Abstract
Objectives:
The expanded quantitative urine culture (EQUC) protocol was used to compare the microbial abundance and diversity of voided urines obtained using a standard urine collection or using the Peezy midstream device versus paired peri-urethral specimens.
Methods:
62 female participants were assigned to 1 of 3 cohorts. One cohort used a standard clean catch mid-stream urine protocol that included a castile soap wipe, the second cohort used a Peezy mid-stream collection device with castile soap wipe and the third used the Peezy device without a castile soap wipe. Each participant watched a video that detailed the collection method. Prior to using the castile soap wipe, a peri-urethral swab was obtained to measure peri-urethral microbial abundance. Demographics and pelvic floor symptoms were assessed by validated questionnaires. Microbes were detected using EQUC. Diversity within each sample was analyzed using alpha diversity measures.
Results:
Paired peri-urethral and urine samples for each woman were analyzed and compared for species abundance, richness, and diversity. Bacterial profiles of Peezy-collected urines differed significantly by multiple diversity indices and had significantly reduced colony-forming units compared to paired peri-urethral swabs. In contrast, within the standard clean catch cohort, voided urine had higher abundance and richness than paired peri-urethral swabs.
Conclusions:
Compared to standard clean catch method, the Peezy urine collection device with and without the castile soap wipe resulted in urine with lower bacterial abundance that was distinct from the peri-urethra. Voided urine collected by Peezy may reduce post-bladder microbial contribution.
Keywords: Urine collection, female urinary microbiome (FUM), bacteria, specimen handling/methods
INTRODUCTION:
Contrary to long-held dogma, the bladder is not sterile; instead, it contains communities of microbes commonly referred to as the bladder microbiota. Transurethral catheterized urine specimens that were deemed “negative” by standard urine culture were shown to contain bacterial DNA via 16S ribosomal RNA gene sequencing [1]. An enhanced urine culture method called EQUC (expanded quantitative urine culture) then showed that these bacteria were alive [2]. This is not surprising because standard urine culture methods preferentially grow Escherichia coli and reproducibly fail to grow many microbes that were identified using 16S ribosomal gene sequencing [3,4]. In contrast to standard urine culture, EQUC involves plating larger amounts of urine onto various culture media and incubating samples in multiple conditions for a longer length of time. As such, EQUC reproducibly cultures more diverse organisms than standard urine culture, making it a more suitable culture technique to use when studying the lower urinary tract microbiota [2]. Analyzing urine samples obtained by transurethral catheterization by 16S ribosomal RNA sequencing and EQUC, our team and others have defined the bladder microbiota and associations of microbes with specific lower urinary tract symptoms [5–11].
Because transurethral catheterization for urine collection is invasive, it has limitations as a universal collection method for research of bladder microbiota, especially for longitudinal studies involving community populations. Although voided urine use is more practical, the typical “clean catch” method has been shown to contain high amounts of microbes associated with the vagina, vulva, and surrounding skin [12–14]. The presence of these contaminants can complicate the accurate depiction of bladder microbiota, as voided urines are more representative of the complete genitourinary tract [15].
The standard clean catch (SCC) method involves using a cleansing castile soap wipe, urinating the initial urine stream into the toilet, and then collecting midstream urine into a sterile cup [12]. Typically, there is no standard instruction for how much urine should be voided before midstream collection. The lack of standardization of the standard clean catch procedure and the varying physical characteristics of women may contribute to the contamination of these urine samples with the microbes residing in the urethra, vagina, vulva, and surrounding skin. Before microbiome researchers can use voided urine, we need to develop or identify a “cleaner” catch voided urine sample. Forte Medical has developed a device (Peezy) that discards approximately ten milliliters of urine prior to collection into a sterile container [16]. Previous studies of Peezy’s efficacy have produced conflicting results using standard urine culture techniques [17,18].
In this study, we used EQUC to compare microbial abundance and diversity of paired peri-urethral specimens to microbial abundance and diversity of voided urines obtained by standard clean catch urine collection method or the Peezy midstream device. We hypothesized that voided urines obtained using Peezy have less contribution from the urethra, peri-urethra, vulva and vagina than voided samples obtained by typical clean catch techniques.
METHODS:
This is a cross-sectional pilot cohort study of participants from two randomized trials with Loyola IRB-approval. The first trial randomized 40 women into one of two arms of a voided urine collection study (standard clean catch technique using a castile soap wipe versus a “modified clean catch” approach that did not involve a castile soap wipe). Only the 20 women enrolled into the standard clean catch method using a castile soap wipe were included in this analysis because our “modified technique” demonstrated no superiority over the standard clean catch technique. The second trial randomized 42 women to use the Peezy device with a castile soap wipe versus using the Peezy device without the castile soap wipe.
Recruitment:
Adult women 18 or older who were employees or students of the Loyola University Hospital System and/or Loyola University Health Sciences Campus were invited to participate in this IRB approved trial. Women were included if they could speak fluent English, were ambulatory, could view video-based instruction for the collection method, and answered yes to the query “do you feel your bladder is full enough to void.” Women were excluded if they had a history of recurrent UTI (3 or more UTI in the last year or 2 in the last six months), pelvic organ prolapse, urinary urgency incontinence or urinary stress incontinence. Other exclusion criteria included current use of antibiotics, post-menopausal status, pregnancy, current menstruation or the use of intermittent self-catheterization. Following a signed informed consent, participants completed three forms: Demographic information, the Pelvic Floor Distress Inventory-20 [19], and the UTISA questionnaire [20] in which the participant rates the severity and bother for seven common UTI symptoms. All participants were enrolled and samples were collected at the Loyola University Outpatient Urogynecology clinic and the Clinical Research Office, and all bacterial detection was performed at the Center for Translational Research and Education. Participants received a $5 gift card.
Sample Collection:
After watching a video detailing proper sample collection, each participant provided a peri-urethral swab and voided urine specimen. For women in the standard clean catch cohort, participants washed their hands with soap and water before collecting a peri-urethral sample with the provided swab. Participants then used a castile soap wipe to cleanse the peri-urethral area by wiping from front to back before partially voiding into the toilet, then collecting midstream urine in a sterile cup. For women in the two Peezy cohorts, participants washed their hands with soap and water before collecting a peri-urethral sample with the provided swab. Half of the participants, randomized to the Peezy with wipe, then used the castile soap wipe to cleanse the peri-urethral area by wiping from front to back before voiding into the device (PZW). The other half voided into the device without using a castile soap wipe (PZ). The Peezy device was held below the perineum and the participants urinated into the device, which allows the initial stream (approximately ten milliliters of urine) to pass through into the toilet (Figure 1). This initial stream causes the expansion of a cellulose sponge that, when engaged, forces the midstream urine into a sterile urine collection tube through a one-way valve. Once finished voiding, participants held the Peezy device over the toilet to allow excess urine to flow through the device. Participants then unscrewed the sterile collection tube from the device and placed the provided cap onto the tube.
Figure 1: Participant flow.
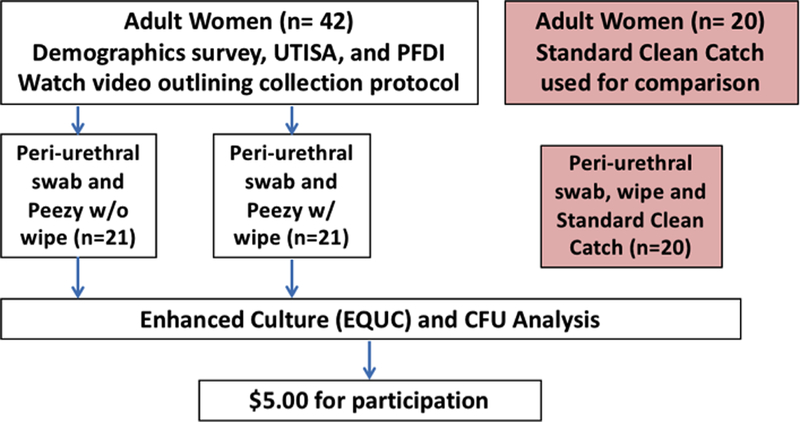
There were no losses or exclusions after randomization. Recruitment began June 2017 and ended February 2018.
Culture Method and Identification of Bacterial Isolates:
Paired urine and peri-urethral samples were cultured using EQUC, which consists of inoculation of 0.1mL urine onto diverse types of media (BAP, chocolate agar, colistin and nalidixic acid (CNA) agar, CDC anaerobe 5% BAP) with incubation in diverse environments (5% CO2, aerobic conditions, Campy gas mixture (5% O2, 10% CO2, 85% N) or anaerobic conditions) all at 35°C for 48 hours. The level of detection for EQUC is 10 CFU/mL, represented by 1 colony of growth on any of the plates. EQUC is designed to isolate a broad array of Gram-negative and Gram-positive bacteria, including anaerobes and fastidious bacteria that grow slowly. Each morphologically distinct colony type was counted and isolated on a fresh plate of the same media to prepare a pure culture that was used for identification with Matrix-Assisted Laser Desorption/Ionization Time-of Flight (MALDI-TOF) mass spectroscopy.
Statistical Analysis:
REDCap (Research Electronic Data Capture) was utilized for the implementation of the random allocation sequence, which was only revealed upon participant enrollment just prior to sample collection. At the conclusion of recruitment, bacterial compositions of voided urines and peri-urethral swabs were analyzed using alpha diversity measures. Richness, or the number of microbial species present, was calculated by comparing the number of observed species for each cohort and by each method of collection. Evenness, or the distribution of microbial species within the sample, was calculated with Pielou’s Index. This index ranks samples from 0 to 1, with 1 being completely even. A sample with a Pielou index score close to 1 contains all species present in nearly equal abundance, while a lower index score indicates that certain species are more abundant than others. Abundance, or the number of each organism present, was calculated with Fisher’s alpha diversity Index. Communities with higher Fisher’s Index values had similar abundances of multiple species. Combined interactions were calculated with the Shannon (richness and evenness) and Simpson (richness and abundance) indices. Higher Shannon diversity values indicate communities with high richness and evenness. Higher Simpson diversity values indicate communities with higher richness and abundance. In all cases, higher index values describe more diverse samples. Patient demographics and clinical characteristics were compared using Wilcoxon rank-sum tests for continuous variables and chi-square or Fisher’s exact tests for nominal variables. Wilcoxon signed rank tests were used to assess for statistical significance of within-group diversity differences in colony forming units and diversity measures. Kruskal-Wallis tests on swab measures were used to assess the statistical significance of differences in colony forming units between groups. P-values < 0.05 were considered statistically significant. Analyses were performed using RStudio 1.1.423 (Boston, MA).
RESULTS:
The 62 female participants were predominantly Caucasian, had a mean age of 28.8 years (range 22–52) and a mean BMI of 25.7 kg/m2 (range 17.2–47.2 kg/m2). There were 20 participants in the standard clean catch cohort (SCC), 21 participants in the Peezy with peri-urethral wipe cohort (PZW) and 21 participants in the Peezy without peri-urethral wipe cohort (PZ). The cohorts did not differ significantly in their demographics, PFDI-20, or UTISA symptoms scores (Table 1). The cohorts also represent healthy participants with no urinary tract symptoms and minimal pelvic floor symptoms.
Table 1.
Participant Demographics
| Participant Demographics (N=62) | |
|---|---|
| Average age (Range) | 28.8 years (22–52 years) |
| Average BMI (Range) | 24.7 kg/m2 (17.23–47.2 kg/m2) |
|
Ethnicity: Caucasian Asian Hispanic African American Other |
57% 20% 11% 9% 3% |
Microbiota abundance:
We investigated the abundance data by comparing the distribution of CFU/ml of voided urines to the mean CFU/ml of the paired peri-urethral swabs (that were obtained prior to the peri-urethral wipe). For both Peezy cohorts, the voided urines had significantly lower CFU/ml than their paired peri-urethral swabs (Table 2). In contrast, there was no difference in CFU/ml of SCC-obtained urines and their paired peri-urethral swabs.
Table 2: Abundance data shows the Peezy device reduces CFUs in voided urine.
Median colony forming units (CFU) per mL and interquartile ranges (IQR) for each cohort. Significant values are bolded.
| CFU/mL | SCC | PZW | PZ |
|---|---|---|---|
|
Swab, median (IQR) |
153500 (106075–23070) |
226000 (140000–305400) |
209500 (121250–246600) |
|
Void, median (IQR) |
115700 (86700–227800) |
118500 (25500–204300) |
109650 (101900–150300) |
| P-value | 0.216 | <0.001 | 0.005 |
PZW versus PZ Microbiome Diversity:
Microbiota of voided urines from both Peezy cohorts had significantly lower diversity values than those of paired peri-urethral swabs in Pielou (evenness), Shannon (richness and evenness), and Simpson (richness and abundance) indices (Figure 2A–C, Supplemental Table 1). Voided urines from both Peezy cohorts did not differ significantly from paired peri-urethral microbiota in species richness or abundance (Fisher’s Index).
Figure 2: Comparison of diversity between Peezy and SCC cohorts.
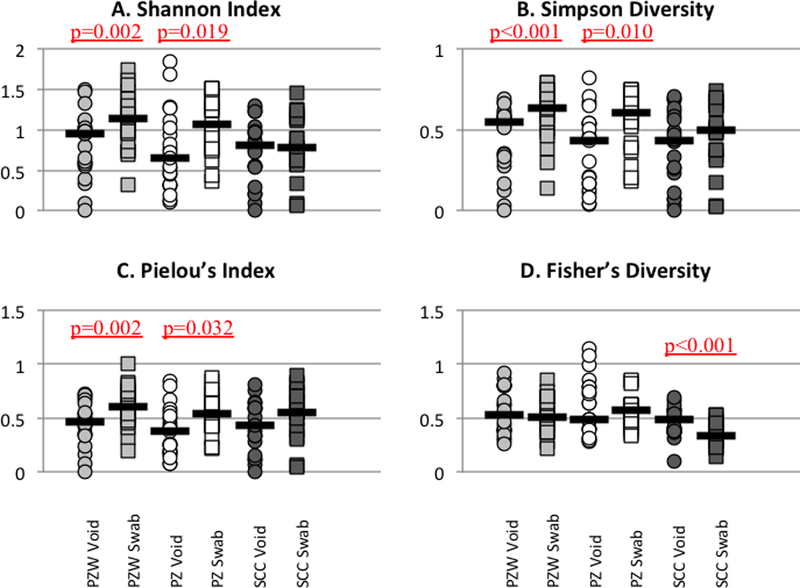
Diversity measures for voided urines were compared to the paired peri-urethral swab. Median values are marked with black lines. Significant differences are denoted with underlined p values (see also Supplemental Table 1).
SCC Microbiome Diversity:
Microbiota from the paired SCC urine and peri-urethral swabs did not differ significantly in diversity values for Pielou, Shannon, or Simpson indices. The only diversity measures in which the microbiota from the paired SCC voided urine and peri-urethral swab significantly differed were Fisher’s Index (Figure 2D, Supplemental Table 1) and species richness, with the microbiota of the voided urine obtained by SCC having significantly greater relative abundance and richness than the paired peri-urethral microbiota.
DISCUSSION:
The aim of this study was to better understand the use of voided urine in microbiome studies. Obtaining catheterized urine is a rate limiting step for many researchers who want to do large community-based studies. Urine in the bladder has a low biomass and as urine from the bladder traverses the urethra, the urethral meatus, peri-urethra and vulvovaginal skin, the biomass increases. This has been previously demonstrated by the greater relative abundance of voided urine compared to catheterized urine [21,22]. Therefore, voided urine that has a higher relative abundance than its matched peri-urethral swab (which was obtained prior to urination and use of the castile wipe) is suggestive of a higher post-bladder contribution to the voided urine. The microbiota abundance data in this study demonstrates that standard clean catch urines produced similar CFU/ml to paired peri-urethral specimens. In contrast, Peezy urines yielded significantly lower CFU/ml than peri-urethral specimens. This contrast suggests that the Peezy device, used with or without peri-urethral cleansing, is more capable of reducing post-bladder contributions to voided urines than the standard clean catch method.
One of the challenges of this study was how to measure “better” voided urine collection. Previous studies have clearly demonstrated that voided and catheterized urine are distinct [21,22]. Since each woman has her own bacterial community, we needed to compare voided urine specimens to possible contamination from the urethra, peri-urethra, vulva and vagina. We used diversity indices to compare the microbial communities of each participant’s peri-urethra and voided urine. These indices are used to describe environmental and bacterial communities. The peri-urethral versus voided urine Shannon, Simpson and Pielou indices were significantly different for Peezy but not for standard clean catch participants. This indicates that the peri-urethral and voided urine microbial communities were distinct for Peezy, but were not distinct for standard clean catch.
Bladder urine microbial composition has been shown to have a lower biomass than the peri-urethral skin and vagina. As a result, voided urine obtained by a “cleaner” catch should yield lower bacterial abundance, corresponding to a lower Fisher alpha diversity value. The Fisher alpha diversity value was significantly higher for the standard clean catch voided urine compared to the peri-urethral specimen, suggestive of microbial contributions to the urine sample from post-bladder structures, such as urethra, vulva and vagina. Significant differences in Fisher diversity values were not seen for the Peezy cohorts’ voided urine versus peri-urethral specimen, suggesting that the standardized removal of the initial urine stream reduces but does not eliminate post-bladder bacterial contribution. Likewise, the castile soap wipe does not appear to reduce peri-urethral contribution to urine for the Peezy cohort; therefore, it may prove to be an unnecessary and possibly confounding step in voided urine collection.
Our study includes some important strengths. The first is the use of EQUC. While other studies have examined the efficacy of Peezy, this is the first to utilize EQUC for sample analysis. The standard urine culture method, utilized in previous studies, preferentially cultures Escherichia coli, whereas EQUC has been shown to capture a wider range of bacterial species [2,18]. A sensitive assay, such as EQUC, is necessary to assess a novel urine collection since the bladder and vaginal microbiome have considerable overlap. In this study, the use of EQUC for analysis of the samples enabled us to identify a broader and more accurate range of bacteria, thus providing a better understanding of bacterial profiles both from the peri-urethra and LUT.
Another strength was our standardized protocol. Urine collection directions are given in a variety of ways to patients; including verbally, written, or sometimes no instruction at all. In this study, the content and delivery of directions for each protocol was controlled through the use of a pre-recorded video lasting approximately two minutes with both written and visual instruction of the collection protocol. Participants were then given an opportunity to ask clarifying questions before sample collection. The Peezy packaging contains visual and written instructions. Because one possible source of vaginal and skin bacterial contribution to standard urine collection may be inappropriate collection technique due to lack of standardized directions, this study sought to minimize this confounding factor through consistent delivery of directions. Additionally, by utilizing written, visual, and verbal communication of directions, the diverse learning needs of participants was addressed. The use of standardized directions does mean that the results of this study may not be generalizable to studies where women are not given specific directions on how to collect their voided urine sample. The Peezy device may provide an additional level of standardization to the urine collection process by controlling the amount of initial voided urine (approximately ten milliliters) discarded prior to collection. Rather than the requirement to start and stop one’s stream of urine, the device allows for one continuous void. This may be the mechanism by which Peezy provides urine with lower bacterial abundance.
Finally, we used a peri-urethral swab as an internal control for vulvo-vaginal flora. Voided urine obtained by standard clean catch has been shown to include microbiota that reside outside of the LUT, most often due to vulvo-vaginal contribution, which cannot be easily distinguished from bladder microbiota. We utilized paired peri-urethral swabs as a control for each individual. By understanding the bacterial profile of the peri-urethral area, we could then compare this sample to the voided urine sample. Through this method we assessed the bacterial contribution of post-urethral bacteria to a given voided urine sample. This allowed us to evaluate methods of collection for reduction in post-bladder contribution. The Peezy device showed a significant reduction in post-bladder contribution.
We acknowledge that our study also had limitations. This study was limited by the number of participants and the use of a “healthy” control population as clearly demonstrated by the low UTISA and PFDI-20 subscales of the participants. Because this was a pilot study, future research should focus on increasing the number and diversity of participants. Because the participants were young and relatively fit (avg. BMI: 24.7 kg/m2), they may have presented few issues with urine collection which is not the case in older urogynecologic patients. The generalizability of Peezy for future LUT microbiome research still needs to be assessed.
Our hope is that, as a research community, we can develop methods that allow us to obtain voided urines with as little post-bladder contamination as possible. We recommend that EQUC be utilized in future research studies due to its increased sensitivity and ability to capture a broad range of bacteria. In this small study, we found that the use of the Peezy device suggests a step towards a “cleaner” catch. We also found the peri-urethral wipe was not a necessary step for use of the Peezy device. Additional studies and methods will validate or refute our findings and build on the current research of using voided urines to study the microbiome of the lower urinary tract.
Supplementary Material
ACKNOWLEDGMENTS:
We kindly thank Mary Tulke RN for her assistance with participant recruitment and sample collection. We thank Giovanna Forte for the donation of Peezy devices.
FUNDING: This study was supported by a grant to AJW from the NIH (R01 DK104718).
Footnotes
CONFLICT OF INTEREST STATEMENT:
Dr. Mueller discloses research support from Astellas, Advisory board position with Boston Scientific and legal consultation with Butler-Snow/Ethicon. Dr. Wolfe discloses research support from Astellas and Kimberly Clark. The remaining authors (Southworth and Hochstedler) report no disclosures.
Literature Cited/References:
- 1.Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50(4):1376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price TK, Dune TD, Hilt EE, et al. The clinical urine culture: enhanced techniques improve detection of clinically relevant microorganisms. J Clin Microbiol. 2016;54:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khasriya R, Sathiananthamoorthy S, Ismail S, Kelsey M, et al. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol. 2013;51(7):2054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. Journal of Translational Medicine. 2012;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas-White KJ, Hilt EE, Fok C, et al. Incontinence medication response relates to the female urinary microbiota. Int Urogynecol J. 2016;27:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce M, Zilliox MJ, Rosenfeld AB, et al. The female urinary microbiome in urgency urinary incontinence. American Journal of Obstetrics & Gynecology. 2015;213(3):347.e1–347.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karstens L, Asquith M, Davin S, et al. Does the urinary microbiome play a role in urgency urinary incontinence and its severity? Front. Cell. Infect. Microbiol. 2016;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerman AL, Underhill DM. The mycobiome of the human urinary tract: potential roles for fungi in urology. Ann Transl Med. 2017;5(2):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fok CS, Gao X, Lin H, et al. Urinary symptoms are associated with certain urinary microbes in urogynelogica surgical patients. Int Urogynecol J. 2018;1433(3032):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Immergut MA, Gilber EC, Frensilli FJ, et al. The myth of the clean catch urine specimen. Urology. 1981;17:339–340. [DOI] [PubMed] [Google Scholar]
- 13.Lifshitz E, Kramer L. Outpatient urine culture: does collection technique matter? Arch Intern Med. 2000;160:2537–2540. [DOI] [PubMed] [Google Scholar]
- 14.Baerheim A, Digranes A, Hunskaar S. Evaluation of urine sampling technique: bacterial contamination of samples from women students. British Journal of General Practice. 1992;42:241–243. [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas-White K, Forster SC, Kumar N, Van Kuiken M, Putoniti C, Stares MD, Hilt EE, Price TK, Wolfe AJ, Lawley TD. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nature Communications. 2018;9:1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards J Peezy mid-stream urine (MSU) usability study. NIHR Trauma MIC. [Google Scholar]
- 17.Jackson SR, et al. A novel midstream urine-collection device reduces contamination rates in urine cultures amongst women. BJU Int. 2005;96:360–4. [DOI] [PubMed] [Google Scholar]
- 18.Collier S, Matijiu F, Jones G, et al. A prospective study comparing contamination rates between a novel mid-stream urine collection device (Peezy) and a standard method in renal patients. J Clin Pathol. 2014;67(2):139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber MD, Walters MD, Bump RC Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders PFDI-20 and PFIQ-7. American Journal of Obstetrics and Gynecology 2005; 193, 103–13 [DOI] [PubMed] [Google Scholar]
- 20.Clayson D, Wild D, Doll H, Keating K, Gondek K. Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): the UTI Symptom Assessment questionnaire. BJU international. 2005;96(3):350–9. [DOI] [PubMed] [Google Scholar]
- 21.Munir T, Lodhi M, Hussain RM, et al. Association between periurethral colonization with Uropathogens and subsequent bacteriuria in catheterized patients. J Coll Physicians Surg Pak. 2009;19(3):169–172. [PubMed] [Google Scholar]
- 22.Cattell WR, McSherry MA, Northeast A, et al. Periurethral enterobacterial carriage in pathogenesis of recurrent urinary infection. British Medical Journal. 1974;4:136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


