Abstract
Forest undergrowth plants are tightly connected with the shady and humid conditions that occur under the canopy of tropical forests. However, projected climatic changes, such as decreasing precipitation and increasing temperature, negatively affect understory environments by promoting light‐demanding and drought‐tolerant species. Therefore, we aimed to quantify the influence of climate change on the spatial distribution of three selected forest undergrowth plants, Dracaena Vand. ex L. species, D. afromontana Mildbr., D. camerooniana Baker, and D. surculosa Lindl., simultaneously creating the most comprehensive location database for these species to date.
A total of 1,223 herbarium records originating from tropical Africa and derived from 93 herbarium collections worldwide have been gathered, validated, and entered into a database. Species‐specific Maxent species distribution models (SDMs) based on 11 bioclimatic variables from the WorldClim database were developed for the species. HadGEM2‐ES projections of bioclimatic variables in two contrasting representative concentration pathways (RCPs), RCP2.6 and RCP8.5, were used to quantify the changes in future potential species distribution.
D. afromontana is mostly sensitive to temperature in the wettest month, and its potential geographical range is predicted to decrease (up to −63.7% at RCP8.5). Optimum conditions for D. camerooniana are low diurnal temperature range (6–8°C) and precipitation in the wettest season exceeding 750 mm. The extent of this species will also decrease, but not as drastically as that of D. afromontana. D. surculosa prefers high precipitation in the coldest months. Its potential habitat area is predicted to increase in the future and to expand toward the east.
This study developed SDMs and estimated current and future (year 2050) potential distributions of the forest undergrowth Dracaena species. D. afromontana, naturally associated with mountainous plant communities, was the most sensitive to predicted climate warming. In contrast, D. surculosa was predicted to extend its geographical range, regardless of the climate change scenario.
Keywords: Dracaena afromontana Mildbr., Dracaena camerooniana Baker, Dracaena surculosa Lindl., ecological niche modeling, forest undergrowth plants, global warming, Maxent model, rainforest, shade‐demanding species
1. INTRODUCTION
The genus Dracaena Vand. ex L. belongs to the family Asparagaceae (APG III, 2009). It is considered to be monophyletic based on molecular studies (Lu & Morden, 2010) and comprises approximately 116 species (Govaerts et al. 2018). Dracaena is distributed in subtropical and tropical regions of the world. Most species are found in Africa, Madagascar, and Asia, and the remainder are found in Socotra, the Mediterranean region, Macaronesia, Central America, Cuba, Micronesia, Australia, and the Pacific Islands (Bos, 1998; Marrero, Almeida, & González‐Martín, 1998; Staples & Herbst, 2005). Approximately 63 species of Dracaena occur in Africa (incl. Madagascar) (Damen, Burg, Wiland‐Szymańska, & Sosef, 2018). Three of them belong to a group of dragon trees found in semiarid and subtropical climates (Marrero et al., 1998). The rest are connected with humid habitats (Bos, 1984; Pierzchalska, Nowak, Wilkin, Mwachala, & Wiland‐Szymańska, 2014).
The species of the genus Dracaena are among the most important ornamental plants in the world due to their various leaf shapes and colorations as well as their resistance to indoor conditions (Bos, 1984; Singh & Dadlani, 2000). Some species of Dracaena produce chemicals valued in medicine and cosmetology, as well as in traditional healing. The best examples are Dracaena draco L., D. cinnabari Balf. f., and D. ombet Heuglin ex Kotschy and Peyr., which produce a resin called “dragon's blood” (Elnoby, Moustafa, & Mansour, 2017; Jura‐Morawiec & Tulik, 2016), as well as D. mannii Baker and D. arborea (Wild.) Link (Okunji, Iwu, Jackson, & Tally, 1996). Dracaena species also play a role as markers of borders and other socially important sites in Africa (Sheridan, 2008). Spontaneously regenerating D. afromontana is utilized by farmers as a plant that provides ecosystem services on coffee plantations in Rwanda (Smith, Gassner, Agaba, Nansamba, & Sinclair, 2018). Fruits of Dracaena are forage for birds and mammals (González‐Castro, Pérez‐Pérez, Romero, & Nogales, 2019). In spite of its importance, the taxonomy and distribution of the genus Dracaena are not fully known, and recent works stress the need for further investigations of this genus (Damen et al., 2018; Pierzchalska et al., 2014). The habitat preferences of African Dracaena species have not been studied except for those of D. ombet (Robiansyah & Hajar, 2017). However, the difficulty of performing a continental‐scale, systematic botanical study in Africa (e.g., due to staff, funding and accessibility limitations) negatively impacts our understanding of the current distribution of Dracaena species on this continent. Increasing anthropogenic impact (e.g., cultivating ornamental plants) also prevents a reliable assessment of the current distribution of plants, especially those connected with fragile and overexploited habitats, for example, the equatorial forest (Ahmed & Mlay, 1998). The scarcity of botanical data acquired during planned botanical surveys can be overcome by historical herbarium materials that have been collected over decades. Gathering records from numerous herbaria worldwide may shed light on the current and future potential distribution of plant species when applying specially designed mathematical methods (Crawford & Hoagland, 2009; Elith & Leathwick, 2007).
Global climate variability is recognized as one of the main causes of changes in the spatial distribution of plants (Costion et al., 2015; Kelly & Goulden, 2008). One of the most susceptible ecosystems is the tropical forest, broadly defined as an area with tree canopy >5 m tall covering at least 10% of more than 0.5 ha surface and located between the tropics of Cancer and Capricorn (FAO, 2001). Tropical forests are global hotspots of biodiversity and significant modulators of climate change but are also among the most vulnerable ecosystems worldwide. Among them are rainforests or equatorial forests which are relatively dense, tall, and evergreen broadleaf forests with a high number of tree species growing in a moist environment (Lewis, 2006; Ter Steege et al., 2003). In recent years, these ecosystems have been decreasing due to logging and mining activities that lead to further land degradation and land use change over time (Laurance & Cochrane, 2001). However, even the apparently intact forest areas have been altered, especially in their physiology and ecology. A “CO2 fertilization” hypothesis suggests that the productivity and growth rate of tropical forests increase due to the higher amount of CO2 (originating from the combustion of fossil fuels) available for photosynthesis (Lloyd & Farquhar, 2008). It has been found that the dry biomass of trees in tropical rainforests increased by approximately 1 Mg ha−1 year−1 in last 20 years of the 20th century (Baker et al., 2004). One of the effects of this biomass increase will be a faster plant life cycle, a higher tree mortality rate, and consequently a more frequent occurrence of canopy gaps. This, in turn, will favor light‐demanding species and would be a limiting factor for shade‐demanding species such as forest undergrowth plants (Lewis, 2006; Lewis, Malhi, & Phillips, 2004). Therefore, there is a risk of the decline in forest undergrowth plant populations, even when located in intact rainforests far from direct human impacts. In this study, we investigated three Dracaena species that belong to this group of plants. They can serve as a proxy for other shade‐tolerant understory species.
The “CO2 fertilization” hypothesis is still broadly discussed worldwide, as there is also evidence that the aboveground net primary productivity in equatorial forests does not increase, despite the distinct increase in CO2 concentration in recent years (Clark, Clark, & Oberbauer, 2013). This may be an effect of recently observed drying, especially in western equatorial Africa (James, Washington, & Rowell, 2013). Additionally, long‐term satellite multisensor measurements of the vegetation conditions in equatorial forests show that forest greenness is declining in most parts of the African equatorial forest (Zhou et al., 2014). This photosynthetic capacity loss can be attributed to the drying tendency, which, consequently, may lead to modification of the forest species composition by favoring drought‐tolerant species (Fauset et al., 2012; Lewis, 2006). In this case, forest undergrowth plants that are typically adjusted to shady, wet environments will be more endangered. Therefore, regardless of whether the future conditions of tropical forests follow the trend of the “CO2 fertilization” hypothesis, the number and strength of threats to forest understory plants seem to be increasing more rapidly than for other ecological groups of plants in equatorial forests.
Therefore, the potential ranges of three Dracaena species growing as understory plants in equatorial forests were modeled in this study. A general purpose of species distribution modeling (SDM) is to estimate the potential geographical distribution of species based on the environmental conditions recorded at species record locations. For presence–absence data, it is possible to apply traditional ecological modeling techniques, such as GLM (generalized linear models), GAM (general additive models), and others (Franklin & Miller, 2009). Presence–absence data could be collected in a systematic, planned botanical study, which is frequently difficult due to time, funding, and staff limitations and to political situations (Elith et al., 2006). In contrast, presence‐only data are much easier to obtain but are more difficult to reliably use in SDM (Fithian & Hastie, 2013). To account for the frequent lack of the absence data, so‐called pseudo‐absence or background data are usually simulated, for example, using a Maxent model that is particularly designed for presence–background data (Phillips, Anderson, & Schapire, 2006; Phillips & Dudik, 2008). A Maxent model is able to predict not only the current species occurrence probability but also the probability in past and future climates (Febbraro et al., 2017; Kukwa & Kolanowska, 2016). Recently, the future climate change scenario generation process has been redesigned from a sequential to a parallel approach. The latter approach starts with the target radiative forcing in the year 2,100 and assumes that different combinations of affecting factors (e.g., policy, economy, land use changes) may contribute to the same target level. There are four main representative concentration pathways (RCPs) that generally reflect radiative forcing continuously rising (RCP 8.5), stabilizing (RCP 6.0 and RCP 4.5) or reaching a peak, and then declining (RCP 2.6) (Moss et al., 2010).
Considering the previously stated need to assess the influence of climate change on a vulnerable ecological group of plants (forest undergrowth species), we specified the following hypothesis for this study: As a representation of forest undergrowth plants, three selected Dracaena species will be dramatically affected by climate change‐induced loss of potential habitats, and therefore, a large proportion of the current potential range will be eliminated in the near future. To assess the future distribution patterns of the species in question, we aimed to (a) create a database with validated species occurrence records for the three Dracaena species in question, (b) identify the most important climate variables affecting the distribution of these Dracaena species, (c) determine the optimum climatic conditions for these Dracaena species, (d) estimate and discuss the current potential geographic range of these Dracaena species in comparison with the collected records, and (e) show future changes in the ranges of these Dracaena species according to two contrasting climate warming scenarios.
2. MATERIALS AND METHODS
2.1. Dracaena spp. occurrence records
For our study, we chose three species of Dracaena characterized by different spatial distributions: D. afromontana Mildbr., D. camerooniana Baker, and D. surculosa Lindl. D. afromontana is a 2–12 m high shrub or tree. It grows in Eastern and Central Africa (Figure 1) (Kelbessa, Kalema, & Crook, 2013). Dracaena camerooniana is a branched shrub with cane‐like shoots, 0.3–8 m in height, growing in Central and West Africa (Figure 1) (Damen et al., 2018). Both species are listed on the IUCN Red List as species of Least Concern (Crook, 2013; Kelbessa et al., 2013). Dracaena surculosa is also a branched shrub, 1–8 m in height naturally, that grows in West Africa (Figure 1) and is widely cultivated as an ornamental plant (Bos, 1984). More detailed information about the species studied can be found in Mwachala (2005).
Figure 1.
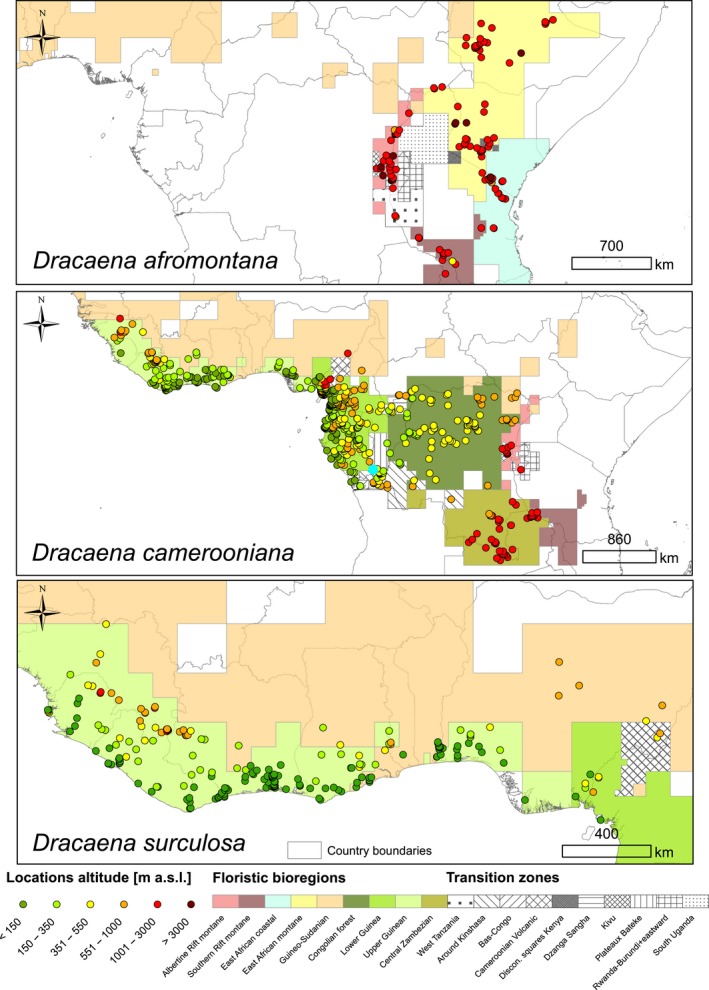
Location and altitude of presence records of three Dracaena species on the background of African floristic bioregions (Droissart et al., 2018)
Occurrence records originated from 93 herbaria around the world (a complete list in Appendix S1; abbreviations according to Thiers, 2018). We only took records of specimens that we had seen and that have an exact specified location in the wild (coordinates available). Finally, we validated the identification of 1,223 herbarium records: 161 D. afromontana, 771 D. camerooniana, and 291 D. surculosa (see Appendix S2). Some of the records were collected in the same location or close to each other (within a distance of 30 arc sec—approximately 1 km2 at the equator—the spatial resolution of bioclimatic variables: see section Environmental variables), which could introduce potential bias resulting from haphazard sampling (Syfert, Smith, & Coomes, 2013). To decrease the spatial autocorrelation, we removed all locations but one, attributed to one grid cell of the bioclimatic layers. This procedure allowed us to prevent the analysis from overweighting locations situated in easy‐to‐access, well‐surveyed areas (Newbold, 2010). Therefore, for modeling purposes, in the end, we used 920 independent locations (presence‐only data), with 122 records for D. afromontana, 588 for D. camerooniana, and 210 for D. surculosa (Figure 1). Altitudinal data used in this study were retrieved from GTOPO30, a global digital elevation model with a spatial resolution of 30 arc sec (the same as the bioclimatic data in this study, see below) and available at the U.S. Geological Survey's Center for Earth Resources Observation and Science (EROS). To ensure the uniformity of information for analysis, we did not use altitudinal data from herbarium labels.
2.2. Species distribution modeling
Maxent, a machine learning technique using a maximum entropy approach, has been used in this study. It generally means that of the different possible probability distribution functions describing and explaining the data, the most appropriate one is the distribution with maximal entropy within a set of constraints (Elith et al., 2011; Jaines, 1957). Maxent model results are presented in a readily interpretable logistic format that indicates areas with higher and lower probabilities of species occurrence. Maxent is considered to have comparable or better quality than other models of this type applied to presence–background data in SDM (Elith et al., 2006). We used the Maxent software, version 3.3.3k (Phillips et al., 2006; Phillips & Dudik, 2008) with the default parameters because they have been validated over many species and environmental variables, various sample sizes, and biases (Elith et al., 2011). The convergence threshold, maximum number of iterations, maximum number of background points for creating background data, and prevalence were set to 10−5, 5 × 102, 104 and 5 × 10−1, respectively.
The goodness‐of‐fit of the SDM models calibrated in this study was tested using the approach of a receiver operating characteristic (ROC) curve that shows the interdependence between true positive (sensitivity) and false positive (1‐specificity) rates of presence/background events of the observed/predicted data. The area under the ROC curve (AUC) was used as a measure for model accuracy. The AUC provides a simple numerical value (varying from 0.5—no predictive power—to 1—perfect discrimination) that quantifies the location of the ROC curve to a diagonal that describes a random model (Hanley & McNeil, 1982). The final testing AUC value for a particular species distribution model was obtained by 10‐fold cross‐validation (Christensen, 2002). The testing AUC values were justified according to the traditional classification of the model performance (Swets, 1988): poor (AUC = 0.5–0.6), fair (0.6–0.7), good (0.7–0.8), very good (0.8–0.9), and excellent (0.9–1).
2.3. Environmental variables
To define the ecological requirements and niches of the three Dracaena species, we used the bioclimatic variables from the Worldclim database v.1.4. (Hijmans, Cameron, Parra, Jones, & Jarvis, 2005, http://www.worldclim.org/) with a 30 arc‐sec spatial resolution. A complete list of the 19 explanatory variables was reduced to 11 bioclimatic variables (Table 1). The 8 redundant variables were removed based on the results of a pairwise Pearson correlation test incorporated in ENMTools (Warren, Glor, & Turelli, 2010) over the study area (Table S3.1 in Appendix S3). Only variables with <0.75 correlation proceeded to further analysis (as described in Ponce‐Reyes et al., 2017). We also reduced the number of variables by not considering altitude and other nonclimatic variables because adding such variables typically does not increase the AUC values (Vedel‐Sørensen, Tovaranonte, Bøcher, Balslev, & Barfod, 2013). Moreover, the additional information carried by such variables as elevation or aspect frequently overlaps with the information stored in climatic variables (Apaydin, Anli, & Ozturk, 2011; Shi, Paull, & Rayburg, 2016). Some authors indicate that the use of only climatic parameters as explanatory variables in SDM can be problematic (e.g., Wiens, Stralberg, Jongsomjit, Howell, & Snyder, 2009) because land use and land cover are very important factors that determine the species distribution. However, this kind of limitation particularly involves studies on smaller spatial scales (Pearson & Dawson, 2003). In contrast, the present study area comprises a large part of the African continent, and the herbarium records came from the entire known range of the selected Dracaena species, which improves the scientific value of the species distribution modeling (Cuyckens, Christie, Domic, Malizia, & Renison, 2016). Thus, we decided to focus on carefully selected bioclimatic variables only.
Table 1.
Description of bioclimatic variables used in this study and retrieved from Worldclim database (Hijmans et al., 2005)
| Variable description | Abbreviation |
|---|---|
| Mean Diurnal Temperature Range (difference between mean monthly maximum and minimum temperature) | Temp_range |
| Isothermality—the relationship between Mean Diurnal Temperature Range and Temperature Annual Range (difference between temp_max and temp_min) | Isotherm |
| Maximum temperature of the warmest month | Temp_max |
| Minimum temperature of the coldest month | Temp_min |
| Mean temperature of the wettest quarter | Temp_wet |
| Mean temperature of the driest quarter | Temp_dry |
| Precipitation seasonality—variation coefficient | Prec_var |
| Precipitation total of wettest quarter | Prec_wet |
| Precipitation total of driest quarter | Prec_dry |
| Precipitation total of the warmest quarter | Prec_warm |
| Precipitation total of the coldest quarter | Prec_cold |
A relative contribution of variables to the overall Maxent model performance has been checked to identify the factors most likely limiting the occurrence of the Dracaena species studied. Two measures of the variable importance were (a) the permutation importance of a bioclimatic variable and (b) jackknife resampling analysis showing AUC with only a particular variable and without this variable. The values of the three variables with the highest contribution (most important) per species were plotted against the probability of occurrence, resulting in partial dependence plots.
2.4. Climate change scenarios, climate model
From four main existing representative concentration pathways (RCPs), we selected the best and the worst scenarios, called RCP 2.6 and RCP 8.5, respectively (Moss et al., 2010). By considering two opposite climate change scenarios, we were able to capture the possible variability (maximum–minimum interval) in future distribution changes of the three species of Dracaena studied.
To predict the future potential distributions of the three Dracaena species, we used projections of the Worldclim bioclimatic variables (Hijmans et al., 2005) calculated from future climate projections within the framework of the Coupled Model Intercomparison Project Phase 5 (CMIP5) (Taylor, Stouffer, & Meehl, 2012). The HadGEM2‐ES model (Collins et al., 2011) was selected for the present study based on the recommendation of this model to predict future temperature and precipitation changes in Africa (Dike et al., 2015). Other studies, such as that of Brands, Herrera, Fernandez, and Gutierrez (2013), confirmed that HadGEM2‐ES outperforms other models gathered in CMIP5 when compared for Africa.
2.5. Environmental niche mapping
The current and future potential distribution of the three Dracaena species was mapped using the maximum sensitivity + specificity logistic threshold (max SSS). This parameter is based on maximizing the sum of sensitivity and specificity and is recommended for use to binarize the occurrence probabilities (Liu, White, & Newell, 2013 and references therein). Gathering all the Maxent model iterations, we obtained a statistical distribution of the selected threshold for training and test datasets for each species (Figure S3.1 in Appendix S3). Finally, we mapped a median current potential distribution (current potential range), minimum and maximum possible range, and 1st and 3rd quartile range based on max SSS values. For future climate change scenarios, we mapped the median and minimum future range over the background of the current potential range. In addition, we calculated the current and future potential range area attributed to a particular country. Comparing this to the number of herbarium records per country may serve as a proxy for the completeness of distribution studies of selected Dracaena species in a country. Both the initial number of records and the final selection of unique locations are provided in the Supporting Information (see Tables S3.1, S3.2, S3.3. in Appendix S3). To provide more details on the future potential area of Dracaena species, we calculated future surface area changes based on two different climate warming scenarios.
3. RESULTS
3.1. Importance of the environmental variables
For D. afromontana, temp_wet (46.0%) and prec_dry (21.2%) contributed most to the model performance (Figure 2). Temp_wet also turned out to be the most important variable when testing the AUC for individual variables (AUC = 0.96) (Figure 3). In D. camerooniana, three variables contributed at least 15%: prec_wet (30.5%), prec_var (28.3%), and temp_range (17.9%) (Figure 2). Using individual variables, temp_range resulted in an AUC higher than 0.8, and precipitation variables reached maximal AUC = 0.735 (prec_wet) (Figure 3). In D. surculosa, the three following variables were important: prec_dry (40.0%), prec_cold (15.7%), and isotherm (15.0%) (Figure 2). This was reflected in the AUC analysis, where prec_cold and prec_dry reached AUC = 0.887 and AUC = 0.845, respectively (Figure 3).
Figure 2.
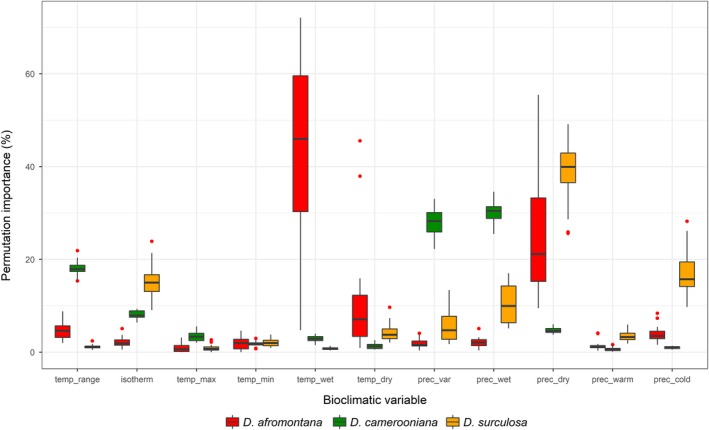
Relative contribution (permutation importance) of environmental variables to the final species distribution models for Dracaena species. Boxplot: central value—median, upper/lower hinges—1st and 3rd quartile, whiskers—extreme values
Figure 3.
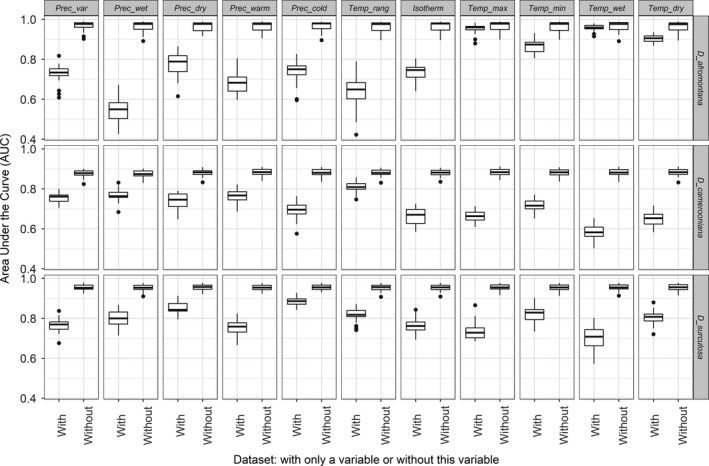
AUC values obtained at jackknife analysis for all the 11 bioclimatic variables used. AUC was calculated for partial models with only a particular variable and without this variable
3.2. Potential optimum conditions
The occurrence of D. afromontana was directly related to temp_wet and temp_dry. Occurrence probability sharply increased when these two variables were lower than 20°C and 15°C, respectively. In turn, the occurrence probability of D. camerooniana distinctly increased when prec_wet was >750 mm, prec_var was <70 mm, and temp_range was 6–8°C. In D. surculosa, the optimum conditions occurred when prec_cold exceeded 1,300 mm and prec_dry exceeded 430 mm (Figure 4).
Figure 4.
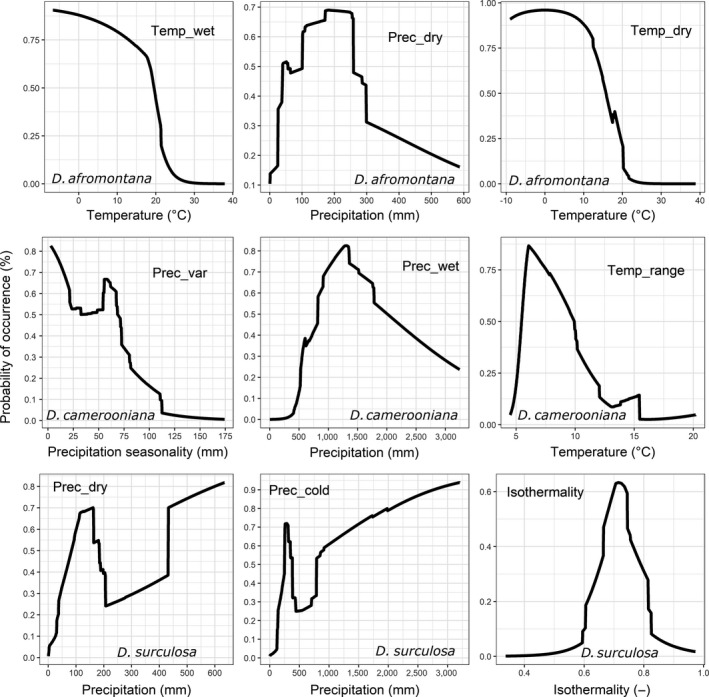
Partial dependence curves showing the response of Dracaena species to three variables mostly responsible for their spatial distribution (assuming that remaining variables are constant). The higher the probability of occurrence (vertical axis), the more suitable conditions for particular Dracaena species
3.3. Current potential distribution
Dracaena afromontana records were located mainly at higher altitudes in East Africa in the following floristic bioregions (Droissart et al., 2018): 2—East African montane, 5—Albertine Rift montane, and 9—Southern Rift montane. D. camerooniana occupies a wide area of Central Africa surrounding the Gulf of Guinea, concentrated mainly on its Eastern bank and in the Congo Basin, in the following bioregions: 3—Guineo‐Congolian, 5—Albertine Rift montane, and 8—Central Zambezian and transition zones. Numerous D. camerooniana records also originated from the areas adjacent to the north coast of the Gulf of Guinea that were sympatric to the D. surculosa locations (Figure 1), which is restricted to the 3—Guineo‐Congolian (including 3a—Upper Guinea and 3b—Lower Guinea) and 1—Guineo‐Sudanian bioregions.
Species distribution models developed in this study have been classified as excellent (D. afromontana and D. surculosa) or very good (D. camerooniana), reaching mean testing AUCs of 0.966, 0.955, and 0.883, respectively (Table 2). The entire modeled D. afromontana current potential range (573,400 km2) is located in the mountainous parts of nine African countries, with Ethiopia, Tanzania, Kenya, and DR Congo occupying 36.1%, 18.9%, 15.9%, and 11.4% of the current potential range, respectively. Most records were located in Tanzania (42) and Ethiopia (35). However, the highest record density (locations per 10,000 km2 of the country) was calculated for Burundi (5.14) (Table 3, Figure 5, Table S3.1 in Appendix S3).
Table 2.
Validation scores of the three Dracaena species final distribution models
| Species name | Training AUC | Testing AUC | ||||
|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | |
| D. afromontana | 0.978 | 0.981 ± 0.002 | 0.984 | 0.901 | 0.966 ± 0.03 | 0.989 |
| D. camerooniana | 0.899 | 0.902 ± 0.002 | 0.906 | 0.837 | 0.883 ± 0.02 | 0.911 |
| D. surculosa | 0.965 | 0.967 ± 0.001 | 0.969 | 0.916 | 0.955 ± 0.02 | 0.978 |
Abbreviation: AUC, area under the receiver operation characteristic curve.
Table 3.
Changes in three Dracaena sp. potential coverage until 2050 according to two contrasting climate change scenarios
| Species | Statistical distribution of a potential rangea | Current potential coverage (×1,000 km2) | Future potential coverage—climate change scenario | |||
|---|---|---|---|---|---|---|
| RCP2.6 | RCP8.5 | |||||
| Area (×1,000 km2) | Relative change (%) | Area (×1,000 km2) | Relative change (%) | |||
| D. afromontana | Median range | 573.4 | 250.1 | −56.3 | 166.5 | −63.7 |
| Minimum range | 209.1 | 107.0 | −48.8 | 76.0 | −70.9 | |
| Maximum range | 1,531.0 | 599.1 | −60.9 | 336.9 | −78.0 | |
| D. camerooniana | Median range | 2,698.5 | 1,954.6 | −27.6 | 1,066.0 | −60.5 |
| Minimum range | 1,160.0 | 1,057.6 | −8.8 | 539.2 | −53.5 | |
| Maximum range | 4,450.7 | 3,102.0 | −30.3 | 1,958.1 | −56.0 | |
| D. surculosa | Median range | 1,028.4 | 1,486.6 | +44.6 | 1,763.1 | +71.5 |
| Minimum range | 578.9 | 820.7 | +41.8 | 853.4 | +47.4 | |
| Maximum range | 2,886.3 | 4,150.7 | +43.8 | 4,853.8 | +68.2 | |
Median range—potential distribution area calculated using median max SSS logistic threshold; Minimum range—potential distribution area calculated using maximum value of max SSS logistic threshold; Maximum range—potential distribution area calculated using minimum value of max SSS logistic threshold.
Figure 5.
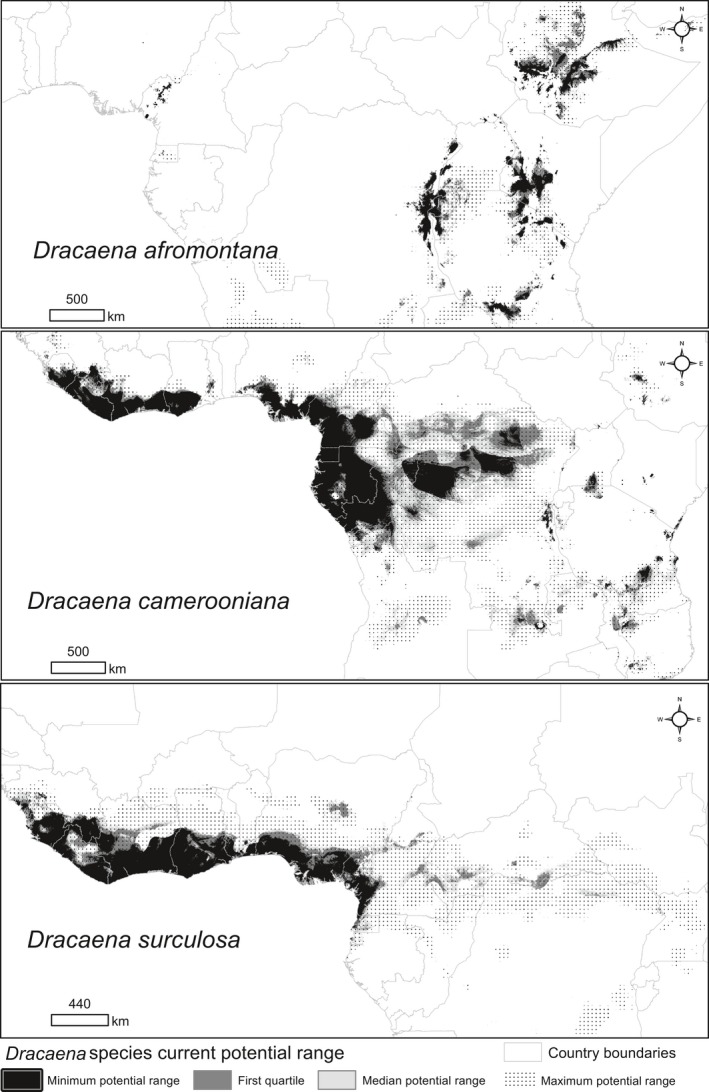
Current potential distribution of three Dracaena species in Africa. Current potential range of a particular species is attributed to median potential range. Maximum and minimum potential ranges were shown on the basis of minimum and maximum (respectively) values of max SSS logistic threshold obtained during the fitting of the models
The current potential range of D. camerooniana covered 2,698,500 km2, mainly in the following countries: DR Congo (35.6% of current potential range), Congo (11.4%), Cameroon (9.6%), and Gabon (9.5%). Cameroon (185), DR Congo (179), and Gabon (129) had the most record locations in their territory, but the highest record density was noted in Equatorial Guinea (7.46 per 10,000 km2 of country area) (Table 3, Figure 5, Table S3.2 in Appendix S3).
The estimated D. surculosa current potential range covered 1,028,400 km2, primarily in Ivory Coast (18.8%), Nigeria (18,8%), Ghana (12,3%), and Cameroon (11.8%). Most herbarium records originated from Ivory Coast, and this country also had the highest record density (4.21 per 10,000 km2 of country area) (Table 3, Figure 5, Table S3.3 in Appendix S3).
3.4. Future potential distribution
Regardless of the climate change scenario, the area potentially occupied by D. afromontana and D. camerooniana is predicted to be markedly reduced by 2050 (Figure 6a–d). The former species seems to be even less resistant to climate warming than the latter; its potential distribution may be reduced by 56.3% in the RCP 2.6 scenario and 63.7% in the RCP 8.5 scenario (Table 3). Considering individual countries, the largest loss of D. afromontana potential range in the future involves Tanzania, where the range area will decrease dramatically regardless of the climate change scenario (at least 75% of the current potential range) (Figure S3.2 in Appendix S3). D. camerooniana will experience a markedly lower loss rate in the potential distribution area if the optimistic RCP 2.6 scenario occurs (27.6%). However, if the pessimistic/negative RCP 8.5 scenario occurs, the loss rate will reach a similar level as for D. afromontana (60.5%) (Table 3). The RCP 2.6 climate change scenario seems to affect the D. camerooniana potential range relatively slightly. In contrast, at RCP 8.5, most countries lose large fractions of this range, especially DR Congo, which is predicted to lose at least 60% of the current potential range of D. camerooniana (Figure S3.2 in Appendix S3).
Figure 6.
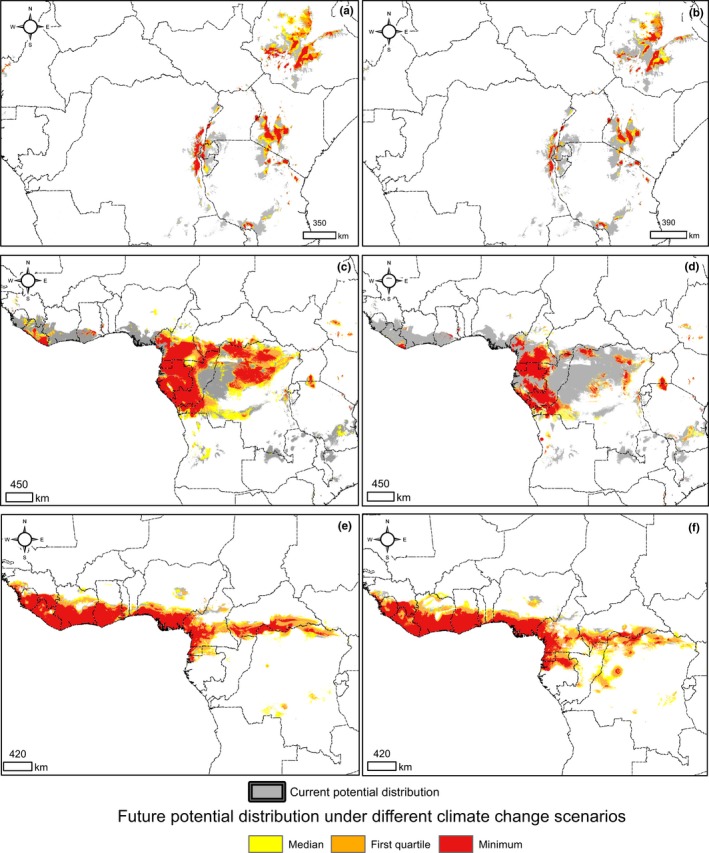
Future potential distribution of Dracaena species. Under two contrasting climate change scenarios (representative concentration pathways, RCPs), RCP 2.6 (left column) and RCP 8.5 (right column): (a and b) Dracaena afromontana; (c and d) Dracaena camerooniana; (e and f) Dracaena surculosa
In contrast, the potential distribution area of D. surculosa is projected to increase until 2050 (Figure 5e–f). Under the RCP 8.5 scenario, the median current potential distribution of this species is predicted to be almost two times larger than at present, with especially high increases in Ivory Coast, Cameroon, and Liberia. In Ghana and Sierra Leone, the D. surculosa area increases only if the RCP 8.5 scenario is applied. In addition, the D. surculosa area in Nigeria is expected to only slightly increase regardless of the climate change scenario (Table 3, Figure S3.2).
4. DISCUSSION AND CONCLUSIONS
4.1. Potential distribution modeling in Dracaena species
To date, the potential impact of climate change on the Dracaena species has been analyzed only for two species from the “dragon tree” group: Dracaena cinnabari Balf.f. from Socotra (Yemen), and Dracaena ombet Kotschy and Peyr. from northeastern Africa (Attorre et al., 2007; Robiansyah & Hajar, 2017). Robiansyah and Hajar (2017) used a Maxent model to estimate the current and future distribution of D. ombet according to the “worst” climate change scenario (RCP 8.5 projections for 2050 and 2070). This species is classified as endangered on the IUCN list, and moreover, the population consists mainly of old specimens, and seedlings are found very rarely. The locations of D. ombet are hardly accessible because they occur on steep slopes in the desert mountains of northeastern Africa, so the geographical distribution of the species is poorly known (Elnoby et al., 2017). Furthermore, although D. ombet is well adjusted to drought conditions, the future potential extent of this species markedly decreases (Robiansyah & Hajar, 2017). These authors obtained a satisfactory model (AUC = 0.979) despite the study being based on only 24 location records. However, the AUC values for the testing set have not been given, and thus, it is difficult to estimate the real predictive power of this model. In contrast, in our study, we used many more locations (at least 122 records for D. afromontana), and our model was of a similar quality at training set (AUC = 0.981 and AUC = 0.966, for test and training set, respectively). A similar methodology, but using regression tree analysis instead of Maxent, has been used to assess the changes in the potential distribution of D. cinnabari in Socotra (Attorre et al., 2007). It turned out that this species is also predicted to lose its potential habitat in the future.
The species D. cinnabari and D. ombet are considered remnants from the Mio‐Pliocene subtropical forests and are considered close to extinction because of the late Pliocene climate changes causing the drying and desertification of northwestern Africa (Mies, 1996). The question is whether the forest understory plants from the Dracaena genus growing in tropical Africa will be affected by such dramatic climate changes, which will possibly lead to their extinction. Until now, Dracaena forest undergrowth plants were not studied in this way (Pierzchalska et al., 2014). In this study, we chose three endemic African forest undergrowth species characterized by different distributions within the continent but connected with humid habitats. We examined the next 30 years of projected climate changes and showed that selected species respond differently to the climatic signal. D. afromontana habitat area will decrease over 50% until 2050 according to both RCP 2.6 and RCP 8.5. We documented that the optimum temperature in the wettest quarter for this species does not exceed 20°C. However, the climatic projections for the region indicate an increase in temperature (Engelbrecht et al., 2015). Thus, the minimum altitude at which D. afromontana could grow is increasing. This phenomenon results in a reduction of the area of potential habitats of D. afromontana. In contrast, there is evidence that D. afromontana may be grown by farmers as a fence or boundary marker (Sheridan, 2008), which may be the reason that the number of locations of this species will not decrease as rapidly as predicted. Nevertheless, we predicted a future loss of or decrease in potential natural D. afromontana habitats. Currently, the status of D. afromontana on the IUCN Red list is of Least Concern because the population is relatively stable in Eastern Africa (Kelbessa et al., 2013). However, there is evidence that the potential distribution area of D. afromontana, the Albertine Rift, is likely to lose many endemic species until 2080 (specifically, up to 80% of the current ranges of endemic plants are projected to be lost) (Ayebare, Plumptre, Kujirakwinja, & Segan, 2018). Therefore, D. afromontana should be treated as a species that is the most vulnerable to projected climate warming.
Potential habitats for D. camerooniana seem to be less vulnerable to climate warming, but the model developed in this study showed a possible slight decrease until 2050. However, changing the scenario from RCP 2.6 to RCP 8.5 greatly increases the loss in potential range area (from 27% to 60%, respectively). Currently, the species distribution area is relatively large, D. camerooniana is frequently found in different types of forests, including secondary and degraded forests, and the population is considered generally stable (Crook, 2013). We suggest that forest loss may exert a stronger impact than climate warming in the near future.
In contrast to the previous species, the range of D. surculosa is predicted to increase under both climate conditions analyzed. In particular, an expansion toward Central Africa is expected. We documented that low temperature and precipitation can limit the growth of D. surculosa, but the predicted future changes result in the opposite conditions, so we assume that this species is not endangered when considering climatic factors only.
4.2. Discrepancies between the number of herbarium records and current potential distribution
In some countries, relatively few locations of Dracaena species were recorded. Nevertheless, the record density per 10,000 km2 was higher than that in the remaining countries. This was the case with D. afromontana in Burundi and Rwanda, D. camerooniana in Equatorial Guinea, and D. surculosa in Liberia and Sierra Leone. This result may imply that these countries have been well surveyed for Dracaena species. The common feature of the mentioned countries is that they are rather small and are located in the center of the known geographic ranges of the particular Dracaena species. Thus, large parts of these countries constitute suitable habitats for the species, which is another explanation of the high record density in these countries.
We noted that the current potential distribution range was also modeled in countries in which Dracaena species location records were scarce or absent. A small number or no records of D. camerooniana originated from Eastern Africa (Ethiopia, Kenia, Uganda, Tanzania, Malawi, and Mozambique) but the Maxent model indicated some isolated, species‐suitable areas in central Ethiopia and southern Tanzania. Similarly, D. surculosa herbarium material came from locations gathered around the Gulf of Guinea, with Cameroon and Nigeria as the easternmost countries. However, the D. surculosa model revealed that a potential range may also occur further to the east, especially in the Central African Republic and DR Congo. All these examples confirm that the modeled potential range area may overestimate the current Dracaena species distribution. Nonetheless, these “unexpected” modeled areas may become new focus territories for future niches and population discoveries. However, one needs to keep in mind that the suitability of an area does not necessarily mean that it is accessible to the species. The current and past distribution of habitat types that are unsuitable for the species in question might create impenetrable biological barriers. Additionally, the dispersal mode might prevent the species from gaining a wider range. Dracaena species generally have fleshy fruits and are assumed to be dependent on primates, birds, and rodents for successful seed dispersal (Bollen & Van Elsacker, 2002; González‐Castro et al., 2019; Mwachala, 2005). Since Africa is known to have undergone dramatic climate changes in its recent geological history (Anhuf, 2000), a lack of suitable paths of migration could have prevented the species from reaching areas suitable for their survival.
Surprisingly, D. camerooniana, having a well‐defined geographic distribution with a primary center located around the Gulf of Guinea and in the Congo Basin, also has a secondary center of distribution that differs markedly in terms of environmental characteristics from the primary center. The secondary center of the range, the Katanga Plateau, is located at a much higher altitude (approximately 2,000 m a.s.l.) and latitude (15°S) than the Congo Basin. Although one important environmental variable for the potential distribution of D. camerooniana, prec_wet, is very similar in the Congo Basin and on the Katanga Plateau, the second crucial variable, temp_range, differs considerably at the Katanga Plateau, being approximately 10°C higher than in the Congo Basin. This suggests that D. camerooniana can survive in various climate conditions. Additionally, D. surculosa can be found outside its geographical distribution center in the Guineo‐Sudanian bioregion but at higher altitudes than in the Guineo‐Congolian bioregion. Further research is recommended to explore possible genetic and ecological adaptations of D. camerooniana on the Katanga Plateau in relation to its major distribution center in the Congo Basin.
5. CONCLUDING REMARKS
An understanding, documentation, and recognition of all plant diversity is one of the targets of the Global Strategy for Plant Conservation, which is a program of the Convention on Biological Diversity (http://www.plants2020.net/about-the-gspc/). There is a need to understand the ecology and habitat requirements of species closely related to cultivated plants, also ornamental plants. The importance of such studies is connected with possible resistance to pests and diseases, which is present in wild species but might not be found in the genomes of the cultivated species. Defining the ecogeographic characteristics of such species can lead to the characterization of such potentially important adaptive traits (Heywood, Casas, Ford‐Lloyd, Kell, & Maxted, 2007). In examining the distribution of Dracaena species, one needs, however, to remember that the cultural significance of this genus in Africa is immense, and some species, for example, D. arborea, are planted on purpose in populated areas to mark boundaries, grave sites, and other important places (Sheridan, 2008). Additionally, D. afromontana is known to occur in farmlands in Rwanda, where this species is used as a source of fiber (Smith et al., 2018). The list of the species used for different cultural and traditional purposes is so far incomplete, except for the information which can be gathered from the herbarium sheets.
To conclude, this is the first study to estimate the current and future (year 2050) potential distribution of the selected forest undergrowth Dracaena Vand. ex L. species. For D. afromontana Mildbr., D. camerooniana Baker, and D. surculosa Lindl., we showed how the probability of species occurrence changes throughout Africa. The outcome shows that species occupying similar habitats may differently respond to similar climatic changes. While the first two species show a decrease in their ranges, the latter actually gains niches with projected climate changes. Moreover, the great importance of Dracaena species in human cultivation and culture might add positively to their distribution in anthropogenic habitats. More studies concerning spatial distribution and its future changes in Dracaena species are needed, as this understanding is necessary for the protection of these important iconic species and the richest diversity centers of these useful plants. This understanding is also potentially helpful to assess the climate change vulnerability of the entire group of forest undergrowth plants.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
PB and JWS conceived the ideas; TD and JWS validated herbarium materials; TD, PB, KP, MN and JP prepared a database; PB and JWS analyzed the data; and PB and JWS led the writing with assistance from TD. All authors contributed to the interpretation of results and provided comments on the manuscript.
Supporting information
ACKNOWLEDGMENTS
We would like to thank the KNOW Leading National Research Centre (Project No. 01/KNOW2/2014) at the Faculty of Biology, Adam Mickiewicz University in Poznań for financial support during the preparation of this manuscript. We are also grateful for numerous herbaria employees and the reviewers of this manuscript whose work also contributed to this study.
Bogawski P, Damen T, Nowak MM, et al. Current and future potential distributions of three Dracaena Vand. ex L. species under two contrasting climate change scenarios in Africa. Ecol Evol. 2019;9:6833–6848. 10.1002/ece3.5251
Data Availability Statement: Climate data from Worldclim database were used (worldclim.org). Sampling locations (Maxent input files) as well as GIS layers showing the locations are stored in Dryad repository (https://doi.org/10.5061/dryad.96240sr).
DATA ACCESSIBILITY
Climate data from Worldclim database were used (worldclim.org). Sampling locations (Maxent input files) as well as GIS layers showing the locations are stored in Dryad repository (https://doi.org/10.5061/dryad.96240sr).
REFERENCES
- Ahmed, A. G. M. , & Mlay, W. (1998). Environment and sustainable development in Eastern and Southern Africa: Some critical issues. New York, NY: St. Martin's Press, Inc, Scholarly and Reference Division. [Google Scholar]
- Angiosperm Phylogeny Group (APG) (2009). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society, 161, 105–121. [Google Scholar]
- Anhuf, D. (2000). Vegetation history and climate changes in Africa North and South of the Equator (10°S to 10°N) during the Last Glacial Maximum In Smolka P., & Volkheimer W. (Eds.), Southern Hemisphere Paleo‐ and Neoclimates (pp. 225–248). Berlin, Heidelberg: Springer. [Google Scholar]
- Apaydin, H. , Anli, A. S. , & Ozturk, F. (2011). Evaluation of topographical and geographical effects on some climatic parameters in the Central Anatolia Region of Turkey. International Journal of Climatology, 31, 1264–1279. 10.1002/joc.2154 [DOI] [Google Scholar]
- Attorre, F. , Francesconi, F. , Taleb, N. , Scholte, P. , Saed, A. , Alfo, M. , & Bruno, F. (2007). Will dragonblood survive the next period of climate change? Current and future potential distribution of Dracaena cinnabari (Socotra, Yemen). Biological Conservation, 138, 430–439. 10.1016/j.biocon.2007.05.009 [DOI] [Google Scholar]
- Ayebare, S. , Plumptre, A. J. , Kujirakwinja, D. , & Segan, D. (2018). Conservation of the endemic species of the Albertine Rift under future climate change. Biological Conservation, 220, 67–75. [Google Scholar]
- Baker, T. R. , Phillips, O. L. , Malhi, Y. , Almeida, S. , Arroyo, L. , Di Fiore, A. , … Vásquez Martínez, R. (2004). Increasing biomass in Amazonian forest plots. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359, 353–365. 10.1098/rstb.2003.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen, A. , & Van Elsacker, L. (2002). Feeding ecology of Pteropus rufus (Pteropodidae) in the Littoral Forest of Sainte Luce, SE Madagascar. Acta Chiropterologica, 4(1), 33–47. [Google Scholar]
- Bos, J. J. (1984). Dracaena in West Africa. Agricultural University Wageningen Papers 84‐1, The Netherlands, pp. 6833–126. [Google Scholar]
- Bos, J. J. (1998). Dracaenaceae. In Kubitzki K. (Ed.), The Families and genera of flowering plants, monocotyledons, lilianae (Except Orchidacea), pp. 238–241. Berlin, Germany: Springer. [Google Scholar]
- Brands, S. , Herrera, S. , Fernandez, J. , & Gutierrez, J. M. (2013). How well do CMIP5 Earth System Models simulate present climate conditions in Europe and Africa? A performance comparison for the downscaling community. Climate Dynamics, 41, 803–817. 10.1007/s00382-013-1742-8 [DOI] [Google Scholar]
- Christensen, R. (2002). Plane answers to complex questions: The theory of linear models. (Springer Texts in Statistics). New York, NY: Springer. [Google Scholar]
- Clark, D. A. , Clark, D. B. , & Oberbauer, S. F. (2013). Field‐quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climatic stress, 1997–2009. Journal of Geophysical Research: Biogeosciences, 118, 783–794. [Google Scholar]
- Collins, W. J. , Bellouin, N. , Doutriaux‐Boucher, M. , Gedney, N. , Halloran, P. , Hinton, T. , … Woodward, S. (2011). Development and evaluation of an Earth‐System model – HadGEM2. Geoscientific Model Development, 4, 1051–1075. 10.5194/gmd-4-1051-2011 [DOI] [Google Scholar]
- Costion, C. M. , Simpson, L. , Pert, P. L. , Carlsen, M. M. , Kress, W. J. , & Crayn, D. (2015). Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biological Conservation, 191, 322–330. 10.1016/j.biocon.2015.07.022 [DOI] [Google Scholar]
- Crawford, P. H. C. , & Hoagland, B. W. (2009). Can herbarium records be used to map alien species invasion and native species expansion over the past 100 years? Journal of Biogeography, 36(4): 651–661. [Google Scholar]
- Crook, V. (2013). Dracaena camerooniana. The IUCN Red List of Threatened Species, 2013, e.T44393573A44477731 10.2305/IUCN.UK.2013-2.RLTS.T44393573A44477731.en [DOI] [Google Scholar]
- Cuyckens, G. A. , Christie, D. A. , Domic, A. I. , Malizia, L. R. , & Renison, D. (2016). Climate change and the distribution and conservation of the world's highest elevation woodlands in the South American Altiplano. Global and Planetary Change, 137, 79–87. 10.1016/j.gloplacha.2015.12.010 [DOI] [Google Scholar]
- Damen, T. H. J. , van der Burg, W. J. , Wiland‐Szymańska, J. , & Sosef, M. S. (2018). Taxonomic novelties in African Dracaena (Dracaenaceae). Blumea ‐ Biodiversity, Evolution and Biogeography of Plants, 63(1), 31–53. 10.3767/blumea.2018.63.01.05 [DOI] [Google Scholar]
- Di Febbraro, M. , Carotenuto, F. , Castiglione, S. , Russo, D. , Loy, A. , Maiorano, L. , & Raia, P. (2017). Does the jack of all trades fare best? Survival and niche width in Late Pleistocene megafauna. Journal of Biogeography, 44(12), 2828–2838. 10.1111/jbi.13078 [DOI] [Google Scholar]
- Dike, V. N. , Shimizu, M. H. , Diallo, M. , Lin, Z. , Nwofor, O. K. , & Chineke, T. C. (2015). Modelling present and future African climate using CMIP5 scenarios in HadGEM2‐ES. International Journal of Climatology, 35, 1784–1799. 10.1002/joc.4084 [DOI] [Google Scholar]
- Droissart, V. , Dauby, G. , Hardy, O. J. , Deblauwe, V. , Harris, D. J. , Janssens, S. , … Couvreur, T. L. P. (2018). Beyond trees: Biogeographical regionalization of tropical Africa. Journal of Biogeography, 45(5), 1153–1167. 10.1111/jbi.13190 [DOI] [Google Scholar]
- Elith, J. , Graham, C. H. , & Anderson, R. P. , Dudík, M. , Ferrier, S. , Guisan, A. , … Zimmermann, E. N. (2006). Novel methods improve prediction of species' distributions from occurrence data. Ecography, 29, 129–151. 10.1111/j.2006.0906-7590.04596.x [DOI] [Google Scholar]
- Elith, J. , & Leathwick, J. (2007). Predicting species distributions from museum and herbarium records using multiresponse models fitted with multivariate adaptive regression splines. Diversity and Distributions, 13(3), 265–275. 10.1111/j.1472-4642.2007.00340.x [DOI] [Google Scholar]
- Elith, J. , Phillips, S. J. , Hastie, T. , Dudík, M. , Chee, Y. E. , & Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17, 43–57. 10.1111/j.1472-4642.2010.00725.x [DOI] [Google Scholar]
- Elnoby, S. K. , Moustafa, A. A. , & Mansour, S. R. (2017). Impact of climate change on the endangered Nubian dragon tree (Dracaena ombet) in the South Eastern of Egypt. Catrina, 16(1), 23–28. [Google Scholar]
- Engelbrecht, F. , Adegoke, J. , Bopape, M.‐J. , Naidoo, M. , Garland, R. , Thatcher, M. , … Gatebe, C. (2015). Projections of rapidly rising surface temperatures over Africa under low mitigation. Environmental Research Letters, 10, 85004 10.1088/1748-9326/10/8/085004 [DOI] [Google Scholar]
- FAO (2001). Forest Resources Assessment 2000. FAO Forestry Papers 140, Rome. [Google Scholar]
- Fauset, S. , Baker, T. R. , Lewis, S. L. , Feldpausch, T. R. , Affum‐Baffoe, K. , Foli, E. G. , … Swaine, M. D. (2012). Drought‐induced shifts in the floristic and functional composition of tropical forests in Ghana. Ecology Letters, 15, 1120–1129. 10.1111/j.1461-0248.2012.01834.x [DOI] [PubMed] [Google Scholar]
- Fithian, W. , & Hastie, T. (2013). Finite‐sample equivalence in statistical models for presence‐only data. Annals of Applied Statistics, 7(4), 1917–1939. 10.1214/13-AOAS667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, J. , & Miller, J. A. (2009). Mapping species distributions: Spatial inference and prediction. New York, NY: Cambridge University Press. [Google Scholar]
- González‐Castro, A. , Pérez‐Pérez, D. , Romero, J. , & Nogales, M. (2019). Unraveling the seed dispersal system of an insular “Ghost” Dragon Tree (Dracaena draco) in the wild. Frontiers in Ecology and Evolution, 7(39), 6833–11. [Google Scholar]
- Govaerts, R. (2018). World Checklist of Asparagaceae In Roskov Y., Abucay L., Orrell T., … Penev L. (Eds.), Species 2000 & ITIS Catalogue of Life, 28th March 2018. World Checklist of Selected Plant Families (version Aug 2017). Digital resource at www.catalogueoflife.org/col. Species 2000. Leiden, The Netherlands: Naturalis. [Google Scholar]
- Hanley, J. A. , & McNeil, B. J. (1982). The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology, 143(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Heywood, V. , Casas, A. , Ford‐Lloyd, B. , Kell, S. , & Maxted, N. (2007). Conservation and sustainable use of crop wild relatives. Agriculture, Ecosystems and Environment, 121(2007), 245–255. 10.1016/j.agee.2006.12.014 [DOI] [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25(15), 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- Jaines, E. T. (1957). Information theory and statistical mechanics. Physics Reviews, 106, 620–630. [Google Scholar]
- James, R. , Washington, R. , & Rowell, D. P. (2013). Implications of global warming for the climate of African rainforests. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20120298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jura‐Morawiec, J. , & Tulik, M. (2016). Dragon's blood secretion and its ecological significance. Chemoecology, 26, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelbessa, E. , Kalema, J. , & Crook, V. (2013). Dracaena afromontana. The IUCN Red List of Threatened Species, 2013, e.T44392757A44413063 10.2305/IUCN.UK.2013-2.RLTS.T44392757A44413063.en [DOI] [Google Scholar]
- Kelly, A. E. , & Goulden, M. L. (2008). Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences of the United States of America, 105(33), 11823–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukwa, M. , & Kolanowska, M. (2016). Glacial refugia and the prediction of future habitat coverage of the South American lichen species Ochrolechia austroamericana . Scientific Reports, 6(1), 38779 10.1038/srep38779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance, W. F. , & Cochrane, M. A. (2001). Special section: Synergistic effects in fragmented landscapes. Conservation Biology, 15, 1488–1489. [Google Scholar]
- Lewis, S. L. (2006). Tropical forests and the changing earth system. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 195–210. 10.1098/rstb.2005.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. L. , Malhi, Y. , & Phillips, O. L. (2004). Fingerprinting the impacts of global change on tropical forests. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359, 437–462. 10.1098/rstb.2003.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. , White, M. , & Newell, G. (2013). Selecting thresholds for the prediction of species occurrence with presence‐only data. Journal of Biogeography, 40, 778–789. 10.1111/jbi.12058 [DOI] [Google Scholar]
- Lloyd, J. , & Farquhar, G. D. (2008). Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 363, 1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P.‐L. , & Morden, C. (2010). Phylogenetics of plant genera Dracaena and Pleomele (Asparagaceae). Botanica orientalis – Journal of Plant Science, 7, 64–72. [Google Scholar]
- Marrero, A. , Almeida, R. S. , & González‐Martín, M. (1998). A new species of the wild dragon tree, Dracaena (Dracaenaceae) from Gran Canaria and its taxonomic and biogeographic implications. Botanical Journal of the Linnean Society, 128, 291–314. [Google Scholar]
- Mies, B. A. (1996). The phytogeography of Socotra: evidence for disjunctive taxa, especially with Macaronesia In Dumont H. J. (Ed.), Proceedings of the First International Symposium on Socotra Island: Present and future (pp. 83–105). New York, NY: United Nations Publications. [Google Scholar]
- Moss, R. H. , Edmonds, J. A. , Hibbard, K. A. , Manning, M. R. , Rose, S. K. , van Vuuren, D. P. , … Wilbanks, T. J. (2010). The next generation of scenarios for climate change research and assessment. Nature, 463, 747–756. 10.1038/nature08823 [DOI] [PubMed] [Google Scholar]
- Mwachala, G. (2005). Systematics and ecology of Dracaena L. (Ruscaceae) in Central, East and Southern Africa. PhD thesis, manuscript. Institute of Biology, University of Koblenz‐Landau. [Google Scholar]
- Newbold, T. (2010). Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Progress in Physical Geography: Earth and Environment, 34, 3–22. 10.1177/0309133309355630 [DOI] [Google Scholar]
- Okunji, C. O. , Iwu, M. M. , Jackson, J. E. , & Tally, J. D. (1996). Biological activity of saponins from two Dracaena species. Advances in Experimental Medicine and Biology, 404, 415–428. [DOI] [PubMed] [Google Scholar]
- Pearson, G. R. , & Dawson, T. P. (2003). Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Global Ecology & Biogeography, 12, 361–371. 10.1046/j.1466-822X.2003.00042.x [DOI] [Google Scholar]
- Phillips, S. J. , Anderson, R. P. , & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259. [Google Scholar]
- Phillips, S. J. , & Dudik, M. (2008). Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography, 31, 161–175. 10.1111/j.0906-7590.2008.5203.x [DOI] [Google Scholar]
- Pierzchalska, J. , Nowak, M. M. , Wilkin, P. , Mwachala, G. , & Wiland‐Szymańska, J. (2014). Geoenvironmental modelling of the geographic range structure of Dracaena aubryana Brongn. Ex C.J. Morren (Asparagaceae) on the African Continent. Badania Fizjograficzne R.V. Seria B Botanika, B63, 7–20. [Google Scholar]
- Ponce‐Reyes, R. , Plumptre, A. J. , Segan, D. , Ayebare, S. , Fuller, R. A. , Possingham, H. P. , & Watson, J. E. M. (2017). Forecasting ecosystem responses to climate change across Africa's Albertine Rift. Biological Conservation, 209, 464–472. 10.1016/j.biocon.2017.03.015 [DOI] [Google Scholar]
- Robiansyah, I. , & Hajar, A. S. (2017). Predicting current and future distribution of endangered tree Dracaena ombet Kotschy and Peyr. under climate change. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 87(1), 225–232. [Google Scholar]
- Sheridan, M. (2008). Tanzanian ritual perimetrics and African landscapes: The case of Dracaena . International Journal of African Historical Studies, 41(3), 491–521. [Google Scholar]
- Shi, H. , Paull, D. , & Rayburg, S. (2016). Spatial heterogeneity of temperature across alpine boulder fields in New South Wales, Australia: Multilevel modelling of drivers of microhabitat climate. International Journal of Biometeorology, 60(7), 965–976. 10.1007/s00484-015-1089-4 [DOI] [PubMed] [Google Scholar]
- Singh, H. P. , & Dadlani, N. K. (2000). Current status of floriculture – National and International Scenario. New Delhi, India: Commercial Floriculture, Malhotra Publishing House. [Google Scholar]
- Smith, D. E. , Gassner, A. , Agaba, G. , Nansamba, R. , & Sinclair, F. (2018). The utility of farmer ranking of tree attributes for selecting companion trees in coffee production systems. Agroforestry Systems, 6833–15. 10.1007/s10457-018-0257-z [DOI] [Google Scholar]
- Staples, G. W. , & Herbst, D. R. (2005). A tropical garden Flora. Honolulu, Hawaii: Bishop Museum Press. [Google Scholar]
- Swets, J. A. (1988). Measuring the accuracy of diagnostic systems. Science, 240(4857), 1285–1293. 10.1126/science.3287615 [DOI] [PubMed] [Google Scholar]
- Syfert, M. M. , Smith, M. J. , & Coomes, D. A. (2013). The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE, 8(2), e55158 10.1371/journal.pone.0055158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, K. E. , Stouffer, R. J. , & Meehl, G. A. (2012). An overview of CMIP5 and the experiment design. Bulletin of American Meteorological Society, 93(4), 485–498. 10.1175/BAMS-D-11-00094.1 [DOI] [Google Scholar]
- Ter Steege, H. , Pitman, N. , Sabatier, D. , Castellanos, H. , Van derHout, P. , Daly, D. C. , … Morawetz, W. (2003). A spatial model of tree alphadiversity and tree density for the Amazon. Biodiversity and Conservation, 12, 2255–2277. [Google Scholar]
- Thiers, B. (2018). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. Retrieved from http://sweetgum.nybg.org/science/ih/ [Google Scholar]
- Vedel‐Sørensen, M. , Tovaranonte, J. , Bøcher, P. K. , Balslev, H. , & Barfod, A. S. (2013). Spatial distribution and environmental preferences of 10 economically important forest palms in western South America. Forest Ecology and Management, 307, 284–292. 10.1016/j.foreco.2013.07.005 [DOI] [Google Scholar]
- Warren, D. L. , Glor, R. E. , & Turelli, M. (2010). ENMTools: A toolbox for comparative studies of environmental niche models. Ecography, 33(3), 607–611. 10.1111/j.1600-0587.2009.06142.x [DOI] [Google Scholar]
- Wiens, J. A. , Stralberg, D. , Jongsomjit, D. , Howell, C. A. , & Snyder, M. A. (2009). Niches, models, and climate change: Assessing the assumptions and uncertainties. Proceedings of the National Academy of Sciences of the United States of America, 106(Suppl. 2), 19729–19736. 10.1073/pnas.0901639106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L. , Tian, Y. , Myneni, R. B. , Ciais, P. , Saatchi, S. , Liu, Y. Y. , … Hwang, T. (2014). Widespread decline of Congo rainforest greenness in the past decade. Nature, 509, 86–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Climate data from Worldclim database were used (worldclim.org). Sampling locations (Maxent input files) as well as GIS layers showing the locations are stored in Dryad repository (https://doi.org/10.5061/dryad.96240sr).


