Abstract
Several OCs have been detected in bald eagle (Haliaeetus leucocephalus) nestling (eaglet) plasma in the upper Midwestern United States. Despite frequent and relatively high concentrations of OCs in eaglets, little is understood about potential biological effects associated with exposure. We screened an existing database of OC concentrations in eaglet plasma collected from the Midwestern United States against bioactivity information from the ToxCast database. ToxCast bioactivity information consists of concentrations expected to elicit responses across a range of biological space (e.g. cellular, developmental, etc.) obtained from a series of high throughput assays. We calculated exposure—activity ratios (EAR) by calculating the ratio of plasma concentrations to concentrations available in ToxCast. Bioactivity data were not available for all detected OCs. Therefore, our analysis provides estimates of potential bioactivity for 19 of the detected OCs in eaglet plasma. Perfluorooctanesulfonic acid (PFOS) EAR values were consistently the highest among all study areas. Maximum EAR values were ≥1 for PFOS, perfluorononanoic acid, and bisphenol A in 99.7, 0.53 and 0.26% of samples, indicating that some plasma concentrations were greater than what may be expected to elicit biological responses. About 125 gene targets, indicative of specific biological pathways, were identified as potentially being affected. Inhibition of several CYP genes, involved in xenobiotic metabolism, were most consistently identified. Other identified biological responses have potential implications for motor coordination, cardiac functions, behavior, and blood circulation. However, it is unclear what these results mean for bald eagles, given that ToxCast data are generated using mammalian-based endpoints. Despite uncertainties and limitations, this method of screening environmental data can be useful for informing future monitoring or research focused on understanding the occurrence and effects of OCs in bald eagles and other similarly-positioned trophic species.
Keywords: perfluorinated chemicals, ToxCast, plasma, in-vitro bioactivity
Capsule:
Comparison of organic contaminant concentrations in bald eaglet plasma to bioactivity data from in-vitro bioassays indicates that PFOS, CYP-related endpoints, and sites near urban areas should be prioritized for future monitoring focused on understanding the effects of organic contaminant exposure in bald eagles.
Introduction
The presence of organic contaminants (OCs) in aquatic environments has been widely documented (Ahrens 2011; Dykstra et al. 2010; Elliott and VanderMeulen 2017; Hites 2006; Loos et al. 2009). Furthermore, many OCs, including legacy and current use, have the potential to elicit biological effects in exposed organisms. There is evidence that some OCs accumulate in organisms such as mussels (Booij et al. 2002; Dodder et al. 2014; Kimbrough et al. 2009) and fish (Flanagan Pritz et al. 2014; Stahl et al. 2014; Zhang et al. 2013) leading to potential transfer up the food chain to higher trophic organisms such as bald eagles (Haliaeetus leucocephalus). In fact, several current-use OCs, such as perfluorinated chemicals (PFCs) and flame retardants have been detected in bald eagle nestling (eaglet) plasma (Dykstra et al. 2010; Route et al. 2014a; Route et al. 2014b; Venier et al. 2010) throughout the upper Midwestern United States. Bald eagle nestlings are particularly useful indicators of local contamination because they are fed from a relatively small territory (1–2 km2; Stalmaster, 1987).
Effects of exposure to legacy OCs, such as DDT, DDE, and PCBs, are widely known and include eggshell thinning and developmental deformities (e.g. Bowerman et al. 2003; Gilbertson and Morris 1976; Kozie and Anderson 1991). Productivity (number of young produced) of bald eagles was inversely correlated with DDE and PCB concentrations in eggs (Wiemeyer et al. 1984) and eaglet blood plasma (Bowerman et al. 2003). With the ban of DDT, many bird populations began to recover during the 1980’s and 1990’s. Furthermore, with declining concentrations of PCBs and other organochlorine chemicals, the relationships between concentrations and reproductive success largely disappeared (Donaldson et al. 1999). Although many bird populations recovered, recovery was not uniform throughout the Great Lakes region (Bowerman et al. 2003; Dykstra et al. 1998; Grim and Kallemeyn 1995; Kozie and Anderson 1991), for reasons that are not fully understood. Productivity and reproductive success appear to be increasing or stable, but OCs are still present in the environment with the potential to pose a hazard to the health of bald eagles.
While there is some information on the biological effects of OCs on organisms using traditional laboratory exposure methods, data currently include a very limited number of current-use chemicals. Furthermore, testing methods and endpoints are not consistent among chemicals making it difficult to compare the relative hazard from different OCs. The U.S. Environmental Protection Agency’s (USEPA) ToxCast database contains bioactivity data (chemical concentrations expected to affect specific gene targets or biological pathways) from a consistent set of assays for thousands of OCs. Data are generated using high-throughput in-vitro assays and provide pathway-specific responses of screened chemicals. Although the assays are primarily targeted toward mammalian processes, the ToxCast database provides an efficient way to screen for potential biological responses in other organisms and provides context for the relative hazards of chemicals found in the environment (Kavlock et al. 2012). Using chemical-endpoint interaction data from ToxCast, initial screening can be used to prioritize future research or management activities to better understand or help mitigate the potential hazards associated with exposure to harmful chemicals. For example, Blackwell et al. (2017) used ToxCast bioactivity data to screen environmental concentrations of various OCs in water samples, providing an indication of which chemicals presented the greatest relative hazard to aquatic biota health, which biological pathways were more likely affected, and which of the sampled sites warrant more study. Use of the ToxCast database in this manner provides an efficient method for screening environmental data for a large number of chemicals in a consistent manner.
Our study evaluated an existing database of OC concentrations in bald eaglet plasma using bioactivity data from USEPA’s ToxCast database to assess potential biological responses from OC exposure in the upper Midwestern United States. Our objective was to provide context for previously reported OC data by screening those data against concentrations suspected to affect specific biological processes. Although some studies have used ToxCast data to screen environmental samples such as water or sediment, few have screened concentrations of contaminants in plasma using this approach. Plasma contaminant concentrations allow for a direct comparison of contaminant burdens with the ToxCast data, removing uncertainties associated with potential trophic transport or biomagnification of OCs from sediment or water. This screening can be used to prioritize future monitoring efforts by identifying the relative importance of: (1) OCs posing a greater hazard to bald eagle health, (2) specific biological responses that may be elicited from OC exposure, and (3) sites where bald eagle health may be more threatened by exposure to OCs. This information can also be used to guide management activities focused on monitoring and minimizing the presence of OCs near areas with active bald eagle nests. For example, identification of a particular OC that may pose a hazard to eagle health could lead to actions that focus on reducing the loading of that OC to the environment.
Methods
Details regarding initial study design and results for most chemical data can be found in Dykstra et al. (2010) and Route et al. (2014a, 2014b). Results not previously reported in the aforementioned papers are provided in Supplementary Information. A brief description of methods follows to provide context for the current analysis.
Study Areas
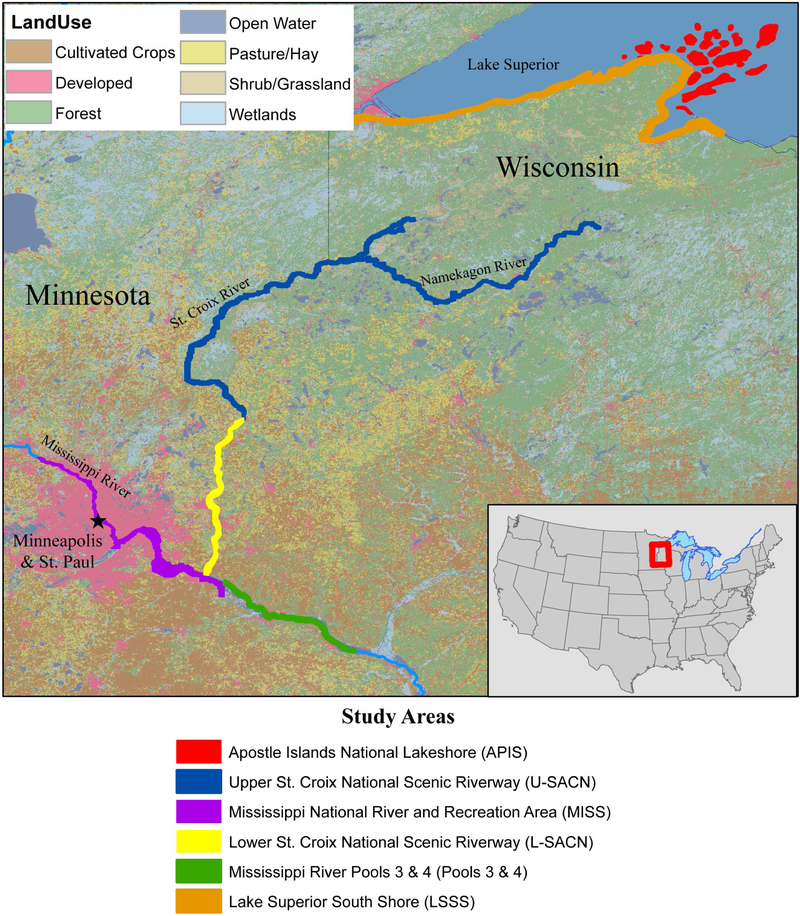
Eaglet plasma was collected from one eaglet at 159 eagle nests (sites) located within six study areas in the Upper Midwestern United States (Fig. 1, Tables 1 and S1) from 2006 to 2015. Several sites (95 of 159, or 60%) were sampled in multiple years (Table S1). From 2006 to 2015, eaglets were sampled at four core study areas: Apostle Islands National Lakeshore (APIS), the upper St. Croix National Scenic Riverway (U-SACN), lower St. Croix National Scenic Riverway (L-SACN), and Mississippi National River and Recreation Area (MISS). Additional resources allowed for sampling along Wisconsin’s Lake Superior South Shore (LSSS) in 2007 and 2008 and downstream from MISS in Pools 3 and 4 of the Mississippi River (Pools 3&4) in 2008 and 2009. Study areas were located within or adjacent to U.S. national parks and were chosen to represent a gradient of land-use characteristics. Because of the proximity of L-SACN, MISS, and Pools 3&4 to the Minneapolis-St. Paul metropolitan area, these study areas are strongly influenced by activities that accompany developed land use such as wastewater treatment plants, urban stormwater runoff, and other direct point sources (e.g. stormwater outfalls, industrial discharges, etc.) (Tables 1 and S1). Land use in the other study areas is mostly forested with some agriculture. However, APIS and LSSS may also be under the influence from the port cities of Duluth, Minnesota and Superior, Wisconsin. Point sources may also contribute to atmospheric loading of some OCs providing a mechanism for transport and dispersion across a broader geographic area.
Figure 1.
Study areas where bald eaglet plasma was collected during 2006–2015.
Table 1.
Summary of select landscape characteristics and spatial distribution of eaglet plasma samples collected throughout the upper Midwestern United States, 2006–15. NPDES, National Pollutant Discharge Elimination System
| Study Area | Average distance to nearest city (population ≥10,000), kilometers |
Average distance to nearest NPDES discharge, kilometers |
Number of sites sampled |
Number of samples collected |
|---|---|---|---|---|
| Apostle Islands National Lakeshore (APIS) | 107.6 | 13.6 | 27 | 58 |
| Lake Superior South Shore (LSSS) | 73.8 | 4.7 | 15 | 16 |
| Upper St. Croix Scenic Riverway (U-SACN) | 72.7 | 11.2 | 31 | 65 |
| Lower St. Croix Scenic Riverway (L-SACN) | 14.2 | 4.4 | 22 | 64 |
| Mississippi National River and Recreation Area (MISS) | 5.3 | 1.8 | 43 | 145 |
| Mississippi River Pools 3 & 4 (Pools 3&4) | 13.7 | 2.9 | 21 | 33 |
| Totals | 159 | 381 | ||
Sample Collection and Analysis
Plasma samples (n=381) were collected from one five- to nine-week old eaglet at each site and analyzed for various contaminants. Sampling and analytical effort varied throughout the study dependent on available funding (Table 1, Table S1). Although analytical effort varied, the primary targeted OCs were 84 polychorinated biphenyl (PCB) congeners, 16 perfluorinated compounds (PFCs), 17 polybrominated diphenyl ether (PBDE) congeners, and DDT and its metabolites, DDE and DDD. Beginning in 2010, a suite of current-use OCs, including bisphenol A (BPA), octylphenol (OP), 4 phthalates, triclosan/triclocarban, and several more pesticides (e.g. chlordane, dieldrin, nonachlor) were added. All samples were analyzed at the Wisconsin State Laboratory of Hygiene. Primary targeted OCs were determined using gas chromatography/mass spectrometry (PBDEs, DDT, DDE, DDD, PCBs, nonachlor, and chlordane) or liquid chromatography/tandem mass spectrometry (PFCs) methods. Analytical methods for determination of primary targeted OCs are detailed in Dykstra et al. (2010) and Route et al. (2014a, 2014b). Current-use OCs were determined using a high-performance liquid chromatography-triple quadrupole mass spectrometric method based on methods detailed in Silva et al. (2003), USEPA (1995, variously dated), and Ye et al. (2008). A volume of 0.5 mL serum was buffered to low pH followed by liquid/liquid extraction with methyl tert-butyl ether. Current-use OCs were extracted on a Phenomenex Strata-X column and eluted using methanol:acetonitrile (1:1) containing 1% acetic acid, evaporated to dryness and reconstituted in 100 μL methanol. Contaminants were then separated using binary gradient elution reversed phase chromatography and analyzed using a triple quadruple mass spectrometer in the atmospheric pressure chemical ionization negative ionization mode.
Quality Assurance/quality control
Laboratory matrix-spike samples were analyzed with environmental samples to monitor method performance (Table S2). With few exceptions, percent recovery of most OCs fell within 70–130%. PCB3 had consistently low percent recoveries (31–68%), perfluorododecanoic acid, perfluorotridecanoic acid, and perfluorotetradecanoic acid had at least one high (>200%) recovery, and percent recovery of isononylphenol was 150% in 2014. Concentrations were not corrected for recovery for this analysis.
ToxCast database
The ToxCast database is a publicly accessible database containing high-throughput screening data for over 9,000 unique chemicals. Chemicals tested in ToxCast come from a single source and are prepared and screened in a consistent, standardized manner (Richard et al. 2016), greatly increasing the comparability of data between assays. All chemicals are analyzed in dose-response, allowing for point-of-departure estimates to be determined for each chemical-assay pair, and chemicals can be ranked in terms of relative potency within a given assay. The current version of the database (v2, October 2015) (USEPA 2015) includes 12 assay batteries encompassing cell-free, biochemical-based in vitro assays, cell-based in vitro assays, and high-throughput whole organism assays (USEPA 2015). ToxCast assays cover a range of biological space, including nonspecific endpoints (cytotoxicity, oxidative stress, cell morphology), and endpoints associated with over 200 unique signaling pathways (e.g. aryl hydrocarbon receptor, androgen receptor, pregnane X receptor). The bioactivity data can be prioritized by specific intended gene targets or broader intended target families (groups of gene targets related to similar biological pathways) to assess potential biological responses. Data in ToxCast are obtained by testing mammalian cells but can be used to provide context for environmental data and an indication of the types of biological responses that may be expected in other biota.
Data Analysis
Comparisons of OC concentrations to bioactivity data available from ToxCast were analyzed in R (v.3.4.0; R Core Team 2015) using the toxEval package (DeCicco et al. 2018). Plasma concentrations were compared against activity cutoff concentration (ACC) values obtained from the ToxCast database (USEPA 2015) to calculate exposure—activity ratios (EAR). The ACC represents the concentration at which a threshold of response is achieved from in vitro tests and was used because it is uniform for all chemicals tested within an assay (Blackwell et al. 2017). Data originating from Apredica and Bioseek assays were excluded from this analysis because they target mostly nonspecific endpoints that we concluded would not be beneficial for evaluating specific biological responses to OCs in bald eagles. Assays classified as “background measurement” were also excluded from analysis for a similar reason. Individual endpoints are annotated by ‘intended target family’ (ITF) in ToxCast, which can be used to group endpoints according to function. We included all ITFs available in ToxCast in our analysis, except for ‘zebrafish’ and ‘undefined’ because we determined them to be unrepresentative for this analysis. Additionally, PCB congener pairs and triplets are not represented in the current version of ToxCast, so were removed from the dataset prior to comparisons with bioactivity data.
Data quality flags indicating false positive OC-endpoint matches in ToxCast were used to further filter the dataset for our analysis. EAR values calculated with an ACC containing any of the following flags were excluded from analysis: only highest concentration above baseline (baseline here refers to the noise of an assay, Filer et al. 2016), active; only one concentration above baseline, active; noisy data; borderline active; gain AC50 < lowest concentration & loss AC50 < mean concentration; and hit-call potentially confounded by overfitting. Several OC-endpoint matches resulted in relatively high (>100) EAR values. The dose-response curves associated with these OC-endpoint matches were examined to assess if the curve followed a logical dose-response relationship. We determined the dose-response curves associated with the high EAR values to be of sufficient quality to include in analysis.
Summary statistics of EAR values were calculated: number of OC-endpoint matches, minimum EAR (EARmin), average EAR (EARmean), and maximum EAR (EARmax). EAR values associated with the same assay within a given sample were summed to provide an indication of total biological response to chemical mixtures (EARmix). This method assumes simple mixture additivity of detected chemicals (i.e. individual chemicals may cause the same biological response). Additionally, a total EAR (EARTot) was calculated for each eaglet sample to identify sites with the greatest overall potential to elicit biological responses. This was calculated by summing EAR values for every chemical-endpoint match identified in each eaglet.
Results and Discussion
Organic contaminant presence
A summary of chemical concentrations for all OCs detected in at least one sample is included in Table S3. Of the detected OCs, 19 (including congeners) had associated bioactivity data in ToxCast and will be the focus of the following discussion (Tables 2, S3). Detected concentrations of the 19 OCs screened against ToxCast ranged from 0.83 (PCB187) to 4,200 (perfluorooctanesulfonic acid or PFOS) μg/L. The greatest concentrations were generally detected for PFCs; PFOS and perfluorononanoic acid (PFNA) were detected at a maximum of 4,200 and 160 μg/L, respectively. Maximum concentrations of PBDEs, phthalates, PCBs, and other PFCs were 20, 22, 5.3, and 110 μg/L, respectively. For comparison, Venier et al. (2010) reported a maximum PBDE concentration of 6.96 μg/L in bald eagle plasma collected across several Great Lakes, substantially lower than observed in our study. Concentrations of total PCBs in plasma detected in our study were often within ranges reported in bald eagles across several Great Lakes (Bowerman et al 2003; Venier et al 2010). In general, OCs were detected more often and in greater concentrations in eaglet plasma collected near urban centers. Specifically, high PFC concentrations reflected known contamination plumes in the Minneapolis-St. Paul metropolitan area where high concentrations have been detected in groundwater, surface water, birds and fish (Custer et al. 2010; Monson 2013; Oliaei et al. 2013; Route et al. 2014b; Yingling 2015).
Table 2.
Select summary statistics for the 19 organic contaminants detected in bald eaglet plasma samples from the upper Midwestern United States that were screened against available bioactivity information in ToxCast. Concentrations are micrograms per liter.
| Chemical | Minimum concentration |
Median concentration |
Maximum concentration |
|---|---|---|---|
| Flame retardant | |||
| PBDE99 | 0.12 | 1.1 | 15 |
| PBDE47 | 0.19 | 3.5 | 20 |
| Alkylphenol | |||
| 4-tert-octylphenol | 2.95 | 2.95 | 2.96 |
| Phthalate | |||
| Bis(2-ethylhexyl) tetrabromophthalate | 0.4 | 0.74 | 1.6 |
| Mono(2-ethylhexyl) phthalate | 0.27 | 1.2 | 22 |
| Antioxidant | |||
| Bisphenol A | 0.53 | 1.05 | 5.8 |
| Paraben | |||
| Butylparaben | 0.27 | 0.27 | 0.28 |
| Propylparaben | 0.51 | 0.73 | 3.57 |
| Pesticide | |||
| Dieldrin | 0.5 | 0.945 | 5.7 |
| Polychlorinated biphenyl | |||
| PCB187 | 0.83 | 1.55 | 5.3 |
| Perfluorinated compound | |||
| Perfluorodecanoic acid | 1.1 | 15 | 85 |
| Perfluoroheptanoic acid | 0.12 | 0.18 | 6.6 |
| Perfluorohexanoic acid | 0.13 | 4.9 | 5.7 |
| Perfluorononanoic acid | 0.82 | 3.7 | 160 |
| Perfluorooctanoic acid | 0.12 | 0.5 | 14 |
| Perfluorooctanesulfonic acid | 7.5 | 335 | 4,200 |
| Perfluoroundecanoic acid | 1.1 | 7.9 | 110 |
| Antimicrobial | |||
| Triclocarban | 0.27 | 0.31 | 0.58 |
| Triclosan | 0.82 | 0.95 | 1.8 |
Organic contaminant prioritization
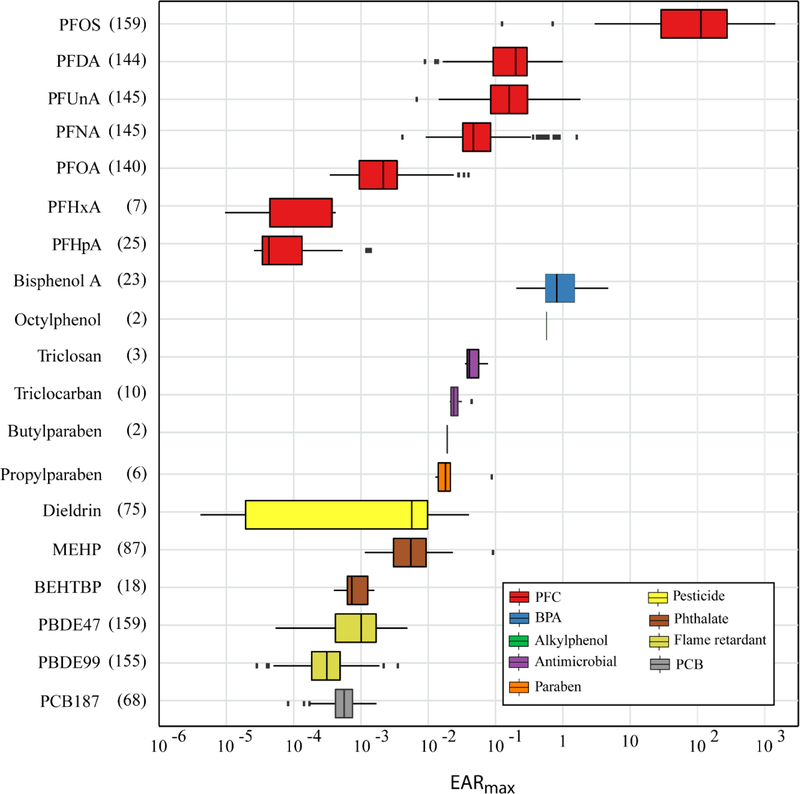
EARmax values for individual OC-endpoint matches ranged from 0.0004 (perfluorohexanoic acid or PFHxA) to 907 (PFOS; Table S4) across all samples. Individual EARmax values were orders of magnitude <1 for 16 of the 19 (84%) OCs. EARmax values for PFHxA and perfluoroheptanoic acid (PFHpA) were consistently low, compared to the other OCs (Fig. 2). However, EARmax was >1 for PFOS, BPA, and PFNA, indicating that plasma concentrations were greater than ACC concentrations in at least one sample at some sites. PFOS consistently had the highest EAR values among the OCs for which chemical-endpoint interaction data exist. Although analyzed in relatively few samples (n=24 among APIS, USACN, L-SACN, and MISS), BPA concentrations resulted in EARmax values similar to those observed for many of the PFCs. Several other OCs had EARmax values that were ≥10% of at least one ACC [e.g. perfluorodecanoic acid = 0.37, perfluoroundecanoic acid (PFUnA) = 0.30, OP = 0.14], indicating that focused work on these specific OCs may be warranted to more fully understand their distributions and potential hazards to eagle health. Taking into account all OC-endpoint matches for a given chemical within a sample, three OCs stand out because the total EARmax is >1: PFOS, PFUNA, and BPA (Fig. 2).
Figure 2.
Chemical summaries of maximum exposure—activity ratios (EARmax) for organic contaminants detected in eaglet plasma collected from six study areas in the upper Midwestern United States, 2006–2015. Values represent the maximum of the sum of EAR for all organic contaminant-endpoint matches for a given chemical within a sample. Numbers in parentheses after chemical name represent the number of sites (eagle nests) at which the chemical was detected. Boxplot whiskers extend to the smaller of the maximum value and 1.5 times the interquartile range, and the larger of the minimum value and 1.5 times the interquartile range. Individual points represent values beyond the ends of the whiskers. PFC, perfluorinated chemical; BPA, bisphenol A; PCB, polychlorinated biphenyl.
Based on EAR magnitudes among the different OCs, PFCs, and in particular PFOS, stand out as potentially the most hazardous to bald eagles. Furthermore, the actual hazard from total PFCs may be greater than what our analysis shows because numerous PFCs that were detected in our study have not been tested in the ToxCast program. Relatively high (>1) EAR values associated with PFOS were observed in eaglet plasma across the entire study area. However, the highest values mostly occurred at sites near the Minneapolis-St. Paul urban area (Table S5) where PFCs are produced. High PFOS concentrations in water, sediment, fish, birds, and other biota have been documented in this area, as well as the dominance of PFOS in relation to detected PFCs (Custer et al. 2010; Custer et al. 2012; Delinsky et al. 2010; Oliaei et al. 2013; Route et al. 2014b). Specifically, Pool 2 in the Mississippi River (within our MISS study area) is known to be a hot spot for PFOS, though concentrations appear to be decreasing over time (Monson 2013; Route et al. 2014b). Additionally, similar mean concentrations of ∑PFCs were detected in tree swallow nestling plasma across the Great Lakes basin (Custer et al. 2017), but no association between PFC concentrations and egg failure was observed (Custer et al. 2012). PFOS concentrations in 7 samples (5 from MISS, 2 from L-SACN) exceeded an avian toxicity reference value of 1,700 μg/L in plasma, a value deemed to be protective of a tertiary avian predator based on gross pathological effects (Newsted et al. 2005). Although relatively few plasma concentrations exceeded this toxicity reference value, exposure to PFCs can cause sub-lethal effects. For example, hepatic PFOS concentrations (mean range 54 to 81 ng/g wet weight) were negatively correlated with expression of genes associated with molecular chaperones, ribonucleic acid (RNA) processes, and carbohydrate transport and metabolism in common cormorants (Nakayama et al. 2008). Similarly, a concentration of 1,500 μg/L caused 0.5 to 2-fold changes in some thyroid gene expression in exposed chicken cells (Vongphachan et al. 2011). Other PFCs such as perfluorobutanesulfonic acid (PFBS), PFHxA, and perfluorohexanesulphonic acid (PFHxS) caused more than two-fold changes in thyroid gene expression at the same concentration. Comparable results were observed for herring gull cells exposed to similar concentrations of perfluorobutyrate, PFBS, PFHxA, PFHxS, PFHpA, and perfluoroheptanesulfonate (Vongphachan et al. 2011), all of which were detected in eaglet plasma in our study.
Similar to PFCs, PCBs and PBDEs are known to frequently occur in the environment and have frequently been detected in biota (Chen and Hale 2010; Wenning et al. 2011; Van Ael et al. 2012). However, bioactivity information for many PCB and PBDE congeners included in this study is limited in the ToxCast database. For example, only 2 of 54 PCBs and 2 of 10 detected PBDEs had available information. A total of 53 PCBs (including congener pairs and triplicates) were detected in at least half of all samples. Considering the frequent detection of PCBs and limited bioactivity information in ToxCast, this analysis potentially greatly underestimates the hazard of PCB exposure to eagles. Because PCBs are legacy chemicals, their occurrence in the environment and potential effects have been fairly well studied (Bowerman et al. 2003; Gilbertson and Morris 1976). Reduced nest site attentiveness was observed in glaucous gulls (Larus hyperboreus) with ∑PCB concentrations in blood as low as 50 μg/kg (Harris and Elliott 2011). Geometric means of ∑PCBs in bald eaglet plasma assessed as part of our study were >50 μg/kg at LSSS, MISS, and L-SACN (Dykstra et al. 2010). Given evidence of frequent exposure, more information related to potential sub-lethal effects from exposure would be valuable for assessing eagle health.
Although there have been mixed results, PBDEs, and their hydroxylated forms, have been implicated in the interference of thyroid circulation in bald eagles (Cesh et al. 2010). Other biological effects such as steroid hormone and retinol production show varied responses among species exposed to PBDEs and other similar flame retardants (Guigueno and Fernie 2017). Additionally, patterns in P450 EROD activity among groups of terns generally followed patterns of contaminant concentrations such as ∑PCBs and ∑PBDEs (i.e. groups with higher contaminant concentrations exhibited greater P450 activity) (Herring et al. 2010). Although results from laboratory exposures of birds to PCBs and PBDEs show inconsistent biological responses, reduced antibody-mediated responses in American kestrels (Falco sparverius) exposed to PBDEs (Fernie et al. 2005) and adverse effects on cardiac development in domestic chickens (Carro et al. 2013) have been observed. The frequent occurrence of PCBs and PBDEs in bald eaglets combined with the limited availability of data related to biological responses in bald eagles and other birds suggests these chemicals should be a priority for research to better understand the potential biological effects resulting from exposure.
Several known or suspected endocrine disruptors (e.g. BPA, OP, phthalates) were detected. Of these, our analysis shows that the relative order in terms of highest potential for eliciting biological responses is BPA>OP>mono(2-ethylhexyl) phthalate>bis(2-ethylhexyl) tetrabromophthalate. The EARmax for BPA was >1 for at least one assay endpoint. Biological effects (e.g. impaired growth and development, reduced thyroid hormone production, impaired reproduction, etc.) of exposure to these OCs have been documented in other vertebrates such as fish, humans, and other birds (e.g. Boas et al. 2010; Flint et al. 2012; Mankidy et al. 2016).
Biological response prioritization
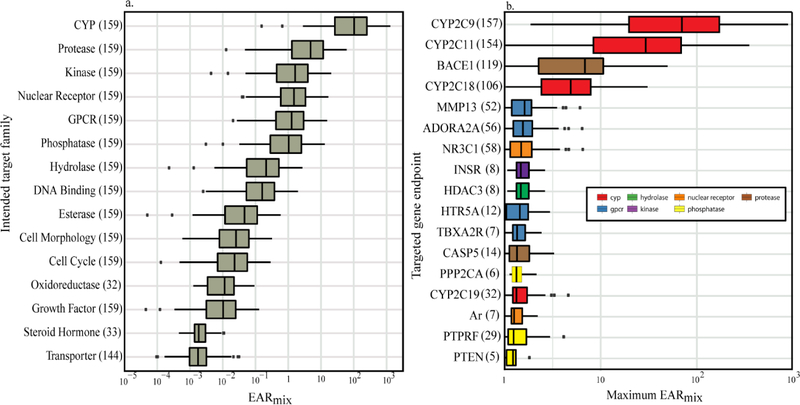
Values of EARmix were used to assess potential biological responses. These values represent exposure to OC mixtures by accounting for every detected chemical associated with a specific endpoint (EAR>0). A total of 125 gene targets were identified as potentially being affected by detected OCs in this study with EARmix values ranging from <0.0001 to 908 (Fig. 3). The following discussion will focus on the 29 gene targets with associated EARmix ≥1 for brevity (Table S5).
Figure 3.
Summaries of mixture exposure—activity ratios (EARmix) for organic contaminants detected in eaglet plasma collected from six study areas in the upper Midwestern United States, 2006–2015. Mixture EAR was calculated by summing the calculated EAR for every chemical identified as potentially affecting specific (a) intended target families and (b) targeted gene endpoints. For brevity, Figure 3b only shows identified gene targets with an associated EARmix ≥1 and sample size ≥5. Numbers in parentheses after target family or endpoint name represent the number of sites (eagle nests) affected. Boxplot whiskers extend to the smaller of the maximum value and 1.5 times the interquartile range, and the larger of the minimum value and 1.5 times the interquartile range. Individual points represent values beyond the ends of the whiskers.
Processes related to several of the cytochrome P450 (CYP) genes were identified as potentially being inhibited; the highest observed EARmix values were associated with CYP gene targets (Fig. 3, Table S5). CYP genes can be related to metabolism of xenobiotic substances (Watanabe et al. 2013) and catalyzation of endogenous steroids in birds (Tsutsui et al. 2013). Although the CYP genes identified in this analysis are associated with the former, other CYP genes are not specifically covered by the available assays. Our results indicate potential for inhibited metabolism of foreign substances in eagles within the study area because of exposure to the measured OCs. Although some evidence suggests no clear orthologous (evolved from a common ancestor, separated by a speciation event, but retains the same function) relationship between bird and human CYP2C genes, these genes are not well-characterized in most birds (Watanabe et al. 2013). Inhibition of other CYP genes (e.g. CYP7B) can lead to reduced reproductive success in birds as a result of decreased sexual behavior (Tsutsui et al. 2013). Though it is unclear if this is the case for the eaglets sampled in this study, this information could be used to prioritize future monitoring efforts focused on identifying biological responses to validate these results.
The BACE1 endpoint was also often identified as potentially being inhibited, with both a high number of OC-endpoint matches and relatively high EARmix values. BACE1 is typically associated with the formation of Alzheimer’s in humans (Dominguez et al. 2005). Although it is unclear what this might mean for other vertebrates, such as eagles, hyperactivity (Dominguez et al. 2005) or more adventurous activity (Harrison et al. 2003) was observed in mice expressing BACE1 compared to those not. Other evidence suggests that BACE1 may be required for formation and maturation of muscle spindles, so inhibition may result in alterations to motor coordination (Cheret et al. 2013).
Four G-protein-coupled receptors (GPCRs; ADORA2A, HTR5A, HTR7, and TBXA2R) were the second most commonly identified target family based on EAR magnitudes. These endpoints are associated with functions such as cardiac rhythm and circulation, blood flow, platelet aggregation, and behavioral functions. There is some evidence of ADORA and HTR7 being conserved in chickens, however an ortholog for TBXA2R is lacking (Lagerström et al. 2006).
Other gene targets with EARmix>1 represent biological pathways associated with steroidal [androgen receptor (AR), glucocorticoid receptor (GR)] and non-steroidal [pregnane-X receptor (PXR) and peroxisome proliferator activated receptor gamma (PPARG)] nuclear receptors, proteases [matrix metallopeptidase 13 (MMP13)], and phosphatases [protein tyrosine phosphatase receptor type F (PTPRF)]. Inhibition of some of these gene targets may result in behavioral, skeletal, or reproductive responses. For example, there is some evidence that AR plays a regulatory role in aggressive behavior in male song sparrows during the pre-breeding season (Sperry et al. 2010), in which an AR antagonist slightly decreased the number of flights associated with aggressive displays. These results indicate that birds exposed to chemicals that affect the AR may be outcompeted for nesting territory or mates because they are less likely to confront other males. MMP13 plays a role in recovery from tibial dyschondroplasia (a skeletal abnormality in birds) through its role in vascularization and ossification processes (Asawakarn and Asawakarn 2012). Therefore, inhibition could result in longer recovery times or other skeletal abnormalities which may affect the ability of eagles to participate in certain behaviors. Although PTPRF has not been widely studied in birds, inhibition in human cells has been linked to proliferation of tumor growth (Bera et al. 2014).
Site prioritization
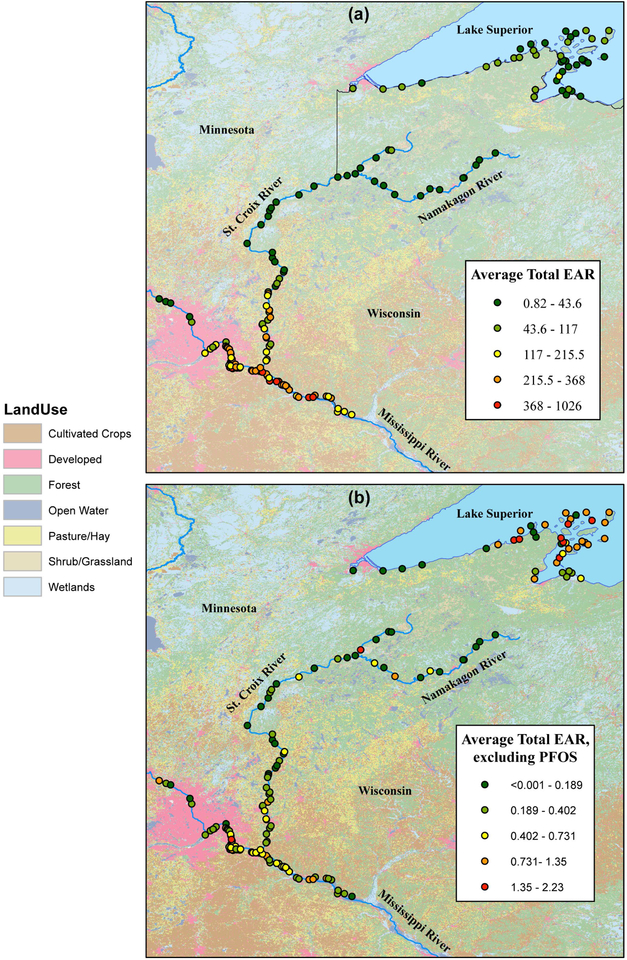
To prioritize sites where eagles may be more affected by exposure to the OCs included in our analysis, we used EARTot, which sums EAR values from all OC-endpoint matches identified in each sample. Average values of EARTot ranged from 0.8 to 1,026. Sites with relatively high EARTot values were consistently high across most samples collected at that site in different years (e.g. WH-012, WH-TX05), indicating that the eagles are being continuously exposed to OCs at these sites over extended periods of time. All but two sites (AS-106 and AS-TX-09, both in APIS) had average EARTot ≥1 (Table S6). Furthermore, 77, 88, and 91% of sites located within L-SACN, MISS, and Pools 3+4 had average EARTot≥100.
Because PFOS was so prevalent throughout the study area, we also calculated EARTot excluding PFOS (EARTot-NoPFOS; Table S6) to explore the underlying variability in the rest of the chemical data. Values for EARTot-NoPFOS exhibited greater heterogeneity within study areas, compared to EARTot, indicating that, for most OCs, contamination may be more site specific and related to local sources. Excluding PFOS substantially reduced average EAR values (EAR ranged from 8.9E-5 to 5.2) however, average EAR values for 21 (13%) of the sites was still ≥1. Almost half (44%) of the sites with EARTot-NoPFOS≥1 were located within APIS (Table S6, Fig. 4b). Additionally, two sites within U-SACN had average EARTot-NoPFOS≥1. In consideration of EARTot, sites within U-SACN were ranked low compared to the rest of the study areas. Using EARTot-NoPFOS values, the overall pattern of higher EAR values within L-SACN, MISS, and Pools 3+4 compared to the other study areas essentially reverses (Fig. 4). Although several sites within the more affected study areas may be given high priority, now sites within APIS and a few within U-SACN would be ranked higher on a priority list for further monitoring. When excluding PFOS, 18 (66%) APIS sites had EARTot-NoPFOS>1, the highest percentage of the six study areas. This was largely driven by BPA, PFNA, and PFUnA EAR values.
Figure 4.
Average total exposure—activity ratios (EAR) (a) including PFOS, and (b) excluding PFOS in bald eaglet plasma samples collected from six study areas in the upper Midwestern United States, 2006–2015. Total EAR values represent all chemicals detected within a sample for which activity concentrations exist.
There was little variation in EARTot values among sites within a study area, indicating that eagles within a given study area are exposed to similar chemical profiles. Despite the low intra-area variability, we found evidence of high inter-area variability indicating that, because of different chemical profiles among sites, the hazards of OC exposure are not similar among different populations (Table S6, Fig. 4). Other researchers have documented the importance of contaminant profile heterogeneity among freshwater systems as factors influencing which OCs may bioaccumulate (Elliott et al. 2009).
Conclusions
We evaluated the potential sub-lethal biological responses of 19 OCs detected in bald eaglet plasma by using bioactivity information (chemical concentrations expected to elicit biological responses) from the USEPA ToxCast database. Concentrations of most OCs were substantially lower than concentrations expected to affect biological processes, but PFOS, PFNA, and BPA concentrations were above activity concentrations in at least one sample. Biological processes such as metabolism, behavior, development, and cardiac functions were identified as potentially being affected by OCs present in bald eaglet plasma. Some patterns emerged pointing to site-specific exposure differences, indicating the importance of local point sources, particularly with respect to PFOS. Although our analysis indicates that among the OCs tested PFOS should be given high priority for future monitoring, this screening was limited by the availability of contaminant occurrence data and bioactivity information in ToxCast. For example, bioactivity information for PCBs and PBDEs was sparse, potentially underestimating the overall hazard to eagle health. Additionally, it must be noted that identified biological responses do not necessarily mean there will be an observed effect. Further research needs to be conducted to assess how and if the identified alterations in biological activity result in gross health effects. Nonetheless, this screening provides information about expected biological responses from specific chemicals. The information can be used to prioritize chemicals and sites for future monitoring or research efforts focused on understanding the effects of OC exposure on bald eagles. This information could ultimately guide management efforts to mitigate OC influence on bald eagles.
Supplementary Material
Acknowledgements
This work was supported by the National Park Service’s Great Lakes Inventory and Monitoring Network with additional funds from the Great Lakes Restoration Initiative, and the Minnesota Pollution Control Agency. The views expressed in this article do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
- Ahrens L, 2011. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. J. Environ Monit 13:20–31. 10.1039/C0EM00373E. [DOI] [PubMed] [Google Scholar]
- Asawakarn S, Asawakarn T, 2012. Role of matrix metalloproteinases in animals. Thai Journal of Veterinary Medicine 42:137–42. [Google Scholar]
- Bera R, Chiou C-Y, Yu M-C, Peng J-M, He C-R, Hsu C-Y, Huang H-L, Ho UY, Lin S-H, Lin Y-J, Hseih S-Y, 2014. Functional genomics identified a novel protein tyrosine phosphatase receptor type F-mediated growth inhibition in hepatocarcinogenesis. Hepatology 59:2238–50. 10.1002/hep.27030. [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Ankley GT, Corsi SR, DeCicco LA, Houck KA, Judson RS, Li S, Martin MT, Murphy E, Schroeder AL, Smith WR, Swintek J, Villeneuve DL, 2017. An ‘EAR’ on environmental surveillance and monitoring: A case study on the use of exposure-activity ratios (EARs) to prioritize sites, chemicals, and bioactivities of concern in Great Lakes Waters. Environ Sci Technol 51:8713–24. 10.1021/acs.est.7b01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebæk NE, Hegedüs L, Hilsted L, Juul A, and Main KM, 2010. Childhood exposure to phthalates: Associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Persp 118 :1458–64. 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij K, Zegers BN, Boon JP, 2002. Levels of some polybrominated diphenyl ether (PBDE) flame retardants along the Dutch coast as derived from their accumulation in SPMDs and blue mussels (Mytilus edulis). Chemosphere 46:683–88. 10.1016/S0045-6535(01)00232-6. [DOI] [PubMed] [Google Scholar]
- Bowerman WW, Best DA, Giesy JP, Shieldcastle MC, Meyer MW, Postupalsky S, Sikarskie JG, 2003. Associations between regional differences in polychlorinated biphenyls and dichlorodiphenyldichloroethylene in blood of nestling bald eagles and reproductive productivity. Environ Toxicol Chem 22:371–76. . [DOI] [PubMed] [Google Scholar]
- Carro T, Dean K, Ottinger MA, 2013. Effects of an environmentally relevant polychlorinated biphenyl (PCB) mixture on embryonic survival and cardiac development in the domestic chicken. Environ Toxicol Chem 32: 1325–31.0 10.1002/etc.2178. [DOI] [PubMed] [Google Scholar]
- Cesh LS, Elliott KH, Quade S, McKinney MA, Maisoneuve F, Garcelon DK, Sandau CD, Letcher RJ, Williams TD, Elliott JE, 2010. Polyhalogenated aromatic hydrocarbons and metabolites: Relation to circulating thyroid hormone and retinol in nestling bald eagles (Haliaeetus leucocephalus). Environ Toxicol Chem 29 :1301–10. 10.1002/etc.165. [DOI] [PubMed] [Google Scholar]
- Chen D, Hale RC, 2010. A global review of polybrominated diphenyl ether flame retardant contamination in birds. Environ Int 36:800–811. 10.1016/j.envint.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Cheret C, Willem M, Fricker FR, Wende H, Wulf-Goldenberg A, Tahirovic S, Nave K-A, Saftig P, Haass C, Garratt AN, Bennett DL, Birchmeier C, 2013. Bace1 and neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO Journal 32:2015–28. 10.1038/emboj.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer CM, Custer TW, Etterson MA, Dummer PM, Goldberg D, Franson. JC, 2012. Reproductive success and contaminant associations in tree swallows (Tachycineta bicolor) used to assess a beneficial use impairment in U.S. and binational Great Lakes’ Areas of Concern. Thai Journal of Veterinary Medicine 42:137–42. 10.1007/s10646-018-1913-9. [DOI] [PubMed] [Google Scholar]
- Custer TW., Kannan K, Tao L, Yun SH, and Trowbridge A, 2010. Perfluorinated compounds and polybrominated diphenyl ethers in great blue heron eggs from three colonies on the Mississippi River, Minnesota. Waterbirds 33:86–95. 10.1675/063.033.0110. [DOI] [Google Scholar]
- Custer CM, Custer TW, Schoenfuss HL, Poganski BH, Solem L, 2012. Exposure and effects of perfluoroalkyl compounds on tree swallows nesting at Lake Johanna in east central Minnesota, USA. Reprod Toxicol 33:556–62. 10.1016/j.reprotox.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Custer TW, Custer CM, Dummer PM, Goldberg D, Franson JC, Erickson RA, 2017. Organic contamination in tree swallow (Tachycineta bicolor) nestlings at United States and binational Great Lakes Areas of Concern. Environ Toxicol Chem 36:735–48. 10.1002/etc.3598. [DOI] [PubMed] [Google Scholar]
- DeCicco LA, Corsi SR, Villeneuve DL, Blackwell BR, Ankley GT, 2018. toxEval: Evaluation of measured concentration data using the toxcast high-throughput screening database or a user-defined set of concentration benchmarks. R package version 1.0.0., https://code.usgs.gov/water/toxEval. 10.5066/P906UQ5I. [DOI] [Google Scholar]
- Delinsky AD, Strynar MJ, Mccann PJ, Varns JL, Mcmillan L, Nakayama SF, Lindstrom AB, 2010. Geographical distribution of perfluorinated compounds in fish from Minnesota lakes and rivers. Environ Sci Technol 44:2549–54. 10.1021/es903777s. [DOI] [PubMed] [Google Scholar]
- Dodder NG, Maruya KA, Ferguson LP, Grace R, Klosterhaus S, La Guardia MJ, Lauenstein GG, Ramirez J, 2014. Occurrence of contaminants of emerging concern in mussels (Mytilus spp.) along the California coast and the influence of land use, storm water discharge, and treated wastewater effluent. Mar Pollut Bull 81:340–46. 10.1016/j.marpolbul.2013.06.041. [DOI] [PubMed] [Google Scholar]
- Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, Roebroek AJM, Schwake M, D’Hooge R, Bach P, Kalinke U, Moechars D, Alzheimer C, Reiss K, Saftig P, De Strooper B, 2005. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280:30797–806. 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- Donaldson GM, Shutt JL, Hunter P, 1999. Organochlorine contamination in bald eagle eggs and nestlings from the Canadian Great Lakes. Arch Environ Con Tox 36:70–80. 10.1007/s002449900444. [DOI] [PubMed] [Google Scholar]
- Dykstra CR, Meyer MW, Warnke DK, Karasov WH, Andersen DE, Bowerman IV WW, Giesy JP, 1998. Low reproductive rates of Lake Superior bald eagles: Low food delivery rates or environmental contaminants? J Great Lakes Res 24:32–44. 10.1016/S0380-1330(98)70797-X. [DOI] [Google Scholar]
- Dykstra CR, Route WT, Meyer MW, Rasmussen PW, 2010. Contaminant concentrations in bald eagles nesting on Lake Superior, the Upper Mississippi River, and the St. Croix River. J Great Lakes Res 36:561–69. 10.1016/j.jglr.2010.06.006. [DOI] [Google Scholar]
- Elliott KH, Cesh LS, Dooley JA, Letcher RJ, Elliott JE, 2009. PCBs and DDE, but not PBDEs, increase with trophic level and marine input in nestling bald eagles. Sci Tot Environ 407:3867–75. 10.1016/j.scitotenv.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Elliott SM, VanderMeulen DD, 2017. A regional assessment of chemicals of concern in surface waters of four Midwestern United States national parks. Sci Tot Environ 579 10.1016/j.scitotenv.2016.11.114. [DOI] [PubMed] [Google Scholar]
- Fernie KJ, Mayne G, Shutt JL, Pekarik C, Grasman KA, Letcher RJ, Drouillard K, 2005. Evidence of immunomodulation in nestling American kestrels (Falco sparverius) exposed to environmentally relevant PBDEs. Environ Pollut 138: 485–93. 10.1016/j.envpol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Filer DL, Kothiya P, Setzer RW, Judson RS, Martin MT, 2016. tcpl: The ToxCast pipeline for high-throughput screening data. Bioinformatics 33: 618–20. 10.1093/bioinformatics/btw680. [DOI] [PubMed] [Google Scholar]
- Flanagan-Pritz CM, Schrlau JE, Massey Simonich SL, Blett TF, 2014. Contaminants of emerging concern in fish from Western U.S. and Alaskan national parks - Spatial distribution and health thresholds. J Am Water Resour As 50:309–23. 10.1111/jawr.12168. [DOI] [Google Scholar]
- Flint S, Markle T, Thompson S, Wallace E, 2012. Bisphenol A exposure, effects, and policy: A wildlife perspective. J Environ Manage 104:19–34. 10.1016/j.jenvman.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Gilbertson M, Morris RD, 1976. Abnormal chicks and PCB residue levels in eggs of colonial birds on the Lower Great Lakes (1971–1973). Auk 93:434–442. [Google Scholar]
- Grim LH, Kallemeyn LW, 1995. Reproduction and distribution of bald eagles in Voyageurs National Park, Minnesota, 1973–1993. Washington D.C. http://www.dtic.mil/docs/citations/ADA322666. [Google Scholar]
- Guigueno MF, Fernie KJ, 2017. Birds and flame retardants: A review of the toxic effects on birds of historical and novel flame retardants. Environ Res 154:398–424. 10.1016/j.envres.2016.12.033. [DOI] [PubMed] [Google Scholar]
- Harris ML, Elliott JE, 2011. Effects of polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans, and polybrominated diphenyl ethers in wild birds In Environmental Contaminants in Biota, edited by Beyer WN and Meador JP, 2nd Edition,751 Boca Raton, FL: Taylor and Francis Group, LLC. [Google Scholar]
- Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, Winter PH, Shilliam CS, Hughes ZA, Dawson LA, Gonzalez MI, Upton N, Pangalos MN, Dingwall C, 2003. BACE1 (β-Secretase) transgenic and knockout mice: Identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci 24:646–55. 10.1016/S1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Herring G, Ackerman JT, Eagles-Smith CA, Adelsbach TL, Melancon MJ, Stebbins KR, Hoffman DJ, 2010. Organochlorine and PBDE concentrations in relation to cytochrome P450 activity in livers of Forster’s terns (Sterna forsteri) and Caspian terns (Hydroprogne caspia), in San Francisco Bay, California. Arch Environ Con Tox 58:863–73. 10.1007/s00244-009-9366-z. [DOI] [PubMed] [Google Scholar]
- Hites RA, 2006. Brominated flame retardants in the Great Lakes In Persistent organic pollutants in the Great Lakes, edited by Hites Ronald A., 5N:355–90. Berlin: Springer Berlin Heidelberg; 10.1007/b13133. [DOI] [Google Scholar]
- Kavlock R, Chandler R, Houck K, Hunter S, Judson R, Kleinstreuer N, Knudsen T, Martin M, Padilla S, Reif D, Richard A, Rotroff D, Sipes N, Dix D, 2012. Update on EPA’s ToxCast program: Providing high throughput decision support tools for chemical risk management. Chem Res in Toxicol 25:1287–1302. 10.1021/tx3000939. [DOI] [PubMed] [Google Scholar]
- Kimbrough KL, Johnson WE, Lauenstein GG, Christensen JD, Apeti DA, 2009. An assessment of polybrominated diphenyl ethers (PBDEs) in sediments and bivalves of the U.S. coastal zone. Silver Spring, MD. NOAA Technical Memorandum NOS NCCOS 94. 87 p. http://aquaticcommons.org/14877/1/PBDEreport.pdf [Google Scholar]
- Kozie KD, Anderson RK, 1991. Productivity, diet, and environmental contaminants in bald eagles nesting near the Wisconsin shoreline of Lake Superior. Arch Environ Con Tox 20:41–48. 10.1007/BF01065326. [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Hellström AR, Gloriam DE, Larsson TP, Schiöth HB, Fredriksson R, 2006. The G protein-coupled receptor subset of the chicken genome. PLoS Computational Biology 2:0493–0507. 10.1371/journal.pcbi.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos R, Gawlik BM, Locoro G, Rimaviciute E, Contini S, Bidoglio G, 2009. EU-wide survey of polar organic persistent pollutants in European River waters. Environ Pollut 157:561–68. 10.1016/j.envpol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Mankidy R, Wiseman S, Ma H, Giesy JP, 2016. Biological impact of phthalates. PubMed Commons 217:1–2. 10.1016/j.toxlet.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Monson B, 2013. Perfluorochemicals in Mississippi River Pool 2: 2012 update https://www.pca.state.mn.us/sites/default/files/c-pfc1-21.pdf. [Google Scholar]
- Nakayama K, Iwata H, Tao L, Kannan K, Imoto M, Kim E-Y, Tashiro K, Tanabe S, 2008. Potential effects of perfluorinated compounds in common cormorants from Lake Biwa, Japan: An implication from the hepatic gene expression profiles by microarray. Environ Toxicol Chem 27:2378–86. [DOI] [PubMed] [Google Scholar]
- Newsted JL, Giesy JP, Jones PD, 2005. Avian toxicity reference values (TRVs) for perfluorooctane sulfonate (PFOS). Organohalogen Compounds 67:830–33. [DOI] [PubMed] [Google Scholar]
- Oliaei F, Kriens D, Weber R, Watson A, 2013. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ Sci Pollut R 20:1977–92. 10.1007/s11356-012-1275-4. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.r-project.org/. [Google Scholar]
- Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadaraiah I, Yang C, Rathman J, Martin MT, Wambaugh JF, Knudsen TB, Kancherla J, Mansouri K, Patlewicz G, Williams AJ, Little SB, Crofton KM, Thomas RS, 2016. ToxCast chemical landscape: Paving the road to 21st Century toxicology. Chem Res Toxicol 29:1225–51. 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- Route WT, Dykstra CR, Rasmussen PW, Key RL, Meyer MW, Mathew J, 2014a. Patterns and trends in brominated flame retardants in bald eagle nestlings from the upper Midwestern United States. Environ Sci Technol 48:12516–24. 10.1021/es501859a. [DOI] [PubMed] [Google Scholar]
- Route WT, Russell RE, Lindstrom AB, Strynar MJ, Key RL, 2014b. Spatial and temporal patterns in concentrations of perfluorinated compounds in bald eagle nestlings in the upper Midwestern United States. Environ Sci Technol 48:6653–60. 10.1021/es501055d. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW, 2003. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Chrom B 789:393–404. [DOI] [PubMed] [Google Scholar]
- Sperry TS, Wacker DW, Wing JC, 2010. The role of androgen receptors in regulating territorial aggression in male song sparrows. Horm Behav 57:86–95. 10.1016/j.yhbeh.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Stahl LL, Snyder BD, Olsen AR, Kincaid TM, Wathen JB, Mccarty HB, 2014. Perfluorinated compounds in fish from U.S. urban rivers and the Great Lakes. Sci Total Environ 499:185–95. 10.1016/j.scitotenv.2014.07.126. [DOI] [PubMed] [Google Scholar]
- Stalmaster MV, 1987. The bald eagle. Universe Publishing, New York. [Google Scholar]
- Tsutsui K, Haraguchi S, Inoue K, Miyabara H, Ubuka T, Hatori M, Hirota T, Fukada O, 2013. New biosynthesis and biological actions of avian neurosteroids. Journal of Experimental Neuroscience 7:15–29. 10.4137/JEN.S11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, 1995. Methods for the determination of organic compounds in drinking water. EPA/600/4–88/039. [Google Scholar]
- USEPA, 2015. ToxCast & Tox21 data summary files from invitrodb_v2. 2015. http://www2.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data. [Google Scholar]
- USEPA, variously dated. Test methods for evaluating solid waste, physical/chemical methods. EPA publication SW-846, Third edition, Final updates I (1993), II (1995), IIA (1994), IIB (1995), III (1997), IIIA (1999), IIIB (2005), IV (2008), and V (2015). [Google Scholar]
- Van Ael E, Covaci A, Blust R, Bervoets L, 2012. Persistent organic pollutants in the Scheldt Estuary: Environmental distribution and bioaccumulation. Environ Int 48:17–27. 10.1016/j.envint.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Venier M, Wierda M, Bowerman WW, Hites RA, 2010. Flame retardants and organochlorine pollutants in bald eagle plasma from the Great Lakes Region. Chemosphere 80:1234–40. 10.1016/j.chemosphere.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Vongphachan V, Cassone CG, Wu D, Chiu S, Crump D, Kennedy SW, 2011. Effects of perfluoroalkyl compounds on mRNA expression levels of thyroid hormone-responsive genes in primary cultures of avian neuronal cells. Toxicol Sci 120:392–402. 10.1093/toxsci/kfq395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe KP, Kawai YK, Ikenaka Y, Kawata M, Ikushiro SI, Sakaki T, Ishizuka M 2013. Avian cytochrome P450 (CYP) 1–3 family genes: Isoforms, evolutionary relationships, and mRNA expression in chicken liver. PLoS ONE 8:1–11. 10.1371/journal.pone.0075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning RJ, Martello L, Prusak-Daniel A, 2011. Environmental contaminants in biota: Interpreting tissue concentrations In Environmental Contaminants in Biota: Interpreting Tissue Concentrations, edited by Nelson Beyer W and Meador James P., Second Edition, 768 Boca Raton: Taylor and Francis Group, LLC. [Google Scholar]
- Wiemeyer SN, Lamont TG, Bunck CM, Sindelar CR, Gramlich FJ, Fraser JD, Byrd MA, 1984. Organochloride pesticide, polychlorobiphenyl, and mercury residues in bald eagle eggs−−1969–1979--and their relationships to shell thinnings and reproduction. Archives Of Environmental Contamination And Toxicology 13:529–49. [DOI] [PubMed] [Google Scholar]
- Ye X, Tou LJ, Needham LL, Calafat AM, 2008. Automated on-line column-switching HPLCMS/MS method for measuring environmental phenols and parabens in serum. Talanta 76:265–871. 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Yingling V, 2015. Karst influence in the creation of a PFC megaplume In proceedings of the 14th Multidisciplinary Conference on Sinkholes and the Engineering and Environmental Impacts of Karst, edited by Doctor Daniel H., Land Lewis, and Stephenson J. Brad, 319–26. National Cave and Karst Research Institute; 10.5038/9780991000951. [DOI] [Google Scholar]
- Zhang X, Xu Q, Man S, Zeng X, Yu Y, Pang Y, Sheng G, Fu J, 2013. Tissue concentrations, bioaccumulation, and biomagnification of synthetic musks in freshwater fish from Taihu Lake, China. Environ Sci Pollut R 20:311–22. 10.1007/s11356-012-1095-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.