Abstract
Chronic, intermittent ethanol (CIE) exposure is known to produce neuroadaptive alterations in excitatory neurotransmission that contribute to the development of dependence. Although activation of protein kinases (e.g., cyclic AMP [cAMP]-dependent protein kinase) is implicated in the synaptic trafficking of these receptors following CIE exposure, the functional consequences of these effects are yet to be fully understood. The present study sought to delineate the influence of protein kinase in regulating cytotoxicity following CIE exposure, as well as to examine the relative roles of ethanol exposure and ethanol withdrawal (EWD) in promoting these effects. Rat hippocampal explants were exposed to a developmental model of CIE with or without co-application of broad-spectrum protein kinase inhibitor KT-5720 (1 mM) either during ethanol exposure or EWD. Hippocampal cytotoxicity was assessed via immunofluorescence (IF) of neuron-specific nuclear protein (NeuN) with thionine staining of Nissl bodies to confirm IF findings. Concomitant application of ethanol and KT-5720 restored the loss of NeuN/Fox-3 IF in pyramidal CA1 and granule DG cell layers produced by CIE, but there was no restoration in CA3. Application of KT-5720 during EWD failed to significantly alter levels of NeuN IF, implying that ethanol exposure activates protein kinases that, in part, mediate the effects of EWD. KT-5720 application during EWD also restored thionine staining in CA1, suggesting kinase regulation of both neurons and non-neuronal cells. These data demonstrate that CIE exposure alters protein kinase activity to promote ethanol withdrawal-associated loss of NeuN/Fox-3 and highlight the influence of kinase signaling on distinct cell types in the developing hippocampus.
Keywords: Ethanol withdrawal (EWD), Neuron specific nuclear protein (NeuN), Chronic Intermittent ethanol (CIE), Immunofluorescence (IF)
Introduction
Patterns of binge-like ethanol consumption and multiple detoxifications (i.e., ethanol withdrawal [EWD]) predict poorer neurologic outcomes. These effects include physical manifestations of EWD (e.g., Veatch & Becker, 2005), neurocognitive perturbations (e.g., Zhao et al., 2013), and hippocampal neurodegeneration (e.g., Corso, Mostafa, Collins, & Neafsey, 1998) in adult rodents. These effects are associated with neuroadaptive changes in excitatory neurotransmission (e.g., Christian, Alexander, Diaz, & McCool, 2013; Nelson et al., 2005; Veatch & Becker, 2005). As an example, a prior study utilizing electrophysiological techniques demonstrated that EWD from CIE produced increased presynaptic glutamate function in the adult rat basolateral amygdala (Christian et al., 2013). Other electrophysiological studies have found amplified N-methyl-D-aspartate (NMDA)-receptor-mediated responses in the pyramidal CA1 cell layer of the hippocampal formation following exposure to CIE, relative to age-matched controls (Nelson et al., 2005). In addition, enhanced mGlu-1 and NMDA GluN signaling within the central nucleus of the amygdala (Cozzoli et al., 2014), as well as increased expression of group 1 mGlu-family and NMDA GluN2 proteins (Cozzoli et al., 2009), are observed in adult C57BL/6J mice subjected to binge-like ethanol administration. These behavioral and neurobiological data suggest that the behavioral effects of EWD are associated with alterations in excitatory neurotransmission.
Western blot and immunoblot analyses revealed that CIE produced selective increases in GluN1 and GluN2B subunit expression on the surface membrane in fetal cultured cortical neurons (Qiang, Denny, & Ticku, 2007). Exposure to KT-5720 (i.e., 1 mM), an inhibitor of cyclic AMP-dependent protein kinase (PKA), and other similar protein kinases (e.g., mitogen-activated protein kinases [MAPK]), prevented increases in GluN1 and partially prevented increases in GluN2B expression in the developing cortex (Qiang et al., 2007). Another study demonstrated that ethanol exposure promotes trafficking of NMDA receptors in developing hippocampal neurons via activity-dependent processes (e.g., protein kinases) (Carpenter-Hyland, Woodward, & Chandler, 2004). Within the nucleus accumbens, activation of the cAMP-dependent protein kinase, PKA, confers the sensitivity of NMDA receptors following ethanol application in modulation of dopaminergic tone in periadolescent (i.e., 3e4 weeks old) rats (Maldve et al., 2002; see Lovinger, 2002 for a brief review). In addition, protein kinases (e.g., MAPK and extracellular signal-regulated kinases [ERK]) are known to phosphorylate group 1 metabotropic glutamate receptors (for a review, see Mao & Wang, 2016). A recent study conducted in our laboratory demonstrated that group 1 metabotropic glutamate (mGlu)-family proteins contribute to cytotoxicity in a developmental model of CIE (Reynolds, Williams, Saunders, & Prendergast, 2015). Taken together, these findings suggest that protein kinase activation might regulate neuroadaptive alterations in glutamatergic neurotransmission observed following CIE, particularly in the developing central nervous system (CNS). However, the functional role of protein kinase activity in promoting hippocampal cytotoxicity following CIE exposure is not clearly understood. Further, the relative roles of ethanol exposure and EWD in activating these intracellular signals to promote the cytotoxic effects of CIE have not been delineated. In the present report, we examined the functional effects of broad-spectrum protein kinase inhibition by the broadspectrum staurosporine analog KT-5720 on the cytotoxic effects of ethanol in a developmental model of CIE.
Methods
Organotypic hippocampal slice culture preparation
Whole brains were aseptically removed from 8-day-old SpragueeDawley rats (Harlan Laboratories; Indianapolis, IN) and transferred to sterile culture dishes containing frozen dissecting medium (Minimum Essential Medium [MEM; Invitrogen, Carlsbad, CA], 25 mM HEPES [Sigma, St. Louis, MO], 10.60 mM Amphotericin B solution [Sigma], and 50 mM streptomycin/penicillin [Invitrogen]). Bilateral hippocampi were extracted and carefully transferred to sterile plates containing chilled culture medium (dissecting medium, distilled water, 36 mM glucose [Fisher, Pittsburgh, PA], 25% Hanks’ Balanced Salt Solution [HBSS; Invitrogen], 25% [v/v] heat-inactivated horse serum [HIHS; Sigma], 0.05% Amphotericin B solution [Sigma], and 0.05% streptomycin/ penicillin [Invitrogen]). Excess hippocampal tissue was carefully removed using a stereoscopic microscope, and unilateral hippocampi were sectioned at 200 mM using a McIlwain Tissue Chopper (Mickle Laboratory Engineering Co. Ltd., Gomshall, UK). Hippocampi with cell layers intact (i.e., CA1, CA3, and DG) were selected under the stereoscopic microscope and then carefully plated using transfer pipettes onto Millicell-CM 0.4-mM biopore membrane inserts placed in a sterile 6-well culture that contained 1 mL of pre-incubated culture medium per well. Each culture well plate generated 18e24 hippocampi. Excess culture medium was extracted off the top of each biopore membrane insert and hippocampi were maintained in a water-jacketed incubator at 37 oC with a gas composition of 5% CO2/95% air for 5 days prior to experimental manipulation for adequate membrane adherence (after Butler et al., 2010). Care of all animals was carried out in agreement with the University of Kentucky’s Institutional Animal Care and Use Committee.
Chronic, intermittent ethanol (CIE) regimen
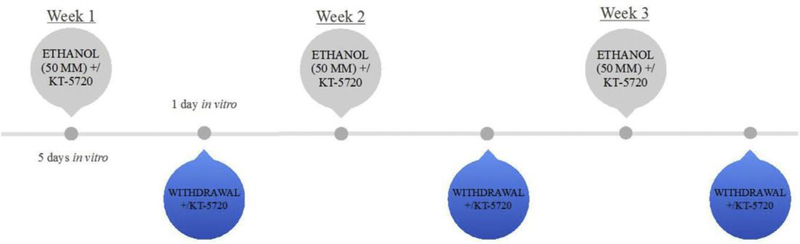
An in vitro model of CIE that has been published previously (Reynolds, Saunders, & Prendergast, 2016) was used to assess the functional role of cAMP-dependent protein kinase activation in promoting the cytotoxic effects of CIE. At 5 days in vitro, hippocampi were randomly transferred to plates containing either 1 mL of the ethanol-naïve culture medium (control) or ethanol-containing medium (i.e., 50 mM) for 5 days with or without the addition of KT-5720 (1 mM), a broad-spectrum protein kinase inhibitor (Bain et al., 2007). During each 5-day exposure period, ethanol and control-treated hippocampi were maintained inside Ziploc® bags filled with 5% CO2/95% air and water bath solutions containing either distilled water (50 mL) for control plates or distilled water (50 mL) containing ethanol (50 mM) for ethanol-treated plates, so as to maintain ethanol at 50 mM. At 11 days in vitro, hippocampi were removed from culture plates and transferred to new plates containing 1 mL of fresh control culture media for a 24-h EWD period with or without the addition of KT-5720 (1 mM). This treatment regimen was repeated a total of three times (see Fig. 1). It is worthwhile to note that KT-5720 was applied either concomitantly with ethanol or during EWD for each of the three cycles of CIE at the same time points in ethanol- and control-treated slices. The concentration of ethanol (i.e., 50 mM) was selected based on prior reports demonstrating ubiquitous decreases of NeuN IR and thionine staining of Nissl bodies in this model of CIE (Reynolds, Berry, Sharrett-Field, & Prendergast, 2015). This concentration has also been shown to reflect patterns of binge drinking (Eckardt et al., 1998). The concentration of KT-5720 (i.e., 1 mM) was selected based on a prior report by Ticku and colleagues showing efficacy for protein kinase inhibition (Qiang et al., 2007). KT-5720 was first dissolved in 100% dimethyl sulfoxide (DMSO; Fisher) to yield a final working concentration of 0.01% DMSO in control and ethanol-treated culture medium.
Fig. 1.
Rat hippocampal explants were exposed to ethanol (50 mM) for 5 days in vitro, followed by a 24-h period of withdrawal, and repeated three times. KT-5720, a broad-spectrum protein kinase inhibitor, was applied to ethanol-enriched medium or ethanol-free medium either discretely during each 5-day ethanol exposure or withdrawal period, in ethanol- and control-treated hippocampi.
Immunohistochemistry
Following the CIE treatment regimen described above, hippocampi were fixed for immunohistochemical procedures by pipetting 1 mL of 10% formalin solution on the top and bottom of each culture plate well, with incubation for 30 min at room temperature. Wells were then washed twice with phosphate-buffered saline (PBS) and then stored at 4 oC until immunohistochemistry was performed. NeuN (Fox-3) is a protein located in nearly all postmitotic neurons (Kim, Adelstein, & Kawamoto, 2009), and is a reliable marker of neuronal integrity (Butler et al., 2010; Reynolds et al., 2016). Immunohistochemistry was performed by transferring hippocampi to plates containing 1 mL of permeabilization (wash) buffer (200 mL PBS [Invitrogen], 200 mL Triton X-100 [Sigma], 0.010 mg bovine serum [Sigma]) on the bottom of each well. One mL of buffer was then added to the top of each well for a 45-min incubation period at room temperature to permeate cell membranes. Hippocampi were then transferred to plates containing 1x PBS on the bottom of each well, and primary monoclonal antibody mouse anti-NeuN (1:200; Sigma) was carefully pipetted on the top of each well as hippocampi were incubated at 4 oC for 24 h. Hippocampi were washed twice with 1x PBS and then incubated for 24 h with goat anti-mouse fluorescein isothiocyanate (FITC; 1:200; Sigma). Hippocampi were washed twice with 1x PBS. NeuN IF was visualized using SPOT software 4.0.2 (advanced version) for Windows (W. Nuhsbahm Inc.; McHenry, IL, USA) through a 5x objective with a Leica DMIRB microscope (W. Nuhsbahm Inc.; McHenry, IL, USA) connected to a computer and captured with a SPOT 7.2 color mosaic camera (W. Nuhsbahm). FITC IR was detected using a band-pass filter at 495 nm (520 nm emission).
Histology
Thionine is a monochromatic dye known to bind to Nissl substance(s) located on cytoplasmic RNA and DNA content of all cell nuclei (Ka da r, Wittmann, Liposits, & Fekete, 2009). Following immunohistochemistry, hippocampi were exposed to a 0.2% thionine stock solution for 5 min followed by a 2-min dehydration period with 70% ethanol before being washed twice and imaged. Thionine staining of Nissl bodies was imaged with SPOT software 4.0.2 (advanced version) for Windows (W. Nuhsbahm Inc.; McHenry, IL, USA) through a 5x objective with a Leica DMIRB microscope (W. Nuhsbahm Inc.; McHenry, IL, USA) connected to a computer and captured with a SPOT 7.2 color mosaic camera (W. Nuhsburg).
Statistical analyses
Statistical analyses were conducted to assess the effects of broad-spectrum staurosporine analog and protein kinase inhibitor KT-5720 on NeuN IF and thionine staining of Nissl bodies. The present experiment was conducted two times using two different rat litters. All data were converted to percent control and then combined for data analyses and ease of interpretation. Immunohistochemical data were analyzed using a two-factor analysis of variance (ANOVA) with media (i.e., control-treated and ethanol-treated) and drug (i.e., no drug and drug) as factors for each examined hippocampal subregion (i.e., CA1, CA3, and DG). Thionine staining of Nissl bodies was used to confirm NeuN findings in control-treated and ethanol-treated hippocampi, as well as hippocampi co-exposed to ethanol and KT-5720 (i.e., during CIE), and ethanol-treated hippocampi exposed to KT-5720 during EWD using a 1-way ANOVA. For graphical representation of thionine data, mean data from these treatment conditions were converted using the formula ([x-100]-100*[−1]) so as to express data on the same scale used for the immunohistochemical data. This statistical strategy is based on a prior report utilizing histological analyses in this model of CIE (Reynolds, Berry, et al., 2015). Post hoc analyses were conducted when appropriate using Tukey’s HSD. Effects were considered significant at p < 0.05. For graphical representation and interpretation, all data are presented as group mean ± the standard error of the mean (SEM).
Results
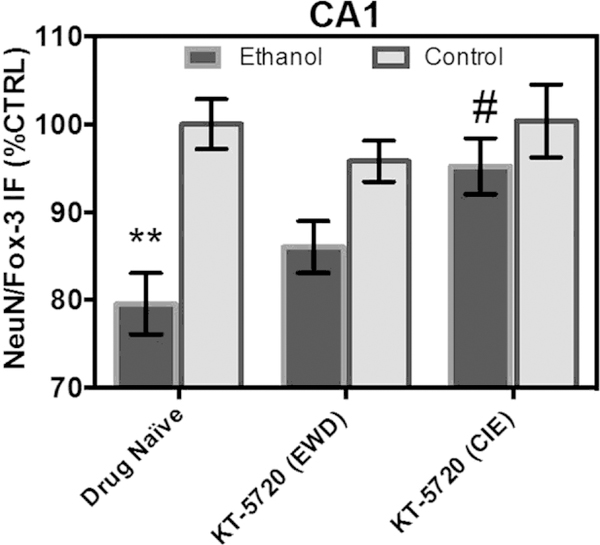
In the pyramidal cell layer of the CA1, ANOVA analyses revealed a significant drug-by-media interaction [F(2,213) ¼ 3.04, p ¼ 0.05], a significant main effect of drug [F(2,213) ¼ 3.47, p ¼ 0.03], and a significant main effect of medium [F(1,213) ¼ 20.46, p < 0.0001]. Fig. 2 shows that exposure to CIE produced a significant 20% decrease in NeuN/Fox-3 IR relative to control-treated tissue. Concomitant application of KT-5720 and ethanol reversed the loss of NeuN produced by CIE by nearly 16% in this subregion (Tukey’s HSD). Application of KT-5720 during EWD failed to alter levels of NeuN IR in ethanol-treated hippocampi (Tukey’s HSD). Fig. 2 shows that application of KT-5720 also failed to significantly alter levels of NeuN IR in control-treated tissue (Tukey’s HSD).
Fig. 2.
Co-application of protein kinase inhibitor KT-5720 (1 mM) and ethanol (i.e., 50 mM) restored the loss of NeuN/Fox-3 IR produced by CIE exposure in the pyramidal cell layer of CA1. Data are presented as percent control of the mean ± SEM. **Statistical significance (p < 0.05) compared to control-treated hippocampi; #statistical significance (p < 0.05) compared to drug-naïve, ethanol-treated hippocampi. N ¼ 40 for ethanol-treated hippocampi; N ¼ 40 for hippocampi treated with ethanol and KT during EWD; N ¼ 40 for hippocampi treated with ethanol and KT concomitantly; N ¼ 38 for control-treated hippocampi; N ¼ 36 for control-treated hippocampi with KT applied during the EWD period; N ¼ 25 for control-treated hippocampi with KT applied during CIE.
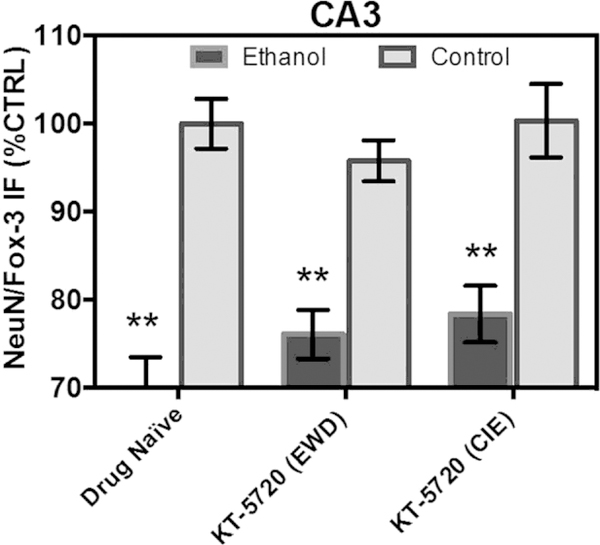
In the pyramidal cell layer of CA3, ANOVA analyses revealed a significant main effect of medium [F(1,213) ¼ 85.13, p < 0.0001], but not a significant main effect of drug (p > 0.05). Fig. 3 shows that exposure to CIE produced a significant 30% decrease in NeuN/Fox-3 IR relative to control-treated tissue (Tukey’s HSD). Fig. 3 shows that neither ethanol-treated hippocampi (p > 0.05; Tukey’s HSD) nor control-treated hippocampi (p > 0.05; Tukey’s HSD) were significantly altered following application of KT-5720 in this sub-region.
Fig. 3.
Neither ethanol-treated hippocampi nor control-treated hippocampi were significantly altered following application of protein kinase inhibitor KT-5720 (1 mM) in the pyramidal cell layer of CA3. Data are presented as percent control of the mean ± SEM. **Statistical significance (p < 0.05) compared to control-treated hippocampi. N ¼ 40 for ethanol-treated hippocampi; N ¼ 40 for hippocampi treated with ethanol and KT during EWD; N ¼ 40 for hippocampi treated with ethanol and KT concomitantly; N ¼ 38 for control-treated hippocampi; N ¼ 36 for control-treated hippocampi with KT applied during the EWD period; N ¼ 25 for control-treated hippocampi with KT applied during CIE.
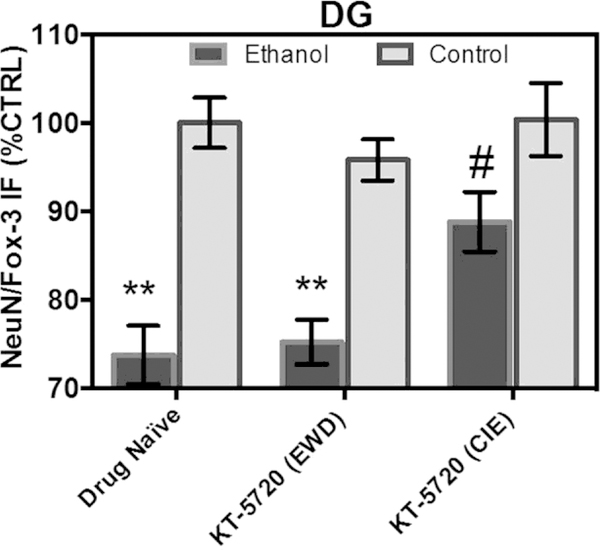
In the granule cell layer of the DG, ANOVA analyses revealed a significant main effect of drug [F(2,213) ¼ 4.63, p ¼ 0.01] and a significant main effect of medium [F(1,213) ¼ 58.91, p < 0.0001]. Fig. 4 shows that exposure to CIE produced a significant 26% decrease in NeuN/Fox-3 IR relative to control-treated tissue (Tukey’s HSD). Concomitant application of KT-5720 and ethanol reversed the loss of NeuN produced by CIE by nearly 15% in this subregion (Tukey’s HSD). Fig. 4 shows that application of KT-5720 during EWD failed to alter levels of NeuN IR in ethanol-treated hippocampi (Tukey’s HSD). Application of KT-5720 also failed to significantly alter levels of NeuN IR in control-treated tissue (Tukey’s HSD).
Fig. 4.
Co-application of protein kinase inhibitor KT-5720 (1 mM) and ethanol (i.e., 50 mM) attenuated the loss of NeuN/Fox-3 IR produced by CIE exposure in the granule cell layer of the DG. Data are presented as percent control of the mean ± SEM. **Statistical significance (p < 0.05) compared to control-treated hippocampi; #statistical significance (p < 0.05) compared to drug-naïve, ethanol-treated hippocampi. N ¼ 40 for ethanol-treated hippocampi; N ¼ 40 for hippocampi treated with ethanol and KT during EWD; N ¼ 40 for hippocampi treated with ethanol and KT concomitantly; N ¼ 38 for control-treated hippocampi; N ¼ 36 for control-treated hippocampi with KT applied during the EWD period; N ¼ 25 for control-treated hippocampi with KT applied during CIE.
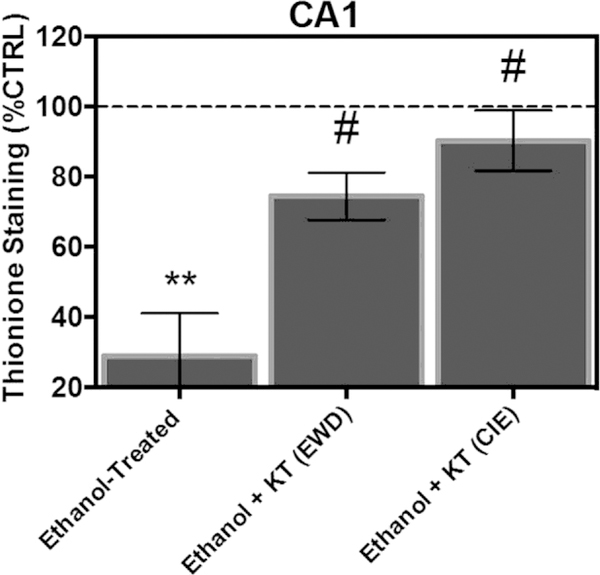
Thionine staining of Nissl bodies was used as a secondary measure of cytotoxicity to confirm significance obtained from immunohistochemical analyses. In the pyramidal cell layer of the CA1, ANOVA analyses revealed a significant main effect of treatment [F(3,85) ¼ 12.06, p < 0.0001]. Fig. 5 shows that exposure to CIE produced a significant 70% decrease in thionine staining of Nissl bodies relative to control-treated tissue (Tukey’s HSD). Concomitant application of KT-5720 and ethanol reversed the loss of Nissl bodies produced by CIE by nearly 61% in this subregion (Tukey’s HSD). Interestingly, application of KT-5720 during EWD also significantly increased thionine levels in ethanol-treated hippo-campi (Tukey’s HSD).
Fig. 5.
Co-application of protein kinase inhibitor KT-5720 (1 mM) and ethanol (i.e., 50 mM) reversed the loss of thionine staining of Nissl bodies produced by CIE exposure in the pyramidal cell layer of the CA1. Thionine staining of Nissl bodies was also spared following application of KT-5720 (1 mM) during EWD in this hippocampal subregion. Data are presented as percent control of the mean ± SEM. **Statistical significance (p < 0.05) compared to control-treated hippocampi; #statistical significance (p < 0.05) compared to drug-naïve, ethanol-treated hippocampi; N ¼ 24 for ethanol-treated hippocampi; N ¼ 23 for hippocampi treated with ethanol and KT during EWD; N ¼ 20 for hippocampi treated with ethanol and KT concomitantly; N ¼ 22 for control-treated hippocampi.
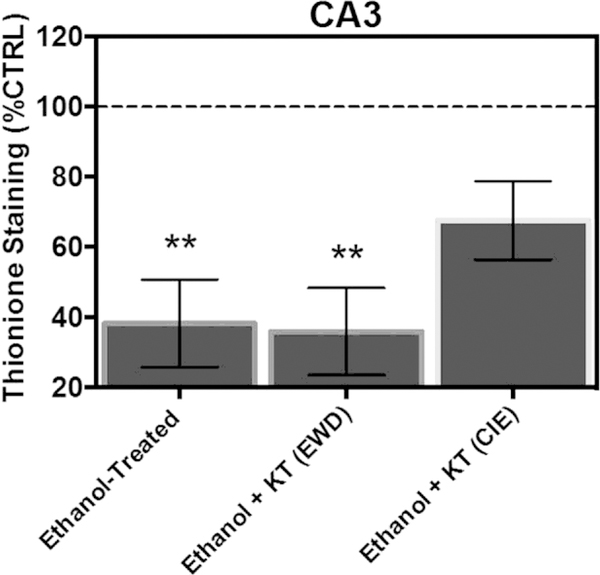
In the pyramidal cell layer of CA3, ANOVA analyses revealed a significant main effect of treatment [F(3,85) ¼ 6.82, p ¼ 0.0004]. Fig. 6 shows that exposure to CIE produced a significant 69% decrease in thionine staining of Nissl bodies relative to control-treated tissue (Tukey’s HSD). Concomitant application of KT-5720 and ethanol significantly attenuated the loss of Nissl bodies by nearly 30% in this subregion (Tukey’s HSD), but a complete reversal of the loss of thionine was not observed (i.e., these effects were not statistically significant from ethanol-treated tissue [Tukey’s HSD]). Application of KT-5720 during EWD failed to significantly alter thionine levels in ethanol-treated hippocampi (Tukey’s HSD).
Fig. 6.
Co-application of protein kinase inhibitor KT-5720 (1 mM) and ethanol (i.e., 50 mM) attenuated the loss of thionine staining of Nissl bodies produced by CIE exposure in the pyramidal cell layer of CA3. **Statistical significance (p < 0.05) compared to control-treated hippocampi; #statistical significance (p < 0.05) compared to drug-naïve, ethanol-treated hippocampi; N ¼ 24 for ethanol-treated hippocampi; N ¼ 23 for hippocampi treated with ethanol and KT during EWD; N ¼ 20 for hippocampi treated with ethanol and KT concomitantly; N ¼ 22 for control-treated hippocampi.
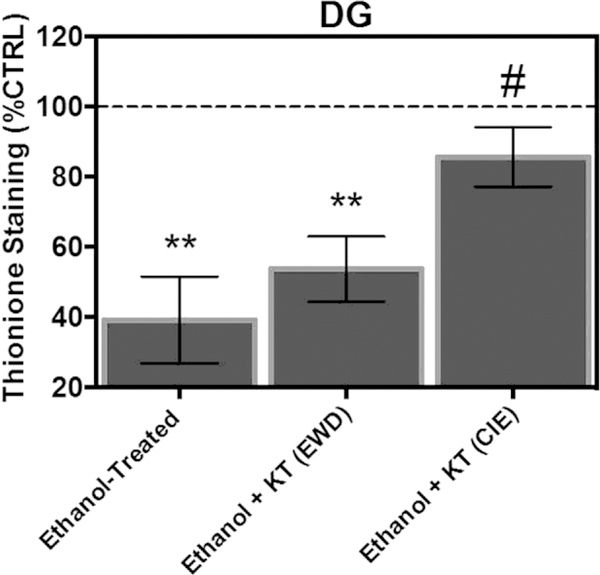
In the granule cell layer of the DG, ANOVA analyses revealed a significant main effect of treatment [F(3,85) ¼ 7.60, p ¼ 0.0001]. Fig. 7 shows that exposure to CIE produced a significant 60% decrease in thionine staining of Nissl bodies relative to control-treated tissue (Tukey’s HSD). Concomitant application of KT-5720 and ethanol reversed the loss of Nissl bodies produced by CIE by nearly 43% in this subregion in a similar manner to immunohistochemical findings (Tukey’s HSD). However, application of KT-5720 during EWD failed to significantly alter thionine levels in ethanol-treated hippocampi (Tukey’s HSD).
Fig. 7.
Co-application of protein kinase inhibitor KT-5720 (1 mM) and ethanol (i.e., 50 mM) attenuated the loss of thionine staining of Nissl bodies produced by CIE exposure in the granule cell layer of the CA3. **Statistical significance (p < 0.05) compared to control-treated hippocampi; #statistical significance (p < 0.05) compared to drug-naïve, ethanol-treated hippocampi; N ¼ 24 for ethanol-treated hippocampi; N ¼ 23 for hippocampi treated with ethanol and KT during EWD; N ¼ 20 for hippocampi treated with ethanol and KT concomitantly; N ¼ 22 for control-treated hippocampi.
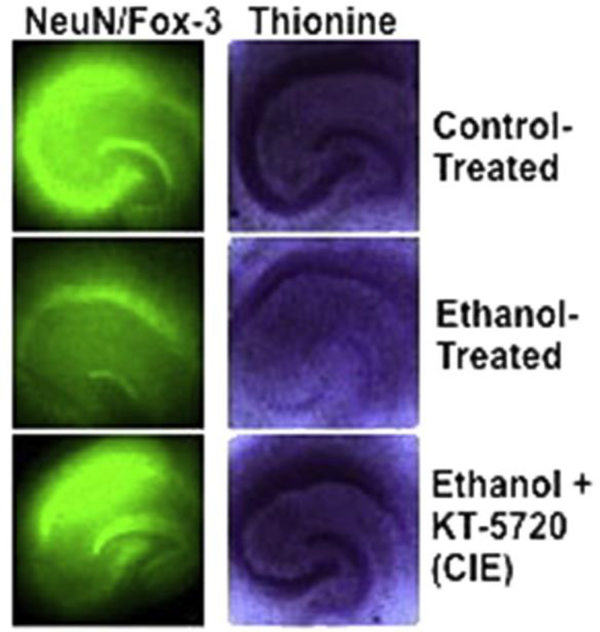
Fig. 8 depicts representative images of NeuN/Fox-3 IR (left panel) and thionine staining of Nissl bodies (right panel) of control-treated hippocampi (top panel), ethanol-treated hippocampi (middle panel), and hippocampi co-exposed to ethanol and KT-5720 (1.0 mM) during CIE.
Fig. 8.
Representative images of NeuN/Fox-3 IR (left panel) and thionine staining of Nissl bodies (right panel) in control-treated hippocampi (top row), ethanol-treated hippocampi (middle row), and hippocampi co-exposed to ethanol and KT-5720 (1 mM). The brightness of all representative images has been increased by 20% in Microsoft PowerPoint so as to better visualize hippocampal cell layers.
Discussion
Exposure to CIE produces neuroadaptations in glutamatergic tone. These effects are mediated, in part, via protein kinase-dependent phosphorylation of synaptic NMDA-receptor complexes in developing hippocampal neurons (Carpenter-Hyland et al., 2004). Subsequent trafficking of NMDA receptors from the endoplasmic reticulum to the synapse can occur in isolated developing hippocampal neurons (Mu, Otsuka, Horton, Scott, & Ehlers, 2003), conferring sensitivity to cytotoxicity. In the present studies, we examined the effects of protein kinase inhibition by the staurosporine analog KT-5720 on promoting withdrawal-associated cytotoxicity following a developmental model of CIE that has been employed in prior reports (e.g., Reynolds et al., 2016). CIE exposure produced significant hippocampal cytotoxicity characterized by significant decreases in NeuN/Fox-3 IR and thionine staining of Nissl bodies in CA1, CA3, and dentate gyrus hippocampal subregions in a similar manner to a prior study conducted in our laboratory (Reynolds, Berry, et al., 2015). In general, these findings are consistent with prior findings in which exposure to CIE produced neurocognitive and neurodegenerative effects, such as cytotoxicity in neocortex (Nagy & Laszlo, 2002) and hippocampal neurodegeneration in adult rats (Collins, Zou, & Neafsey, 1998; Zhao et al., 2013). Although the neurodegeneration produced by CIE in the adult hippocampus is notable in the granule cell layer of the dentate gyrus (e.g., Collins et al., 1998), we observed robust cytotoxicity in the CA1 and dentate gyrus in a developmental model of CIE. The reasons for these effects are unknown, but could reflect the developmental expression of glutamatergic (e.g., NMDA) receptors in hippocampal subregions. For example, Brewer et al. (2007) found that exposure to glutamate (i.e., 30 and 100 mM) produced increased cell death in aged fetal cultures (23e24 days in vitro) as compared to immature fetal cultures (8e9 days in vitro) (Brewer et al., 2007), demonstrating age-related developmental differences in regard to excitatory neurotransmission.
In the present studies, KT-5720 effectively reversed loss of NeuN/Fox-3 in the pyramidal cell layer of the CA1 and dentate granule cell layer, but not in CA3. A prior study conducted in our laboratory has demonstrated a selective vulnerability of immature hippocampal CA1 pyramidal cells to excitotoxic insult (Butler et al., 2010). Although hippocampal CA1 subregion neurotoxicity produced by EWD requires activation of intrinsic polysynaptic hippocampal pathways and function of NMDA receptors, CA3 and dentate gyrus subregions are typically more resistant to insult in developing hippocampal explants exposed to a single period of EWD (Prendergast et al., 2004). Given that the CIE treatment regimen employed in the present report consists of multiple ethanol and EWD exposure periods, and thereby was maintained in vitro for a longer duration of time, we propose that spreading of cytotoxicity (and reversal of the cytotoxic effects of CIE) from the CA1 to the dentate gyrus may regulate these region-specific effects of protein kinase inhibition. Consistent with this notion is a prior study demonstrating re-organization and de novo sprouting of mossy fiber tracts from granule cells to pyramidal cells in developing hippocampal explants due to loss of afferent innervation (Gutie rrez & Heinemann, 1999).
In the present report, we found that concomitant application of ethanol and staurosporine analog KT-5720 restored NeuN/Fox-3 IR in pyramidal CA1 and granule DG cell layers in a developmental model of CIE. Thionine staining of Nissl bodies was employed to confirm immunohistochemical findings in the present report. In general, similar effects were observed with thionine staining in each subregion, but KT-5720 application during EWD also restored thionine staining in the CA1. Given that thionine binds to Nissl bodies located on cytoplasmic RNA and DNA content of all cell nuclei (Ka da r et al., 2009), the differences between NeuN/Fox-3 and thionine findings with regard to protein kinase inhibition during EWD within the CA1 is probably due to the ability of KT-5720 to restore integrity of astrocytes in addition to neurons. Consistent with this notion, adult and fetal astrocytes express NMDA receptors, and are vulnerable to excitotoxic insult in a similar manner (Lee et al., 2010). Astrocytes in the developing hippocampus are also sensitive to EWD (Wilkins et al., 2006). Interactions between neuron growth and glial development are known to occur in response to ethanol and are regulated in part, via protein kinase activity (Pascual & Guerri, 2007).
Worthwhile to note is that there are a number of protein kinases that are inhibited by KT-5720 in addition to PKA, such as phosphorylase kinase, phosphoinositide-dependent kinase-1 (PDK1), and mitogen-activated protein kinase (for a review, see Murray, 2008). The present findings demonstrate that ethanol activates protein kinases, in general, prior to EWD to promote loss of NeuN/ Fox-3 expression in developing rat hippocampal explants. However, definitive conclusions about the influence of PKA in promoting the cytotoxic effects of CIE, in particular, cannot be drawn. For example, Ticku and colleagues found that KT-5720 inhibited the increased NMDA receptor clustering following CIE in immature cortical neurons (Qiang et al., 2007). It is possible that inhibition of phosphoinositide-dependent kinase-1 (PDK1), in addition to cAMP-dependent protein kinases, regulated these effects. As another example, a recent study conducted in our laboratory demonstrates endoplasmic reticulum inositol triphosphate and sigma receptors are stimulated by CIE during development to promote withdrawal-associated loss of neuron-specific nuclear protein/Fox-3 (Reynolds et al., 2016). Consistent with this notion, intracellular Ca2þ is implicated in activation of presynaptic protein kinase in modulation of spontaneous g-Aminobutyric acid (GABA) release following ethanol application in the periadolescent rat cerebellum (Kelm, Criswell, & Breese, 2007). Others have shown that intracellular Ca2þ mobilization is required for ethanol-induced increases in activity of protein kinases in the mature hippocampus (Balinõ, Ledesma, & Aragon, 2014), suggesting that intracellular Ca2þ may also modulate these effects upstream of protein kinase activity.
Collectively, these findings suggest that protein kinase activity prior to EWD regulates the cytotoxic effects of CIE. These effects are likely mediated, in part, via mobilization of intracellular calcium from endoplasmic reticulum-bound inositol triphosphate- and sigma chaperone-proteins prior to withdrawal. These neuro-adaptive changes in protein kinase activity during development may confer the sensitivity to withdrawal-associated cytotoxicity following CIE and perhaps contribute to the sedative effects of ethanol and voluntary ethanol consumption in mature rodents (Thiele et al., 2000; Wand, Levine, Zweifel, Schwindinger, & Abel, 2001).
Acknowledgments
This research was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (grant number: AA013388) and by the National Institute on Drug Abuse (NIDA) (grant number: T32 DA016176).
References
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. (2007). The selectivity of protein kinase inhibitors: A further update. The Biochemical Journal, 408, 297e315 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balinõ P, Ledesma JC, & Aragon CM (2014). In vivo study of ethanol-activated brain protein kinase a: Manipulations of Ca2þ distribution and flux. Alcoholism: Clinical and Experimental Research, 38, 629e640 10.1111/acer.12289. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, et al. (2007). Increased vulnerability of hippocampal neurons with age in culture: Temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Research, 1151, 20e30 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, et al. (2010). Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-aspartate-type glutamate receptors. Neuroscience, 165, 525e534 10.1016/j.neuroscience.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, & Chandler LJ (2004). Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. The Journal of Neuroscience, 24, 7859e7868 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, & McCool BA (2013). Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology, 65, 134e142 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Zou JY, & Neafsey EJ (1998). Brain damage due to episodic alcohol exposure in vivo and in vitro: Furosemide neuroprotection implicates edema-based mechanism. FASEB Journal, 12, 221e230 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9472987. [DOI] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, & Neafsey EJ (1998). Brain neuronal degeneration caused by episodic alcohol intoxication in rats: Effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcoholism: Clinical and Experimental Research, 22, 217e224 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9514310. [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, et al. (2014). Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology, 39, 435e444 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. (2009). Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: Functional implications for alcoholism. The Journal of Neurosciences, 29, 8655e8668 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. (1998). Effects of moderate alcohol consumption on the central nervous system. Alcoholism: Clinical and Experimental Research, 22, 998e1040 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9726269. [DOI] [PubMed] [Google Scholar]
- Gutie rrez R, & Heinemann U (1999). Synaptic reorganization in explanted cultures of rat hippocampus. Brain Research, 815, 304e316 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9878801. [DOI] [PubMed] [Google Scholar]
- Kadar A, Wittmann G, Liposits Z, & Fekete C (2009). Improved method for combination of immunocytochemistry and Nissl staining. Journal of Neuroscience Methods, 184, 115e118 10.1016/j.jneumeth.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, & Breese GR (2007). Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. The Journal of Pharmacology and Experimental Therapeutics, 323, 356e364 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, & Kawamoto S (2009). Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. The Journal of Biological Chemistry, 284, 31052e31061 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Ting KK, Adams S, Brew BJ, Chung R, & Guillemin GJ (2010). Characterisation of the expression of NMDA receptors in human astrocytes. PLoS One, 5, e14123 10.1371/journal.pone.0014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM (2002). NMDA receptors lose their inhibitions. Nature Neuroscience, 5, 614e616 10.1038/nn0702-614. [DOI] [PubMed] [Google Scholar]
- Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, et al. (2002). DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nature Neuroscience, 5, 641e648 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- Mao LM, & Wang JQ (2016). Regulation of group I metabotropic glutamate receptors by MAPK/ERK in neurons. Journal of Nature and Science, 2, e268. [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, & Ehlers MD (2003). Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron, 40, 581e594 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14642281. [DOI] [PubMed] [Google Scholar]
- Murray AJ (2008). Pharmacological PKA inhibition: All may not be what it seems.Science Signaling, 1, re4 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- Nagy J, & La szlo L (2002). Increased sensitivity to NMDA is involved in alcohol-withdrawal induced cytotoxicity observed in primary cultures of cortical neurones chronically pre-treated with ethanol. Neurochemistry International, 40, 585e591 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11900853. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, & Gruol DL (2005). Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Research, 1048, 69e79 10.1016/j.brainres.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Pascual M, & Guerri C (2007). The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. Journal of Neurochemistry, 103, 557e568 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA 2nd, Gibson DA, Holley RC, et al. (2004). Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. Neuroscience, 124, 869e877 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny AD, & Ticku MK (2007). Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Molecular Pharmacology, 72, 95e102 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Berry JN, Sharrett-Field L, & Prendergast MA (2015). Ethanol withdrawal is required to produce persisting N-methyl-D-aspartate receptordependent hippocampal cytotoxicity during chronic intermittent ethanol exposure. Alcohol, 49, 219e227 10.1016/j.alcohol.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Saunders MA, & Prendergast MA (2016). Ethanol stimulates endoplasmic reticulum inositol triphosphate and sigma receptors to promote withdrawal-associated loss of neuron-specific nuclear protein/Fox-3. Alcoholism: Clinical and Experimental Research, 40, 1454e1461 10.1111/acer.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Williams LA, Saunders MA, & Prendergast MA (2015). Group 1 mGlu-family proteins promote neuroadaptation to ethanol and withdrawal-associated hippocampal damage. Drug and Alcohol Dependence, 156, 213e220 10.1016/j.drugalcdep.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, & McKnight GS (2000). High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. The Journal of Neuroscience, 20, RC75 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10783399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM, & Becker HC (2005). Lorazepam and MK-801 effects on behavioral and electrographic indices of alcohol withdrawal sensitization. Brain Research, 1065, 92e106 10.1016/j.brainres.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Wand G, Levine M, Zweifel L, Schwindinger W, & Abel T (2001). The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. The Journal of Neuroscience, 21, 5297e5303 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11438605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins LH Jr., Prendergast MA, Blanchard J, Holley RC, Chambers ER, & Littleton JM (2006). Potential value of changes in cell markers in organotypic hippocampal cultures associated with chronic EtOH exposure and withdrawal: Comparison with NMDA-induced changes. Alcoholism: Clinical and Experimental Research, 30, 1768e1780 10.1111/j.1530-0277.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, & Wu CF (2013). Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behavioural Brain Research, 236, 270e282 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]