Abstract
Background:
Germline genetic testing increasingly identifies advanced prostate cancer (PCa) patients who are candidates for precision therapies. The Prostate Cancer Clinical Trials Consortium (PCCTC) established the Germline Genetics Working Group to provide guidance and resources to expand effective use of germline genetic testing.
Methods:
A 14-item questionnaire was emailed to academic oncologists at 43 PCCTC sites to collect information on germline genetic testing patterns, including patients considered, choice of assays, barriers slowing adoption, and actions to overcome barriers.
Results:
26 genitourinary oncologists from 19 institutions responded. Under 40% reported referring patients to a genetics department, while the remainder take personal responsibility for genetic testing and counseling; 62% consider testing all metastatic PCa patients, while 11% consider testing all patients with high-risk local disease; and 27% use multi-gene comprehensive pan-cancer panels while 54% use smaller or targeted cancer gene panels. Barriers to widespread use are (1) delayed or limited access to genetic counseling, (2) no insurance coverage, (3) lack of effective workflows, (4) insufficient educational materials, and (5) time and space constraints in busy clinics. The primary limitation was the <50% response from PCCTC sites and no coverage of non-academic cancer treatment facilities.
Conclusion:
Joint efforts by urologists, oncologists, genetics counselors, insurers, and cancer centers can accelerate implementation of integrated germline genetic services for personalized treatment and clinical trial eligibility for PCa patients.
Keywords: prostate cancer, survey, BRCA, Lynch, clinical trials, genetic testing, germline, somatic, metastatic, DNA repair, PARP inhibitors, pembrolizumab
MICRO ABSTRACT
Over 10% of patients with advanced prostate cancer carry inherited genetic mutations that may amplify their response to targeted therapies, but barriers, including a shortage of genetic counselors, limit patient access to testing that would enable targeted therapy. This study of practices in nineteen U.S. comprehensive cancer centers found that a shortage of genetic counselors and 4 other barriers limit adoption of this important advance. The paper also catalogues germline genetic testing practices and illuminates initiatives that may expand testing availability.
INTRODUCTION
A significant number of prostate cancer (PCa) cases have a heritable component. Germline DNA damage repair (DDR) defects are present in over 10% of patients who develop metastatic PCa (mPCa), with BRCA2 found in more than 5% and BRCA1, ATM, and CHEK2 each found in 1%-2%. Prevalence of germline mutations in DDR genes in men with mPCa exceeded the observed 5% prevalence in men with localized PCa and 3% prevalence in men without a known cancer diagnosis.1, 2 In recent years, the treatment landscape for mPCa has been refined by the discovery of DDR deficiency as predictive biomarkers for response to targeted therapies. For example, the presence of homologous recombination deficiency may predict response to poly (ADP-ribose) polymerase (PARP) inhibitors as well as to other DNA-damaging chemotherapy agents (platinum chemotherapy).3–6 Similarly, the presence of germline mutations in mismatch repair genes (MMR) may identify candidates for treatment with immune checkpoint inhibitors.7–10 Thus, it has become progressively important to assess practice patterns and needs regarding germline genetic testing and counseling for men with PCa. Urologists are increasingly ordering germline testing for their PCa patients in light of recent evidence that BRCA1/2 and ATM mutation status is associated with grade reclassification or PCa patients undergoing active surveillance.11 Urology involvement in germline genetic testing will grow as the area evolves to include high risk localized and earlier disease states.12–14
The Germline Genetics Working Group (GGWG) of the Prostate Cancer Clinical Trials Consortium (PCCTC) was established in June 2017 in response to the need to better inform and advise clinicians of the increasing evidence that germline alterations in DDR genes may identify additional options for prostate cancer therapy. The objectives of the GGWG are to work together with clinicians and researchers around topical challenges of treatment selection and eligibility for trials of investigational therapeutics, and to enable more streamlined and effective use of germline genetic testing in PCa patients in the face of a rapidly evolving genetics-informed therapeutic landscape.
In June 2018, the GGWG produced a White Paper that presented a framework to address unique challenges and therapeutic opportunities regarding germline testing for precision therapy in patients with advanced PCa and identified areas of future research.15 The White Paper recommended that clinicians: (1) consider expanding germline genetic testing beyond cancer risk assessment to inform treatment selection and eligibility for clinical trials, (2) work with genetic counselors to ensure pre-test informed decision-making through education or counseling and post-testing counseling and, (3) where appropriate, ensure mechanisms for offering cascade germline genetic testing to family members. However, barriers and challenges to broader implementation of these recommendations require attention.
To elucidate practice patterns and challenges in germline genetic testing of PCa patients in oncology, the GGWG surveyed medical oncologists from institutions that are members of the PCCTC. Here, we report survey data from responding oncologists from nineteen PCCTC cancer centers identifying commonalities and differences in practice patterns. The survey results were used to support recommendations for addressing barriers to germline testing for men with PCa and are placed in the context of current NCCN guidelines for genetic testing of PCa patients.
MATERIALS AND METHODS
Survey.
A 14-question survey was developed by the GGWG to capture the practice patterns and needs of oncologists at PCCTC institutions. The survey was refined after pilot testing and then distributed to oncologists at their institutions using RedCap, a web-based survey tool, with an email (12/20/17) asking members to complete the survey and to ask other investigators at their institutions to respond. A reminder email was sent on 1/16/18 to GGWG members who had not completed the survey. PCCTC principal investigators were reminded of the survey during two sequential monthly conference calls that took place between the two email-based survey distributions (12/21/17 and 1/8/18).
The survey questions offered multiple choice answer selections on personal practices around genetics services, on patient characteristics oncologists consider for germline testing (metastatic disease, advanced stage, family history), on cascade genetic testing processes, and on gene panels. The remainder were free-form questions asking for clarifications of answers to the multiple-choice questions and also asking participants to describe their approaches to integrating germline testing with therapeutic clinical trials and to cascade testing. The full survey may be found in the Supplementary Materials.
Analysis:
Data were gathered in the RedCap package and exported to a comma separated value file. Data were imported into Excel, where they were summarized, tabulated, and graphed.
RESULTS
Between 12/20/2017 and 4/3/2018, representatives of the 43 PCCTC participating and affiliate sites received the germline genetic testing current practice survey and an email encouraging redistribution to institutional colleagues, and twenty-six PCa oncologists from nineteen sites (44%) completed the survey (Table 1)
Table 1:
Cancer Centers Providing Germline Genetic Testing Survey Responses
| Cancer Center | Responses |
|---|---|
| Fred Hutchinson Cancer Research Institute | 3 |
| Johns Hopkins Sidney Kimmel Comprehensive Cancer Center | 3 |
| Lurie Comprehensive Cancer Center | 2 |
| Rush University Medical Center | 2 |
| Weill Cornell Medical College | 2 |
| Beth Israel Deaconess Cancer Center | 1 |
| Carbone Cancer Center | 1 |
| Dana-Farber Cancer Institute | 1 |
| Duke Comprehensive Cancer Center | 1 |
| Jonsson Comprehensive Cancer Center | 1 |
| Lineberger Comprehensive Cancer Center | 1 |
| Masonic Cancer Center | 1 |
| Memorial Sloan Kettering Cancer Center | 1 |
| Moores Cancer Center | 1 |
| Oregon Health and Science University Knight Cancer Institute | 1 |
| Sidney Kimmel Cancer Center at Thomas Jefferson University | 1 |
| University of Chicago Medicine Comprehensive Cancer Center | 1 |
| Wayne State Karmanos Cancer Institute | 1 |
| Yale Cancer Center | 1 |
Personal Practices of PCa Oncologists regarding Genetics Services for PCa Patients
Whereas 38% (10/26) of participating oncologists reported that they refer patients to a separate department for genetic testing and counseling, more than half reported taking personal responsibility for some or all genetic education and testing of their patients. Fifteen percent (4/26) reported personally performing pre-test counseling, ordering germline testing, and performing post-test counseling. Those who reported using a combination of approaches generally referred to genetic counselors for post-test counseling. Variation in practice appears to depend on factors such as (1) patient’s insurance coverage for genetic testing and counseling, (2) availability of testing and counseling resources within the oncology group, (3) testing and counseling resources in a separate genetics department, and (4) wait times for referrals.
Patients Considered for Germline Genetic Testing
Metastatic PCa
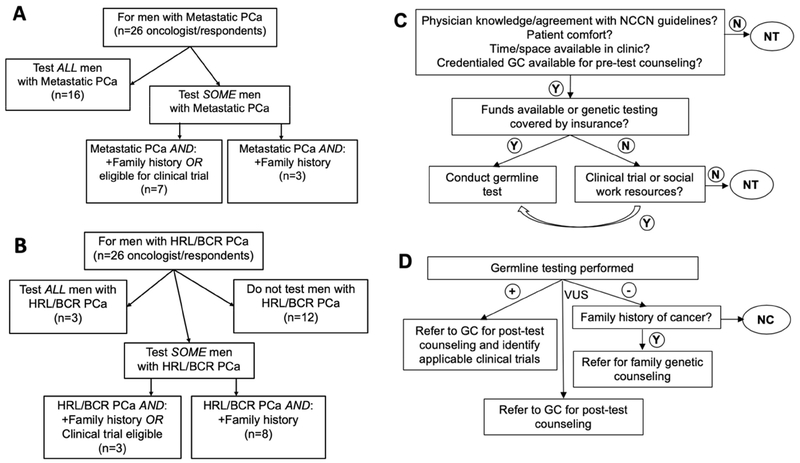
All twenty-six participating oncologists reported considering some mPCa patients for germline genetic testing: 62% reported considering all mPCa patients; 27% considered testing mPCa patients with a family history and/or who were eligible for clinical trials; and 12% considered testing only for patients with a family history for germline genetic testing (Fig. 1A).
Figure 1: Provider Considerations of Germline Testing Among Men with Prostate Cancer.
Note: Panel A: Men with mPCa considered for germline testing; Panel B: Men with high-risk localized and biochemically recurrent PCa considered for germline testing; Panel C: Factors affecting decision to test; Panel D: Factors affecting decisions on results.
Abbreviations: +, positive results; −, negative results; BCR, biochemically recurrent; GC, genetic counselor; HRL, high risk localized; N, no; NC, no post-test genetic counseling; NT, no germline genetic testing; PCa, prostate cancer; VUS, variant of uncertain significance; Y, yes.
High-risk Localized PCa Patients and Non-metastatic PCa
More than half of the participating oncologists (54%) considered germline genetic testing for some PCa patients with high-risk localized or non-metastatic disease (HRL/nmPCa), while twelve (46%) did not consider germline testing for these patients. Three (12%) reported testing all HRL/nmPCa patients; 3 (12%) reported considering testing only for patients with a family history for germline genetic testing; and 8 (31%) reported considering testing only those with a family history of cancer (Fig. 1B).
Operational Barriers Faced by Oncologists Considering Germline Genetic Testing for PCa Patients
Based on participant responses and free text comments, five barriers in streamlining genetic testing were identified: access to genetic counselors, insurance coverage and cost, clinic workflow, time and space availability, and access to resources for provider and patient education. Fig. 1C shows the role of those operational barriers and the flow of considerations on whether to conduct germline genetic testing reported by responding oncologists, while Fig. 1D shows how referral decisions are made on the basis of those results.
Reported Germline Testing Approaches
Participating oncologists reported their approaches for germline cancer predisposition testing. Twenty-seven percent (7/26) reported using only “comprehensive pan-cancer panels,” while 54% (14/26) listed only “expanded cancer panels (e.g., Lynch and BRCA1/2 and hereditary breast and ovarian cancer genes),” and 4 others reported using more than one type of panel. One participant did not answer this question (Table 2).
Table 2.
Approaches to Germline Cancer Predisposition Testing
| Respondent | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General approach to germline cancer predisposition testing* | 11 | 6 | 10 | 17 | 21 | 1 | 22 | 18 | 20 | 16 | 19 | 13 | 2 | 8 | 12 | 3 | 15 | 7 | 24 | 5 | 25 | 26 | 23 | 4 | 9 |
| Comprehensive, pan-cancer panel | • | • | • | • | • | • | • | • | |||||||||||||||||
| Expanded cancer panel (e.g., Lynch and BRCA1/2 and hereditary breast and ovarian cancer genes) | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||||||
| Limited prostate-cancer-specific panel | • | ||||||||||||||||||||||||
| Specific individual genes (e.g., BRCA1, BRCA2) | • | • | |||||||||||||||||||||||
| Clinical-trial focused panel | • | • | |||||||||||||||||||||||
Sites that listed multiple approaches (e.g., comprehensive and limited) explained that lack of insurance or limited patient capacity to pay sometimes causes them to select a more limited panel.
Resources for Patients and Family Members Regarding Genetic Testing
The GGWG aggregated a list of websites that it has found valuable for educating patients and their families about germline genetic testing for PCa (Table 3). Two participants reported they are developing local resources (a video and an information sheet for patients).
Table 3:
Web Resources for Educating PCa Patients and Families about Germline Genetic Testing
| Source | URL | Content |
|---|---|---|
| Color | https://www.color.com/leam/can-cancer-be-inherited | Patient-friendly discussion and data about cancer due to inherited (germline) genetic mutations |
| Dana Farber | https://www.dana-farber.org/cancer-genetics-and-prevention/videos/ | Videos explaining topics in cancer genetics and testing |
| Invitae | https://www.invitae.com/en/patients/genetic-diagnosis/ | Brief descriptions of genetic testing |
| Memorial Sloan Kettering | https://www.mskcc.org/cancer-care/risk-assessment-screening/hereditary-genetics/genetic-counseling/inherited-risk-prostate | Very brief discussion of inherited risk of PCa |
| National Society of Genetic Counselors | https://www.nsgc.org/ | Comprehensive guidance for patients, including help finding counselors |
| Prostate Cancer Foundation | https://www.pcf.org/news/genetic-screening-guidelines-for-prostate-cancer/ | High-level overview of genetics-related knowledge helpful to patients with PCa and their families |
| https://www.pcf.org/patient-resources/family-cancer-risk/genetic-testing-prostate-cancer/ | Recommendation to speak with physician about whether patients need genetic testing, with five more detailed sub-pages | |
| Sidney Kimmel Cancer Center of Thomas Jefferson University | https://prostategenetics.jeffersonhealth.org/ | Prostate-cancer-focused and patient-friendly, web-based resource explaining genetic counseling, genetic testing, family history, genes, and cancer risks |
DISCUSSION
The relevance of germline genetic testing in PCa is emerging today as it did in breast cancer three decades ago, although in PCa it is accompanied by concurrent therapeutic relevance. These rapid and exciting changes have resulted in challenges illustrated by the 2018 PCCTC GGWG survey of medical PCa oncologists at academic institutions in the PCCTC consortium, around their practices in germline genetic testing of PCa patients. The survey identified common themes across nineteen institutions as well as substantial variation in those practices. Five barriers to obtaining genetic testing were identified: lack of timely access to genetic counselors, lack of insurance coverage or high patient out-of-pocket costs, lack of integrated clinic workflow, time and space constraints, and insufficient resources for provider and patient education. Our survey found that more than half of oncologists reported taking part or full responsibility for germline genetic testing and education/counseling, despite the fact that most oncologists are not trained in genetic counseling.16 Nearly 40% of the participating oncologists reported that among mPCa patients, they considered germline genetic testing mainly for those patients with a family history of cancer and those who were eligible for a clinical trial with genetic eligibility criteria, rather than all mPCa patients. Germline testing results are increasingly important for consideration of clinical trial eligibility. PCCTC sites reported ten therapeutic trials for PCa patients with relevance to germline mutations and four trials testing new models of genetics delivery in prostate cancer (Table 4). Similarly, a little more than half of respondents reported considering germline genetic testing for patients with high-risk localized PCa and non-metastatic PCa. These responses may reflect a period of limited resources and substantial logistical barriers and the need for triaging and prioritization of genetic counseling and testing until barriers can be better addressed.
Table 4.
Therapeutic and Delivery Model Clinical Trials in PCa with Relevance for Germline Genetic Eligibility Criteria
| Phase | Title | Disease State | Abbreviated Title | Clinicaltrials.gov |
|---|---|---|---|---|
|
Therapeutic
Trials | ||||
| III | Study of Olaparib versus Enzalutamide or Abiraterone Acetate in Men with Metastatic Castration-Resistant Prostate Cancer | mCRPC | PROFOUND | |
| III | A Study of Rucaparib versus Physician’s Choice of Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer and Homologous Recombination Gene Deficiency | mCRPC | TRITON3 | |
| II | A Phase 2 Efficacy and Safety Study of Niraparib in Men with Metastatic Castration-Resistant Prostate Cancer and DNA-Repair Anomalies | mCRPC | GALAHAD | |
| II | A Multicenter, Open-Label Phase 2 Study of Rucaparib in Patients with Metastatic Castration-Resistant Prostate Cancer Associated with Homologous Recombination Deficiency | mCRPC | TRITON2 | |
| II | Response Rate Study of Talazoparib in Men with DNA Repair Defects and Metastatic Castration-Resistant Prostate Cancer Who Previously Received Taxane-Based Chemotherapy and Progressed on at Least 1 Novel Hormonal Agent (Enzalutamide and/or Abiraterone Acetate/Prednisone) | mCRPC | ||
| II | Olaparib in Men with High-Risk Biochemically-Recurrent Prostate Cancer Following Radical Prostatectomy, with Integrated Biomarker Analysis | BCR | ||
| II | Abiraterone/Prednisone, Olaparib, or Abiraterone/Prednisone + Olaparib in Patients with Metastatic Castration-Resistant Prostate Cancer with DNA Repair Defects | mCRPC | BRCAaway | |
| Pilot | Docetaxel and Carboplatin in Treating Patients with Metastatic, Hormone-Resistant Prostate Cancer Containing Inactivated Genes in the BRCA 1/2 Pathway | mCRPC | ABCD | |
| II | Docetaxel and Carboplatin for Patients with mCRPC and DNA-Repair Deficiencies | mCRPC | V-ABCD | |
| II | Trial of Rucaparib in Patients with Metastatic Hormone-Sensitive Prostate Cancer Harboring Germline DNA Repair Gene Mutations | mHSPC | TRIUMPH | |
|
Delivery Model
Trials | ||||
| N/A | Evaluating an Alternative Clinical Genetics Cancer Care Delivery Model: A Pilot Study of Patient Outcomes: Evaluating greater oncologist participation in genetic testing | PCa | ||
| - | ||||
| - | ||||
| N/A | Genetic Evaluation of Men: Registry of PCa patients with increased risk with family history and biospecimen bank | PCa | GEM Registry | |
| - | ||||
| N/A | Genetic Counseling Processes and Outcomes Among Males with Prostate Cancer: Testing pre-genetic-test video versus traditional pre-test counseling | PCa | ProGen | |
| - | ||||
| N/A | Genetic Testing for Men with Metastatic Prostate Cancer: Assess germline homologous recombination variants and family history, and identify men who might benefit from research and treatment options | mPCa | GENTleMEN | |
Abbreviations: PCa, prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; mPCa, metastatic prostate cancer; mHSPC, metastatic hormone-sensitive prostate cancer; BCR, biochemical recurrence
At the time the survey was distributed, the National Comprehensive Cancer Network (NCCN) guidelines did not include recommendations for germline testing for most PCa patients. Based on recent data of germline mutations in men with PCa, before all GGWG survey responses were returned, NCCN Prostate Guidelines (Version 4.2018) were expanded to recommend consideration of germline genetic testing for most metastatic and high-risk localized PCa patients.17 Thus, the survey captured change in action.
The current guidelines recommend consideration of germline testing for all patients with high-risk and very high-risk local disease, regional disease, and metastatic disease. Further, with more sensitive techniques for early identification of metastatic disease such as PSMA, fluciclovine,18 and choline,19, 20 more patients may be classified as metastatic than in the past, further increasing the numbers of patients to be considered for germline genetic testing. This expansion of patient populations to be considered for genetic testing, together with the barriers reflected in our findings, highlight the need for dedicated education and training for radiation oncologists, urologists and medical oncologists. In the localized disease setting with risk levels that are very low, low, favorable intermediate, and unfavorable intermediate, the NCCN Prostate guidelines suggest consideration for germline testing based on a strong family history of prostate cancer and/or other primary cancers, or for patients with a relative with a known familial cancer risk syndrome.
The current NCCN Prostate guidelines also suggest that patients whose tumor testing is positive for MSI-high or deficient-MMR (indicating potential use of pembrolizumab in treatment for mCRPC), also be referred for genetic counseling to assess for the possibility of Lynch syndrome. Finally, the current NCCN guidelines recommend that physicians consider testing tumors of patients with mCRPC for germline and somatic mutations in BRCA1, BRCA2, ATM, PALB2, and FANCA.
Approximately half of survey participants indicated that they would refer patients for genetic counseling and dedicated confirmatory germline testing if a tumor mutation was potentially germline in nature (thus with family counseling implications21) consistent with the recommendation in the GGWG White Paper15. As tumor sequencing for targeted treatment opportunities increase in PCa, the likelihood of identifying mutations that are potentially germline may increase, raising the need for distinct workflows to address this specific clinical scenario.
Several respondents noted that patient willingness to undergo germline genetic testing could be affected by concerns about genetic discrimination for life, disability, and long-term care insurance. The Genetic Information Nondiscrimination Act (GINA) of 200822 provides protection from genetic discrimination in health insurance and employment in most employment scenarios, but does not cover life insurance, long-term care, disability insurance, Indian Health Service, federal employees enrolled in the Federal Employee Health Benefits Plan, and other specific VA or U.S. military plans. Given these gaps in protection by the GINA law and potential changes over time, patients approached about germline testing need to consider these issues and their own financial situations prior to proceeding with germline testing. Thus, providers and patients can benefit from educational and practice-ready resources to help address the need to discuss genetic discrimination laws.
There are some limitations to consider in our results. Urologists were not surveyed, as metastatic disease has been a key driver of genetic testing up to the present time. However, since guidelines for testing is expanding to earlier stage disease, including urologists in future surveys will add important information. Our analysis does not account for institutional limitations that may have informed physician decisions regarding whom to test. In addition, there were site-specific differences in clinical trial availability with germline genetic eligibility criteria, including in the non-metastatic PCa setting which would have influenced consideration of genetic testing for that group. Another limitation is the response rate (oncologists from only nineteen of forty-three PCCTC sites responded), and the composition of respondents being largely oncologists with specific focus on prostate cancer in academic centers. Nevertheless, we feel that the general concepts around the clinical need for better integration of germline genetic testing in prostate cancer care and the current barriers to implementation will be broadly applicable across oncology practice settings.
CONCLUSION
The NCCN and other professional organizations advocate informed decision-making for patients in the pre-test setting.23–27 Research to improve delivery of pre-test education and optimization of informed decision-making is key to streamlining genetic testing for men with PCa. In the post-test setting, discussion with a genetic counselor is important for patients with germline mutations, variants of uncertain significance, and with no mutations but with a family history of cancer to ensure understanding of results and appropriate follow up with regards to additional cancer screening and cascade testing recommendations. Physicians ordering genetic testing need to be well-versed in cancer risk guidelines for screening, genetic results interpretation, GINA laws, and population-level cancer screening guidelines. While referral to a genetic counselor is preferred when possible, there is a recognized shortage of genetic counselors that is predicted to worsen,28, 29 suggesting a role for subspecialty oncologists with training in genetics as well as for genetics training for oncology providers who perform aspects of genetic counseling themselves.30 Registries that include germline data, family history, treatments, and outcomes such as those being developed in the GEM, GENTLeMEN, and ProGEN trials, along with systems to address barriers to genetic testing, will help inform future guidelines and facilitate integrated genetic testing and counseling services into busy clinical practices.
Supplementary Material
CLINICAL PRACTICE POINTS.
In the context of an evolving therapeutic landscape for men with mPCa and expanding NCCN guidelines for germline testing for patients with earlier stage disease, oncologists and urologists will increasingly need to consider incorporating genetic education, counseling, and germline testing for men with PCa. Providing guideline-concordant care now requires that practices and institutions prioritize including germline genetic testing as part of optimal care delivery. Physicians, mid-level providers, genetic counselors, practice managers, and other team members must work in a concerted manner to overcome these barriers in practice- and resource-specific ways to this evolving care model.
ACKNOWLEDGEMENTS
We gratefully acknowledge support from the Prostate Cancer Foundation, the Institute for Prostate Cancer Research, the Pacific Northwest Prostate Cancer NIH SPORE CA097186, NCI Center Core Grants (P30 CA008748 and P30 CA006973), NCI K23 CA197526, and UL1 TR002319. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Prostate Cancer Research Program under Award Numbers W81XWH-17-2-0043, W81XWH-17-2-0018, W81XWH-17-2-0017, W81XWH-17-2-0020, W81XWH-17-2-002, W81XWH-17-2-00221, W81XWH-17-2-0027, and W81XWH-15-2-0018. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Giri VN, Obeid E, Gross L, Bealin L, Hyatt C, Hegarty SE, et al. Inherited Mutations in Men Undergoing Multigene Panel Testing for Prostate Cancer: Emerging Implications for Personalized Prostate Cancer Genetic Evaluation, JCO Precision Oncology 2017. :1, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. The New Englandjournal of medicine. 2016;375:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. The New England journal of medicine. 2015;373:1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic Inactivation of BRCA2 in Platinum-sensitive Metastatic Castration-resistant Prostate Cancer. European urology. 2016;69:992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudadi K, Suzman DL, Anagnostou V, et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget. 2018;9:28561–28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bono JS GJ, Ojamaa K, Rodriguez JM, Drake CG, Hoimes CJ, et al. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC)., Journal of Clinical Oncology 2018. 36:15_suppl, 5007–5007. . [Google Scholar]
- 11.Carter HB, Helfand B, Mamawala M, et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. European urology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giri VN, Hegarty SE, Hyatt C, et al. Germline genetic testing for inherited prostate cancer in practice: Implications for genetic testing, precision therapy, and cascade testing. The Prostate. 2019;79:333–339. [DOI] [PubMed] [Google Scholar]
- 13.Nicolosi P, Ledet E, Yang S, et al. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. European urology. 2015;68:186–193. [DOI] [PubMed] [Google Scholar]
- 15.Carlo MI, Giri VN, Paller CJ, et al. Evolving Intersection Between Inherited Cancer Genetics and Therapeutic Clinical Trials in Prostate Cancer: A White Paper From the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. JCO Precision Oncology. 2018:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zon RT, Goss E, Vogel VG, et al. American Society of Clinical Oncology policy statement: the role of the oncologist in cancer prevention and risk assessment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohler JL, Armstrong AJ, D’Amico AV, et al. NCCN Clinical Practice Guidelines in Oncology Prostate Cancer, Version 1.20182018. [Google Scholar]
- 18.Parent EE, Schuster DM. Update on (18)F-Fluciclovine PET for Prostate Cancer Imaging. J Nucl Med 2018;59:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantiello F, Crocerossa F, Russo GI, et al. Comparison Between (64)Cu-PSMA-617 PET/CT and (18)F-Choline PET/CT Imaging in Early Diagnosis of Prostate Cancer Biochemical Recurrence. Clin Genitourin Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 20.Miyahira AK, Pienta KJ, Morris MJ, et al. Meeting report from the Prostate Cancer Foundation PSMA-directed radionuclide scientific working group. The Prostate. 2018;78:775–789. [DOI] [PubMed] [Google Scholar]
- 21.Giri VN, Knudsen KE, Kelly WK, et al. Role of Genetic Testing for Inherited Prostate Cancer Risk: Philadelphia Prostate Cancer Consensus Conference 2017. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health, National Human Genome Research Project. The Genetic Information Nondiscrimination Act of 2008. https://www.genome.gov/27568492/the-genetic-information-nondiscrimination-act-of-2008/ Accessed 12/3/2018.
- 23.National Comprehensive Cancer Network. Prostate Cancer (Version 4.2018). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf Accessed 12/3/2018.
- 24.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 2.2019). https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf Accessed 12/3/2018.
- 25.Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21:151–161. [DOI] [PubMed] [Google Scholar]
- 26.Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:3660–3667. [DOI] [PubMed] [Google Scholar]
- 27.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. The Journal of urology. 2018;199:683–690. [DOI] [PubMed] [Google Scholar]
- 28.Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the Supply and Demand for Certified Genetic Counselors: a Workforce Study. J Genet Couns. 2018;27:16–20. [DOI] [PubMed] [Google Scholar]
- 29.Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. Am J Med Genet C Semin Med Genet 2018;178:24–37. [DOI] [PubMed] [Google Scholar]
- 30.https://www.who.int/genomics/professionals/education/en/ Accessed 12/3/2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.