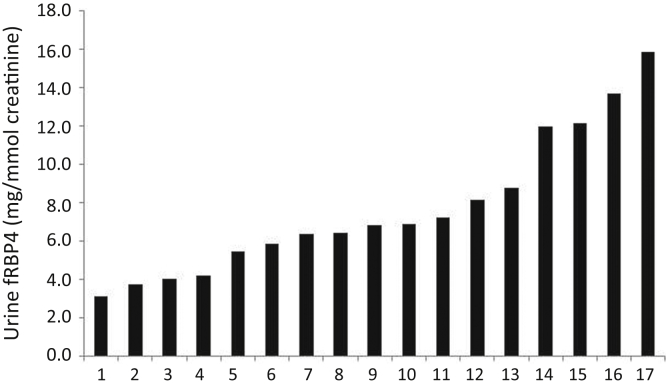
A new, sensitive, and accurate assay for the free form of RBP4 in urine (UfRBP) has recently been developed, and we have used it to reexamine the GSC of fRBP4.9 The approach we use is similar to that previously reported, but it has yielded markedly different results based on the new and more accurate measurement of UfRBP4. Patients with Dent 1 disease, due to a CLCN5 mutation, were studied. In these patients, there appears to be a “knockout” of reabsorption of proteins by the proximal renal tubule, and this is supported by experimental work.S1–S4 This reabsorption normally relies on the function of CLC5, the gene product of CLCN5, which is required for megalin/cubilin-dependent receptor-mediated endocytosis. As a result, assuming a complete knockout and absence of other pathways for addition or loss of protein, the composition of the patient`s urine will include all the plasma proteins filtered at the renal glomerulus. The estimated urine content of a protein in 24 hours will be the same as the quantity appearing in the glomerular ultrafiltrate in 24 hours.8 An analogous approach has recently been used to estimate the GSC of albumin in mice.S5 Dent 1 patients with well-preserved glomerular filtration rate were studied so that glomerular filtration could be assumed to be normal. Results of measurements of UfRBP4 in 17 patients with Dent1 disease are shown in Figure 1. These gave a median urine concentration of 6.65 mg/mmol creatinine (range: 3.1–15.9 mg/mmol). One outlying UfRBP4 result, 47.4 mg/mmol, is excluded from calculations because this one measurement is far beyond the distribution of the other 17 results (see Figure 1).
Figure 1.
Excretion of urine free-retinol-binding protein 4 (fRBP4) in 17 patients with Dent 1 disease.
Using the mathematical method described, this yields a new value for the GSC of UfRBP4 of 0.097 ± 0.017 (± SEM).8 This new value should be contrasted with the previous value of 0.38 ± 0.057 and clearly shows that plasma fRBP4 is not freely filtered. The GSC of fRBP4 is, therefore, about one-tenth that of β2-microglobulin. The change in measured GSC is solely due to the more accurate values for the urine concentrations in Dent 1 patients. Previous measurements of the very high concentrations of RBP4 present in the urine of Dent 1 patients were questionable. This is due to assay nonlinearity upon dilution of the very high levels of URBP4 in renal Fanconi syndrome and uncertainty about the immunoassay targets, as discussed previously in more depth.9 The similarity of the estimates for the GSC of β2-microglobulin obtained using data from Dent 1 patients and from experimental work in rats suggests that the approach used here for fRBP4 is valid. Plasma fRBP4 is still, on a molar basis, expected to contribute 8.5% of the proteins in the glomerular ultrafiltrate, compared with some 16.8% for albumin (see Supplementary Methods). No change is suggested to the concept that proximal tubular impairment will cause very large changes to the urinary excretion of fRBP4. However, this finding does suggest that increased glomerular permeability to plasma fRBP4 will increase its excretion in urine, just as it is believed to cause microalbuminuria in diabetes mellitus and certain cardiovascular conditions.S6,S7 Such changes are expected to be smaller than those seen in the proximal tubular disease of renal Fanconi syndrome, but they nevertheless should be readily detectable. Indeed, we hypothesize that the poorly understood pathologic process that causes microalbuminuria in diabetes and certain cardiovascular conditions will also affect excretion of more readily filtered, but not freely filtered, proteins such as fRBP4, and even more dramatically than albumin itself. Studies are in progress to examine this hypothesis.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by the Cambridge Biomedical Research Centre and by Addenbrooke's Hospital Charities.
Footnotes
Supplementary Material
Supplementary Methods.
Supplementary References.
References
- 1.Bernard A.M., Vyskocil A.A., Mahieu P., Lauwerys R.R. Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin Chem. 1987;33:775–779. [PubMed] [Google Scholar]
- 2.Norden A.G.W., Lapsley M., Unwin R.J. Urine retinol-binding protein 4: a functional biomarker of the proximal renal tubule. Adv Clin Chem. 2014;63:85–122. doi: 10.1016/b978-0-12-800094-6.00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Chan A., Park L., Collins L.F. Correlation between tenofovir drug levels and the renal biomarkers RBP-4 and ß2M in the ION-4 study cohort. Open Forum Infect Dis. 2019;6:ofy273. doi: 10.1093/ofid/ofy273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard A., Vyskocyl A., Mahieu P., Lauwerys R. Effect of renal insufficiency on the concentration of free retinol-binding protein in urine and serum. Clin Chim Acta. 1988;171:85–93. doi: 10.1016/0009-8981(88)90293-8. [DOI] [PubMed] [Google Scholar]
- 5.Laube G.F., Russell-Eggitt I.M., van’t Hoff W.G. Early proximal tubular dysfunction in Lowe’s syndrome. Arch Dis Child. 2004;89:479–480. doi: 10.1136/adc.2003.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumpio B.E., Maack T.L. Kinetics, competition, and selectivity of tubular absorption of proteins. Am J Physiol. 1982;243:F379–F392. doi: 10.1152/ajprenal.1982.243.4.F379. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier C., Nguyen-Simonnet H., Vincent C. Renal tubular absorption of beta 2 microglobulin. Kidney Int. 1984;26:170–175. doi: 10.1038/ki.1984.151. [DOI] [PubMed] [Google Scholar]
- 8.Norden A.G.W., Lapsley M., Lee P.J. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 2001;60:1885–1892. doi: 10.1046/j.1523-1755.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 9.Burling K.A., Cutillas P.R., Church D. Analysis of molecular forms of urine retinol-binding protein in Fanconi syndrome and design of an accurate immunoassay. Clin Chim Acta. 2012;413:483–489. doi: 10.1016/j.cca.2011.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods.
Supplementary References.