Background
While teleophthalmology is not a novel technique or method of care, its application is set to undergo a transformation due to the implementation of artificial intelligence (AI). The field of AI has recently experienced significant advancements in image recognition due to a technique called deep learning, and is increasingly being investigated in ophthalmic image segmentation, analysis, and clinical decision making [1–5].
Review and challenges
For teleophthalmology to be successfully adopted on a larger scale, it must address a need. This can be accomplished by either a substantial improvement in the quality of care, or by increasing access to timely care. The number of ophthalmologists is growing at half the rate of our population aged over 60 years, and there are 23 countries with less than one ophthalmologist per million population [6]. The rate of prematurity in the United States has increased to 13% but only 54% of eligible retina or pediatric specialists are willing to manage retinopathy of prematurity (ROP) [7]. Regarding screening for diabetic retinopathy (DR), 58% of patients with diabetes fail to obtain follow-up dilated fundus exams [8]. Understandably, in its current state teleophthalmology has found the most use in DR and ROP screening to address these significant shortages of care. Notably, the Joslin Vision Network (JVN) has participated in one of the largest clinically validated studies employing teleophthalmology. The group demonstrated a method to triage and grade DR in a non-ophthalmic setting for further referral and management, and achieved “high concordance between management decisions based on JVN imaging, clinical examination, and fundus photography” [9, 10]. The English National Screening Programme for DR managed to screen and triage 83% (over 2,000,000) of the eligible patients with diabetes in England, and has significantly contributed to DR no longer being the leading cause of blindness in England and Wales [11]. Additionally, in a large prospective multicenter study, Biten et al. showed no difference between telemedicine and ophthalmoscopy in overall accuracy for detecting clinically significant ROP [12]. Such validation studies will be increasingly important as teleophthalmology continues to expand.
Developments and future potential
AI-assisted triage and clinical decision-making holds great potential for increasing efficiency and access to care. However, in order to use these technologies effectively, a modification of clinic flow may be necessary. Korteum et al. demonstrated a proof-of-concept, rapid-access, virtual medical retina clinic providing care for over 1700 patients. These referrals had optical coherence tomography (OCT) and color fundus imaging, basic undilated eye exams, and histories taken by trained nursing staff, with subsequent image evaluation by qualified graders. The virtual clinic model was successful in reducing costs and increasing space in “face to face” clinics [13]. Similar virtual clinics have been successfully established for low-risk glaucoma screening, and had relatively low clinically significant false negative rate of 4% when reviewed by an ophthalmologist “face to face” audit [14].
The application of AI to home-based monitoring is being validated in screening for DR, and is gaining Food and Drug Administration (FDA) approval [15]. Furthermore, promising technologies such as automated whole-eye binocular OCT may soon enable ophthalmologists to remotely acquire increasingly detailed imaging along with pupillometry, perimetry, motility, and visual acuity [16]. Novel models of remote care delivery including “telephotocoagulation” as shown by Kozak et al. demonstrated the feasibility of creating image-based and fluorescein angiogram-based treatment plans for remote navigated retinal photocoagulation [17]. Telephotocoagulation and other tele-treatments may provide methods of decoupling diagnosis and treatment locations. As experts are frequently concentrated urban locations, such decoupling may evolve from convenience to necessity for providing care to a dispersed population and to patients in developing countries.
The recent rapid advancement of technology in image acquisition such as ultra-widefield imaging has changed the treatment paradigms of common posterior segment pathology such as proliferative diabetic retinopathy. When used in a teleophthalmology platform, nonmydriatic ultra widefield imaging can reduce the rates of ungradable images, decrease image evaluation time, and increase the rate of identification of DR compared to traditional fundus photography [18].
Discussion/conclusion
The infusion of artificial intelligence (AI) into ophthalmology is occurring, and point of care AI may improve image quality, reduce the number of ungradable images, and more effectively triage care in the setting of limited availability of subspecialists [19]. Eventually, clinically validated AI algorithms may augment virtual clinics that triage care to specialized physicians as necessary (Fig. 1).
Fig. 1.
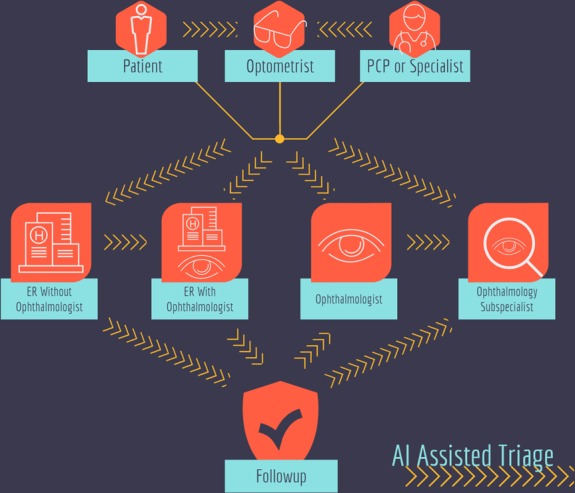
Current complicated clinical pathways in the United States for patients to be seen by an ophthalmic subspecialist. Arrowed lines represent potential impact points of artificial intelligence for increased triage efficiency. Current research includes AI integration into patient facing eye exam kiosks, ophthalmic point of care imaging devices, and remote image reading
As ophthalmologists, we must perform due diligence to clinically validate such increasingly diverse telemedicine tools. There are numerous considerations involving legal, reimbursement, security, and ethical issues which must be addressed. Whether an algorithm is AI-assisted with diagnostic decision-making in the hands of physicians, or AI-driven without human intervention, there are significant legal ramifications. For example, a primary care physician can potentially rely on AI-driven algorithms to dictate escalation of care. However, during a potential delay in care or false negative diagnosis, the liability becomes unclear. AI based on supervised learning must be trained on labeled images, and there exists a risk of a false negative diagnosis if the algorithm is presented a disease process it was not trained with. Reimbursement with emergent technologies can be equally ambiguous. IDX, LLC (Coralville, IA) recently received FDA approval for its diabetic retinopathy screening algorithm that is physician independent (https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm604357.htm), using the FDA designation of breakthrough technology. Time savings and removal of professional fees for interpretation of exams may become a major consideration for payer reimbursement structure. Reimbursement will be an evolving process, with implications for further development of the technology. Additionally, while there is potential for cost savings, we must consider the often costly investment in IT and security infrastructure to support this new technology.
With any emergent technology that has the potential of significantly altering healthcare delivery, we must consider issues of ethics and access. Through AI, telemedicine has the potential to increase access, but must be implemented in such a way that it is done equitably to a large population, with such considerations at the forefront of FDA approval. Ultimately, the evolution of telemedicine through technologies such as AI will inevitably lead to wider adoption. Therefore, the authors encourage proactively engendering such dialogue regarding ethics, laws, reimbursement, security, and equitable access to increase the probability of safe and effective future implementation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ting DSW, Cheung CYL, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–23. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlegl T, Waldstein SM, Bogunovic H, et al. Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology. 2018;125:549–58. doi: 10.1016/j.ophtha.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Lee CS, Tyring AJ, Deruyter NP, et al. Deep-learning based, automated segmentation of macular edema in optical coherence tomography. Biomed Opt Express. 2017;8:3440–8. doi: 10.1364/BOE.8.003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen JC, Lee CS, Keane PA, Xiao S, Wu Y, Rokem A, et al. Forecasting Future Humphrey Visual Fields Using Deep Learning. arXiv [cs.CV]. 2018. Available at: https://www.arxiv.org/abs/1804.04543. [DOI] [PMC free article] [PubMed]
- 5.De Fauw Jeffrey, Ledsam Joseph R., Romera-Paredes Bernardino, Nikolov Stanislav, Tomasev Nenad, Blackwell Sam, Askham Harry, Glorot Xavier, O’Donoghue Brendan, Visentin Daniel, van den Driessche George, Lakshminarayanan Balaji, Meyer Clemens, Mackinder Faith, Bouton Simon, Ayoub Kareem, Chopra Reena, King Dominic, Karthikesalingam Alan, Hughes Cían O., Raine Rosalind, Hughes Julian, Sim Dawn A., Egan Catherine, Tufail Adnan, Montgomery Hugh, Hassabis Demis, Rees Geraint, Back Trevor, Khaw Peng T., Suleyman Mustafa, Cornebise Julien, Keane Pearse A., Ronneberger Olaf. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nature Medicine. 2018;24(9):1342–1350. doi: 10.1038/s41591-018-0107-6. [DOI] [PubMed] [Google Scholar]
- 6.Resnikoff S, Felch W, Gauthier TM, et al. The number of ophthalmologists in practice and training worldwide: a growing gap despite more than 200,000 practitioners. Br J Ophthalmol. 2012;96:783–7. doi: 10.1136/bjophthalmol-2011-301378. [DOI] [PubMed] [Google Scholar]
- 7.Richter GM, Williams SL, Starren J, et al. Telemedicine for retinopathy of prematurity diagnosis: evaluation and challenges. Surv Ophthalmol. 2009;54:671–85. doi: 10.1016/j.survophthal.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murchison AP, Hark L, Pizzi LT, et al. Non-adherence to eye care in people with diabetes. BMJ Open Diabetes Res Care. 2017;5:e000333. doi: 10.1136/bmjdrc-2016-000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavallerano AA, Cavallerano JD, Katalinic P, et al. A telemedicine program for diabetic retinopathy in a Veterans Affairs Medical Center--the Joslin Vision Network Eye Health Care Model. Am J Ophthalmol. 2005;139:597–604. doi: 10.1016/j.ajo.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 10.Bursell SE, Cavallerano JD, Cavallerano AA, et al. Stereo nonmydriatic digital-video color retinal imaging compared with Early Treatment Diabetic Retinopathy Study seven standard field 35-mm stereo color photos for determining level of diabetic retinopathy. Ophthalmology. 2001;108:572–85. doi: 10.1016/S0161-6420(00)00604-7. [DOI] [PubMed] [Google Scholar]
- 11.Scanlon PH. The English National Screening Programme for diabetic retinopathy 2003-2016. Acta Diabetol. 2017;54:515–25. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biten H, Redd TK, Moleta C, Campbell JP, Ostmo S, Jonas K, et al. Diagnostic accuracy of ophthalmoscopy vs telemedicine in examinations for retinopathy of prematurity. JAMA Ophthalmol. 2018;136:498. doi: 10.1001/jamaophthalmol.2018.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kortuem Karsten, Fasler Katrin, Charnley Amanda, Khambati Hussain, Fasolo Sandro, Katz Menachem, Balaskas Konstantinos, Rajendram Ranjan, Hamilton Robin, Keane Pearse A, Sim Dawn A. Implementation of medical retina virtual clinics in a tertiary eye care referral centre. British Journal of Ophthalmology. 2018;102(10):1391–1395. doi: 10.1136/bjophthalmol-2017-311494. [DOI] [PubMed] [Google Scholar]
- 14.Kotecha A, Brookes J, Foster PJ. A technician-delivered ‘virtual clinic’ for triaging low-risk glaucoma referrals. Eye. 2017;31:899–905. doi: 10.1038/eye.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Heijden AA, Abramoff MD, Verbraak F, et al. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 2018;96:63–8. doi: 10.1111/aos.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra R, Mulholland PJ, Dubis AM, et al. Human factor and usability testing of a binocular optical coherence tomography system. Transl Vis Sci Technol. 2017;6:16. doi: 10.1167/tvst.6.4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozak I, Payne JF, Schatz P, et al. Teleophthalmology image-based navigated retinal laser therapy for diabetic macular edema: a concept of retinal telephotocoagulation. Graefes Arch Clin Exp Ophthalmol. 2017;255:1509–13. doi: 10.1007/s00417-017-3674-1. [DOI] [PubMed] [Google Scholar]
- 18.Silva PS, Cavallerano JD, Tolls D, et al. Potential efficiency benefits of nonmydriatic ultrawide field retinal imaging in an ocular telehealth diabetic retinopathy program. Diabetes Care. 2014;37:50–5. doi: 10.2337/dc13-1292. [DOI] [PubMed] [Google Scholar]
- 19.Wen JC, Lee CS, Keane PA, Xiao S, Wu Y, Rokem A, et al. Forecasting Future Humphrey Visual Fields Using Deep Learning. bioRxiv. 2018: 293621. Available at: https://www.biorxiv.org/content/early/2018/04/02/293621 [Accessed April 9, 2018]. [DOI] [PMC free article] [PubMed]


