Abstract
Background:
Prior studies demonstrate that ethanol exposure induces the release of intracellular calcium (CA2+) in modulation of γ-Aminobutyric acid (GABA)-ergic tone and produces concomitant alterations in sigma (σ)-1 protein expression that may contribute to the development ethanol dependence. However, the influence of CA2+ released from endoplasmic reticulum (ER)-bound inositol triphosphate (IP3) and σ−1 receptors in regulating hippocampal function has yet to be delineated.
Methods:
Rat hippocampal explants were subjected to chronic intermittent ethanol exposure (CIE) with or without the addition of IP3 inhibitor xestospongin C (0–0.5 μM) or σ−1 receptor antagonist BD-1047 (0–80 μM). Hippocampal viability was assessed via immunohistochemical labeling of neuron specific nuclear protein (NeuN)/Fox-3 in CA1, CA3, and DG sub-regions.
Results:
Exposure to CIE produced consistent and significant decreases of NeuN/Fox-3 in each primary cell layer of the hippocampal formation. Co-exposure to xestospongin reversed these effects in the CA1 sub-region, and significantly attenuated these effects in the CA3 and DG regions. Xestospongin application also significantly increased NeuN/Fox-3 immunofluorescence in ethanol-naïve hippocampi. Co-exposure to 20 μM BD-1047 also reversed the loss of NeuN/Fox-3 during CIE exposure in each hippocampal cell layer, whereas exposure to 80 μM BD-1047 did not alter NeuN-Fox-3 in ethanol-treated hippocampi. By contrast, 80 μM BD-1047 application significantly increased NeuN/Fox-3 immunofluorescence in ethanol-naïve hippocampi in each sub-region.
Conclusions:
These data suggest that ethanol stimulates ER IP3 and σ−1 receptors to promote hippocampal loss of NeuN/Fox-3 during CIE.
Keywords: ethanol withdrawal, neuron specific nuclear protein, calcium, inositol triphosphate, sigma-1
INTRODUCTION
Calcium (CA2+) is a ubiquitous intracellular signal known to control a cadre of cellular processes. Internal CA2+ stores are held within membrane systems of the endoplasmic reticulum (ER) or sarcoplasmic reticulum of muscle cells, as well as mitochondria (Berridge, 1993). Release of intracellular CA2+ is controlled by various channels, such as the inositol-1,4,5-trisphosphate (IP3) receptor and ryanodine receptor families (Clapper et al., 1987). Ethanol application is known to produce concomitant alterations in ER-bound protein complexes (e.g., decreases in IP3 mRNA [Rodríguez et al., 1996]), ionotropic glutamatergic (e.g., inhibition of N-methyl-D-aspartate [NMDA] receptors [Lovinger et al., 1989]) and group-1 metabotropic glutamate-family proteins (i.e., decreases in mGlu1 mRNA [Simonyi et al., 1996]). These studies demonstrate that ethanol alters polyphosphoinositide signaling, but the functional effects of these neuroadaptive changes are not yet fully understood. For example, prior studies utilizing whole-cell voltage-clamp recordings demonstrate that presynaptic release of intracellular CA2+ is required for spontaneous release of γ-Aminobutyric acid (GABA) neurotransmission in cerebellar neurons following ethanol exposure (Kelm et al., 2007). Others demonstrate that application of pharmacologically relevant concentrations of ethanol (i.e., 25–50 mM) facilitates release of CA2+ from IP3-insensitive pools in brain microsomes (Daniell & Harris, 1989). Simultaneous application of ethanol and IP3, however, produced an additive release of intracellular CA2+. Although these findings suggest that ethanol may stimulate other ER-bound proteins in addition to IP3 receptors, the etiology of these effects has yet to be delineated.
Located in close proximity to IP3 receptors are sigma (σ)-1 receptors, which are CA2+-sensitive, ligand-regulated chaperones of the ER implicated in potentiation of prolonged IP3 receptor-mediated CA2+ signaling (Hayashi & Su, 2007). Sigma-1 receptors are highly concentrated in the hippocampus (Alonso et al., 2000) and therefore involved in mediation of synaptic plasticity that may contribute to the development of ethanol dependence. For example, a prior study found that chronic, intermittent ethanol (CIE) administration produced an upregulation of σ−1 receptor protein expression in the hippocampus (Sabeti, 2011). These increases in σ−1 polypeptide levels were attenuated by exposure to a σ−1 receptor antagonist (10 mg/kg BD-1047). In particular, prior studies demonstrate that σ−1 receptors mediate intrinsic plasticity in cornu ammonis (i.e., CA1) hippocampal neurons following exposure to CIE (Meunier et al., 2006). These effects contribute to the neurocognitive consequences produced by CIE (Meunier et al., 2006) and their inhibition reduced ethanol consumption in Sardinian alcohol-preferring rats (Sabino et al., 2009). Functional polymorphisms in the σ−1 receptor gene were also associated with a propensity for alcoholism in a case-controlled study (Miyatake et al., 2004) suggesting that σ−1 receptors may regulate development of dependence in the clinical population.
Collectively, these findings suggest that ethanol exposure may stimulate release of intracellular CA2+ that may contribute to the neurocognitive consequences of CIE. However, the role of CA2+ released from ER-bound IP3 and σ−1 receptors in regulating hippocampal viability has yet to be delineated. The purpose of the present studies, therefore, is to examine the effects of intracellular CA2+ release from IP3 and σ−1 receptor sensitive stores on withdrawal-associated loss of neuron specific nuclear protein (NeuN)/Fox-3 in rat hippocampal explants.
MATERIALS AND METHODS
Organotypic Hippocampal Slice Culture Preparation
Whole brains from eight-day-old Sprague-Dawley rats (Harlan Laboratories; Indianapolis, IN) were aseptically collected, transferred to culture dishes containing frozen dissecting media composed of Minimum Essential Medium (MEM; Invitrogen, Carlsbad, CA), 25 mM HEPES (Sigma, St. Louis, MO), 10.60 μM Amphotericin B solution (Sigma), and 50 μM streptomycin/penicillin (Invitrogen), and bilateral hippocampi were removed and then carefully transferred to sterile plates containing chilled culture media composed of dissecting media, distilled water, 36 mM glucose (Fisher, Pittsburg, PA), 25% Hanks’ Balanced Salt Solution (HBSS; Invitrogen), 25% (v/v) heat-inactivated horse serum (HIHS; Sigma), 0.05% Amphotericin B solution (Sigma), and 0.05% streptomycin/penicillin (Invitrogen). A stereoscopic microscope was used to detach excess hippocampal tissue prior to sectioning of unilateral hippocampi at 200 μM using a McIlwain Tissue Chopper (Mickle Laboratory Engineering Co. Ltd., Gomshall, UK). Hippocampi were selected under the stereoscopic microscope for intact cell layers prior to careful placement onto Millicell-CM 0.4 μM biopore membrane inserts that contained 1 mL of pre-incubated culture media, and were then placed in a sterile six-well culture plate. Each culture well plate generated eighteen intact hippocampal explants. Excess culture media was extracted off the top of each biopore membrane insert using the stereoscopic microscope. Explants were maintained in an incubator at 37°C with a gas composition of 5% CO2/95% air for five days in order to allow tissue to adhere to the membrane inserts prior to the first media change (after Butler et al., 2010). Care of all animals was carried out in agreement with the University of Kentucky’s Institutional Animal Care and Use Committee.
Ethanol Exposure and Withdrawal
The present study used an in vitro model of CIE that has been published previously (Reynolds et al., 2015A; Reynolds et al., 2015B) to delineate the influence of intracellular CA2+ from ER-bound receptors in promoting withdrawal-associated loss of hippocampal NeuN/Fox-3. In brief, male and female rat hippocampal explants were randomly transferred at 5 days in vitro to plates containing either 1 ml of the ethanol-naïve culture media (control) or ethanol-containing media (i.e., 0 and 50 mM) for five days with or without the addition of xestospongin C (0.5 μM), a membrane-permeant inhibitor of IP3-mediated CA2+ release or BD-1047 (20 and 80 μM), a selective antagonist at the σ−1 receptor (Kim et al., 2010; Taylor & Tovey, 2010). At 11 days in vitro, explants were removed from their respective treatment groups and transferred to plates containing 1 ml of fresh ethanol-naïve culture media for a 24-hour ethanol withdrawal period. During each withdrawal period, tissue was drug naïve. This treatment regimen was repeated a total of three times. Xestospongin C was first dissolved in 100% dimethyl sulfoxide (DMSO; Fisher) to yield a final working concentration of 0.01% DMSO in the ethanol-naïve and ethanol-containing culture media (after Reynolds et al., 2015B). Concentrations of xestospongin C (i.e., 0–0.5 μM) and BD-1047 (i.e., 0–80 μM) were selected based on prior reports showing efficacy in reducing withdrawal-associated neurotoxicity from other drugs of abuse, such as methamphetamine when applied acutely (Smith et al., 2008; Smith et al., 2010). The concentration of ethanol (i.e., 50 mM) was selected in the present report as it produces a consistent loss of NeuN in this model of CIE (Reynolds et al., 2015A; Reynolds et al., 2015B), as well as reflects binge-like ethanol consumption in the clinical population (Eckardt et al., 1998).
Immunohistochemistry
NeuN (Fox-3) is a protein found in nearly all post-mitotic neurons (Kim et al., 2009; Mullen et al., 1992), and was selected in the present study because it is a reliable marker of neuronal integrity using this model of CIE (Reynolds et al., 2105A; Reynolds et al., 2105B). Rat hippocampal explants were fixed by placing 1 ml of 10% formalin solution on the top and bottom of each culture plate well, incubated at room temperature for 30 minutes, washed twice with phosphate buffered saline (PBS), and then stored at 4°C until conducting immunohistochemistry. Immunohistochemical labeling with NeuN/Fox-3 was conducted by transferring formalin-fixed explants to a plate containing 1 ml of permeabilization (wash) buffer (200 ml PBS [Invitrogen], 200 μL Triton X-100 [Sigma], 0.010 mg Bovine Serum [Sigma]). One ml of buffer was added to the top of each well, and explants were incubated at room temperature for 45 minutes in order to permeate cell membranes. Explants were then incubated at 4°C for 24 hours with the primary monoclonal antibody mouse anti-NeuN (1:200; Sigma). Explants were then washed with PBS and incubated for 24 hours with goat anti-mouse fluorescein isothiocyanate (FITC; 1:200; Sigma), and imaged with SPOT software 4.0.2 (advanced version) for Windows (W. Nuhsbahm Inc.; McHenry, IL, USA) through a 5x objective with a Leica DMIRB microscope (W. Nuhsbahm Inc.; McHenry, IL, USA) connected to a computer and captured with a SPOT 7.2 color mosaic camera (W. Nuhsbahm). A band-pass filter at 495 nm (520 nm emission) detected FITC immunofluorescence, allowing for NeuN/Fox-3 optical density determination after Butler et al., 2010.
Statistical analyses
Statistical analyses were conducted to delineate the influence of intracellular CA2+ release from ER-bound receptors in regulating hippocampal integrity following exposure to CIE. Studies included in the present report were conducted two times using two different rat litters. All data were converted to percent control and then combined for ease of interpretation. Data were analyzed using a two-factor analysis of variance (ANOVA) with media (i.e., ethanol-containing and ethanol-naïve) and drug (i.e., no drug and drug) as the factors for each hippocampal cell layer (i.e., CA1, CA3, and DG). Pairwise comparisons of means were conducted using Holm-Sidak’s multiple comparisons test. Effects were considered significant at p<0.05. For graphical representation and interpretation, data are presented as group mean +/ the standard error of the mean (SEM).
RESULTS
Inhibitor of IP3-mediated CA2+ release by Xestospongin C
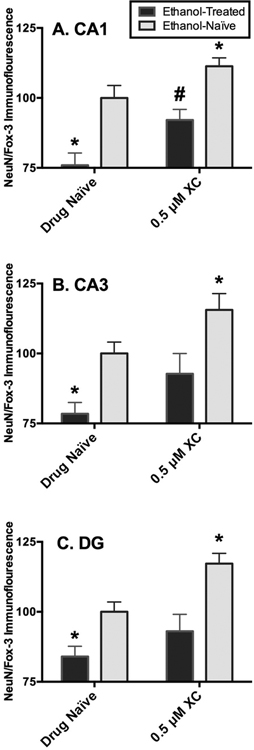
In the pyramidal cell layer of the CA1, ANOVA analyses revealed significant main effects of media (F[1,115= 29.49, p=0.0001) and drug (F[1,115= 11.88, p<0.0008). CIE exposure produced a 24% decrease in NeuN/Fox-3 protein immunofluorescence relative to ethanol-naïve tissue. Exposure to xestospongin C (0.5 μM) attenuated the loss of NeuN/Fox-3 immunofluorescence produced by three cycles of CIE by 16% (p=0.007; Holm-Sidak’s multiple comparisons) (Figure 1A). Application of xestospongin C resulted in significant increases of NeuN/Fox-3 immunofluorescence by 11% (p=0.0009; Holm-Sidak’s multiple comparisons) in ethanol-naïve tissue (Figure 1A).
Fig. 1.
Effects of IP3 inhibitor6 xestospongin C (0.5 lM) application on NeuN/Fox-3 immunofluorescence in ethanol (EtOH)-exposed and EtOHna€ıve hippocampi within the pyramidal cell layer of the CA1 (A), CA3 (B), and the granule cell layer of the dentate gyrus (DG) (C). Data are presented as percent control of the mean SEM. *Statistical significance (p < 0.05) compared to EtOH- and -drug-na€ıve hippocampi; #statistical significance (p < 0.05) compared to EtOH-treated, but drug-na€ıve hippocampi. N = 27 for EtOH-treated hippocampi; N = 29 for hippocampi co-exposed to EtOH and xestospongin C; N = 33 for EtOH- and drug-na€ıve hippocampi; N = 30 for EtOH-na€ıve hippocampi exposed to xestospongin C.
In the pyramidal cell layer of the CA3, ANOVA analyses revealed significant main effects of media (F[1,115= 11.95, p<0.0001) and drug (F[1,115= 5.39, p=0.007). In this sub-region, CIE exposure produced a 21% decrease in NeuN/Fox-3 protein immunofluorescence relative to ethanol-naïve tissue. Exposure to xestospongin C (0.5 μM) attenuated the loss of NeuN/Fox-3 immunofluorescence produced by three cycles of CIE as expression of NeuN/Fox-3 detected in ethanol-treated hippocampi co-exposed to xestospongin C was not altered significantly as compared to ethanol and drug-naive tissue (p>0.05; Holm-Sidak’s multiple comparisons) (Figure 1B). In addition, application of xestospongin C resulted in significant increases of NeuN/Fox-3 immunofluorescence by 15% (p=0.03; Holm-Sidak’s multiple comparisons) in ethanol-naïve tissue (Figure 1B).
A similar pattern was observed in the granule cell layer of the DG as ANOVA analyses revealed significant main effects of media (F[1,115= 21.28, p<0.0001) and drug (F[1,115= 9.09, p=0.003). CIE exposure produced a 15% decrease in NeuN/Fox-3 protein immunofluorescence relative to ethanol-naïve tissue in this sub-region. Exposure to xestospongin C (0.5 μM) attenuated the loss of NeuN/Fox-3 immunofluorescence produced by three cycles of CIE in ethanol-treated hippocampi in a similar manner to the CA3 sub-region (p>0.05; Holm-Sidak’s multiple comparisons) (Figure 1C). Application of xestospongin C resulted in significant increases of NeuN/Fox-3 immunofluorescence by 17% (p=0.001; Holm-Sidak’s multiple comparisons) in ethanol-naive tissue (Figure 1C).
σ−1 receptor antagonist BD-1047
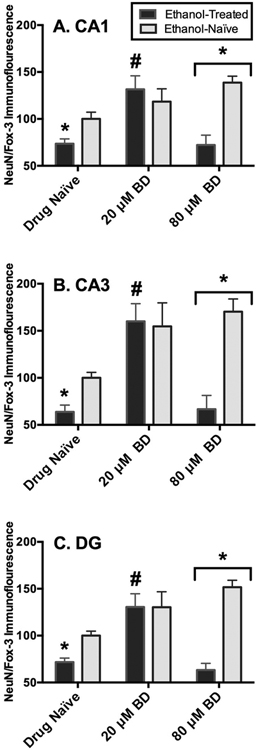
In the pyramidal cell layer of the CA1, ANOVA analyses revealed a significant media-by-drug interaction (F[2,66= 7.01, p=0.002), a significant main effect of media (F[2,66= 6.08, p=0.004), and a significant main effect of drug (F[2,66= 8.79, p=0.004). CIE exposure produced a 26% decrease in NeuN/Fox-3 protein immunofluorescence relative to ethanol-naïve tissue. Exposure to the lower concentration of BD-1047 (20 μM) reversed these effects by 58% (p=0.003; Holm-Sidak’s multiple comparisons), whereas exposure to higher BD-1047 concentrations (i.e., 80 μM) failed to restore levels of NeuN/Fox-3 protein immunofluorescence in ethanol-exposed hippocampi (p>0.05; Holm-Sidak’s multiple comparisons) (Figure 2A). Application of BD-1047 at the lower concentration (i.e., 20 μM) did not significantly alter levels of NeuN/Fox-3 protein immunofluorescence in ethanol-naïve tissue (p>0.05; Holm-Sidak’s multiple comparisons) whereas exposure to higher concentrations of BD-1047 (i.e., 80 μM) resulted in significant increases of NeuN/Fox-3 immunofluorescence by 36% (p=0.0009; Holm-Sidak’s multiple comparisons) in ethanol-naive tissue (Figure 2A).
Fig. 2.
Effects of r-1 chaperone protein antagonist BD-1047 (20 and 80 lM) application on NeuN/Fox-3 immunofluorescence in ethanol (EtOH) treated and EtOH naive within the CA1 (A), CA3 (B), and dentate gyrus (DG) (C). Data are presented as percent control of the mean SEM. *Statistical significance (p < 0.05) compared to EtOH- and drug-naive hippocampi; #statistical significance (p < 0.05) compared to EtOH-treated, but drug-naive hippocampi. N = 10 for EtOH-treated hippocampi; N = 14 for hippocampi co-exposed to EtOH and BD-1047 (20 lM); N = 11 for hippocampi co-exposed to EtOH and BD-1047 (80 lM); N = 11 for EtOHand drug-naive hippocampi; N = 13 for EtOH-naive hippocampi exposed to BD-1047 (20 lM); N = 13 for EtOH-naive hippocampi exposed to EtOH and BD-1047 (80 lM).
In the pyramidal cell layer of the CA3, ANOVA analyses revealed a significant media-by-drug interaction (F[2,66= 5.65, p=0.006), a significant main effect of media (F[2,66= 10.64, p=0.002), and a significant main effect of drug (F[2,66= 10.09, p=0.002). CIE exposure produced a 36% decrease in NeuN/Fox-3 protein immunofluorescence relative to ethanol-naïve tissue (p=0.0009; Holm-Sidak’s multiple comparisons). Exposure to the lower concentration of BD-1047 (i.e., 20 μM) reversed these effects by 96% (p=0.0004; Holm-Sidak’s multiple comparisons), whereas exposure to higher BD-1047 concentrations (i.e., 80 μM) failed to restore levels of NeuN/Fox-3 protein immunofluorescence in ethanol-exposed hippocampi (p>0.05; Holm-Sidak’s multiple comparisons) (Figure 2B). Application of BD-1047 at the lower concentration (20 μM) did not significantly alter levels of NeuN/Fox-3 protein immunofluorescence in ethanol-naïve tissue (p>0.05; Holm-Sidak’s multiple comparisons), whereas exposure to higher concentrations of BD-1047 (i.e., 80 μM) resulted in significant increases of NeuN/Fox-3 immunofluorescence by 36% (p=0.0002; Holm-Sidak’s multiple comparisons) in ethanol-naïve tissue (Figure 2B).
A similar pattern was observed in the granule cell layer of the dentate gyrus as ANOVA analyses revealed a significant media-by-drug interaction (F[2,66= 8.70, p=0.0008), a significant main effect of media (F[2,66= 18.75, p=0.0001), and a significant main effect of drug (F[2,66= 8.04, p=0.0008). CIE exposure produced a 28% decrease in NeuN/Fox-3 protein immunofluorescence relative to ethanol-naïve tissue. Exposure to the lower concentration of BD-1047 (20 μM) reversed these effects by 58% (p=0.003; Holm-Sidak’s multiple comparisons), whereas exposure to higher BD-1047 concentrations (i.e., 80 μM) failed to restore levels of NeuN/Fox-3 protein immunofluorescence in ethanol-exposed hippocampi (p>0.05; Holm-Sidak’s multiple comparisons) (Figure 2C). Application of BD-1047 at the lower concentration (20 μM) did not alter levels of NeuN/Fox-3 protein immunofluorescence in ethanol-naive tissue (p>0.05; Holm-Sidak’s multiple comparisons), whereas exposure to higher concentrations of BD-1047 (i.e., 80 μM) resulted in significant increases of NeuN/Fox-3 immunofluorescence by 51% (p=0.0001); Holm-Sidak’s multiple comparisons in ethanol-naive tissue (Figure 2C).
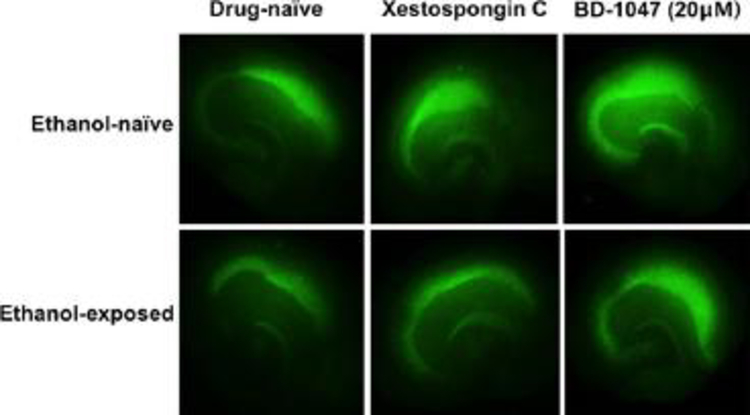
Depicted in Figure 3 are representative images of NeuN/Fox-3 immunofluorescence of ethanol and drug-naïve hippocampi (top left), ethanol-naïve hippocampi exposed to xestospongin C (0.5 μM) (top middle), ethanol-naïve hippocampi exposed to BD-1047 (20 μM) (top right), ethanol–exposed drug-naïve hippocampi (bottom left), hippocampi co-exposed to ethanol and xestospongin C (0.5 μM) (bottom middle), hippocampi co-exposed to ethanol and BD-1047 (20 μM) (bottom right).
Fig. 3.
Representative images of NeuN/Fox-3 immunofluorescence of ethanol (EtOH)- and drug-na€ıve hippocampi (top left), EtOH-na€ıve hippocampi exposed to xestospongin C (0.5 lM) (top middle), EtOH-na€ıve hippocampi exposed to BD-1047 (20 lM) (top right), EtOH-treated, but drug-na€ıve hip-pocampi (bottom left), hippocampi co-exposed to EtOH and xestospongin C (0.5 lM) (bottom middle), and hippocampi coexposed to EtOH and BD-1047 (20 lM) (bottom right).
DISCUSSION
The present study examined the influence of ethanol-induced activity of ER-bound receptors and presumptive intracellular CA2+ release on hippocampal viability, as reflected in NeuN immunoreactivity after Reynolds et al. 2015A. We first assessed the efficacy of xestospongin C, a macrocyclic bis-1-oxaquinolizidine and potent blocker of IP3-mediated CA2+ release on the vesicles of the ER membrane (Gafni et al., 1997), to prevent ethanol withdrawal-associated loss of NeuN in hippocampi exposed to a CIE regimen. CIE produced significant decreases in NeuN/Fox-3 immunofluorescence in each of the primary cell layers of the hippocampal formation by 15–24%. These results are not unexpected and are consistent with the magnitude of effects typically observed following this CIE treatment regimen (Reynolds et al., 2015A; Reynolds et al., 2015B). Although we have previously shown that the loss of NeuN using this model of CIE corresponds to loss of cellular marker thionine (Reynolds et al., 2015A), these effects could reflect either a decrease of NeuN protein expression or adverse effects on “physiological status” (Weyer & Schilling, 2003) rather than loss of neurons. However, prior studies suggest that NeuN loss correlates strongly with other measures of neuronal loss (Chen-Roetling et al., 2013; Liu et al., 2009). Liu et al. 2009, for example, demonstrate that NeuN is a more sensitive marker of neuronal loss than measuring infarct volume in a stroke model because the NeuN loss occurs before the obvious infarction. Nonetheless, co-application of ethanol and xestospongin C (0.5 μM) for three consecutive cycles of CIE significantly attenuated levels of NeuN/Fox-3 immunofluorescence in each examined hippocampal sub-region, indicating that ethanol acutely induces activity of IP3-dependent signaling. Others have shown that release of intracellular CA2+ from IP3-sensitive stores plays a critical role in the alcohol-mediated death of cerebellar granule neurons (Kouzoukas et al., 2013). This is consistent with prior studies demonstrating that ethanol application engages IP3 receptors to release intracellular CA2+ in the hippocampus (Daniell & Harris; 1989) in a protein-kinase C (PKC)-dependent manner (Mironov & Hermann, 1996), and advances our understanding of the functional consequences of this release. These data suggest that ethanol may mobilize intracellular CA2+ via upstream activation of second messenger effectors, such as protein kinases, prior to withdrawal to promote the decreases of NeuN/Fox-3 observed in the present report. Given the notion that ethanol acutely inhibits NMDA receptor function (Lovinger et al., 1989) and CIE exposure produces decreased hippocampal viability via over-activation of NMDA receptors during period of withdrawal (Reynolds et al., 2015A), perhaps NMDA receptors may be activated down-stream of IP3 receptors prior to withdrawal to produce the loss of NeuN/Fox-3 observed in the present report. Additionally, the accumulation of CA2+ intracellularly may perturb neuronal integrity via myriad effects unrelated to glutamatergic tone, including mitochondrial swelling.
We further examined the influence of intracellular CA2+ release in mediating hippocampal function using the selective antagonist of the chaperone σ−1 protein BD-1047. Stimulation of these ER-bound, ligand-regulated receptors stabilizes IP3 receptors to prevent proteasome degradation (Hayashi & Su, 2007). Co-application of ethanol and BD-1047 (20 μM) for three consecutive cycles of CIE reversed the loss of NeuN/Fox-3 immunofluorescence in the CA1, CA3, and DG sub-regions of the hippocampal formation. This ability of BD-1047 to mitigate the effects of CIE on NeuN loss is consistent with a prior study conducted by Sabeti (2011) suggesting that CIE exposure blunts intrinsic neuronal plasticity in a σ−1-dependent manner in the adolescent hippocampus. In general, it is unknown whether the ability of BD-1047 to restore hippocampal integrity in the present report is mediated via interactive effects of σ−1 chaperone proteins, NMDA receptors, or mGlu-containing proteins, which are known to be important in producing decreased hippocampal viability in this model (Reynolds et al., 2015B). In one study, application of BD-1047 restored activity-induced changes in synaptic strength (LTP) to basal levels in hippocampal slices withdrawn from a CIE treatment regimen in an NMDA-independent manner (Sabeti & Gruol, 2008). In another study, blocking intracellular CA2+ release increased GluN1 subunit expression in a similar manner to the observed ethanol-associated increases in GluN1 polypeptide levels (Chen et al., 1999). Thus, the role of intracellular CA2+ function in modulating NMDA receptor function is not well understood.
Others have found that NMDA-dependent neuronal firing is mediated via σ−1 receptors of hippocampal pyramidal neurons (Monnet et al., 1990; Monnet et al 1992A) coupled to G-proteins (i.e., Gi/o [Monnet et al 1992B]). In vivo administration of ifenprodil, a selective NMDA-receptor antagonist for the GluN2B complex and σ−1 receptor agonist (Hanner et al., 1996) has been shown to alleviate seizures during periods of ethanol withdrawal (Malinowska et al., 1999; for a review, see Debonnel & de Montigny, 1996). Consistent with this notion, we have previously found that group 1 G-coupled family-proteins (i.e., Gq) promote neuroadaptation to ethanol and withdrawal-associated hippocampal damage following CIE exposure in vitro and physical dependence in vivo (Reynolds et al., 2015B), suggesting a role for ethanol’s activation of Gq-linked polyphosphoinositide signaling prior to CA2+ mobilization from internal stores. In addition, prior studies have demonstrated that BD-1047 administration restored cognitive impairments induced by blocking glutamatergic receptors (Maurice & Su, 2009) and chronic ethanol administration (Meunier et al., 2006), as well as attenuated ethanol-induced conditioned place preference (Maurice et al., 2003) and reduced ethanol consumption (Sabino et al., 2009). Therefore, the influence of IP3 and σ−1 receptors in regulating hippocampal integrity following CIE in the present report may have implications for the development of neurocognitive deficits associated with hippocampal integrity produced by CIE exposure (e.g., impaired cognitive flexibility [Bandanich et al., 2011] and working memory perturbations [Zhao et al., 2013]).
Interestingly, we found that the lower concentration of BD-1047 (20 μM) reversed CIE-induced loss of NeuN in ethanol-treated hippocampi whereas the higher concentration (80 μM) increased levels of NeuN immunofluorescence in ethanol-naïve hippocampi but failed to alter loss of NeuN in ethanol-treated hippocampi. Given that intracellular CA2+ enhances the sensitivity of ethanol on the GluN1-containing NMDA receptors (Mirshahi et al., 1998), the interaction observed in the present report could reflect inherent differences in NMDA receptor subunit stoichiometry produced by ethanol that confers sensitivity to agonist and/or antagonist application, such as fractional CA2+ permeability (Burnashev et al., 1995) though not yet confirmed. By contrast xestospongin application increased levels of NeuN in both ethanol-treated and ethanol-naïve hippocampi. The reasons for these effects are unknown, but likely reflect inherent characteristics of organotypic hippocampal slice culture preparations (i.e., ER stress) (for a review see Berridge et al., 2003; Verkhratsky, 2002), even in the absence of ethanol. For example, the preparation of hippocampal explants itself is known to produce basal neurotoxicity associated with influx of CA2+ (Mayer et al., 2002) likely through activation of glutamatergic mGlu5-containing proteins, NMDA receptors (Mayer et al., 2002; Driscoll et al., 1991), and extracellular glutamate accumulation (Driscoll et al., 1993). In addition, pharmacological blockade of intracellular CA2+ at the ER using antagonists at relatively low concentrations (e.g., 5-di(tert-butyl)-1,4-benzohydroquinone [BHQ]), modestly but significantly enhances survival by ~10% in primary cultures of cerebellar neurons (Mhyre et al., 2000), suggesting a neuroprotective role for blunting CA2+ mobilization in cell culture. The present findings taken together with prior studies, therefore, suggest that blocking intracellular CA2+ release at the ER may protect hippocampal neurons from CA2+-associated loss of NeuN due to preparation and maintenance (i.e., medium-change toxicity) of hippocampal explants.
Taken together, these findings suggest that ethanol mobilizes intracellular CA2+ via ER-bound IP3 and σ−1 receptors in possible modulation of glutamatergic tone to promote withdrawal-associated loss of NeuN/Fox-3. In addition, these ER-bound proteins are involved in regulation of hippocampal pyramidal and granule cell function and perhaps synaptic plasticity. These concomitant alterations in intracellular CA2+ signaling may contribute to the development ethanol dependence in vivo (Veatch & Becker, 2002; 2005) and in the clinical population (Ballenger & Post, 1978; Duka et al., 2004).
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by AA013388 from the National Institute on Alcohol and Alcoholism (NIAAA) awarded to MAP and T32 DA035200 awarded to CRR from the National Institute on Drug Abuse (NIDA).
Role of Funding Source: AA013388, T32 DA035200
LITERATURE CITED
- ALONSO G, PHAN V, GUILLEMAIN I, SAUNIER M, LEGRAND A, ANOAL M & MAURICE T 2000. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience, 97, 155–70. [DOI] [PubMed] [Google Scholar]
- BALLENGER JC & POST RM 1978. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry, 133, 1–14. [DOI] [PubMed] [Google Scholar]
- BADANICH KA, BECKER HC & WOODWARD JJ 2011. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci, 125, 879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE MJ 1993. Inositol trisphosphate and calcium signalling. Nature, 361, 315–25. [DOI] [PubMed] [Google Scholar]
- BERRIDGE MJ, BOOTMAN MD & RODERICK HL 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol, 4, 517–29. [DOI] [PubMed] [Google Scholar]
- BURNASHEV N, ZHOU Z, NEHER E & SAKMANN B 1995. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol, 485 (Pt 2), 403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER TR, SELF RL, SMITH KJ, SHARRETT-FIELD LJ, BERRY JN, LITTLETON JM, PAULY JR, MULHOLLAND PJ & PRENDERGAST MA 2010. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neuroscience, 165, 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN X, MOORE-NICHOLS D, NGUYEN H & MICHAELIS EK 1999. Calcium influx through NMDA receptors, chronic receptor inhibition by ethanol and 2-amino-5-phosponopentanoic acid, and receptor protein expression. J Neurochem, 72, 1969–80. [DOI] [PubMed] [Google Scholar]
- CHEN-ROETLING J, LU X, REGAN KA & REGAN RF 2013. A rapid fluorescent method to quantify neuronal loss after experimental intracerebral hemorrhage. J Neurosci Methods, 216, 128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAPPER DL, WALSETH TF, DARGIE PJ & LEE HC 1987. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem, 262, 9561–8. [PubMed] [Google Scholar]
- DANIELL LC & HARRIS RA 1989. Ethanol and inositol 1,4,5-trisphosphate release calcium from separate stores of brain microsomes. J Pharmacol Exp Ther, 250, 875–81. [PubMed] [Google Scholar]
- DEBONNEL G & DE MONTIGNY C 1996. Modulation of NMDA and dopaminergic neurotransmissions by sigma ligands: possible implications for the treatment of psychiatric disorders. Life Sci, 58, 721–34. [DOI] [PubMed] [Google Scholar]
- DRISCOLL BF, DEIBLER GE, LAW MJ & CRANE AM 1993. Damage to neurons in culture following medium change: role of glutamine and extracellular generation of glutamate. J Neurochem, 61, 1795–800. [DOI] [PubMed] [Google Scholar]
- DRISCOLL BF, LAW MJ & CRANE AM 1991. Cell damage associated with changing the medium of mesencephalic cultures in serum-free medium is mediated via N-methyl-D-aspartate receptors. J Neurochem, 56, 1201–6. [DOI] [PubMed] [Google Scholar]
- DUKA T, GENTRY J, MALCOLM R, RIPLEY TL, BORLIKOVA G, STEPHENS DN, VEATCH LM, BECKER HC & CREWS FT 2004. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res, 28, 233–46. [DOI] [PubMed] [Google Scholar]
- ECKARDT MJ, FILE SE, GESSA GL, GRANT KA, GUERRI C, HOFFMAN PL, KALANT H, KOOB GF, LI TK & TABAKOFF B 1998. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res, 22, 998–1040. [DOI] [PubMed] [Google Scholar]
- GAFNI J, MUNSCH JA, LAM TH, CATLIN MC, COSTA LG, MOLINSKI TF & PESSAH IN 1997. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron, 19, 723–33. [DOI] [PubMed] [Google Scholar]
- HANNER M, MOEBIUS FF, FLANDORFER A, KNAUS HG, STRIESSNIG J, KEMPNER E & GLOSSMANN H 1996. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A, 93, 8072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI T & SU TP 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell, 131, 596–610. [DOI] [PubMed] [Google Scholar]
- KELM MK, CRISWELL HE & BREESE GR 2007. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther, 323, 356–64. [DOI] [PubMed] [Google Scholar]
- KIM FJ, KOVALYSHYN I, BURGMAN M, NEILAN C, CHIEN CC & PASTERNAK GW 2010. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Pharmacol, 77, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM KK, ADELSTEIN RS & KAWAMOTO S 2009. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem, 284, 31052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUZOUKAS DE, LI G, TAKAPOO M, MONINGER T, BHALLA RC & PANTAZIS NJ 2013. Intracellular calcium plays a critical role in the alcohol-mediated death of cerebellar granule neurons. J Neurochem, 124, 323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU F, SCHAFER DP & MCCULLOUGH LD 2009. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods, 179, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVINGER DM, WHITE G & WEIGHT FF 1989. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science, 243, 1721–4. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B, NAPIORKOWSKA-PAWLAK D, PAWLAK R, BUCZKO W & GOTHERT M 1999. Ifenprodil influences changes in mouse behaviour related to acute and chronic ethanol administration. Eur J Pharmacol, 377, 13–9. [DOI] [PubMed] [Google Scholar]
- MAURICE T, CASALINO M, LACROIX M & ROMIEU P 2003. Involvement of the sigma 1 receptor in the motivational effects of ethanol in mice. Pharmacol Biochem Behav, 74, 869–76. [DOI] [PubMed] [Google Scholar]
- MAURICE T & SU TP 2009. The pharmacology of sigma-1 receptors. Pharmacol Ther, 124, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER S, HARRIS BR, GIBSON DA, BLANCHARD JA, PRENDERGAST MA, HOLLEY RC & LITTLETON J 2002. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol Clin Exp Res, 26, 1468–78. [DOI] [PubMed] [Google Scholar]
- MEUNIER J, DEMEILLIERS B, CELERIER A & MAURICE T 2006. Compensatory effect by sigma1 (sigma1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav Brain Res, 166, 166–76. [DOI] [PubMed] [Google Scholar]
- MHYRE TR, MAINE DN & HOLLIDAY J 2000. Calcium-induced calcium release from intracellular stores is developmentally regulated in primary cultures of cerebellar granule neurons. J Neurobiol, 42, 134–47. [PubMed] [Google Scholar]
- MIRSHAHI T, ANDERS DL, RONALD KM & WOODWARD JJ 1998. Intracellular calcium enhances the ethanol sensitivity of NMDA receptors through an interaction with the C0 domain of the NR1 subunit. J Neurochem, 71, 1095–107. [DOI] [PubMed] [Google Scholar]
- MIRONOV SL & HERMANN A 1996. Ethanol actions on the mechanisms of Ca2+ mobilization in rat hippocampal cells are mediated by protein kinase C. Brain Res, 714, 27–37. [DOI] [PubMed] [Google Scholar]
- MIYATAKE R, FURUKAWA A, MATSUSHITA S, HIGUCHI S & SUWAKI H 2004. Functional polymorphisms in the sigma1 receptor gene associated with alcoholism. Biol Psychiatry, 55, 85–90. [DOI] [PubMed] [Google Scholar]
- MONNET FP, BLIER P, DEBONNEL G & DE MONTIGNY C 1992A. Modulation by sigma ligands of N-methyl-D-aspartate-induced [3H]noradrenaline release in the rat hippocampus: G-protein dependency. Naunyn Schmiedebergs Arch Pharmacol, 346, 32–9. [DOI] [PubMed] [Google Scholar]
- MONNET FP, DEBONNEL G & DE MONTIGNY C 1992B. In vivo electrophysiological evidence for a selective modulation of N-methyl-D-aspartate-induced neuronal activation in rat CA3 dorsal hippocampus by sigma ligands. J Pharmacol Exp Ther, 261, 123–30. [PubMed] [Google Scholar]
- MONNET FP, DEBONNEL G, JUNIEN JL & DE MONTIGNY C 1990. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur J Pharmacol, 179, 441–5. [DOI] [PubMed] [Google Scholar]
- MULLEN RJ, BUCK CR & SMITH AM 1992. NeuN, a neuronal specific nuclear protein in vertebrates. Development, 116, 201–11. [DOI] [PubMed] [Google Scholar]
- REYNOLDS AR, BERRY JN, SHARRETT-FIELD L & PRENDERGAST MA 2015A. Ethanol withdrawal is required to produce persisting N-methyl-D-aspartate receptor-dependent hippocampal cytotoxicity during chronic intermittent ethanol exposure. Alcohol, 49, 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS AR, WILLIAMS LA, SAUNDERS MA & PRENDERGAST MA 2015B. Group 1 mGlu-family proteins promote neuroadaptation to ethanol and withdrawal-associated hippocampal damage. Drug Alcohol Depend, 156, 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ FD, LUNDQVIST C, ALLING C & GUSTAVSSON L 1996. Ethanol and phosphatidylethanol reduce the binding of [3H]inositol 1,4,5-trisphosphate to rat cerebellar membranes. Alcohol Alcohol, 31, 453–61. [DOI] [PubMed] [Google Scholar]
- SABETI J 2011. Ethanol exposure in early adolescence inhibits intrinsic neuronal plasticity via sigma-1 receptor activation in hippocampal CA1 neurons. Alcohol Clin Exp Res, 35, 885–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABETI J & GRUOL DL 2008. Emergence of NMDAR-independent long-term potentiation at hippocampal CA1 synapses following early adolescent exposure to chronic intermittent ethanol: role for sigma-receptors. Hippocampus, 18, 148–68. [DOI] [PubMed] [Google Scholar]
- SABINO V, COTTONE P, ZHAO Y, STEARDO L, KOOB GF & ZORRILLA EP 2009. Selective reduction of alcohol drinking in Sardinian alcohol-preferring rats by a sigma-1 receptor antagonist. Psychopharmacology (Berl), 205, 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMONYI A, ZHANG JP, SUN AY & SUN GY 1996. Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. Neuroreport, 7, 2115–8. [DOI] [PubMed] [Google Scholar]
- SMITH KJ, BUTLER TR & PRENDERGAST MA 2010. Inhibition of sigma-1 receptor reduces N-methyl-D-aspartate induced neuronal injury in methamphetamine-exposed and -naive hippocampi. Neurosci Lett, 481, 144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH KJ, BUTLER TR, SELF RL, BRADEN BB & PRENDERGAST MA 2008. Potentiation of N-methyl-D-aspartate receptor-mediated neuronal injury during methamphetamine withdrawal in vitro requires co-activation of IP3 receptors. Brain Res, 1187, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR CW & TOVEY SC 2010. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol, 2, a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEATCH LM & BECKER HC 2002. Electrographic and behavioral indices of ethanol withdrawal sensitization. Brain Res, 946, 272–82. [DOI] [PubMed] [Google Scholar]
- VEATCH LM & BECKER HC 2005. Lorazepam and MK-801 effects on behavioral and electrographic indices of alcohol withdrawal sensitization. Brain Res, 1065, 92–106. [DOI] [PubMed] [Google Scholar]
- VERKHRATSKY A 2002. The endoplasmic reticulum and neuronal calcium signalling. Cell Calcium, 32, 393–404. [DOI] [PubMed] [Google Scholar]
- WEYER A & SCHILLING K 2003. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res, 73, 400–9. [DOI] [PubMed] [Google Scholar]
- ZHAO YN, WANG F, FAN YX, PING GF, YANG JY & WU CF 2013. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav Brain Res, 236, 270–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.