Abstract
The consequences of childhood maltreatment are profound and long lasting. Not only does the victim of abuse suffer as a child, but there is mounting evidence that a history of maltreatment places the next generation at risk for significant psychopathology. Research identifies postnatal factors as affecting this intergenerational transmission of trauma. However, emerging evidence suggests that part of this risk may be transmitted before birth, passed on via abuse-related alterations in the in utero environment that are as yet largely unidentified. To date, no study has directly assessed the influence of pregnant women’s abuse history on fetal neurobehavioral development, nor considered trauma-associated poor sleep quality as a mediator reflecting established physiological dysregulation. Using data from 262 pregnant adolescents (ages 14–19), a population at elevated risk for childhood maltreatment, the current study examined maternal emotional abuse history and sleep quality in relation to third-trimester fetal resting heart rate variability, an index of parasympathetic nervous system functioning. The results indicate that maternal emotional abuse history is indirectly associated with lower fetal heart rate variability via abuse-related sleep disturbances. These data demonstrate an association between maternal abuse histories and fetal development, showing that at least part of the intergenerational transmission of risk occurs during pregnancy.
The consequences of childhood maltreatment are profound and long lasting (Cicchetti & Carlson, 1989; Korbin & Krugman, 2014; Myers, 2002). Not only does the victim of abuse suffer as a child, but there is mounting evidence that a history of maltreatment places the next generation at risk. Children of mothers with a history of abuse have more difficulty regulating their emotions and are at greater risk for psychopathology (Bifulco et al., 2002; Roberts, O’Connor, Dunn, & Golding, 2004). Based on a sample of 8,292 children of mothers with and without a childhood abuse history, Roberts et al. (2004) reported that children of abused mothers displayed significantly more conduct problems, emotional problems, and symptoms of hyperactivity. Studies conducted with rodents report similar results, such that the pups of dams that were exposed to chronic and unpredictable emotional stress prior to pregnancy have been shown to exhibit neural and behavioral indicators of risk for psychopathology (Li et al., 2010; Ryzhavskii, et al., 2002), demonstrating intergenerational effects of mothers’ early adverse experiences.
Research investigating how mothers’ experiences with abuse affect the next generation’s psychobiological development and associated risk for psychopathology has identified several influencing factors, including parenting practices (Dixon, Browne, & Hamilton-Giachritsis, 2005; Pears & Capaldi, 2001; Sroufe, Egeland, Carlson, & Collins, 2009) and postnatal parental mental health (e.g., Plant, Barker, Waters, Pawlby, & Pariant, 2013). However, based on studies that find that offspring developmental differences continue to be observed (and in some cases, are strengthened) after controlling for these postnatal variables, several authors (e.g., Danieli, 1998; Sotero, 2006; Yehuda et al., 2007) have suggested that part of the intergenerational transmission of abuse may occur prior to birth, shaping the neurobehavioral development of the child via abuse-associated alternations in pregnant women’s functioning and, consequently, in the in utero environment. Studies of emotional abuse, the most common and arguably most pernicious form of child maltreatment (Berzenski, Yates, & Egeland, 2014; Spertus, Yehuda, Wong, Halligan, & Seremetis, 2003; Teicher, Samson, Polcari, & McGreenery, 2006), may be particularly important for understanding the mechanisms through which a mother’s abuse history has the ability to influence her child’s development.
The Developmental Origins of Health and Disease (DOHaD) Model
According to the DOHaD model, experience-associated alterations in the in utero environment can influence fetal development in ways that may confer risk for mental and physical health problems later in life. Evidence from human and animal studies show that by-products of maternal health and health-related behaviors (e.g., maternal hormones and cytokines) can cross the placenta and influence fetal development (Seckl, 2004; Talge, Neal, Glover, & Early Stress, Translational Research and Prevention Science Network, 2007; Wadhwa, 2005; Weinstock, 2005; Welberg & Seckl, 2001); it is through biological mechanisms of this nature that qualities of pregnant women’s lives are thought to impact the fetus. Support for this model comes from a range of research designs, including studies that show that pregnancy-specific states and experiences are associated with (a) infant and child development, even after controlling for confounding postnatal factors (Davis et al., 2007; Davis & Sandman, 2010; O’Connor, Heron, Golding, Glover, & ALSPAC Study Team, 2003); (b) adverse pregnancy outcomes that indicate differences in fetal growth and development, including preterm birth and lower birth weight (Dole et al., 2003; Spicer et al., 2013; Wadhwa, Sandman, Porto, Dunkel-Schetter, & Garite, 1993); and (c) variations in fetal neurobehavioral development (DiPietro, 2012; DiPietro, Hilton, Hawkins, Costigan, & Pressman, 2002; Glover, 2011; Monk et al., 2000, 2004; Wadhwa, Sandman, & Garite, 2001), including indices of autonomic nervous system regulation such as fetal heart rate variability (HRV; DiPietro, Bornstein, Hahn, Costigan, & Achy-Brou, 2007; DiPietro, Hodgson, Costigan, Hilton, & Johnson, 1996; Doyle et al., 2015). Most of this data comes from studies investigating the prenatal effects of maternal psychological distress (see Glover, 2011; Kinsella & Monk, 2009, for reviews), yet research examining the consequences of women’s abuse histories on children’s development shows distinctly similar results. As reviewed above, children of women with childhood abuse histories are at greater risk for emotional and psychological difficulties, even when they are not exposed directly to any form of maltreatment. In addition, women who suffered abuse during childhood are at greater risk for adverse pregnancy outcomes, including lower birth weight and premature delivery (Gavin, Hill, Hawkins, & Maas, 2011; Grimstad & Schei, 1999; Stevens-Simons & McAnarney, 1994). These studies are suggestive of an in utero effect of maternal abuse histories on child outcomes, though to date that hypothesis has not been tested.
Differences in Fetal Heart Rate Variability May Underlie the Second Generation’s Risk
Studies of fetal neurobehavioral development are unique in their capacity to give insight into whether certain maternal behaviors or experiences (e.g., childhood abuse history) influence the second generation. Fetal studies allow for an examination of effects during the developmental period during which they are presumed to occur, and eliminate many of the inherent confounds in studies of prenatal influences on postnatal functioning. Given that mothers who were abused as children have been shown to display less sensitive and more harsh and controlling parenting behaviors (Dixon, Browne, & Hamilton-Giachritsis, 2005; Pears & Capaldi, 2001; Sroufe et al., 2009), and that these same dimensions of parenting have been shown to influence children’s developmental outcomes, including HRV (e.g., Perry, Mackler, Calkins, & Keane, 2014; Propper et al., 2008), which continues to show rapid development in infancy and early childhood, this is not an insignificant contribution.
Studies of fetal HRV have particular potential to inform our understanding of the consequences of maternal childhood abuse. An index of parasympathetic nervous system functioning, HRV is considered a physiological substrate of emotion regulation (Porges, 2001, 2007; Porges, Doussard-Roosevelt, & Maiti, 1994). In correspondence with the maturation of the parasympathetic innervation of the heart (Dalton, Dawes, & Patrick, 1983; Freeman, Garite, Nageotte, & Miller, 2012), fetal HRV has been shown to increase across gestation (DiPietro et al., 1996, 2004; Doyle et al., 2015); alterations in the in utero environment have been associated with individual differences in both level and change in fetal HRV across pregnancy (DiPietro et al., 1996, 2004; Doyle et al., 2015). Maternal contextual and experiential factors, including psychological distress, socioeconomic status, and culture, have been associated with differences in both level and developmental trajectories of fetal HRV (DiPietro et al., 1996, 2004; Pressman, DiPietro, Costigan, Shupe, & Johnson, 1998). These factors are assumed to be related to differences in the intrauterine milieu, which, in turn, shape the development of the fetal autonomic nervous system. Most studies investigating the impact of these factors demonstrate a negative association between maternal stressors and fetal HRV; however, some have found evidence of accelerated development in the context of in utero stressors (e.g., DiPietro et al., 2002), and others report no direct association (e.g., Doyle et al., 2015), though indirect effects via placental gene methylation have been observed (Monk et al., 2016).
Past research with children and adults has shown that resting HRV is associated with emotional, behavioral, and psychological functioning, such that higher resting HRV is associated with better emotional responding and regulation, whereas lower resting HRV is associated with greater risk for psychopathology and emotional problems (Appelhans & Luecken, 2006; Beauchaine, 2001; Beauchaine, Gatzke-Kopp, & Mead, 2007; Calkins, Graziano, & Keane, 2007). Fetal HRV during the third trimester, the developmental period when fetal HRV is most reliably measured and when the autonomic nervous and cardiovascular systems are most mature, also has been related to postnatal regulation such that lower resting fetal HRV predicts more difficult infant temperament (DiPietro, Ghera, & Costigan, 2008). Resting HRV also has been shown to demonstrate good rank-order predictability to 2 and 10 years of age (Dipietro et al., 2007; DiPietro, Costigan, Pressman, & Doussard-Roosevelt, 2000; Thomas, Haslum, MacGillivray, & Golding, 1989), suggesting that it is a relevant index of future child functioning. We are unaware of research that has assessed maternal abuse history in relation to her child’s HRV; however, the behavioral, emotional, and psychological difficulties that have been reported in children of previously abused women are consistent with individual differences in parasympathetic regulatory capacities.
Sleep as a Mediator
There are data indicating that resting fetal HRV likely will vary as a function of maternal abuse history, yet the mechanisms through which this difference may occur are unclear. Across the range of DOHaD studies, disrupted sleep has received little attention as a potential mediator shaping fetal development despite its strong associations with mood disturbance (Adrien, 2002; Morrison, McGee, & Stanton, 1992), and, relevant here, abuse histories (Glod, Teicher, Hartman, Harakal, & McGreenery, 1997; Greenfield, Lee, Friedman, & Springer, 2011; Sadeh et al., 1995).
Children and adolescents who are the victims of abuse have significant difficulties with sleep, as evidenced by increased nocturnal activity and impaired sleep maintenance (Beitchman et al., 1992; Glod et al., 1997; Moore, 1989; Sadeh et al., 1995). Sleep problems are common among individuals who have suffered abuse as a child (Briere & Runtz, 1987; Kendall-Tackett, 2002; Teegen, 1999), even in the absence of other known sequelae, including depression and obesity. These sleep disturbances have been shown to persist into adulthood (Greenfield et al., 2011; Teegen, 1999) and can contribute to significant physical and mental health problems (Atkinson & Davenne, 2007; Morrison et al., 1992). Believed to be the result of both psychological factors (e.g., feeling that sleep is not a safe state; Moore, 1989) and biological ones (e.g., increased activation of the sympathetic adrenergic system results in difficulties initiating and maintaining sleep; Mellman & Hipolito, 2006; Ouyang, Hellman, Abel, & Thomas, 2004), maternal sleep may have significant relevance for fetal development. Past research investigating sleep quality during pregnancy has found that sleep disturbances are associated with heightened risk for preterm delivery, longer labors, and a greater rate of cesarean sections (Chang, Pien, Duntley, & Macones, 2010; Lee & Gay, 2004; Okun, Roberts, Marsland, & Hall, 2009), indicating that maternal prenatal sleep quality affects the in utero environment, and thus possibly, fetal development.
The Current Study
Using data from a sample of pregnant adolescents, a population of women at heightened risk for childhood abuse (Hillis et al., 2004), the present study sought to examine associations among maternal childhood abuse history, maternal sleep disturbances during pregnancy, and fetal HRV. Specifically, we tested the following research questions: (a) is emotional abuse that the mother suffered as a child associated with resting fetal HRV during the third trimester and (b) is this association mediated by maternal sleep disturbances during pregnancy? We hypothesized that greater emotional abuse would be associated with lower fetal HRV, and that this association would be mediated by greater maternal sleep disturbances. The current study focused on emotional abuse because it is relatively common in nonclinical samples such as the one investigated here.
Past research has identified a number of correlates of maternal emotional abuse, maternal sleep disturbances, and fetal neurobehavioral development. For example, maternal age (DiPietro et al., 1996), ethnicity (Ogueh & Steer, 1998), gestational age at assessment (DiPietro et al., 2004), family income, and fetal sex (Pressman et al., 1998) each have been associated with fetal neurobehavioral development. To rule out the possibility that these variables are exclusively responsible for any observed associations, we incorporated these as covariates in our investigation. Given that women with histories of interpersonal psychological trauma are at heightened risk for psychiatric difficulties during pregnancy (Seng, D’Andrea, & Ford, 2014), that depressed pregnant women tend to have more disturbed sleep (Field et al., 2007), and that prenatal depression has been associated with fetal neurobehavioral development (Field, Diego, & Hernandez-Reif, 2006; Monk et al., 2000, 2004), we also considered the role that maternal depressive symptoms may play in the association between women’s childhood history of emotional abuse and fetal HRV.
Method
Participants
Participants were part of an ongoing research project examining the effects of prenatal stress and nutrition on child development (N = 292). Pregnant adolescents, ages 14–19, were recruited through the Departments of Obstetrics and Gynecology at Columbia University Medical Center and Weill Cornell Medical College. All had a healthy pregnancy at the time of recruitment. Participants were excluded if they acknowledged smoking tobacco or use of recreational drugs, or lacked fluency in English. Participants also were excluded on the basis of frequent use of the following: nitrates, steroids, beta blockers, and triptans. None of these participants were taking psychiatric medications.
Recruitment procedures allowed for participant enrollment in either the first or second trimester of pregnancy. Most participants enrolled when they were 13–16 weeks pregnant (75.34%); the remaining women were 24–27 weeks pregnant. Individuals who were consented for enrollment but did not complete the first-trimester protocol (n = 17), and those who did not complete the abuse questionnaire (n = 13) were excluded from analyses. This yielded a final analytic sample of n = 262. Pregnant adolescents included in the current analyses were on average 17.81 years (SD = 1.29) at enrollment; 88.28% self-identified as Latina. The median household income was $16,000-$25,000 (range = $0-$15,000 to $101,000-$250,000). Forty-three percent of the fetuses were female.
Procedures
Participants completed laboratory sessions when they were 13–16, 24–27, and 34–37 weeks pregnant. At each session, participants completed a number of self-report questionnaires, including a measure of prenatal sleep quality. They also reported on household demographic variables and elements of their medical history. At enrollment, participants completed a retrospective report of childhood abuse history. During the third trimester of pregnancy (34–37 weeks), participants completed a fetal monitoring session that occurred in the late morning or afternoon; they also self-reported depressive symptoms during this session. Medical records were culled for relevant medical information, including the baby’s sex. See Doyle et al. (2015) for additional details about the study design and procedures. All enrolled participants provided written informed consent, and all procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute/Columbia University Medical Center.
Measures
Demographic covariates
At enrollment, participants reported on the following information: the participant’s age at enrollment (in years), her ethnicity (0 = not Latina, 1 = Latina), the child’s sex (0 = female, 1 = male), and their household annual income (1 = $0-$15,000, 2 = $15,000-$25,000, 3 = $26,000-$50,000, 4 = $51,000-$100,000, 5 = $101,000-$250,000, and 6 = above $250,000). The mother’s gestational age at the fetal session was measured in weeks.
Maternal history of emotional abuse
When participants enrolled in the study, they completed the Childhood Trauma Questionnaire—Short Form (CTQ-SF; Bernstein & Fink, 1998). This 28-item retrospective self-report measure indexes abuse and neglect during childhood. Respondents are asked to rate on a 5-point Likert-type scale (1 = never and 5 = very often) how frequently they experienced certain events. This scale yields five subscales: emotional abuse, emotional neglect, sexual abuse, physical neglect, and physical neglect; the five-item emotional abuse scale was used in the current study (α = 0.84). An example item reads “[When I was growing up] people in my family called me things like ‘stupid,’ ‘lazy,’ or ‘ugly.’” For the majority of participants (n = 199), this questionnaire was completed at the 13–16 week session; participants who enrolled in the second trimester (n = 64) completed this questionnaire when they were 24–27 weeks pregnant. Past research with both adolescent and adult samples has demonstrated that the CTQ-SF has good sensitivity and excellent convergent and discriminant validity (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997; Bernstein et al., 1994, 2003; Scher, Stein, Asmundson, McCreary, & Forde, 2001). The continuous emotional abuse score was used in all analyses; however, 89 participants (33.97%) scored greater than an 8 on the emotional abuse subscale, the CTQ-SF criterion for emotional abuse.
Maternal depressive symptoms
Maternal depressive symptoms were assessed using the Reynolds Adolescent Depression Scale (Reynolds, 1987) at study enrollment and again at the 34–37 week session. This 30-item self-report measure asks respondents to rate on a 4-point Likert-type scale (0 = almost never and 3 = most of the time) how frequently in the past week they felt a number of different depressive symptoms. An example item reads “I felt like crying” (α = 0.89 for enrollment and α = 0.86 for the 34–37 week assessment). The reliability and validity of this measure is well established (e.g., Reynolds, 1994; Reynolds & Mazza, 1998). The two assessments of women’s depressive symptoms were used as indicators of the maternal depressive symptoms latent variable.
Maternal sleep disturbances
Maternal sleep disturbances were measured at 13–16, 24–27, and 34–37 weeks gestation using the Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), a self-rated questionnaire that indexes sleep quality and duration over the previous month. In addition to being asked questions relevant to sleep times and duration, participants were asked to rate on a 4-point Likert-type scale (where 0 = not during the past month and 3 = three or more times a week), how frequently they had difficulty sleeping due to a number of reasons. The 9-item sleep disturbances subscale was used in the current study (e.g., “During the past month, how often have you had trouble sleeping because you wake up in the middle of the night or early morning?”; α = 0.66, 0.71, and 0.74 at the 13–16, 24–27, and 34–37 week sessions, respectively). Higher scores on this subscale indicate greater sleep problems. Building on past research that demonstrates that women’s sleep profiles tend to remain stable across pregnancy (Field et al., 2007; Lee, 1998; Okun & Coussons-Read, 2007), we planned to use the sleep disturbance scores from the 13–16, 24–27, and 34–37 weeks gestation assessments as three indicators of the latent variable, sleep disturbances, though we also confirmed this approach based on investigation of our data (see below). Previous research with pregnant women has shown that the Pittsburgh Sleep Quality Index is a valid and psychometrically sound measure of prenatal sleep quality (Carpenter & Andrykowski, 1998).
Fetal heart rate variability
When women were 34–37 weeks pregnant, they completed a fetal monitoring session. During this 20-min session, participants reclined in a semirecumbent position as fetal movement and fetal heart rate were acquired using a Toitu MT 325 fetal actocardiograph (Toitu Co., Ltd, Tokyo). The Toitu detects fetal heart rate and fetal movement via a single transabdominal Doppler transducer and processes this signal through a series of filters. For the duration of the monitoring session, women remained alone in the quiet laboratory room (without cellular phones or other distractions), and were asked to relax as much as possible without falling asleep.
Fetal heart rate and fetal movement data were collected from the output port of the Toitu MT 325 and digitized at 50 Hz using a 16-bit A/D card (National Instruments 16XE50). Data were analyzed offline using custom MATLAB 8.3 scripts (Mathworks Inc., Natrick, MA) developed for this project. As a first step in preprocessing, fetal heart rate below 80 beats per minute (bpm) or above 200 bpm was linearly interpolated and then low-pass filtered at 3 Hz using a 16-point finite impulse response filter. Mean and standard deviation of the resulting fetal heart rate were taken over noninterpolated values. Filtered fetal heart rate was further examined for artifact in the following way: times at which the absolute sample to sample (20 ms) change in fetal heart rate exceeded 5 bpm were found, and fetal heart rate was marked as an artifact until it returned to within 5 bpm of the previous value. The resultant gaps were linearly interpolated. Following previously published reports (DiPietro et al., 2007; Doyle et al., 2015; Fleisher, DiPietro, Johnson, & Pincus, 1997), the standard deviation of the fetal heart rate over this 20-min period was used as this study’s measure of resting fetal HRV.
Analytic strategy
Structural equation modeling was used to test the proposed models (Schumacker & Lomax, 1996). Models were parameterized using the Mplus 7.4 software package (Muthén & Muthén, 1998–2012), using the robust maximum likelihood estimator. This estimator accommodates nonnormal data by adjusting standard errors using the Huber-White sandwich estimator. Model fit was examined using a number of fit indices, including the comparative fit index (CFI; Bentler, 1990), the Tucker-Lewis index (TLI; Tucker & Lewis, 1973), and the root mean square error of approximation (RMSEA; Browne & Cudeck, 1993). CFI and TLI values above 0.95 and RMSEA values below 0.06 indicate good model fit (Hu & Bentler, 1999). Mediation was tested using the model indirect command in Mplus.
Of the 262 women included in this study, 76 (29.01%) did not complete the third-trimester fetal monitoring session. Much of this missing data is because the study protocol initially only administered the fetal monitoring session to a random sample of participants; this resulted in 24 of these missing data points. In addition, a few of our participants missed the session because they were on bed rest or because they delivered before the fetal monitoring session occurred (n = 10); they aborted or miscarried the child (n = 3); they had maternal discomfort during the monitoring session (n = 1); they missed their appointments for unknown reasons (n = 11); or they withdrew their enrollment in the study prior to this session (n = 27). Sixteen participants (6.11%) were identified as outliers (defined as 2 SD above or below the mean) based on either their fetal heart rate (n = 5) or their fetal HRV values (n = 11); the fetal HRV values for these participants were set to missing. One participant’s (.5%) fetal data file was lost during the data transfer process, and fetal data was not collected for two participants (1%) because of an equipment failure. Participants who missed the fetal monitoring session did not differ from the complete sample on any of the variables included in the current study. Rather than eliminating these observations when testing our research questions, we conducted all analyses using full information maximum likelihood (Arbuckle, 1996), a missing data technique that uses all available information to produce estimates that have been shown to be unbiased and more efficient than those produced via other methods of handling data that are missing at random (e.g., listwise deletion and pairwise deletion; Allison, 2003). To strengthen our confidence in the robustness of our findings, we repeated all analyses without these observations included (i.e., we used listwise deletion). Both methods of handling missing data yielded the same pattern of results. Thus, we present the results from the models in which full information maximum likelihood was used, given its demonstrated strengths.
To test our first research question (i.e., is emotional abuse that the mother suffered as a child associated with fetal resting HRV during the third trimester of pregnancy?), our measure of fetal HRV was regressed on the maternal emotional abuse variable. To examine the mediating role of maternal sleep (i.e., the second research question), the sleep disturbances latent variable was added to this model, as a mediator of the association between maternal emotional abuse and fetal HRV. That is, a path was estimated from maternal emotional abuse to the sleep disturbances latent variable, as was a path from the sleep disturbances latent variable to fetal HRV. In addition, fetal HRV was regressed on maternal emotional abuse. Paths were estimated from each of the demographic covariates (i.e., the mother’s age at enrollment, maternal ethnicity, family income, and the child’s sex) to fetal HRV. Nonsignificant paths from these demographic control variables were removed from the final model to preserve model parsimony. To examine the role that maternal depressive symptoms plays in these associations, fetal HRV was regressed on the maternal depressive symptoms latent variable. In addition, the maternal depressive symptoms latent variable was regressed on maternal emotional abuse.
Results
Descriptive statistics and bivariate correlations
Means, standard deviations, and bivariate correlations between study variables are presented in Table 1. Associations among variables were largely as expected, such that maternal emotional abuse as a child was positively associated with maternal sleep disturbances at 13–16 (r = .29, p < .01), 24–27 (r = .22, p < .05), and 34–37 (r = .23, p < .01) week gestation. The various measures of maternal sleep disturbances were positively associated with one another (rs = .44 to .50, p < .01). The two measures of maternal depressive symptoms also were significantly associated with one another (r = .64, p < .01). Maternal sleep disturbances (rs = −.10 to −.27) and emotional abuse (r = −.12) were negatively correlated with resting fetal HRV; not all of these associations were statistically significant.
Table 1.
Descriptive statistics and bivariate correlations among study variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Maternal EA | — | |||||||||||
| 2. PSQI at 13–16 weeks | .29** | — | ||||||||||
| 3. PSQI at 24–27 weeks | .22** | .44** | — | |||||||||
| 4. PSQI at 34–37 weeks | .23** | .50** | .46** | — | ||||||||
| 5. Fetal resting HRV | −.12 | −.27** | −.12 | −.10 | — | |||||||
| 6. RADS at enrollment | .53** | .41** | .26** | .26** | −.19* | — | ||||||
| 7. RADS at 34–37 weeks | .36** | .22* | .30** | .27** | −.16 | .64** | — | |||||
| 8. Maternal age | .06 | .04 | −.03 | −.01 | −.09 | −.04 | −.07 | — | ||||
| 9. Maternal ethnicitya | −.19** | −.06 | −.05 | −.07 | .15 | −.06 | −.07 | −.07 | — | |||
| 10. GA at fetal session | −.07 | .14 | −.03 | .08 | −.20** | −.06 | −.05 | .07 | .12 | — | ||
| 11. Family income | .04 | .05 | .12 | .05 | .08 | .07 | −.05 | −.04 | −.11 | −.09 | — | |
| 12. Child sexb | .01 | .08 | −.08 | −.10 | .07 | .10 | .07 | .03 | −.01 | −.04 | .03 | — |
| Mean | 8.34 | 1.64 | 1.55 | 1.71 | 7.69 | 64.24 | 68.06 | 17.81 | — | 35.38 | 1.81 | — |
| SD | 4.29 | 0.62 | 0.69 | 0.70 | 2.24 | 13.87 | 11.55 | 1.29 | — | 1.84 | 0.87 | — |
| Range | 5–24 | 0–3 | 0–3 | 0–3 | 3.26–14.45 | 34–110 | 44–96 | 14–20 | — | 30–42 | 1–5 | — |
| n | 262 | 202 | 206 | 185 | 159 | 256 | 162 | 258 | 256 | 229 | 163 | 221 |
Note: EA, Emotional abuse subscale of the Childhood Trauma Questionnaire—Short Form; PSQI, sleep disturbances subscale of the Pittsburgh Sleep Quality Index; HRV, heart rate variability; RADS, Reynolds Adolescent Depression Scale; GA, gestational age.
0 = Not Latina, 1 = Latina.
0 = Female, 1 = Male.
p < .05.
p < .01.
Results from models testing our research questions
To test our first research question, fetal HRV was regressed on the mother’s emotional abuse score. Contrary to expectation, there was no direct association between maternal abuse history and fetal HRV, though the association was in the predicted direction (β = −0.06, p = .13).
To test our second research question, the sleep disturbances latent variable was added to the previous model, as a potential mediator of the association between emotional abuse and fetal HRV. Because it is now recognized that significant mediation can be observed in the absence of significant total effects (and in accordance with the advice of quantitative experts, who recommend that investigators not require a significant total effect before proceeding with tests of mediation; Hayes, 2009; Mackinnon, Krull, & Lockwood, 2000; Mathieu & Taylor, 2006; Rucker, Preacher, Tormala, & Petty, 2011; Shrout & Bolger, 2002), we proceeded to test whether there was an indirect effect of emotional abuse on fetal HRV, operating through maternal sleep disturbances.
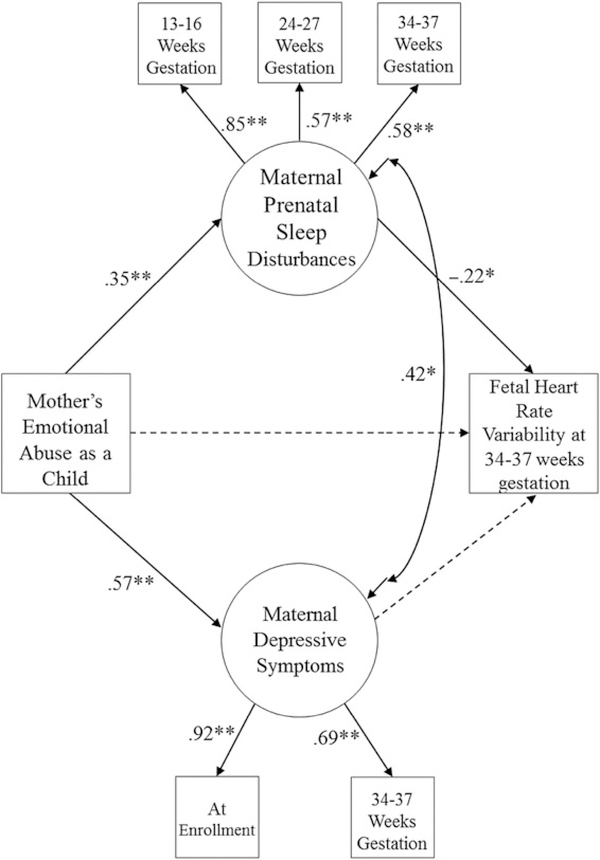
Figure 1 presents the results from this final model. As can be seen in this figure, maternal emotional abuse as a child was associated with greater sleep disturbances across pregnancy (β = 0.35, p < .01), which in turn were associated with lower resting fetal HRV (β = −0.22, p < .05). This indirect effect (from emotional abuse, through sleep disturbances, to fetal HRV) was statistically significant (β = −0.08, p < .05). As described above, this significant indirect effect (even in the absence of a significant total effect) can be interpreted as evidence of mediation (Hayes, 2009; Rucker et al., 2011; Shrout & Bolger, 2002). Maternal emotional abuse was associated with greater maternal depressive symptoms (β = 0.57, p < .01), but maternal depressive symptoms were not associated with fetal HRV when considered in a model with maternal sleep disturbances. This model fit the data well, χ2 (8) = 10.12, p = .26, CFI = 0.99, TLI = 0.98, RMSEA = 0.03. None of the demographic control variables (i.e., maternal age, ethnicity, gestational age at the fetal assessment, family income, and fetal sex) were significantly related to fetal HRV, and thus were not retained in the final model.
Figure 1.
Final model relating maternal childhood emotional abuse, sleep disturbances during pregnancy, and fetal heart rate variability; χ2 (8) = 10.12, p = .26, comparative fit index = 0.99, Tucker-Lewis index = 0.99, root mean square error of approximation = 0.03. *p < .05, **p < .01. All presented parameter estimates are standardized. The dashed line in this figure indicates a path that was estimated but that was not statistically significant. The indirect effect of maternal childhood emotional abuse through maternal prenatal sleep disturbances to fetal heart rate variability is statistically significant (β = −0.08, p < .05).
Discussion
Using data from a large sample of pregnant adolescents, the current study investigated whether pregnant women’s experiences of prior emotional abuse were associated with their fetus’ resting HRV, an index of parasympathetic regulation that is thought to underlie the many psychological and emotion regulatory difficulties identified in children of abused mothers. Contrary to expectation, this study did not find evidence of a direct association between maternal emotional abuse and variation in fetal HRV during the third trimester of pregnancy. However, there was a significant indirect effect of abuse, operating via maternal sleep disturbances across pregnancy. This effect was observed above and beyond the influence of maternal depressive symptoms, which are a common correlate of disrupted sleep and a history of childhood abuse. Although several authors have hypothesized an in utero effect of maternal abuse history, this is the first study to show maternal abuse-related differences in children’s regulatory capacities prior to birth. These findings suggest that pregnant women’s sleep quality, a modifiable health-related behavior, may be an important target for future research, as sleep disturbances appear to be part of how mothers’ experiences with abuse impact the next generation.
Our findings add to a growing body of literature that supports the DOHaD model, extending previous research by considering an alternative influence on fetal development, maternal childhood history of emotional abuse, and by examining an underexplored mediator of this association, maternal sleep. Uniquely, this study provides evidence that maternal experiences prior to conception can have an impact on the developing child, operating through her experience-related behaviors during pregnancy. Although past research guided by the DOHaD model has shown that other aspects of maternal mental and physical health (e.g., psychological distress and nutrition) are related to fetal and child development, this is the first study to our knowledge to relate either abuse history or maternal sleep with fetal neurobehavioral development. In identifying sleep as a mediator of the association between maternal childhood emotional abuse and lower fetal HRV, we provide additional support for the DOHaD model, though these data do not give insight into the specific biological mechanisms through which sleep may alter the in utero environment and, thereby, impact the developing child. This is an important limitation of the current study. Elevated levels of maternal glucocorticoid production may play a role in this association. Past research examining the impact of abuse on child and adolescent hypothalamus-pituitary-adrenal axis functioning has found that cortisol is dysregulated in the context of past abuse (Cicchetti, Rogosch, Gunnar, & Toth, 2010; Gunnar & Vazquez, 2001; Yehuda & Bierer, 2007) and that this dyregulation often persists into adulthood (Heim, Newport, Bonsall, Miller, & Nemeroff, 2003; Stein, Yehuda, Koverola, & Hanna, 1997). Although glucocorticoids play a vital role in supporting healthy fetal development (Harris & Seckl, 2011; Liggins, 1994), elevated maternal cortisol levels have been associated with alterations in fetal (e.g., changes in fetal HRV trajectories; Doyle et al., 2015) and infant (e.g., more difficult temperament; O’Connor, Bergman, Sarkar, & Glover, 2013; Werner et al., 2013) development, as well as epigenetic changes in the placenta (Monk, Spicer, & Champagne, 2013; Monk et al., 2016). Maternal systemic inflammation secondary to sleep problems also may account for the association between maternal sleep disturbances and lower fetal HRV. Sleep disruptions during pregnancy have been associated with elevated levels of circulating cytokines (Okun & Coussons-Read, 2007; Okun et al., 2009); in a separate body of literature, maternal inflammation during pregnancy has been shown to shape fetal brain development (Ponzio, Servatius, Beck, Marzouk, & Kreider, 2007; Smith, Li, Garbett, Mirnics, & Patterson, 2007). Furthermore, systemic inflammation has been posited to be a causal mechanism in preterm birth (Wadhwa, Culhane, Rauh, & Barve, 2001; Wadhwa, Entringer, Buss, & Lu, 2011). Though these are plausible explanations for the associations observed in the current study, more attention needs to be paid to maternal sleep, its biological correlates during pregnancy, and fetal development.
This study is the first to our knowledge to identify maternal nighttime functioning as a mediator in DOHaD models. Previous studies examining the mechanisms through which maternal experiences shape fetal development have largely utilized indices of daytime functioning (e.g., diurnal or daytime cortisol; Kivlighan, DiPietro, Costigan, & Laudenslager, 2008; Monk et al., 2011) or those collapsed over day and night (e.g., blood pressure; Spicer et al., 2013), possibly obscuring unique nighttime effects that are apparent in these data. Given that past research has shown that nighttime-specific biological functioning (e.g., the presence or absence of a nighttime drop in blood pressure; O’Brien et al., 2000) has unique and important implications for adult health, it seems that further investigation of nighttime mediators like sleep could give specific insight into the ways through which pregnant women’s experiences are transmitted to the developing child.
Strengths of this study include consideration of a number of covariates identified by previous research, including demographic (e.g., maternal age) and psychological factors (e.g., maternal depressive symptoms). That our findings remained even after controlling for these confounding variables strengthens our confidence in this study’s conclusion that maternal childhood emotional abuse is associated with fetal resting HRV in part via maternal prenatal sleep, independent of other factors such as maternal depression. The fact that this association persisted even after controlling for maternal depressive symptoms has implications for future research aimed at identifying the biological mechanisms through which sleep impacts fetal development. Specifically, these findings suggest that sleep disturbances exert an effect on fetal HRV via mechanism that are unrelated to associations between depression and fetal development, which may help narrow the field of possible mechanisms for future research.
These findings have a number of implications for intervention, including the following. That differences in regulatory capacities are observable in utero suggests that intervening after the child is born may miss aspects of the intergenerational transmission of trauma that already occur prior to birth and potentially contribute to the psychological and emotional regulatory difficulties for which children of abused mothers are at heightened risk. Given that lower resting fetal HRV has been associated with more difficult infant temperament (DiPietro et al., 2008), this study’s findings also suggest that children of abused mothers may enter the world more physiologically reactive and more difficult to soothe. Preventive programs that focus on teaching mothers behavioral techniques that can help to settle and soothe reactive infants (e.g., Werner et al., 2016) may be particularly high yield when implemented in this population. Child HRV continues to undergo development throughout childhood (and has been shown to be influenced by contextual factors, including parenting behaviors; e.g., Perry et al., 2014), suggesting that interventions of this nature may help children compensate for lower fetal HRV.
Despite its strengths, this study has some limitations. For example, participants self-reported their abuse history and their sleep disturbances, and thus there may be a common reporter bias. Although both of these widely used measures have been shown to have good reliability and validity, future research should examine whether these associations vary when utilizing more objective measures of sleep and abuse (e.g., data obtained via actigraphy, momentary ambulatory measures of sleep, or a laboratory-based sleep study and independent reports of abuse). An additional limitation of the current study is that the measure of childhood emotional abuse did not provide very detailed information about the timing or chronicity of the abuse. Given that these mothers are still quite young (i.e., 14–19 years at enrollment), it is possible that the reported emotional abuse did not occur very long ago (or potentially is still ongoing); the current study was unfortunately unable to examine whether variables of this nature moderate the associations reported in the current manuscript. Future research should collect more detailed information about women’s abuse histories and should examine whether these and other characteristics of the abuse moderate any of the associations described here. The current study focused on emotional abuse because it has been argued to be the most pernicious form of childhood maltreatment (Berzenski et al., 2014; Spertus et al., 2003; Teicher et al., 2006); however, the CTQ-SF also assesses other types of abuse that the current study did not consider in its primary analyses. In post hoc sensitivity analyses, we reran all models using the total abuse score of the CTQ-SF, and found similar results. This effect, however, seems to be driven by participants’ reports of emotional abuse, as the other subscales were not related to maternal sleep disturbances when considered in models together or by themselves. As one of the most common forms of childhood abuse (Spertus et al., 2003), these results suggest that emotional abuse found in nonclinical, community samples such as ours may have consequences across generations. Although one of the strengths of the current study is that it identifies a mediator of the association between emotional abuse and fetal heart rate variability (i.e., sleep), there are many other potential mediators of this association, including maternal nutrition and obesity, and stress. Future research should consider the role that these variables may play in explaining or modifying the association between abuse history and fetal development. In a similar vein, emotional abuse is only one of many potential contributors to sleep disturbance; additional research, focusing on other influences on sleep quality is needed. Finally, the results of this study can only be generalized to a specific population, that is, adolescent mothers residing in a similar urban area to New York City. This population was ideally suited for the current study (given that they are at heightened risk for childhood abuse history); however, these findings may not extend to other populations, such as adult women, whose pregnancy and childhood abuse experiences may be separated by a larger number of years. Future research should examine the extent to which these associations replicate when using data from adult women with or without childhood abuse histories.
The current study adds an in utero time point to the growing body of literature demonstrating that women’s adverse childhood experiences can exert effects on the next generation. Utilizing novel data collected from pregnant teenagers and their fetuses, this study is the first to provide evidence that at least part of the legacy of childhood abuse is passed down to the next generation prior to birth. These results point to a modifiable mechanism, maternal sleep quality, through which maternal abuse history impacts fetal parasympathetic nervous system functioning, and suggests that improving maternal sleep in those with abuse histories may help the pregnant woman, as well as, potentially, protect the neurobehavioral development of the next generation.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R01MH077144 and R01MH093677) and by a postdoctoral fellowship in behavioral medicine awarded by the Herbert H. and Ruth S. Reiner fellowship fund.
References
- Adrien J (2002). Neurobiological bases for the relation between sleep and depression. Sleep Medicine Reviews, 6, 341–351. [PubMed] [Google Scholar]
- Allison PD (2003). Missing data techniques for structural equation modeling. Journal of Abnormal Psychology, 112, 545–557. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. ReviewofGeneralPsychology,10,229–240. [Google Scholar]
- Arbuckle JL (1996). Full information estimation in the presence of incomplete data In Marcoulides GA & Schumacker RE (Eds.), Advanced structural equation modeling (pp. 243–277). Mahwah, NJ: Erlbaum. [Google Scholar]
- Atkinson G, & Davenne D (2007). Relationships between sleep, physical activity and human health. Physiology & Behavior, 90, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, & Mead HK (2007). Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology, 74, 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitchman JH, Zucker KJ, Hood JE, DaCosta GA, Akman D, & Cassavia E (1992). A review of the long-term effects of child sexual abuse. Child Abuse and Neglect, 16, 101–118. [DOI] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107, 238–246. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, & Handelsman L (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 340–348. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, & Fink L (1998). Childhood Trauma Questionnaire: A retrospective self-report: Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, … Ruggiero J (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry, 151, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, … Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect, 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Berzenski SR, Yates TM, & Egeland B (2014). A multidimensional view of continuity in intergenerational transmission of child maltreatment In Korbin JE & Krugman RD (Eds.), Handbook of child maltreatment (pp. 115–129). Dordrecht: Springer. [Google Scholar]
- Bifulco A, Moran PM, Ball C, Jacobs C, Baines R, Bunn A, & Cavagin J (2002). Childhood adversity, parental vulnerability and disorder: Examining inter-generational transmission of risk. Journal of Child Psychology and Psychiatry, 43, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Briere J, & Runtz M (1987). Post sexual abuse trauma data and implications for clinical practice. Journal of Interpersonal Violence, 2, 367–379. [Google Scholar]
- Browne MW, & Cudeck R (1993). Alternative ways of assessing model fit In Bollen KA & Long JS (Eds.), Testing structural equation models (pp. 136–162). Newbury Park, CA: Sage. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, & Keane SP (2007). Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology, 74, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research, 45, 5–13. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Pien GW, Duntley SP, & Macones GA (2010). Sleep deprivation during pregnancy and maternal and fetal outcomes: Is there a relationship? Sleep Medicine Reviews, 14, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Carlson V (Eds.) (1989). Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. New York: Cambridge University Press. [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, & Toth SL (2010). The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development, 81, 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KJ, Dawes GS, & Patrick JE (1983). The autonomic nervous system and fetal heart rate variability. American Journal of Obstetrics and Gynecology, 146, 456–462. [DOI] [PubMed] [Google Scholar]
- Danieli Y (Ed.). (1998). International handbook of multigenerational legacies of trauma. New York: Plenum Press. [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46, 737–746. [DOI] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81, 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro JA (2012). Maternal stress in pregnancy: Considerations for fetal development. Journal of Adolescent Health, 51, S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Bornstein MH, Hahn CS, Costigan K, & Achy-Brou A (2007). Fetal heart rate and variability: Stability and prediction to developmental outcomes in early childhood. Child Development, 78, 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield L, Costigan KA, Merialdi M, Nguyen RH, Zavaleta N, & Gurewitsch ED (2004). Fetal neurobehavioral development: A tale of two cities. Developmental Psychology, 40, 445–456. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Pressman EK, & Doussard-Roosevelt JA (2000). Antenatal origins of individual differences in heart rate. Developmental Psychobiology, 37, 221–228. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Ghera MM, & Costigan KA (2008). Prenatal origins of temperamental reactivity in early infancy. Early Human Development, 84, 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Hilton SC, Hawkins M, Costigan KA, & Pressman EK (2002). Maternal stress and affect influence fetal neurobehavioral development. Developmental Psychology, 38, 659–668. [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Hilton SC,& Johnson TR (1996). Fetal neurobehavioral development. Child Development, 67, 2553–2567. [PubMed] [Google Scholar]
- Dixon L, Browne K, & Hamilton-Giachritsis C (2005). Risk factors of parents abused as children: A mediational analysis of the intergenerational continuity of child maltreatment (Part I). Journal of Child Psychology and Psychiatry, 46, 47–57. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, & Buekens P (2003). Maternal stress and preterm birth. American Journal of Epidemiology, 157, 14–24. [DOI] [PubMed] [Google Scholar]
- Doyle C, Werner E, Feng T, Lee S, Altemus M, Isler JR, & Monk C (2015). Pregnancy distress gets under fetal skin: Maternal ambulatory assessment and sex differences in prenatal development. Developmental Psychobiology. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RK, Garite TJ, Nageotte MP, & Miller LA (Eds.). (2012). Fetal heart rate monitoring. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Field T, Diego M, & Hernandez-Reif M (2006). Prenatal depression effects on the fetus and newborn: A review. Infant Behavior and Development, 29, 445–455. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Figueiredo B, Schanberg S, & Kuhn C (2007). Sleep disturbances in depressed pregnant women and their newborns. Infant Behavior and Development, 30, 127–133. [DOI] [PubMed] [Google Scholar]
- Fleisher LA, Dipietro JA, Johnson TR, & Pincus S (1997). Complementary and non-coincident increases in heart rate variability and irregularity during fetal development. Clinical Science, 92(Pt. 4), 345–349. [DOI] [PubMed] [Google Scholar]
- Gavin AR, Hill KG, Hawkins JD, & Maas C (2011). The role of maternal early-life and later-life risk factors on offspring low birth weight: Findings from a three-generational study. Journal of Adolescent Health, 49, 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glod CA, Teicher MH, Hartman CR, Harakal T, & McGreenery CE (1997). Enduring effects of early abuse on locomotor activity, sleep, and circadian rhythms. Annals of the New York Academy of Sciences, 821, 465–467. [DOI] [PubMed] [Google Scholar]
- Glover V (2011). Annual Research Review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. Journal of Child Psychology and Psychiatry, 52, 356–367. [DOI] [PubMed] [Google Scholar]
- Greenfield EA, Lee C, Friedman EL, & Springer KW (2011). Childhood abuse as a risk factor for sleep problems in adulthood: Evidence from a US national study. Annals of Behavioral Medicine, 42, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstad H, & Schei B (1999). Pregnancy and delivery for women with a history of child sexual abuse. Child Abuse and Neglect, 23, 81–90. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13, 515–538. [DOI] [PubMed] [Google Scholar]
- Harris A, & Seckl J (2011). Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior, 59, 279–289. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, & Nemeroff CB (2003). Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Focus, 1, 282–289. [DOI] [PubMed] [Google Scholar]
- Hillis SD, Anda RF, Dube SR, Felitti VJ, Marchbanks PA, & Marks JS (2004). The association between adverse childhood experiences and adolescent pregnancy, long-term psychosocial consequences, and fetal death. Pediatrics, 113, 320–327. [DOI] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. [Google Scholar]
- Kendall-Tackett K (2002). The health effects of childhood abuse: Four pathways by which abuse can influence health. Child Abuse & Neglect, 26, 715–729. [DOI] [PubMed] [Google Scholar]
- Kinsella MT, & Monk C (2009). Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clinical Obstetrics and Gynecology, 52, 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivlighan KT, DiPietro JA, Costigan KA, & Laudenslager ML (2008). Diurnal rhythm of cortisol during late pregnancy: Associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology, 33, 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbin JE, & Krugman RD (2014). Handbook of child maltreatment. New York: Springer. [Google Scholar]
- Lee KA (1998). Alterations in sleep during pregnancy and postpartum: A review of 30 years of research. Sleep Medicine Reviews, 2, 231–242. [DOI] [PubMed] [Google Scholar]
- Lee KA, & Gay CL (2004). Sleep in late pregnancy predicts length of labor and type of delivery. American Journal of Obstetrics and Gynecology, 191, 2041–2046. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang L, Fang Z, Lin L, Wu C, & Huang Q (2010). Behavioral and neurobiological studies on the male progeny of maternal rats exposed to chronic unpredictable stress before pregnancy. Neuroscience Letters, 469, 278–282. [DOI] [PubMed] [Google Scholar]
- Liggins GC (1994). The role of cortisol in preparing the fetus for birth. Reproduction, Fertility and Development, 6, 141–150. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, & Lockwood CM (2000). Equivalence of the mediation, confounding, and suppression effect. Prevention Science, 1, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu JE, & Taylor SR (2006). Clarifying conditions and decision points for mediational type inferences in organizational behavior. Journal of Organizational Behavior, 27, 1031–1056. [Google Scholar]
- Mellman TA, & Hipolito MMS (2006). Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectrums, 11, 611–615. [DOI] [PubMed] [Google Scholar]
- Monk C, Feng T, Lee S, Krupska I, Champagne FA, & Tycko B (2016). Distress during pregnancy: Epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. American Journal of Psychiatry, 173, 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Bagiella E, Duong JK, Chen IS, … Altincatal A (2011). Effects of maternal breathing rate, psychiatric status, and cortisol on fetal heart rate. Developmental Psychobiology, 53, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Sloan RP, Trien L, & Hurtado A (2000). Maternal stress responses and anxiety during pregnancy: Effects on fetal heart rate. Developmental Psychobiology, 36, 67–77. [PubMed] [Google Scholar]
- Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, … Fifer WP (2004). Fetal heart rate reactivity differs by women’s psychiatric status: An early marker for developmental risk? Journal of the American Academy of Child & Adolescent Psychiatry, 43, 283–290. [DOI] [PubMed] [Google Scholar]
- Monk C, Spicer J, & Champagne FA (2012). Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Development and Psychopathology, 24, 1361–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS (1989). Disturbed attachment in children: A function in sleep disturbance, altered dream production and immune dysfunction. Part 1. Not safe to sleep: Chronic sleep disturbances in anxious attachment. Journal of Child Psychotherapy, 15, 99–111. [Google Scholar]
- Morrison DN, McGee R, & Stanton WR (1992). Sleep problems in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 31, 94–99. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2012). Mplus user’s guide (7th ed.). Los Angeles: Author. [Google Scholar]
- Myers JE (Ed.). (2002). American Professional Society on the Abuse of Children handbook on child maltreatment (3rd ed.). Thousand Oaks, CA: Sage. [Google Scholar]
- O’Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, … Mee F (2000). Use and interpretation of ambulatory blood pressure monitoring: Recommendations of the British Hypertension Society. British Medical Journal, 320, 1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Bergman K, Sarkar P, & Glover V (2013). Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental Psychobiology, 55, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V, & ALSPAC Study Team (2003). Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. Journal of Child Psychology and Psychiatry, 44, 1025–1036. [DOI] [PubMed] [Google Scholar]
- Ogueh O, & Steer PJ (1998). Ethnicity and fetal heart rate variation. Obstetrics and Gynecology, 91, 324–328. [DOI] [PubMed] [Google Scholar]
- Okun ML, & Coussons-Read ME (2007). Sleep disruption during pregnancy: How does it influence serum cytokines? Journal of Reproductive Immunology, 73, 158–165. [DOI] [PubMed] [Google Scholar]
- Okun ML, Roberts JM, Marsland AL, & Hall M (2009). How disturbed sleep may be a risk factor for adverse pregnancy outcomes a hypothesis. Obstetrical and Gynecological Survey, 64, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Hellman K, Abel T, & Thomas SA (2004). Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. Journal of Neurophysiology, 92, 2071–2082. [DOI] [PubMed] [Google Scholar]
- Pears KC, & Capaldi DM (2001). Intergenerational transmission of abuse: A two-generational prospective study of an at-risk sample. Child Abuse and Neglect, 25, 1439–1461. [DOI] [PubMed] [Google Scholar]
- Perry NB, Mackler JS, Calkins SD, & Keane SP (2014). A transactional analysis of the relation between maternal sensitivity and child vagal regulation. Developmental Psychology, 50, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant DT, Barker ED, Waters CS, Pawlby S, & Pariante CM (2013). Intergenerational transmission of maltreatment and psychopathology: The role of antenatal depression. Psychological Medicine, 43, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio NM, Servatius R, Beck K, Marzouk A, & Kreider TIM (2007). Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Annals of the New York Academy of Sciences, 1107, 118–128. [DOI] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42, 123–146. [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74, 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, & Maiti AK (1994). Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development, 59(2–3), 167–186. [PubMed] [Google Scholar]
- Pressman EK, DiPietro JA, Costigan KA, Shupe AK, & Johnson TRB (1998). Fetal neurobehavioral development: Associations with socioeconomic class and fetal sex. Developmental Psychobiology, 33, 79–91. [PubMed] [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, … Cox M (2008). Gene-environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Development, 79, 1377–1394. [DOI] [PubMed] [Google Scholar]
- Reynolds WM (1987). Reynolds Adolescent Depression Scale (RADS-20). Lutz, FL: PAR. [Google Scholar]
- Reynolds WM, & Mazza JJ (1998). Reliability and validity of the Reynolds Adolescent Depression Scale with young adolescents. Journal of School Psychology, 36, 295–312. [Google Scholar]
- Roberts R, O’Connor T, Dunn J, & Golding J (2004). The effects of child sexual abuse in later family life: Mental health, parenting and adjustment of offspring. Child Abuse and Neglect, 28, 525–545. [DOI] [PubMed] [Google Scholar]
- Rucker DD, Preacher KJ, Tormala ZL, & Petty RE (2011). Mediation analysis in social psychology: Current practices and new recommendations. Social and Personality Psychology Compass, 5, 359–371. [Google Scholar]
- Ryzhavskii BY, Sokolova TV, Uchakina RV, Fel’dsherov YI, Sapozhnikov YA, Vasil’eva EV, & Eremenko IR (2002). Effect of emotional stress experienced by female rats before pregnancy on brain development in their offspring. Bulletin of Experimental Biology and Medicine, 134, 126–129. [DOI] [PubMed] [Google Scholar]
- Sadeh A, McGuire JP, Sachs H, Seifer R, Tremblay A, Civita R, & Hayden RM (1995). Sleep and psychological characteristics of children on a psychiatric inpatient unit. Journal of the American Academy of Child & Adolescent Psychiatry, 34, 813–819. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, & Forde DR (2001). The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress, 14, 843–857. [DOI] [PubMed] [Google Scholar]
- Schumacker RE, & Lomax RG (1996). A beginner’s guide to structural equation modeling. Mahwah, NJ: Erlbaum. [Google Scholar]
- Seckl JR (2004). Prenatal glucocorticoids and long-term programming. European Journal of Endocrinology, 151(Suppl. 3), U49–U62. [DOI] [PubMed] [Google Scholar]
- Seng JS, D’Andrea W, & Ford JD (2014). Complex mental health sequelae of psychological trauma among women in prenatal care. Psychological Trauma: Theory, Research, Practice, and Policy, 6, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods, 7, 422–445. [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, & Patterson PH (2007). Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience, 27, 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotero M (2006). A conceptual model of historical trauma: Implications for public health practice and research. Journal of Health Disparities Research and Practice, 1, 93–108. [Google Scholar]
- Spertus IL, Yehuda R, Wong CM, Halligan S, & Seremetis SV (2003). Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse and Neglect, 27, 1247–1258. [DOI] [PubMed] [Google Scholar]
- Spicer J, Werner E, Zhao Y, Choi CW, Lopez-Pintado S, Feng T, … Monk C (2013). Ambulatory assessments of psychological and peripheral stress-markers predict birth outcomes in teen pregnancy. Journal of Psychosomatic Research, 75, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson EA, & Collins WA (2009). The development of the person: The Minnesota study of risk and adaptation from birth to adulthood. New York: Guilford Press. [Google Scholar]
- Stein MB, Yehuda R, Koverola C, & Hanna C (1997). Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biological Psychiatry, 42, 680–686. [DOI] [PubMed] [Google Scholar]
- Stevens-Simon C, & McAnarney ER (1994). Childhood victimization: Relationship to adolescent pregnancy outcome. Child Abuse and Neglect, 18, 569–575. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V, & Early Stress, Translational Research and Prevention Science Network. (2007). Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? Journal of Child Psychology and Psychiatry, 48, 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegen F (1999). Childhood sexual abuse and long-term sequelae In Maercker A, Schutzwohl M, & Solomon Z (Eds.), Post-traumatic stress disorder: A lifespan developmental perspective (pp. 97–112). Seattle, WA: Hogrefe & Huber. [Google Scholar]
- Teicher MH, Samson JA, Polcari A, & McGreenery CE (2006). Sticks, stones, and hurtful words: Relative effects of various forms of childhood maltreatment. American Journal of Psychiatry, 163, 993–1000. [DOI] [PubMed] [Google Scholar]
- Thomas PW, Haslum MN, MacGillivray I, & Golding MJ (1989). Does fetal heart rate predict subsequent heart rate in childhood? Early Human Development, 19, 147–152. [DOI] [PubMed] [Google Scholar]
- Tucker LR, & Lewis C (1973). A reliability coefficient for maximum likelihood factor analysis. Psychometrika, 38, 1–10. [Google Scholar]
- Wadhwa PD (2005). Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology, 30, 724–743. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, & Barve SS (2001). Stress and preterm birth: Neuroendocrine, immune/inflammatory, and vascular mechanisms. Maternal and Child Health Journal, 5, 119–125. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Entringer S, Buss C, & Lu MC (2011).The contribution of maternal stress to preterm birth: Issues and considerations. Clinics in Perinatology, 38, 351–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, & Garite TJ (2001). The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Progress in Brain Research, 133, 131–142. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, & Garite TJ (1993). The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. American Journal of Obstetrics and Gynecology, 169, 858–865. [DOI] [PubMed] [Google Scholar]
- Weinstock M (2005). The potential influence of maternal stress hormones on developmental and mental health of the offspring. Brain Behavior and Immunity, 19, 296–308. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, & Seckl JR (2001). Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology, 13, 113–128. [DOI] [PubMed] [Google Scholar]
- Werner EA, Gustafsson HC, Lee S, Feng T, Jiang N, Desai P, & Monk C (2016). PREPP: Postpartum depression prevention through the mother-infant dyad. Archives of Women’s Mental Health, 19, 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner EA, Zhao Y, Evans L, Kinsella M, Kurzius L, Altincatal A, … Monk C (2013). Higher maternal prenatal cortisol and younger age predict greater infant reactivity to novelty at 4 months: An observation-based study. Developmental Psychobiology, 55, 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, & Bierer LM (2007). Transgenerational transmission of cortisol and PTSD risk. Progress in Brain Research, 167, 121–135. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, & Bierer LM (2007). Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of Holocaust survivors. Archives of General Psychiatry, 64, 1040–1048. [DOI] [PubMed] [Google Scholar]