Abstract
Objective.
To assess treatment patterns, outcomes, and costs for women with low-(LIR) and high-intermediate risk endometrial cancer (HIR) who are treated with and without adjuvant radiotherapy.
Methods.
All patients with stage I endometrioid endometrial cancer who underwent surgery from 2000 to 2011 were identified from the SEER-Medicare database. LIR was defined as G1–2 tumors with <50% myometrial invasion or G3 with no invasion. HIR was defined as G1–2 tumors with ≥50% or G3 with <50% invasion. Patients were categorized according to whether they received adjuvant radiotherapy (vaginal brachytherapy [VBT], external beam radiotherapy [EBRT], or both) or no radiotherapy. Outcomes were analyzed and compared (primary outcome was overall survival).
Results.
10,842 patients met inclusion criteria. In the LIR group (n = 7609), there was no difference in 10-year overall survival between patients who received radiotherapy and those who did not (67% vs 65%, adjusted HR 0.95, 95% CI 0.81–1.11). In the HIR group (n = 3233), patients who underwent radiotherapy had a significant increase in survival (60% vs 47%, aHR 0.75, 95% CI 0.67–0.85). Radiotherapy was associated with increased costs compared to surgery alone ($26,585 vs $16,712, p < .001). Costs for patients receiving VBT, EBRT, and concurrent VBT/EBRT were $24,044, $27,512, and $31,564, respectively (p < .001). Radiotherapy was associated with an increased risk of gastrointestinal (7 vs 4%), genitourinary (2 vs 1%), and hematologic (16 vs 12%) complications (p < .001).
Conclusions.
Radiotherapy was associated with improved survival in women with HIR, but not in LIR. It also had increased costs and a higher morbidity risk. Consideration of observation without radiotherapy in LIR may be reasonable.
Keywords: Low-intermediate risk endometrial cancer, High-intermediate risk endometrial cancer, Endometrial cancer, Radiotherapy, Overall survival, Cost
1. Introduction
Endometrial carcinoma is the most common gynecologic malignancy in the United States, with an estimated 63,230 new cases in 2018 [1]. Although surgery at diagnosis is the cornerstone of management for women with suspected early-stage disease, the use of adjuvant radiation therapy is controversial [2]. Despite the fact that two randomized trials (Gynecologic Oncology Group 99 [GOG-99] and Post-Operative Radiation Therapy in Endometrial Carcinoma 1 [PORTEC-1]) have shown no overall survival advantage for adjuvant radiation in patients with Stage I cancer, evidence suggests a recurrence-free survival benefit for certain intermediate-risk subgroups [3,4].
In an effort to standardize management recommendations for radio-therapy, the American Society for Radiation Oncology (ASTRO) has developed treatment guidelines, which were endorsed by the American Society of Clinical Oncology (ASCO) with minor modifications [5,6]. Specifically, the guidelines recommended the following: 1) patients with early stage grade 1–2 tumors with <50% myometrial invasion (and features such as age > 60 and/or lymphovascular space invasion), or those with grade 3 tumors with no invasion ‘may be treated with or without’ vaginal brachytherapy (VBT); 2) patients with grade 1–2 tumors with ≥50% invasion (and features such as age > 60 and/or lymphovascular space invasion) ‘may benefit from’ external beam radiation therapy [EBRT] (ASTRO recommendation) or VBT (ASCO recommendation); and 3) patients with grade 3 tumors with <50% invasion ‘should be treated’ with VBT.
In 2016, national health expenditures comprised 17.9% of the gross domestic product (GDP) in the United States. Projections from the Center for Medicare and Medicaid Services suggest that medical costs will reach 20% of the GDP by 2026 [7]. As medical costs continue to rise, it is essential to scrutinize not only the benefit of different treatment approaches but also their costs. This is especially relevant in endometrial cancer, the incidence of which has been steadily increasing over the last few decades, likely related to the obesity epidemic [1]. Different medical societies have defined value in cancer care by emphasizing three critical elements: clinical benefit, complications, and cost [8]. The objective of our study was to assess treatment patterns, outcomes, and costs for women with low and high-intermediate risk histologic features who were treated with and without adjuvant radiation, using a national healthcare claims database.
2. Methods
2.1. Data source
We conducted a retrospective population-based cohort study of patients who underwent surgery for endometrioid endometrial cancer using the linked Surveillance, Epidemiology, and End Results registry (SEER)–Medicare database. The SEER registry of the National Cancer Institute contains approximately 97% of all incident cancer cases from tumor registries that cover 26% of the United States population. The Medicare claims database includes billed claims and services data on patients with Medicare Part A (inpatient) and Part B (outpatient) [9]. Claims include all inpatient hospitalizations, outpatient visits, physician and supplier data, and drugs administered. The SEER–Medicare linked database was developed by matching the records of 93% of persons aged >65 years in the SEER registry to the Medicare claims database [10]. We used a combination of International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes, Common Procedural Terminology (CPT) codes, and Healthcare Common Procedure Coding System (HCPCS) codes to identify relevant covariates, treatments, and outcomes, which are shown in Supplementary Tables S1 and S2. Institutional Review Board approval was obtained.
2.2. Cohort selection
All patients aged ≥66 years with endometrioid endometrial cancer who underwent surgery from January 2000 to December 2011 were identified from the SEER -Medicare database. Low-intermediate risk endometrial cancer (LIR) was defined as stage I grade 1–2 tumors with <50% myometrial invasion or grade 3 with no invasion (histologic sub-types for which the ASTRO/ASCO guidelines suggested adjuvant radio-therapy was optional). High-intermediate risk endometrial cancer (HIR) was defined as stage I grade 1–2 tumors with ≥50% invasion (guidelines suggested benefit from radiotherapy) or grade 3 with <50% invasion (guidelines recommended radiotherapy). Using SEER-based pathologic definitions, we identified a total of 43,281 patients who had a diagnosis of endometrial carcinoma during the study period. We sequentially excluded 11,360 patients who did not have endometrioid histology, 1033 who did not have pathologic confirmation or who were diagnosed at autopsy or by death certificate, 9865 who either didn’t have full Medicare Part A and Part B coverage or had HMO enrollment from 12 months before diagnosis till death or till the end of the follow-up period, 303 who did not have surgery, and 9878 who did not fit the clinical/histologic criteria for either LIR or HIR.
2.3. Outcomes and covariates
Patients were categorized according to whether they received adjuvant radiotherapy (vaginal brachytherapy [VBT], external beam radiation therapy [EBRT], or both) or no radiotherapy. The primary outcome was overall survival (assessed individually for the LIR and the HIR groups). Secondary outcomes were costs at up to 6 months post-surgery, and complications at up to 2-years post-surgery for the entire cohort. We chose to assess costs at up to 6 months postoperatively as we wanted to limit costs to the primary treatment of endometrial cancer, and a longer time-period would risk including costs for the treatment of progression, recurrence, or other medical admissions. We chose to assess the 2-year limit for complications as several of them occur in the long-term and typically manifest further out from the treatment period [3].
Patient and tumor characteristics were collected from SEER. Demographic and tumor variables assessed included age, marital status, race or ethnicity, area of residence, year of diagnosis, percentage of high school graduates in census tract, median household income in census tract, region of treatment, tumor size, and grade. We used the Klabunde modification of the Charlson comorbidity index to assess patient comorbidity, using claims in the 12 months prior to cancer diagnosis [11]. ICD-9 and CPT codes used to identify procedures (surgery and radiation therapy) are shown in supplementary Table S1. Complications were derived from a literature review of morbidity associated with radiation therapy, and codes used to identify them are listed in supplementary Table S2 [3,12].
2.4. Statistical analysis
Outcomes were analyzed and compared between patients who underwent surgery alone, and those who also received radiotherapy. Categorical variables were compared using the χ2 test, and continuous variables were compared using the Mann-Whitney U test. Overall survival was defined as the time interval from the date of surgery to the date of death or last follow-up. The Kaplan-Meier method was used to estimate 10-year overall survival rates, and survival between the two groups was compared using the log-rank test. A multivariable Cox proportional hazards regression model was used to calculate differences in survival, after adjusting for variables that were significantly associated with survival on univariate analysis (stepwise model selection with a p-value cut-off of 0.1 was used, and variables with a p-value <0.1 were included in the model). These variables were age, year of diagnosis, race, region of care, modified Charlson comorbidity index, receipt of lymphadenectomy, and tumor grade. Costs included all inpatient and outpatient insurance payments incurred up to 6 months from the date of surgery. All cost estimates were normalized to 2017 U.S. dollar using the medical care component of the consumer price index [13]. Due to the skewed and non-normal distribution of cost, a generalized linear model with a gamma family and log link function was used to adjust for covariates associated with cost variability (region of treatment, area of residence, and percent of high school graduates). All statistical tests were two-sided, with a p < .05 considered significant. Analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC).
3. Results
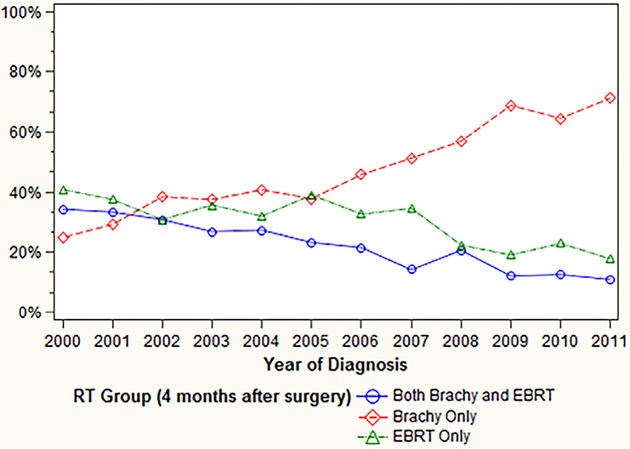
We identified 10,842 women who underwent surgery for endometrial cancer and met our inclusion criteria during the study period. Of these patients, 7609 (70%) were LIR and 3233 (30%) were HIR. Nine percent (n = 660) of patients with LIR had radiation therapy, compared to 46% (n = 1478) of those with HIR. The use of adjuvant radiation in the entire cohort did not change over time (19.9% in 2000 to 19.5% in 2011). However, among all patients who underwent radiation, the following trends were noted from 2000 to 2011 (Fig. 1): VBT use increased from 25% to 71%; EBRT use declined from 41% to 18%; and concurrent VBT/EBRT declined from 34% to 11% (p < .001). Patient characteristics are shown in Table 1. Among the entire cohort, age, geographic region, area of residence, tumor grade, tumor size, and receipt of lymphadenectomy were associated with receiving radiotherapy. Patient characteristics stratified by the LIR and HIR groups are shown in supplementary Tables S3 and S4.
Fig. 1.
Trend in radiotherapy modality use over time.
Table 1.
Patient characteristics (n = 10,842).
| Characteristic | Total n = 10,842 | Surgery group n = 8704 | Surgery and radiation group n = 2138 | p |
|---|---|---|---|---|
| Age | ||||
| 66–70 | 3616 (33%) | 2927 (81%) | 689 (19%) | <0.001 |
| 71–75 | 2937 (27%) | 2308 (79%) | 629 (21%) | |
| 76–80 | 2290 (21%) | 1798 (79%) | 492 (21%) | |
| ≥81 | 1999 (18%) | 1671 (84%) | 328 (16%) | |
| Race/ethnicity | ||||
| African American, non-Hispanic | 534 (5%) | 437 (82%) | 97 (18%) | 0.32 |
| Hispanic | 468 (4%) | 368 (79%) | 100 (21%) | |
| White, non-Hispanic | 9517 (88%) | 7630 (80%) | 1887 (20%) | |
| Other | 323 (3%) | 269 (83%) | 54 (17%) | |
| Region of treatment | ||||
| Northeast | 2715 (25%) | 1912 (70%) | 803 (30%) | <0.001 |
| Midwest | 1502 (14%) | 1209 (81%) | 293 (19%) | |
| South | 2251 (21%) | 1830 (81%) | 421 (19%) | |
| West | 4374 (40%) | 3753 (86%) | 621 (14%) | |
| Area of residence | ||||
| Metropolitan | 9040 (83%) | 7217 (80%) | 1823 (20%) | 0.009 |
| Urban/Rural | 1802 (17%) | 1487 (83%) | 315 (17%) | |
| Year of diagnosis | ||||
| 2000–2003 | 4060 (37%) | 3230 (80%) | 830 (20%) | 0.34 |
| 2004–2007 | 3600 (33%) | 2904 (81%) | 696 (19%) | |
| 2008–2011 | 3182 (29%) | 2570 (81%) | 612 (19%) | |
| Marital status | ||||
| Married | 5018 (47%) | 4055 (80%) | 1026 (20%) | 0.31 |
| Not married | 5358 (49%) | 4316 (81%) | 1042 (19%) | |
| Unknown | 403 (4%) | 333 (83%) | 70 (17%) | |
| Household income, census tract (Median, ± SD) ($US) | $53,311 ± $25,317 | $53,316 ± $25,316 | $54,021 ± $25,311 | 0.17 |
| High school graduates, census tract (Mean %, ± SD) | 82.9% ± 12.1% | 82.8% ± 12.3% | 83.1% ± 11.5% | 0.34 |
| Charlson comorbidity index | ||||
| 0 | 7046 (65%) | 5663 (80%) | 1383 (20%) | 0.08 |
| 1 | 2537 (23%) | 2007 (79%) | 530 (21%) | |
| ≥2 | 1259 (12%) | 1034 (82%) | 225 (18%) | |
| Tumor grade | ||||
| 1 | 5423 (50%) | 4800 (89%) | 623 (11%) | <0.001 |
| 2 | 4362 (40%) | 3193 (73%) | 1169 (27%) | |
| 3 | 1057 (10%) | 711 (67%) | 346 (33%) | |
| Tumor size | ||||
| 0–2.5 cm | 2459 (23%) | 2088 (85%) | 371 (15%) | <0.001 |
| 2.6–5.0 cm | 2558 (24%) | 1899 (74%) | 659 (26%) | |
| >5cm | 585 (5%) | 424 (73%) | 161 (28%) | |
| Unknown | 5240 (48%) | 4293 (82%) | 947 (18%) | |
| Lymphadenectomy | ||||
| Yes | 6416 (59%) | 4944 (77%) | 1472 (23%) | <0.001 |
| No | 4426 (41%) | 3760 (85%) | 666 (15%) | |
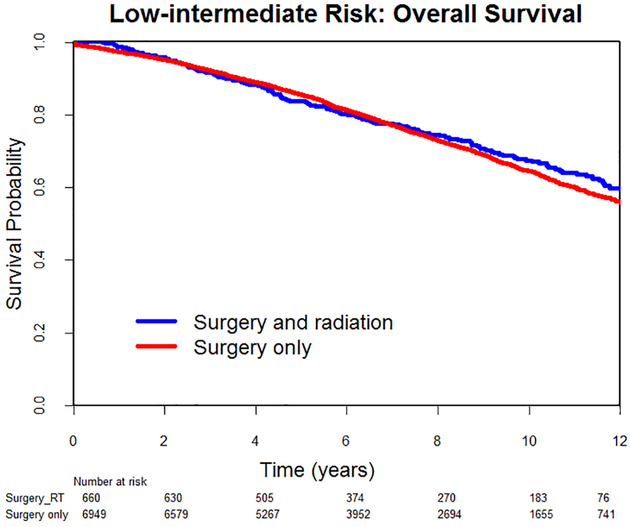
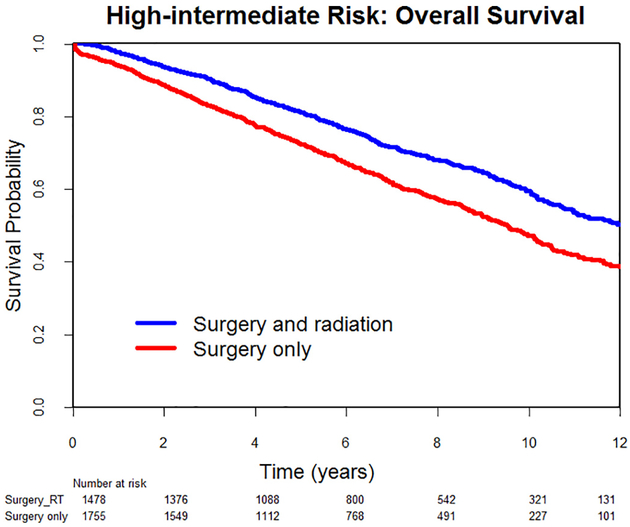
Median follow-up was 81 months (range, 1–168) for the LIR group and 70 months (range, 1–168) for the HIR group. Overall, there were 2176 deaths in the LIR group and 1209 in the HIR group. In the LIR group, there was no difference in 10-year overall survival between patients who had radiotherapy and those who did not (67% vs 65%, HR0.94, 95% CI 0.81–1.09, p = .41) (Fig. 2). On multivariate analysis, after adjusting for age, year of diagnosis, race, region of care, modified Charlson comorbidity index, receipt of lymphadenectomy, and tumor grade, the difference in survival remained non-significant (adjusted HR 0.95, 95% CI 0.81–1.11, p = .55). In the HIR group, patients who underwent radiotherapy had a significant increase in 10-year overall survival (60% vs 47%, HR 0.68, 95% CI 0.61–0.76, p < .001) (Fig. 3). On multivariate analysis, that difference remained significant (adjusted HR 0.75, 95% CI 0.67–0.85, p < .001).
Fig. 2.
Low-intermediate risk endometrial cancer: Overall survival.
Fig. 3.
Low-intermediate risk endometrial cancer: Overall survival.
We performed a secondary analysis comparing survival outcomes for patients who underwent surgery alone and those who received each individual radiation modality, with similar conclusions. In the LIR group, there was no difference in overall survival between patients who only underwent surgery and those who also had VBT (adjusted HR 0.82, 95% CI 0.66–1.02), those who had EBRT (adjusted HR 1.31, 95% CI 0.99–1.72), and those who had concurrent VBT/EBRT (adjusted HR 0.94, 95% CI 0.68–1.30), respectively. In the HIR group, all subgroups who underwent radiotherapy had a significant increase in overall survival: compared to patients who had surgery alone, adjusted HR were 0.61 (95% CI 0.51–0.74) for those who had VBT, 0.84 (95% CI 0.71–0.98) for those who had EBRT, and 0.82, (95% CI 0.68–0.99) for those who had concurrent VBT/EBRT.
Table 2 shows costs incurred up to 6 months postoperatively. Patients who underwent radiation therapy had significantly higher mean adjusted total costs ($26,585) compared to those who only had surgery ($16,712), with a difference of $9873 (p < .001). Costs for patients receiving concurrent VBT/EBRT were the highest ($31,564) followed by EBRT ($27,512), and VBT ($24,044) (p < .001).
Table 2.
Costs incurred up to 6 months post-surgery.
| Treatment group | Adjusted mean | 95% CI | p |
|---|---|---|---|
| Surgery | $16,712 | $16,524–$16,901 | <0.001 |
| Surgery and radiation | $26,585 | $25,984–$27,200 | |
| Radiation modality | |||
| Vaginal brachytherapy | $24,044 | $23,473–$24,629 | <0.001 |
| External beam radiation therapy | $27,512 | $26,724–$28,322 | |
| Concurrent brachytherapy/external beam radiation therapy | $31,564 | $30,531–$32,631 | |
Complications up to two years post-surgery are listed in Table 3. Radiotherapy was associated with an increased risk of gastrointestinal (6.7% vs 3.9%, p < .001), genitourinary (1.6% vs 0.7%, p < .001), thromboembolic (10.4 vs 8.6%, p = .008) and hematologic (16 vs 12.1%, p < .001) 2-year complications.
Table 3.
Complications up to 2 years post-surgery (n = 10,842).
| Complication | Total n = 10,842 | Surgery group n = 8704 | Surgery and radiation group n = 2138 | p |
|---|---|---|---|---|
| Gastrointestinal | 485 (4.5%) | 341 (3.9%) | 144 (6.7%) | <0.001 |
| Lymphedema | 2723 (25.1%) | 2155 (24.8%) | 568 (26.6%) | 0.08 |
| Genitourinary | 94 (0.9%) | 59 (0.7%) | 35 (1.6%) | <0.001 |
| Cutaneous | 82 (0.8%) | 63 (0.7%) | 19 (0.9%) | 0.43 |
| Hematologic | 1400 (12.9%) | 1057 (12.1%) | 343 (16.0%) | <0.001 |
| Venous thromboembolism | 971 (9.0%) | 748 (8.6%) | 223 (10.4%) | 0.008 |
4. Discussion
In this study, we sought to assess treatment patterns, outcomes, and costs in patients with intermediate-risk endometrial cancer. Radiation therapy was associated with improved 10-year overall survival in women with HIR, but not in the LIR cohort. Adjuvant radiation also had significantly increased costs and a higher morbidity risk.
As the costs of cancer care continue to rise, it is paramount to assess the value and cost-effectiveness of different treatment strategies and not just their effect on survival [14]. Although there are many definitions regarding value in healthcare, there is growing consensus that costs and the toxicity of treatment are essential components of this definition [8,15]. In addition, evidence suggests that patients are increasingly responsible for a higher share of their healthcare costs [17]. Cost-shifting occurs through an increasing use of larger copayments and high-deductible policies, with many patients experiencing financial distress or personal bankruptcy as a result of their treatment [18]. Our LIR cohort consisted of patients with stage I grade 1–2 tumors with <50% myometrial invasion or grade 3 with no invasion (all had high-risk features present since all were aged ≥66 years). The ASTRO/ASCO guidelines have suggested that these patients ‘may be treated with or without’ VBT [5,6]. Given the lack of survival benefit in our analysis, and especially in the context of added morbidity and costs, our study suggests no benefit to adjuvant radiation in this setting.
On the other hand, our HIR cohort consisted of patients with stage I grade 1–2 tumors with ≥50% myometrial invasion or grade 3 with <50% invasion. The guidelines proposed that patients with grade 1–2 tumors with ≥50% invasion ‘may benefit from’ EBRT (ASTRO recommendation) or VBT (ASCO recommendation). Our study confirms this benefit from radiation. We did not specifically assess if there was any difference in complications between VBT and EBRT, however the PORTEC-2 study showed that VBT was as effective in ensuring vaginal control as EBRT with fewer gastrointestinal side effects, and that it should be the treatment of choice in patients with HIR endometrial cancer [12]. In the context of added cost for EBRT ($27,512 compared to $24,044 for VBT), our results suggest that VBT may be the better option in this setting. With regards to patients with grade 3 tumors with <50% invasion, the guidelines advised that they ‘should be treated’ with VBT. Our study again confirms a benefit from radiation.
Among the cohort who received adjuvant radiation, the use of VBT increased from 25% in 2000 to 71% in 2011, EBRT use declined from 41% to 18%, and concurrent VBT/EBRT declined from 34% to 11%. These trends are commensurate with the longitudinal reporting of data regarding these different modalities in the literature [2,3,12]. As providers became more aware of the morbidity risk with radiation, the use of both EBRT and concurrent VBT/EBRT decreased over time, presumably because VBT was considered a safer alternative with no difference in clinical outcomes [19]. Indeed, the ASTRO/ASCO guidelines state that there is limited evidence for a benefit to VBT following EBRT [5,6,20]. In our study, concurrent VBT/EBRT had the highest costs ($31,564). In the absence of clinical benefit, our results suggest that the use of concurrent VBT/EBRT in these patients would not be cost-effective.
In a National Cancer Database study, Rydzewski et al. showed that VBT was independently associated with a reduction in mortality in all patients with stage I endometrioid endometrial cancer, irrespective of grade or depth of myometrial invasion [21]. In another analysis using the same database, Wong and colleagues found that adjuvant radiation was associated with improved 5-year overall survival in patients with ≥50% myometrial invasion but no survival benefit for any patients with <50% invasion (irrespective of grade) [22]. An older analysis of patients from 1998 to 2001 using the SEER database also found a 5-year overall survival benefit to adjuvant radiation in patients with grade 1 endometrial cancer with ≥50% myometrial invasion [23]. Importantly, all these studies mainly focused on survival differences, with no evaluation of complications or costs. The main strength of our study lies in the fact that we not only assessed survival outcomes, but also the impact of radiation on healthcare costs and morbidity, factors that are critical to the value equation. The large sample size of our cohort and the length of the study period further strengthen our analysis. We also assessed 10-year overall survival, which is important in a population with early-stage endometrial cancer, as these patients tend to have prolonged survival. Additionally, the SEER-Medicare database captures a broad geographic area, which allowed us to assess patterns and outcomes on a national level [9].
Limitations include the potential for selection and reporting bias, which is inherent to all retrospective, population-based analyses. Complications were based on ICD-9 and CPT codes entered into the database, not a medical review of patient charts. Moreover, the time frame for assessing costs and complications was different. We used a 6-month postoperative limit for costs as we only wanted to assess the costs of primary treatment. It is therefore possible that we are underestimating costs, given that costs of long-term complications were not fully reflected in our analysis. SEER-Medicare lacks information on lymphovascular space invasion, which precluded us from assessing its impact on outcomes. However, since all patients in our cohort were 66 years and older, they all qualified for the LIR and HIR subgroups if they fit the other grade and invasion criteria. Additionally, costs were based on Medicare reimbursements, which may or may not reflect commercial insurance reimbursement, or the cost of delivering care from a hospital or provider perspective.
In conclusion, our results showed that adjuvant radiotherapy was associated with improved survival outcomes in women with HIR endometrial cancer, with no difference in patients with LIR. Radiation therapy was also associated with an increased risk of complications and higher costs. Our results highlight that there exists a range of practice patterns and many women may be receiving radiation therapy when not indicated, or both VBT/EBRT when un-indicated. In the context of cost/value and added morbidity, and especially given the lack of survival benefit, consideration of observation without radiation in LIR may be reasonable if other risk factors are absent.
Supplementary Material
HIGHLIGHTS.
Radiotherapy had no impact on survival in low-intermediate risk endometrial cancer.
Radiotherapy was associated with improved survival in high-intermediate risk endometrial cancer.
Adjuvant radiation had significantly increased costs and a higher morbidity risk.
Acknowledgement
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Conflict of interest statements
This research is supported in part by the Duncan Family Institute, and a National Cancer Institute grant (#P30 CA016672).
Rudy Suidan — Research support: National Institutes of Health T32 grant (#5T32 CA101642).
Larissa Meyer — Research support: Cancer Prevention and Research Institute of Texas grant (#RP140020); National Cancer Institute K award (#K07 CA201013).
Grace Smith — Research support: National Cancer Institute K award (#K07 CA211804).
Sharon Giordano — Research support: Cancer Prevention and Research Institute of Texas grant (#RP160674) and Komen grant (#SAC150061).
Relevant financial activities outside the supported work
Larissa Meyer
Research support: AstraZeneca
Consultant: Clovis Oncology
Charlotte Sun:
Research support: AstraZeneca
Footnotes
The other authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2018.11.005.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin 68 (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Kong A, Johnson N, Kitchener HC, Lawrie TA, Adjuvant radiotherapy for stage I endometrial cancer, Cochrane Database Syst. Rev (2012) Cd003916. [DOI] [PubMed] [Google Scholar]
- [3].Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. , A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study, Gynecol. Oncol 92 (2004) 744–751. [DOI] [PubMed] [Google Scholar]
- [4].Creutzberg CL, van Putten WLJ, Koper PCM, Lybeert MLM, Jobsen JJ, Wárlám-Rodenhuis CC, et al. , Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial, Lancet 355 (2000) 1404–1411. [DOI] [PubMed] [Google Scholar]
- [5].Klopp A, Smith BD, Alektiar K, Cabrera A, Damato AL, Erickson B, et al. , The role of postoperative radiation therapy for endometrial cancer: executive summary of an American Society for Radiation Oncology evidence-based guideline, Pract. Radiat. Oncol 4 (2014) 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meyer LA, Bohlke K, Powell MA, Fader AN, Franklin GE, Lee LJ, et al. , Postoperative radiation therapy for endometrial cancer: American Society of Clinical Oncology clinical practice guideline endorsement of the American Society for Radiation Oncology evidence-based guideline, J. Clin. Oncol 33 (2015) 2908–2913. [DOI] [PubMed] [Google Scholar]
- [7].Centers for Medicare and Medicaid Services, CMS.gov, Available at https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet.html 2018, Accessed date: 12 July 2018. [PubMed]
- [8].Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. , American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options, J. Clin. Oncol 33 (2015) 2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF, Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population, Med. Care 40 (2002) Iv–3–18. [DOI] [PubMed] [Google Scholar]
- [10].SEER-Medicare: brief description of the SEER-Medicare database, Available at: https://healthcaredelivery.cancer.gov/seermedicare/overview/, Accessed date: 21 July 2018.
- [11].Klabunde CN, Potosky AL, Legler JM, Warren JL, Development of a comorbidity index using physician claims data, J. Clin. Epidemiol 53 (2000) 1258–1267. [DOI] [PubMed] [Google Scholar]
- [12].Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. , Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial, Lancet 375 (2010) 816–823. [DOI] [PubMed] [Google Scholar]
- [13].Measuring price change for medical care in the CPI. Consumer price index , Division of Consumer Prices and Price Indexes, U.S. Bureau of Labor Statistics, Washington, DC, 2018, Available at: https://www.bls.gov/cpi/factsheets/home.htm, Accessed date: 21 July 2018. [Google Scholar]
- [14].Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML, Projections of the cost of cancer care in the United States: 2010–2020, J. Natl. Cancer Inst. 103 (2011) 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pendleton RC, We Won’t Get Value-Based Health Care Until We Agree on What “Value” Means, Available at: https://hbr.org/2018/02/we-wont-get-value-based-health-care-until-we-agree-on-what-value-means 2018, Accessed date: 18 July 2018. [Google Scholar]
- [17].Zafar SY, Peppercorn JM, Schrag D, Taylor DH, Goetzinger AM, Zhong X, et al. , The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience, Oncologist 18 (2013) 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramsey S, Blough D, Kirchhoff A, Kreizenbeck K, Fedorenko C, Snell K, et al. , Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis, Health Aff. 32 (2013) 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, et al. , Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial, J. Clin. Oncol 27 (2009) 3547–3556. [DOI] [PubMed] [Google Scholar]
- [20].Greven KM, D’Agostino RB Jr., Lanciano RM, Corn BW, Is there a role for a brachytherapy vaginal cuff boost in the adjuvant management of patients with uterine-confined endometrial cancer? Int. J. Radiat. Oncol. Biol. Phys 42 (1998) 101–104. [DOI] [PubMed] [Google Scholar]
- [21].Rydzewski NR, Strohl AE, Donnelly ED, Kanis MJ, Lurain JR, Nieves-Neira W, et al. , Receipt of vaginal brachytherapy is associated with improved survival in women with stage I endometrioid adenocarcinoma of the uterus: a National Cancer Data Base study, Cancer 122 (2016) 3724–3731. [DOI] [PubMed] [Google Scholar]
- [22].Wong AT, Rineer J, Schwartz D, Weiner J, Safdieh J, Choi K, et al. , Patterns of adjuvant radiation usage and survival outcomes for stage I endometrial carcinoma in a large hospital-based cohort, Gynecol. Oncol 144 (2017) 113–118. [DOI] [PubMed] [Google Scholar]
- [23].Lee CM, Szabo A, Shrieve DC, Macdonald OK, Gaffney DK, Frequency and effect of adjuvant radiation therapy among women with stage I endometrial adenocarcinoma, JAMA 295 (2006) 389–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.