Abstract
Rapid adaptation can aid invasive populations in their competitive success. Resource allocation trade‐off hypotheses predict higher resource availability or the lack of natural enemies in introduced ranges allow for increased growth and reproduction, thus contributing to invasive success. Evidence for such hypotheses is however equivocal and tests among multiple ranges over productivity gradients are required to provide a better understanding of the general applicability of these theories.
Using common gardens, we investigated the adaptive divergence of various constitutive and inducible defence‐related traits between the native North American and introduced European and Australian ranges, while controlling for divergence due to latitudinal trait clines, individual resource budgets, and population differentiation, using >11,000 SNPs.
Rapid, repeated clinal adaptation in defence‐related traits was apparent despite distinct demographic histories. We also identified divergence among ranges in some defence‐related traits, although differences in energy budgets among ranges may explain some, but not all, defence‐related trait divergence. We do not identify a general reduction in defence in concert with an increase in growth among the multiple introduced ranges as predicted trade‐off hypotheses.
Synthesis: The rapid spread of invasive species is affected by a multitude of factors, likely including adaptation to climate and escape from natural enemies. Unravelling the mechanisms underlying invasives' success enhances understanding of eco‐evolutionary theory and is essential to inform management strategies in the face of ongoing climate change.
OPEN RESEARCH BADGES

This article has been awarded Open Materials, Open Data, Preregistered Research Designs Badges. All materials and data are publicly accessible via the Open Science Framework at https://doi.org/10.6084/m9.figshare.8028875.v1, https://github.com/lotteanna/defence_adaptation,https://doi.org/10.1101/435271.
Keywords: constitutive defence, evolution of increased competitive ability hypothesis, growth‐defence trade‐offs, inducible defence, invasive species, latitudinal adaptation, phenolic compounds, resource allocation
1. INTRODUCTION
Biological invasions are occurring at an accelerating pace due to the globalization of anthropogenic activity (Ricciardi, 2007). Individuals colonizing new ranges likely face environments different from those previously experienced (Allendorf & Lundquist, 2003; Chown et al., 2014; Sax & Brown, 2000). Nonetheless, alien populations often display enhanced performance compared to their native counterparts (Blossey & Notzold, 1995; Parker et al., 2013; Thébaud & Simberloff, 2001), and this can be facilitated by rapid adaptation (Chown et al., 2014; Colautti & Lau, 2015; Dlugosch, Anderson, Braasch, Cang, & Gillette, 2015). Therefore, in the face of ongoing environmental change, studies on introduced species are imperative to provide insight into invasive success as well as contemporary evolutionary processes.
Resource allocation trade‐offs between life‐history traits, such as growth rate and reproductive output, feature prominently in evolutionary theories developed to explain the success of invasive species (e.g., Hodgins & Rieseberg, 2011; Kumschick, Hufbauer, Alba, & Blumenthal, 2013; Turner, Hufbauer, & Rieseberg, 2014; Colautti & Lau, 2015). For instance, if abiotic stressors are mitigated upon introduction because of increased resource availability, increased investment in colonization or competitive ability could facilitate invasion success (Bossdorf et al., 2005; Davis, Grime, & Thompson, 2000; Dlugosch, Cang, et al., 2015; Grime, 1977; He, Thelen, Ridenour, & Callaway, 2010). Similarly, the evolution of increased competitive ability hypothesis (EICA) postulates that release from specialist herbivores within the introduced range favors genotypes allocating resources to growth and reproduction in lieu of defence (Blossey & Notzold, 1995). Evidence for such adaptive divergence of invasive populations is however equivocal (Felker‐Quinn, Schweitzer, & Bailey, 2013) perhaps due to allocation trade‐offs among multiple competing functions (Mole, 1994; Züst & Agrawal, 2017), variation in resource availability or acquisition (Uesugi, Connallon, Kessler, & Monro, 2017; Züst & Agrawal, 2017), interplay with nonadaptive processes (Estoup et al., 2016; Facon et al., 2006; Lee, 2002; Prentis, Wilson, Dormontt, Richardson, & Lowe, 2008; Rius & Darling, 2014), or other selective factors, such as climate, playing an important role in governing patterns of trait variation within and between ranges (Lachmuth, Durka, & Schurr, 2011; Turner, Fréville, & Rieseberg, 2015).
Biotic and abiotic clines impacting plant resistance within ranges (Endara & Coley, 2011; Moles, Bonser, Poore, Wallis, & Foley, 2011) can obscure the adaptive underpinnings of trait divergence governed by growth‐defence trade‐offs in response to changes during invasion in herbivory. For instance, herbivore pressure in the native range is expected to increase toward lower latitudes and potentially drive clines in plant defence in some species (Moles, Bonser, et al., 2011). This clinal pattern may be absent in the introduced range due to overall lack of herbivory, resulting in nonparallel defence gradients between ranges (e.g., Cronin, Bhattarai, Allen, & Meyerson, 2015; Allen et al., 2017). Moreover, high‐resource environments support plant species with faster growth that are more vulnerable to herbivores (Coley, Bryant, & Chapin, 1985; Endara & Coley, 2011; Zandt, 2007), resulting in latitudinal clines in defence traits. Latitudinal clines in resource availability could subsequently lead to the evolution of high growth and reduced chemical defences at lower latitudes (Moreira et al., 2014; Woods, Hastings, Turley, Heard, & Agrawal, 2012), although this interspecific pattern may have limited application to intraspecific variation (Hahn & Maron, 2016, but see Woods et al., 2012). However, taken together, these patterns suggest that the evolutionary consequences of herbivore escape could change along latitudinal gradients (Blumenthal, 2006). Geographical clines therefore need to be considered in tests of adaptive divergence between ranges (Colautti, Maron, & Barrett, 2009).
The complex interplay between the evolutionary mechanisms shaping phenotypic divergence could also confound inferences of adaptation. Distinct demographic processes, including founder effects, genetic drift, and admixture, often characterize introduction and alone can lead to divergence between native and introduced populations (Estoup et al., 2016; Facon et al., 2006; Lee, 2002; Prentis et al., 2008; Rius & Darling, 2014). Dissection of the various evolutionary processes that can contribute to trait divergence is required to advance our understanding of rapid spread in invasive species. In addition, the repeatability of evolutionary patterns associated with introductions is unclear, as the majority of studies examining trait evolution following introduction focus on a single invaded range (e.g., Blossey & Notzold, 1995; Joshi & Vrieling, 2005; Hodgins & Rieseberg, 2011; Uesugi & Kessler, 2016, but see Colomer‐Ventura et al., 2015). Repeatable trait divergence across multiple invaded ranges would provide support for adaptive divergence of traits during in invasion as well as insight into selective mechanisms contributing to invasion success (van Boheemen, Atwater, & Hodgins, 2018; Hodgins, Bock, & Rieseberg, 2018).
The frequency and level of attack can impact the evolution of defence traits (Bixenmann, Coley, Weinhold, & Kursar, 2016; Orrock et al., 2015), which might also be expected to trade off due to their costs and redundancy (Agrawal, Conner, & Rasmann, 2010; Koricheva, Nykänen, & Gianoli, 2004). Predictable and strong attack should favor constitutive defence, whereas low, infrequent herbivory would favor no, or an inducible response (Agrawal & Karban, 1999; Ito & Sakai, 2009). These responses have been shown to vary over latitudinal clines within ranges (Moreira et al., 2014; Rasmann & Agrawal, 2011). However, the studies exploring evolutionary shifts of constitutive and inducible defences between native and introduced ranges showed mixed results (e.g., Cipollini, Mbagwu, Barto, Hillstrom, & Enright, 2005; Eigenbrode et al., 2008). Various variable outcomes could result from a decrease in the intensity and frequency of herbivory following introduction (Agrawal & Kotanen, 2003; Maron & Vilà, 2001), including an increase in plasticity (Cipollini et al., 2005; Lande, 2015) or high variability in inducible response among populations (Eigenbrode et al., 2008). Testing such shifts in invasive species would provide insight into factors governing the evolution of induced/constitutive trait defence more generally.
Ambrosia artemisiifolia is a highly suitable system to study adaptive divergence in defence‐related traits during invasion. This native North American weed has successfully established globally (Oswalt & Marshall, 2008), including recent introductions to Europe (~160 years ago Chauvel, Dessaint, Cardinal‐Legrand, & Bretagnolle, 2006) and Australia (~80 years ago; Palmer & McFadyen, 2012; van Boheemen et al., 2017). Repeated clinal associations were found in A. artemisiifolia populations included in the current study, with declines in size and increase in SLA at higher latitudes (van Boheemen et al., 2018), though differences occurred among ranges. At comparable latitudes, European plants were bigger and had lower SLA than natives, while Australian plants had higher SLA leaves (van Boheemen et al., 2018).
We test quantitative trait divergence in (a) physical defence (trichome density), (b) chemical defence (phenolic compounds concentration and richness), and (c) inducibility of chemical defence among the native North American and introduced European and Australian ranges in a series of common garden experiments. Trichomes are found on the leaves and the stems of plants and deter herbivores (Dalin, Ågren, Björkman, Huttunen, & Kärkkäinen, 2008; Kessler & Baldwin, 2002; Tian, Tooker, Peiffer, Chung, & Felton, 2012). Phenolics are secondary metabolites that are often thought to confer resistance against herbivores (Bhattacharya, Sood, & Citovsky, 2010; War et al., 2018, 2012). These compounds are also known to be inducible in response to herbivore damage, as well as simulated herbivory treatments including wounding and methyl jasmonate (MeJA) applications (e.g., Lee, Vogt, Schmidt, Parthier, & Löbler, 1997; Constabel & Ryan, 1998; Keinänen, Oldham, & Baldwin, 2001; Heredia & Cisneros‐Zevallos, 2009). We accounted for population structure, which could potentially drive patterns in traits that are nonadaptive, using >11,000 double‐digest genotype‐by‐sequencing SNPs. Moreover, we controlled for defence‐related trait variation along latitudinal clines.
We predict reduced constitutive defence within the introduced ranges together with elevated inducible response due to lower certainty of attack (Cipollini et al., 2005) and a more plastic (inducible) response in recent colonizations (Lande, 2015). We expect nonparallel defence gradients between native and introduced ranges due to divergence of clines in herbivory (Moles, Wallis, et al., 2011) and/or variable resource gradients (Blumenthal, 2006; Hahn & Maron, 2016). Finally, we explored the association between defence‐related trait divergence and divergence in growth and SLA among ranges as a growth‐defence trade‐off would result in greater growth in conjunction with reduced defence. However, greater defence could be facilitated by genotypes with enhanced resource acquisition resulting in a positive correlation in traits. By considering the complex interplay of the evolutionary mechanisms impacting defence divergence among multiple ranges, we test evolutionary changes in herbivore defence likely shaped by selection.
2. METHODS
2.1. Study species
Ambrosia artemisiifolia is a highly invasive, monoecious, self‐incompatible annual plant (Brandes & Nitzsche, 2006), most commonly found in disturbed habitats (Bassett & Crompton, 1975; Lommen et al., 2017) and is expected to expand its range with ongoing climate change (Chapman, Haynes, Beal, Essl, & Bullock, 2014). It is the leading cause of hayfever worldwide (Taramarcaz, Lambelet, Clot, Keimer, & Hauser, 2005) and has a significant impact on crop yields (Kazinczi, Béres, Novák, Bíró, & Pathy, 2008). Within Europe, admixture following multiple introductions from distinct native sources has been suggested to have contributed to the success of introduced populations, and genetic variation equals levels observed in North America (van Boheemen et al., 2017; Chun, Fumanal, Laitung, & Bretagnolle, 2010; Gaudeul, Giraud, Kiss, & Shykoff, 2011; Gladieux et al., 2010). A subsequent single bottlenecked introduction from Europe has been determined as the origin of the Australian invasion, although the exact European source is unknown (van Boheemen et al., 2017).
Within the native range, around 450 herbivores have been associated with Ambrosia species, of which about 30% are specific to the Ambrosia genus (Gerber et al., 2011). The North American native specialist Ophraella communa is shown to exert high levels of damage (Throop, 2005). Up to 50 polyphagous insect species have been associated with A. artemisiifolia in Europe, yet most cause little damage (Essl et al., 2015; Gerber et al., 2011). Ophraella communa has been sighted in Southern Switzerland and Northern Italy since 2013 (Müller‐Schärer et al., 2014), where it greatly affects A. artemisiifolia seedling survival and growth (Cardarelli et al., 2018). In Australia, generalists Zygogramma bicolorata (leaf‐feeding) and Epiblema strenuana (stem‐boring) are widespread and seemingly exert some control (Palmer & McFadyen, 2012).
2.2. Experimental set‐up
To explore the divergence of constitutive quantitative defence traits between native and introduced ranges (“constitutive‐defence experiment”), while accounting for divergence along latitudinal clines, we collected Ambrosia artemisiifolia seeds in 2013–2014 from broad geographical scales within the native North America and introduced Europe and Australia. We raised seedlings in a common garden (for detailed methods, see Supporting Information). Briefly, we stratified seeds for 6 weeks at 4°C (Willemsen, 1975). After a 2‐week germination at 30°C with 12 hr light/dark cycle, we randomly transplanted the seedlings into 100 ml kwikpot trays with Debco mix, followed by a second transplant to 0.7 L pots containing Debco and 1.5 ml slow‐release fertilizer (Osmocote Pro, 8–9 months) 1 month later. We top‐watered all plants and artificially manipulated daylight following the light cycle at the median latitude for all populations (47.3°N). To explore constitutive defence, we selected a seedling from four maternal lines, originating from 28 North American, 32 European, and 20 Australian locations (Table S1).
A separate greenhouse experiment was conducted to test whether the inducibility of defence response varied among plant origins (hereafter, “induction experiment”). We used a subset of populations used in the constitutive experiment (10 North American, 17 European, and 12 Australian locations, Table S1). For each population, we selected four maternal lines, and grew two seedlings per line as above. One seedling per maternal line was allocated to either the control or simulated herbivory treatment. We simulated herbivory by vertically cutting off half of the newest fully formed leaf (wounding) and subsequently spraying the whole plant with 1 mM methyl jasmonate (MeJA) (Campos‐Vargas & Saltveit, 2002; Heredia & Cisneros‐Zevallos, 2009; Hodgins & Rieseberg, 2011; Jordan, Ally, & Hodgins, 2015). Control plants were not wounded and were sprayed with distilled water.
2.3. Trait measurements
For the constitutive experiment, we recorded trichome density at the mid‐point of each plant under a dissecting microscope (Olympus, SZ‐PT) using a 1 cm × 0.3 cm stem area at the mid‐point of each plant, 9 weeks after the second transplant. Three weeks later, we scanned one young, fully expanded leaf from each plant and calculated leaf area using ImageJ and the R package LeafArea (Katabuchi, 2015). We dried leaves at 45°C for 7 days and an addition 12 hr prior to weighing and weighed to the closest milligram. We calculated specific leaf area (SLA) by dividing leaf area by dry leaf weight (mm2/mg). We deconstructed plants for biomass measurements once the majority of seeds had ripened. We placed aboveground components in paper bags and dried these in ovens at 45°C for at least 36 hr. Before dry weight biomass measures, we dried materials for an additional minimum of 24 hr to ensure the dry weight was constant at the time of measuring and it was not variable due to humidity in the air or incomplete drying. We weighed this shoot biomass to the closest 0.1 g.
Leaf samples for phenolic analyses were collected 4 weeks after the second transplant by clipping approximately 200 mg of the newest fully expanded leaf, which was flash frozen in liquid nitrogen and stored in a −80 °C. In the induction experiment, we collected leaf samples 24 hr after the final treatment. Samples were extracted in 1 ml of 80% methanol (% by volume in water) using a Qiagen TissueLyser II for 30 s at 30 rps twice and centrifuged for 30 min at 570 g. Phenolic samples from the constitutive‐defence experiment were analyzed using HPLC Agilent 1200 series (Agilent Technologies Australia, Mulgrave, VIC, Australia) equipped with C18 reverse‐phase column (Waters, 5.0 μm, 250 mm × 4.6 mm; Alltech Australia, Baulkham Hills NSW, Australia). The elution system consisting of solvents (A) 0.25% H3PO4 in water (pH 2.2) and (B) acetonitrile was: 0–6 min, 0%–12% of B; 6–10 min, 12%–18% of B, and 10–30 min, 18%–58% of B, with a flow rate of 1 ml/min and injection volume of 15 µl (Keinänen et al., 2001). Samples from the induction experiment were analyzed with Agilent Infinity 1260 equipped with C18 reverse‐phase column (Poroshell 120 EC‐C18, 2.7 μm, 150 mm × 3.0 mm; Agilent Technologies Australia, Mulgrave, VIC, Australia). The elution method was modified from above and was: 0–2 min, 0%–12% of B; 2–3.3 min, 12%–18% of B, and 3.3–10 min, 18%–58% of B, with a flow rate of 0.5 ml/min and injection volume of 5 µl. In both experiments, phenolic compound peaks were identified to their compound classes using UV spectra and relative abundance was quantified at 320 nm. To estimate phenolic compound richness, we counted the number of detectable peaks. The relative concentration of eight major phenolic peaks was estimated as area under each peak divided by sample fresh weight. Results could not be directly compared as the two experiments were performed in different greenhouses and samples from each experiment were run using different HPLC machines.
2.4. Statistical analyses
To test whether constitutive defence differed among ranges (the constitutive experiment), we examined individual phenolic compound composition in a multivariate analysis of covariance (MANCOVA) and the concentration of individual phenolic compounds, phenolic compound richness, total phenolic concentration, and trichome density in univariate mixed models. To account for latitudinal variation within and among ranges, each multi‐ and univariate model included range, latitude, their interaction and a latitude2 effect as fixed factors. To control for neutral population structure, possibly shaping trait variation between populations, univariate models included q‐values as a random effect, as obtained from STRUCTURE analysis performed on genetic data. For the multi‐ and univariate analyses, we improved normality of the data by square‐root‐ or log‐transforming traits where appropriate. For the MANCOVA, we included the concentration of eight major phenolic compounds (Spearman's ρ among peaks <0.75) and calculated Wilks' λ (multivariate F‐value) to measure the strength of the associations. To measure the variance explained by the fixed effects or the full model within the univariate models, we calculated the marginal and conditional coefficients of determination using the MuMIn package (Bartón, 2018). We computed type III Wald F‐values with Kenward–Roger degrees of freedom and step‐wise removed nonsignificant effects, starting with the highest order interaction. For univariate models, we plotted the partial residuals of each response variable by ranges, thus accounting for latitudinal clines and neutral population genetic structure and reported these adjusted means and standard errors for each range, calculated using the phia packaged (De Rosario‐Martinez, 2015).
To explore the variation in inducibility among ranges (the induction experiment), we repeated the steps for the constitutive experiment, now including treatment and its interactions with range and latitude as fixed effects. For the MANCOVA, we included five peaks (excluding three with Spearman's ρ > 0.75) to increase power of this test (Scheiner, 2001). We retained treatment in these models, as this was the variable of interest. Here, a significant treatment effect would signify an inducible response, whereas a treatment x range interaction would imply this response differs between ranges. A treatment x latitude interaction would indicate different inducibility at different latitudes. To test whether variation in induction differed between ranges (Eigenbrode et al., 2008), we compared the coefficient of variation (cv) using the modified signed‐likelihood ratio test for equality with 104 simulations in the cvequality package (Krishnamoorthy & Lee, 2014; Marwick & Krishnamoorth, 2018).
To examine associations between defence‐related traits and plant growth and to assess whether divergence in individual resource budgets could have resulted in range differences in defence‐related trait investment, we tested responses of phenolic richness, phenolic concentrations. or trichome density to shoot biomass or SLA. Each model included a defence‐related trait as response, with shoot biomass or SLA, range and their interaction as predictors. We used individual trait values and included individual STRUCTURE q‐values and sampling location as random factors. We explored significant range x defence interactions using a Holm p‐value correction in the phia package (De Rosario‐Martinez, 2015). In these models, a negative association between defence‐related traits and shoot biomass would suggest a trade‐off, while a positive one might indicate differences in resource acquisition. Range differences at similar values of shoot biomass or SLA would indicate defence‐related trait divergence independent of genotypic differences in individual resource budgets.
To explore whether constitutive and inducible defence trade‐off, we first calculated the induced level of total phenolics for each maternal line as the difference between damage and control treatments of the two half‐sibs. This estimate of induction is thought to reduce correlations with control treatment estimates and thus the collinear associations (e.g., due to genotypic biases) will not mask the trade‐off associations (Morris, Traw, & Bergelson, 2006). We included population of origin and individual q‐values as random factor in these models. A significant negative association between induced and constitutive levels of phenolic concentration and richness would indicate the presence of a trade‐off. All statistical analyses were conducted in R v3.4.3 (R Core Team, 2018).
3. RESULTS
3.1. Constitutive defence trait divergence between ranges
We found significant range divergence in constitutive phenolic composition (Table 1a), resulting from differences between the introduced Europe and the native North America (F 8,66 = 3.280, p = 0.010, Wilks' λ = 0.716; Table 1b). Total phenolic concentration was similar between the native and European populations, but 28% lower in Australia (Table 1b,c; Figure 1). Phenolic peak richness differed among ranges (Table 1): it was highest in the introduced European range (adjusted mean of 43 peaks) followed by the native North American (40 peaks) and introduced Australian ranges (33 peaks). Trichome density showed no differences between ranges (Table 1a, Figure 1). The composition of individual phenolic compounds and peak richness depended on latitude, though no such effect was found for the total phenolic concentration or trichome density (Table 1a, Figure 2). We did not observe range x latitude interactions for any of the defence‐related traits (Table 1a), suggesting latitudinal clines, when present, did not differ between ranges.
Table 1.
Ambrosia artemisiifolia defence‐related trait responses (population means) to range, latitude, their interaction and latitude2 (to account for non‐linear relationship) in the constitutive experiment in multivariate (individual phenolic compounds) and univariate analyses (a), with dissection of significant range effects (p < 0.05) in post hoc tests (b). We reported Wald type III F (a) or χ 2 test values (b), Kenward–Roger degrees of freedom (subscript), significance (symbols). In the multivariate analysis, Wilk's λ measure the strength of the association, in univariate analyses, marginal (R 2m) and conditional (R 2c) coefficients measure the variance explained by fixed effects or full models. Models were step‐wise reduced starting with the highest order nonsignificant interaction and univariate analyses included neutral population genetic structure as a random effect
| (a) | Range | Latitude | Latitude2 | Range: Latitude | R 2m | R 2c |
|---|---|---|---|---|---|---|
| Individual phenolic compounds composition |
4.52016,132***, λ = 0.417 |
6.9288,66***, λ = 0.544 |
2.8148,66*, λ = 0.746 |
0.84916,128 (ns), λ = 0.817 |
||
| Phenolic concentration | 8.6011,71.918*** | 0.9341,73.244 (ns) | 0.0361,72.973 (ns) | 0.1271,70.054 (ns) | 0.189 | 0.290 |
| Phenolic richness | 7.6152,58.48** | 7.791,71.66** | 0.0461,53.51 (ns) | 2.0272,69.64 (ns) | 0.177 | 0.688 |
| Trichome density | 0.6632,71.183 (ns) | 1.8251,66.941 (ns) | 3.1481,74.991# | 0.1212,69.141 (ns) | 0.055 | 0.055 |
| (b) | North America–Europe | North America–Australia | Europe–Australia |
|---|---|---|---|
| Individual phenolic compounds composition |
3.2808,66**, λ = 0.716 |
1.5808,66 (ns), λ = 0.840 |
1.9948,66 (ns), λ = 0.805 |
| Phenolic concentration | 1.3971 (ns) | 9.3111** | 17.3211*** |
| Phenolic richness | 4.7831* | 12.7251*** | 15.8431*** |
ns, p > 0.1; #p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 1.
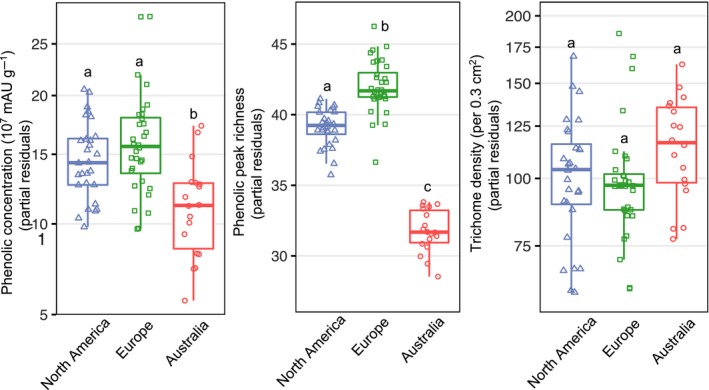
Partial residual defence trait responses (phenolic concentration, peak richness, and trichome density) of Ambrosia artemisiifolia populations to range, accounting for latitudinal clines and neutral population structure. Different letters indicate significance for pairwise range comparisons (Table 1)
Figure 2.
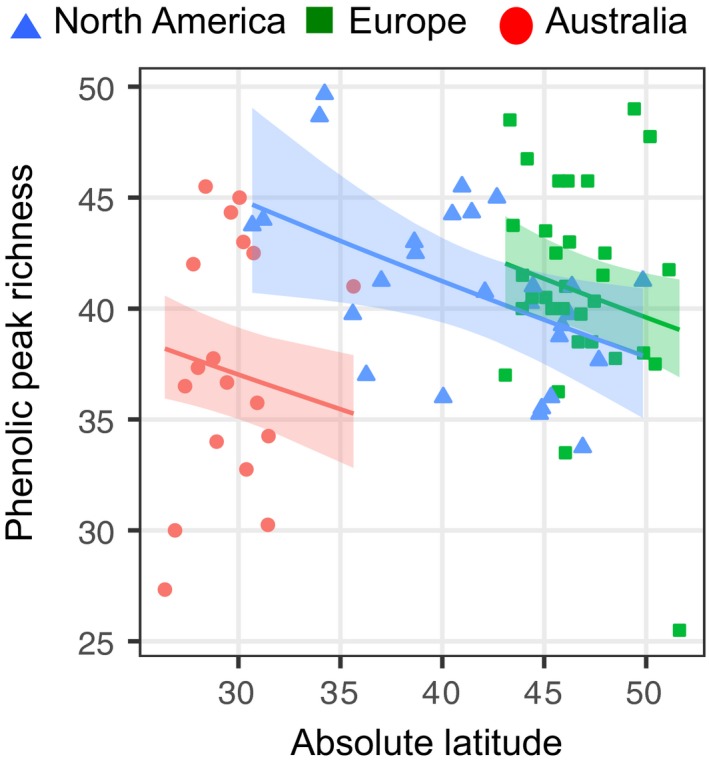
Population mean response of phenolic peak richness to range (native North America, blue triangles; introduced Europe, green squares; introduced Australia, red circles) and latitude in Ambrosia artemisiifolia, with predicted latitudinal clines (±95% confidence interval) corrected for neutral population structure
3.2. Inducible defence trait divergence between ranges
We found a significant treatment effect on individual phenolic compound composition in the induction experiment (F 5,59 = 12.014, p < 0.001, Wilks' λ = 0.496; Table 2). The total phenolic concentration was slightly suppressed in the herbivory‐simulating treatment (Table 2, Figure 3), but the phenolic peak richness did not show a response to experimental treatment (Table 2, Figure 3). We identified no treatment x range x latitude interactions (Table 2, Figure 3), suggesting there is no range difference in inducibility clines. Also, the absence of treatment × latitude interactions (Table 2, Figure 3) suggests an overall lack of latitudinal clines in inducibility. Moreover, no treatment × range interactions (Table 2, Figure 3) suggests the inducible response did not differ between ranges. We did not find range differences in the variation of inducible phenolic peak richness (cv = 1.401, p = 0.496) or concentration (cv = 2.297, p = 0.317).
Table 2.
Ambrosia artemisiifolia defence‐related trait responses (population means) to range, latitude, treatment, their interactions and latitude2 in the inducible experiment in multivariate (individual phenolic compounds) and univariate analyses. Range, latitude, their interaction, or latitude2 were included as covariates and significant results were not explored further. We reported Wald type III F, Kenward–Roger degrees of freedom (subscript), significance (symbols) (a). In the multivariate analysis, Wilk's λ measure the strength of the association, in univariate analyses, marginal (R 2m) and conditional (R 2c) coefficients measure the variance explained by fixed effects or full models (a). Models were step‐wise reduced starting with the highest order nonsignificant interaction and univariate analyses included neutral population genetic structure as a random effect
| Range | Latitude | Latitude2 | Range: Latitude | Treatment | Treatment: Range | Treatment: Latitude | Treatment: range: Latitude | R 2m | R 2c | |
|---|---|---|---|---|---|---|---|---|---|---|
|
Individual phenolic compounds concentration |
7.59110,118***, λ = 0.370 |
10.6375,59***, λ = 0.526 |
4.8185,59**, λ = 0.710 |
2.31110,118*, λ = 0.699 |
12.0145,59***, λ = 0.496 |
0.32610,112 (ns), λ = 0.944 |
0.9775,58 (ns), λ = 0.922 |
1.35710,108 (ns), λ = 0.789 |
||
| Phenolic concentration | 4.9702,30.905* | 5.9321,31.505* | 1.5772,28.556 (ns) | 1.7451,31.505 (ns) | 4.2411,35.628* | 0.0052,32.285 (ns) | 1.0771,35.428 (ns) | 1.4172,31.192 (ns) | 0.201 | 0.457 |
| Phenolic richness | 3.7642,29.93* | 4.7001,31.08* | 1.3402,28.24 (ns) | 6.0301,30.95* | 0.8501,35.33 (ns) | 0.0912,32.1 (ns) | 0.8251,34.89 (ns) | 1.9232,30.78 (ns) | 0.323 | 0.723 |
ns, p > 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 3.
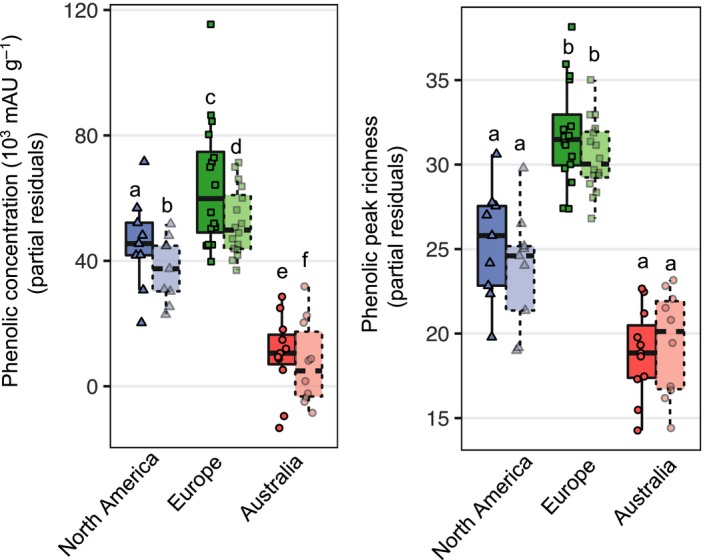
Partial residual defence trait responses (phenolic concentration and peak richness) of Ambrosia artemisiifolia populations to control (solid symbols) and herbivore simulating treatment (wounding + MeJA, dashed transparent symbols), with covariates of range, accounting for latitudinal clines and neutral population structure. Letters indicate significance of effect (Table 2)
3.3. Associations between defence, biomass, and specific leaf area
Within each range, phenolic concentration and richness was positively correlated with shoot biomass, whereas we found a negative association between trichome density and shoot biomass (Table 3, Figure 4). We found high‐SLA leaves had lower phenolic concentration and peak richness, yet higher trichome density (Table 3, Figure 4). No interactions were significant between range and predictor variables (shoot biomass or SLA), suggesting these associations among traits were consistent between ranges (Table 3, Figure 4). These results emphasize the close relationship between plant growth, physiology, and defence.
Table 3.
Constitutive defence trait response of Ambrosia artemisiifolia individuals to shoot biomass, specific leaf area and their interaction with range (a), with dissection of significant range effects (p < 0.05) in post hoc tests (b). We reported corresponding figure, Wald type III F (a) or χ2 test values (b), Kenward–Roger degrees of freedom (subscript) and significance (symbols). Marginal (R 2m) and conditional (R 2c) coefficients measure the variance explained by fixed effects or full models (a). Models were step‐wise reduced starting with the highest order nonsignificant interaction and included population origin and neutral population genetic structure as random effects
| (a) Predictor | Response | Figure 4 | Range | Predictor | Range: Predictor | R 2m | R 2c |
|---|---|---|---|---|---|---|---|
| Shoot biomass | Phenolic concentration | A | 19.3212,79.435*** | 31.0981,181.299*** | 1.4412,184.26 (ns) | 0.160 | 0.189 |
| Phenolic richness | B | 18.962,79.29*** | 53.3891,180.51*** | 1.5652,187.82 (ns) | 0.199 | 0.224 | |
| Trichome density | C | 10.2422,79.4*** | 18.491,174.06*** | 0.5252,180.84 (ns) | 0.093 | 0.103 | |
| Specific leaf area | Phenolic concentration | D | 6.1622,71.167** | 38.4641,208.912*** | 0.982,202.53 (ns) | 0.202 | 0.269 |
| Phenolic richness | E | 5.3492,69.16** | 42.6921,217.97*** | 0.322,202.6 (ns) | 0.215 | 0.331 | |
| Trichome density | F | 1.8282,71.7 (ns) | 10.9941,204.66** | 1.1962,206.12 (ns) | 0.049 | 0.121 |
| (b) Predictor | Response | Figure 4 | North America–Europe | North America–Australia | Europe–Australia |
|---|---|---|---|---|---|
| Shoot biomass | Phenolic concentration | A | 5.8461* | 22.1551*** | 40.0871*** |
| Phenolic richness | B | 2.5461 (ns) | 26.6671*** | 37.9641*** | |
| Trichome density | C | 2.2551 (ns) | 13.0491*** | 21.0471*** | |
| Specific leaf area | Phenolic concentration | D | 2.1521 (ns) | 5.0321* | 12.4851** |
| Phenolic richness | E | 0.4591 (ns) | 6.6551* | 10.6431** | |
| Trichome density | F | – | – | – |
ns, p > 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 4.
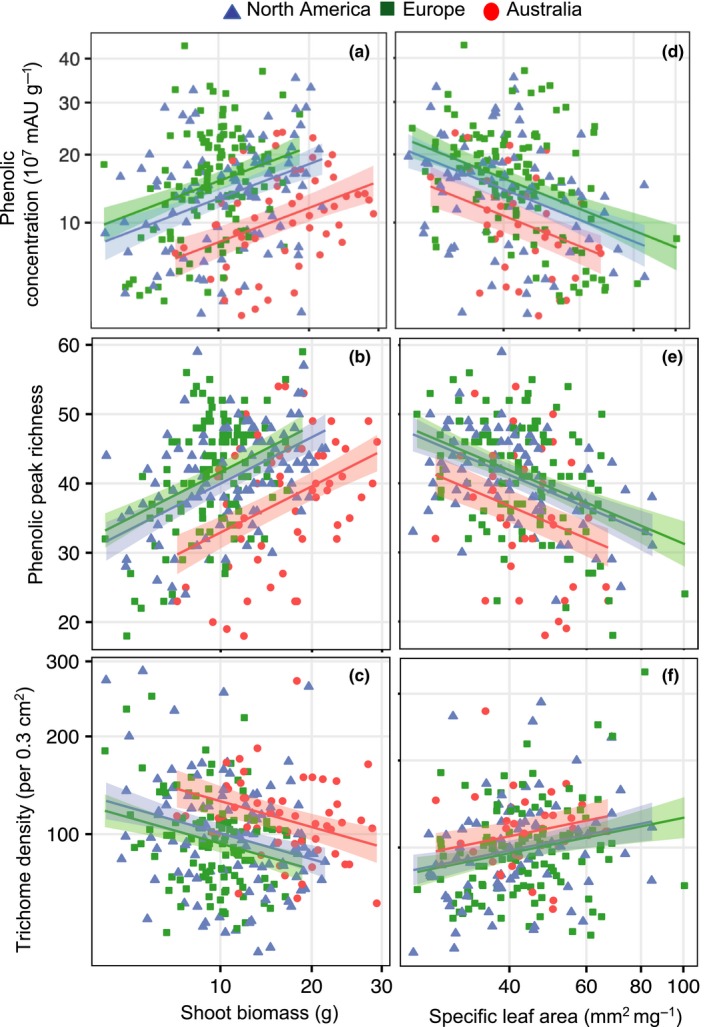
Defence trait responses (phenolic concentration, peak richness, and trichome density) of Ambrosia artemisiifolia individuals to range (native North America (blue triangles); Europe (green squares); Australia (red circles)), shoot biomass (left panels) or specific leaf area (right panels) with model predictions (±95% confidence interval, Table 3)
When controlling for shoot biomass or SLA, total phenolic concentration in European plants was higher compared to North American individuals of comparable weight (Table 3, Figure 4), whereas no difference existed in latitude models (Table 1, Figure 1). Conversely, phenolic peak richness was no longer significantly different between North America and Europe (Table 3, Figure 4) compared to range comparisons accounting for latitude (Table 1, Figure 1). Australian plants exhibited lower phenolic concentration and peak richness compared to native or European plants of comparable weight or SLA. Yet, at the same plant weight, Australian plants had higher trichome densities than in the other ranges (Table 3, Figure 4). These patterns match previous analyses including latitude (Table 1, Figure 1).
3.4. Constitutive‐inducible trade‐offs
Induced levels of phenolic concentration and richness, the response variables, were negatively associated with the predictors, the constitutive levels (concentration: F 1,141.88 = 76.286, p < 0.001; richness: F 1,123.81 = 78.126, p < 0.001; Figure 5). We found no range differences in either induced response trait (concentration: F 2,31.07 = 0.265, p = 0.769; richness: F 2,31.518 = 1.719, p = 0.196; Figure 5), nor did we identify interactions between range and the predictor constitutive phenolic concentration (F 2,141.11 = 0.866, p = 0.423). Range × constitutive phenolic peak richness (F 2,129.82 = 3.045, p = 0.051) was marginally significant. These results suggest that constitutive and inducible defence trade‐off, although there is no difference between ranges.
Figure 5.
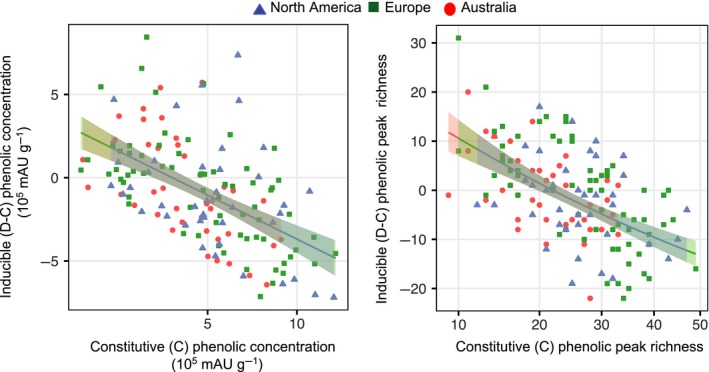
Inducible (D: wounding + MeJA; C: control) versus constitutive (control) defence trait responses (phenolic concentration and peak richness) of Ambrosia artemisiifolia populations among ranges (native North America: blue triangles; Europe: green squares; Australia: red circles) with model predictions (±95% confidence interval).
4. DISCUSSION
In this study, we found evidence for divergence in defence‐related traits within and between ranges. Repeated latitudinal clines in phenolic richness and individual phenolic composition were identified, suggesting rapid adaptation of phenolics to local environments following invasion. Though we observed reduced phenolic concentration and richness in introduced Australia compared to the native plants while controlling for genetic structure, levels were similar or slightly higher in the introduced Europe compared to native populations at comparable latitudes and energy budgets. In addition, trichome density did not differ among ranges. These patterns are inconsistent with the Evolution of Increased Competitive Ability (EICA) hypothesis. In line with predictions, however, a trade‐off between the constitutive and inducible phenolics was observed together with similar phenolic inducibility among ranges. To our knowledge, this is the first study testing the evolution of defence‐related traits across multiple introductions while exploring the predicted confounding of latitudinal clines, population substructure, or genotypic differences in individual resource budgets. Therefore, the apparent absence of the predicted repeated selection against high defence investment following introduction is unlikely to be entirely masked by these factors. We examine these processes in detail and suggest alternative mechanisms driving defence trait divergence within and among native and introduced ranges.
4.1. Divergence in constitutive defence‐related traits
The rapid and repeated latitudinal divergence in phenolic compound composition and richness populations suggests direct or indirect selection of latitude‐associated factors. Corresponding to our findings, typical reported patterns include high growth and low defence at more productive high‐resource (Coley et al., 1985; Endara & Coley, 2011; Zandt, 2007), low‐latitude environments (Blumenthal, 2006; Hahn & Maron, 2016; Moreira et al., 2014; Woods et al., 2012). Native clines in herbivore load could result in such observations, though the predicted herbivore reduction following introduction should lead to nonparallel defence clines among native and introduced ranges (Allen et al., 2017; Cronin et al., 2015). However, in our data, latitudinal clines in defence‐related traits (phenolic compound composition and peak richness) were parallel, which could reflect consistent patterns of selection with latitude in all three ranges. The absence of the predicted patterns could result from parallel clines in herbivore loads in each range or the presence of alternative evolutionary forces driving latitudinal trait divergence in the multiple ranges. Indeed, clinal variation in herbivory is not as common as previously thought (Moles, Bonser, et al., 2011), although geographic information on A. artemisiifolia herbivore pressure is needed.
Alternatively, latitudinal clines could arise through direct selection on the alternative functions of phenolic compounds, or indirect selection through genetic covariance with traits under climate‐mediated selection. Climate was previously shown to be a more important driver of trait divergence compared to enemy release (Colautti & Barrett, 2013; Colautti et al., 2009; Colomer‐Ventura et al., 2015). Along these lines, climatic differences between the ranges not captured by latitude could contribute to patterns of divergence in Australian defence‐related traits. For instance, trichomes protect plants from UV (Bassman, 2004; Hauser, 2014) and selection for this alternate function in high‐UV Australia (WHO, 1998) could potentially explain the higher density of trichomes in this range when controlling for plant size. Herbivore exclusion experiments at various latitudes and environments would be important for to disentangling how resource availability, herbivory, and other climatic factors might interact during invasion and impact the evolution of growth and defence traits.
When correcting for these latitudinal clines, we found conflicting patterns of defence‐related trait divergence between the native and two introduced ranges. Genotypic differences in resource acquisition (Agrawal, 2011; Van Noordwijk & de Jong, 1986; Züst & Agrawal, 2017) and historical contingency (Estoup et al., 2016; Facon et al., 2006; Lee, 2002; Prentis et al., 2008; Rius & Darling, 2014) can obscure trade‐offs predicted under resource allocation trade‐off hypotheses. Accordingly, we show trichome density, phenolic concentration, and peak richness were strongly associated with plant biomass and specific leaf area (SLA; Figure 4). Contrary to EICA predictions, phenolic peak concentration was significantly higher in Europe compared to native North America at comparable shoot biomass, although this difference disappeared when controlling for latitude or SLA. Similarly, phenolic richness was significantly higher in Europe than North America at equivalent latitudes, but this likely reflects the larger size and lower SLA of European plants at similar latitudes (van Boheemen et al., 2018). However, lower phenolic concentration and peak richness in Australia was still present at similar latitude, biomass, or SLA compared to North America. Invasion history is unlikely a major factor in this observed defence‐related trait divergence as we accounted for population genetic structure in our analysis.
An adaptive reduction of constitutive defence traits following introduction to Europe and Australia was predicted due to a general release from natural enemies. However, levels of chemical defence‐related traits (phenolic concentration and richness) were not consistently lower in introduced ranges compared to native populations. Such unexpected findings could have resulted from variation in contemporary herbivory among introduced ranges. Of particular relevance to the EICA hypothesis are specialist herbivores, as herbivory by specialists, but not necessarily generalists, is hypothesized to consistently decline during invasion (Felker‐Quinn et al., 2013; Joshi & Vrieling, 2005; Müller‐Schärer, Schaffner, & Steinger, 2004). Indeed, introduced Japanese A. artemisiifolia populations re‐exposed to specialist leaf beetle Ophraella communa for >10 years were more resistant than herbivore‐free populations (Fukano & Yahara, 2012). However, rapid adaptation to O. communa is unlikely to have led to the observed elevated European phenolic concentration and richness, as the seeds used in our experiment were collected in 2014 and this beetle is constrained to southern Europe since introduction in 2013 (Sun et al., 2017).
Alternatively, differences in generalist load between introduced ranges could have resulted in variation in quantitative digestibility‐reducing chemicals (e.g., phenolics), which defend against both generalist and specialists (Müller‐Schärer et al., 2004). Surveys describe a high diversity of generalist species in Europe (Essl et al., 2015; Gerber et al., 2011) but not in Australia (Palmer & McFadyen, 2012) suggesting herbivory in this species is higher in Europe than Australia. However, Genton, Kotanen, Cheptou, Adolphe, and Shykoff (2005) previously found that compared to native Ontario, the most common forms of damage (chewing and perforation) together with the generalist herbivore load was reduced in introduced France populations consistent with enemy escape in Europe compared to native North America. Contradicting EICA expectations, but consistent with our findings for Europe, the French plants showed no evolutionary loss of defence (Genton et al., 2005). Therefore, although reductions in both specialists and generalist herbivores have been documented in both introduced ranges, we did not find parallel changes in defence‐related traits as predicted by EICA, suggesting such predictions are perhaps too simplistic. Nevertheless, a more detailed survey of herbivory, resistance, and the mechanisms of resistance across all three ranges is warranted, particularly given the contrasting patterns of divergence in phenolics identified among the two introduced ranges. Moreover, a more detailed analysis of the alternative functions of these phenolics (e.g., allelopathic interactions and plant structure; Bhattacharya et al., 2010; Li, Wang, Ruan, Pan, & Jiang, 2010) is required.
A key assumption of EICA is a resource allocation trade‐off between defence and growth. However, even when these traits have evolved in the EICA predicted direction, negative genetic correlations have yet to be detected (Franks, Pratt, Dray, & Simms, 2008; Hodgins et al., 2018; Schrieber et al., 2017). Furthermore, a direct trade‐off might not be evident as resource reallocation from other traits, drawing from the same resource pool, could allow for the elevated investment in defence‐related traits and growth simultaneously (Hodgins et al., 2018; Züst & Agrawal, 2017). For instance, an analysis of climate niche shifts in A. artemisiifolia has revealed that Eurasian and Australasian ranges on average experience warmer, wetter climates compared to the North American range (van Boheemen et al., 2018). Therefore, reduced investment in abiotic stress tolerance could have allowed for resource reallocation to defence and growth simultaneously. These recently acknowledged complex dynamics underlying competitive ability call for more integrative tests of invasive spread.
4.2. Constitutive versus induced range divergence
We observed a negative association between constitutive and inducible defence‐related traits suggesting a trade‐off (Agrawal et al., 2010; Koricheva et al., 2004). A decrease in the level and predictability of attack in the introduced range is expected to cause a reduction in constitutive defence and the maintenance or increase in inducible defence (Cipollini et al., 2005; Lande, 2015; Orians & Ward, 2010). In agreement with this prediction, constitutive phenolic levels were reduced in Australia, while inducible response did not differ among ranges. Such maintenance of mean inducibility could result from insufficient herbivore pressure, where a selection‐drift imbalance could increase inducible variability (Eigenbrode et al., 2008). Although analysis of neutral markers suggests genetic drift has been particularly strong in Australia (van Boheemen et al., 2017), we did not reveal any increase in inducible variation. The growing body of literature testing constitutive versus inducible defence in native and introduced ranges frequently report inconsistent results varying from reductions, to maintenance, to increases in either defence (Agrawal et al., 2015; Beaton, Zandt, Esselman, & Knight, 2011; Carrillo, Wang, Ding, Klootwyk, & Siemann, 2012; Cipollini & Lieurance, 2012; Cipollini et al., 2005; Eigenbrode et al., 2008; Fortuna et al., 2014; Gu et al., 2014; Macel et al., 2017; Wang et al., 2013, 2012) and calls for more detailed research on the cost‐benefit trade‐offs of the various responses.
Remarkably, we found evidence of a suppression of phenolics in response to herbivore simulation for some populations, especially those with high constitutive levels, in contrast to some previous studies (Constabel & Ryan, 1998; Heredia & Cisneros‐Zevallos, 2009; Keinänen et al., 2001; Lee et al., 1997). Conversely, cardenolide suppression was found in various Asclepias species at high constitutive levels (Rasmann, Agrawal, Cook, & Erwin, 2009), though the mechanistic cause was not discussed (Agrawal et al., 2010). We propose that the retraction of phenolics from damaged leaves could indicate a cost‐reducing response when the inducible phenolic compounds have alternative functions (Bhattacharya et al., 2010; Li et al., 2010), or function only in particular aspects of defence response, not induced by the treatment. Similarly, perhaps for those individuals already heavily defended with phenolic compounds increased investment in this chemical defence, which failed to deter an attacking herbivore would have diminishing returns, leading to the potential activation of other defence strategies by the plant. Nevertheless, gaining insight into such cost‐benefit associations might prove difficult due to, for instance, issues identifying and addressing all factors influencing the investment of defence‐related traits (Neilson, Goodger, Woodrow, & Møller, 2013).
5. CONCLUSION
We demonstrate divergence of growth and defence traits within multiple introduced ranges that is consistent with rapid adaptation during introduction. Furthermore, we do not find evidence to support the hypothesis that escape from specialist enemies drives the evolution of increased competitive ability in this invasive, as enhanced growth in European populations was not in lieu of defence‐related trait reduction. The evolution of growth and defense traits in Australian populations, derived from European founders, occurred rapidly (~80 generations), seemingly unconstrained by strong genetic bottleneck identified in this range (van Boheemen et al., 2017), as measured traits in these two invaded ranges are primarily on opposing ends of the phenotypic spectrum of values. Evidence is growing that adaptation to climate might explain the alarming spread and success of non‐natives to a greater extent than release from natural enemies (Colautti & Barrett, 2013; Colautti et al., 2009; Colomer‐Ventura et al., 2015). Indeed, we identified repeated latitudinal patterns in phenolics in all three ranges consistent with climate‐mediated selection, perhaps through corresponding shifts in the biotic community or through direct or indirect selection on phenolics by climate variables. This study emphasizes that intraspecific multi‐introduction tests of trait divergence of invasive species provide important insight into contemporary evolutionary process during range expansion.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
All authors developed the project, with data collection and analyses carried out by LAB and SB, refined by AU and KH. All authors discussed the results, contributed to the MS writing, and gave final approval for publication.
Supporting information
ACKNOWLEDGMENTS
We would like to thank J. Stephens and A. Wetherhill for sample collection, M. Kourtidou and J. Taylor for greenhouse assistance, K. Nurkowski for genomic analyses and R. Andrew for comments on the MS. A Monash University Dean's International Postgraduate Research Scholarship was provided to LAB, a Monash University Startup Grant and an ARC grant (DP180102531) to KAH.
van Boheemen LA, Bou‐Assi S, Uesugi A, Hodgins KA. Rapid growth and defence evolution following multiple introductions. Ecol Evol. 2019;9:7942–7956. 10.1002/ece3.5275
Data Availability Statement: Sequence data are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive under Bioproject PRJNA449949. Scripts are available on https://github.com/lotteanna/defence_adaptation. Data are available on https://doi.org/10.6084/m9.figshare.8028875.v1.
DATA AVAILABILITY
Sequence data are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive under Bioproject PRJNA449949. Scripts are available on https://github.com/lotteanna/defence_adaptation. Data are available on https://doi.org/10.6084/m9.figshare.8028875.v1.
REFERENCES
- Agrawal, A. A. (2011). Current trends in the evolutionary ecology of plant defence. Functional Ecology, 25, 420–432. 10.1111/j.1365-2435.2010.01796.x [DOI] [Google Scholar]
- Agrawal, A. A. , Conner, J. K. , & Rasmann, S. (2010). Tradeoffs and negative correlations in evolutionary ecology In Bell M. A., Futuyma D. J., Eanes W. F., & Levinton J. S. (Eds.), Evolution since darwin: The first 150 years (pp. 243–268). Sunderland, MA: Sinauer Associates. [Google Scholar]
- Agrawal, A. A. , Hastings, A. P. , Bradburd, G. S. , Woods, E. C. , Züst, T. , Harvey, J. A. , & Bukovinszky, T. (2015). Evolution of plant growth and defense in a continental introduction. The American Naturalist, 186, E1–E15. 10.1086/681622 [DOI] [PubMed] [Google Scholar]
- Agrawal, A. A. , & Karban, R. (1999). Why induced defenses may be favored over constitutive strategies in plants In Tollrian R., & Harvell C. D. (Eds.), The ecology and evolution of inducible defenses (pp. 45–61). Princeton, NJ: Princeton University Press. [Google Scholar]
- Agrawal, A. A. , & Kotanen, P. M. (2003). Herbivores and the success of exotic plants: A phylogenetically controlled experiment. Ecology Letters, 6, 712–715. 10.1046/j.1461-0248.2003.00498.x [DOI] [Google Scholar]
- Allen, W. J. , Meyerson, L. A. , Cummings, D. , Anderson, J. , Bhattarai, G. P. , & Cronin, J. T. (2017). Biogeography of a plant invasion: Drivers of latitudinal variation in enemy release. Global Ecology and Biogeography, 26, 435–446. 10.1111/geb.12550 [DOI] [Google Scholar]
- Allendorf, F. W. , & Lundquist, L. L. (2003). Introduction: Population biology, evolution, and control of invasive species. Conservation Biology, 17, 24–30. 10.1046/j.1523-1739.2003.02365.x [DOI] [Google Scholar]
- Bartón, K. (2018). Multi‐model inference. CRAN, The R Foundation for Statistical Computing: R package. Retrieved from https://cran.r-project.org/web/packages/MuMIn/index.html [Google Scholar]
- Bassett, I. J. , & Crompton, C. W. (1975). The biology of canadian weeds. Canadian Journal of Plant Science, 55, 463–476. [Google Scholar]
- Bassman, J. H. (2004). Ecosystem consequences of enhanced solar ultraviolet radiation: Secondary plant metabolites as mediators of multiple trophic interactions in terrestrial plant communities. Photochemistry and Photobiology, 79, 382–398. [DOI] [PubMed] [Google Scholar]
- Beaton, L. L. , Van Zandt, P. A. , Esselman, E. J. , & Knight, T. M. (2011). Comparison of the herbivore defense and competitive ability of ancestral and modern genotypes of an invasive plant, Lespedeza cuneata . Oikos, 120, 1413–1419. 10.1111/j.1600-0706.2011.18893.x [DOI] [Google Scholar]
- Bhattacharya, A. , Sood, P. , & Citovsky, V. (2010). The roles of plant phenolics in defence and communication during agrobacterium and rhizobium infection. Molecular Plant Pathology, 11, 705–719. 10.1111/j.1364-3703.2010.00625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixenmann, R. J. , Coley, P. D. , Weinhold, A. , & Kursar, T. A. (2016). High herbivore pressure favors constitutive over induced defense. Ecology and Evolution, 6, 6037–6049. 10.1002/ece3.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blossey, B. , & Notzold, R. (1995). Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. Journal of Ecology, 83, 887–889. 10.2307/2261425 [DOI] [Google Scholar]
- Blumenthal, D. M. (2006). Interactions between resource availability and enemy release in plant invasion. Ecology Letters, 9, 887–895. 10.1111/j.1461-0248.2006.00934.x [DOI] [PubMed] [Google Scholar]
- Bossdorf, O. , Auge, H. , Lafuma, L. , Rogers, W. E. , Siemann, E. , & Prati, D. (2005). Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia, 144, 1–11. 10.1007/s00442-005-0070-z [DOI] [PubMed] [Google Scholar]
- Brandes, D. , & Nitzsche, J. (2006). Biology, introduction, dispersal and distribution of common ragweed (Ambrosia artemisiifolia l.) with special regard to germany. Nachrichtenblatt Des Deutschen Planzenschutzdienstes, 58, 286–291. [Google Scholar]
- Campos‐Vargas, R. , & Saltveit, M. E. (2002). Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce. Physiologia Plantarum, 114, 73–84. 10.1034/j.1399-3054.2002.1140111.x [DOI] [PubMed] [Google Scholar]
- Cardarelli, E. , Musacchio, A. , Montagnani, C. , Bogliani, G. , Citterio, S. , & Gentili, R. (2018). Ambrosia artemisiifolia control in agricultural areas: Effect of grassland seeding and herbivory by the exotic leaf beetle Ophraella communa . NeoBiota, 38, 1. [Google Scholar]
- Carrillo, J. , Wang, Y. , Ding, J. , Klootwyk, K. , & Siemann, E. (2012). Decreased indirect defense in the invasive tree, Triadica sebifera . Plant Ecology, 213, 945–954. 10.1007/s11258-012-0055-z [DOI] [Google Scholar]
- Chapman, D. S. , Haynes, T. , Beal, S. , Essl, F. , & Bullock, J. M. (2014). Phenology predicts the native and invasive range limits of common ragweed. Global Change Biology, 20, 192–202. 10.1111/gcb.12380 [DOI] [PubMed] [Google Scholar]
- Chauvel, B. , Dessaint, F. , Cardinal‐Legrand, C. , & Bretagnolle, F. (2006). The historical spread of Ambrosia artemisiifolia l. In France from herbarium records. Journal of Biogeography, 33, 665–673. [Google Scholar]
- Chown, S. L. , Hodgins, K. A. , Griffin, P. C. , Oakeshott, J. G. , Byrne, M. , & Hoffmann, A. A. (2014). Biological invasions, climate change and genomics. Evolutionary Applications, 8, 23–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, Y. J. , Fumanal, B. , Laitung, B. , & Bretagnolle, F. (2010). Gene flow and population admixture as the primary post‐invasion processes in common ragweed (Ambrosia artemisiifolia) populations in france. New Phytologist, 185, 1100–1107. 10.1111/j.1469-8137.2009.03129.x [DOI] [PubMed] [Google Scholar]
- Cipollini, D. , & Lieurance, D. M. (2012). Expression and costs of induced defense traits in Alliaria petiolata, a widespread invasive plant. Basic and Applied Ecology, 13, 432–440. 10.1016/j.baae.2012.06.007 [DOI] [Google Scholar]
- Cipollini, D. , Mbagwu, J. , Barto, K. , Hillstrom, C. , & Enright, S. (2005). Expression of constitutive and inducible chemical defenses in native and invasive populations of Alliaria petiolata . Journal of Chemical Ecology, 31, 1255–1267. 10.1007/s10886-005-5284-3 [DOI] [PubMed] [Google Scholar]
- Colautti, R. I. , & Barrett, S. C. (2013). Rapid adaptation to climate facilitates range expansion of an invasive plant. Science, 342, 364–366. 10.1126/science.1242121 [DOI] [PubMed] [Google Scholar]
- Colautti, R. I. , & Lau, J. A. (2015). Contemporary evolution during invasion: Evidence for differentiation, natural selection, and local adaptation. Molecular Ecology, 24, 1999–2017. 10.1111/mec.13162 [DOI] [PubMed] [Google Scholar]
- Colautti, R. I. , Maron, J. L. , & Barrett, S. C. H. (2009). Common garden comparisons of native and introduced plant populations: Latitudinal clines can obscure evolutionary inferences. Evolutionary Applications, 2, 187–199. 10.1111/j.1752-4571.2008.00053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley, P. D. , Bryant, J. P. , & Chapin, F. S. (1985). Resource availability and plant antiherbivore defense. Science, 230, 895–899. 10.1126/science.230.4728.895 [DOI] [PubMed] [Google Scholar]
- Colomer‐Ventura, F. , Martínez‐Vilalta, J. , Zuccarini, P. , Escolà, A. , Armengot, L. , & Castells, E. (2015). Contemporary evolution of an invasive plant is associated with climate but not with herbivory. Functional Ecology, 29, 1475–1485. 10.1111/1365-2435.12463 [DOI] [Google Scholar]
- Constabel, C. P. , & Ryan, C. A. (1998). A survey of wound‐and methyl jasmonate‐induced leaf polyphenol oxidase in crop plants. Phytochemistry, 47, 507–511. 10.1016/S0031-9422(97)00539-6 [DOI] [Google Scholar]
- Cronin, J. T. , Bhattarai, G. P. , Allen, W. J. , & Meyerson, L. A. (2015). Biogeography of a plant invasion: Plant–herbivore interactions. Ecology, 96, 1115–1127. 10.1890/14-1091.1 [DOI] [PubMed] [Google Scholar]
- Dalin, P. , Ågren, J. , Björkman, C. , Huttunen, P. , & Kärkkäinen, K. (2008). Leaf trichome formation and plant resistance to herbivory In Schaller A. (Ed.), Induced plant resistance to herbivory (pp. 89–105). Dordrecht, the Netherlands: Springer. [Google Scholar]
- Davis, M. A. , Grime, J. P. , & Thompson, K. (2000). Fluctuating resources in plant communities: A general theory of invasibility. Journal of Ecology, 88, 528–534. 10.1046/j.1365-2745.2000.00473.x [DOI] [Google Scholar]
- De Rosario‐Martinez, H. (2015). Phia: Post‐hoc interaction analysis (v0.2‐1). CRAN, The R Foundation for Statistical. Computing: R package. Retrieved from https://cran.r-project.org/web/packages/phia/index.html [Google Scholar]
- Dlugosch, K. M. , Anderson, S. R. , Braasch, J. , Cang, F. A. , & Gillette, H. D. (2015). The devil is in the details: Genetic variation in introduced populations and its contributions to invasion. Molecular Ecology, 24, 2095–2111. 10.1111/mec.13183 [DOI] [PubMed] [Google Scholar]
- Dlugosch, K. M. , Cang, F. A. , Barker, B. S. , Andonian, K. , Swope, S. M. , & Rieseberg, L. H. (2015). Evolution of invasiveness through increased resource use in a vacant niche. Nature Plants, 1, 1–5. 10.1038/nplants.2015.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrode, S. D. , Andreas, J. E. , Cripps, M. G. , Ding, H. , Biggam, R. C. , & Schwarzländer, M. (2008). Induced chemical defenses in invasive plants: A case study with Cynoglossum officinale l. Biological Invasions, 10, 1373–1379. 10.1007/s10530-007-9212-z [DOI] [Google Scholar]
- Endara, M. J. , & Coley, P. D. (2011). The resource availability hypothesis revisited: A meta‐analysis. Functional Ecology, 25, 389–398. 10.1111/j.1365-2435.2010.01803.x [DOI] [Google Scholar]
- Essl, F. , Biró, K. , Brandes, D. , Broennimann, O. , Bullock, J. M. , Chapman, D. S. , … Guisan, A. (2015). Biological flora of the british isles: Ambrosia artemisiifolia . Journal of Ecology, 103, 1069–1098. [Google Scholar]
- Estoup, A. , Ravigné, V. , Hufbauer, R. , Vitalis, R. , Gautier, M. , & Facon, B. (2016). Is there a genetic paradox of biological invasion? Annual Review of Ecology, Evolution, and Systematics, 47, 51–72. 10.1146/annurev-ecolsys-121415-032116 [DOI] [Google Scholar]
- Facon, B. , Genton, B. J. , Shykoff, J. , Jarne, P. , Estoup, A. , & David, P. (2006). A general eco‐evolutionary framework for understanding bioinvasions. Trends in Ecology & Evolution, 21, 130–135. 10.1016/j.tree.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Felker‐Quinn, E. , Schweitzer, J. A. , & Bailey, J. K. (2013). Meta‐analysis reveals evolution in invasive plant species but little support for evolution of increased competitive ability (EICA). Ecology and Evolution, 3, 739–751. 10.1002/ece3.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna, T. M. , Eckert, S. , Harvey, J. A. , Vet, L. E. , Müller, C. , & Gols, R. (2014). Variation in plant defences among populations of a range‐expanding plant: Consequences for trophic interactions. New Phytologist, 204, 989–999. 10.1111/nph.12983 [DOI] [PubMed] [Google Scholar]
- Franks, S. J. , Pratt, P. D. , Dray, F. A. , & Simms, E. L. (2008). Selection on herbivory resistance and growth rate in an invasive plant. The American Naturalist, 171, 678–691. 10.1086/587078 [DOI] [PubMed] [Google Scholar]
- Fukano, Y. , & Yahara, T. (2012). Changes in defense of an alien plant Ambrosia artemisiifolia before and after the invasion of a native specialist enemy Ophraella communa . PLoS ONE, 7, e49114 10.1371/journal.pone.0049114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudeul, M. , Giraud, T. , Kiss, L. , & Shykoff, J. A. (2011). Nuclear and chloroplast microsatellites show multiple introductions in the worldwide invasion history of common ragweed, Ambrosia artemisiifolia . PLoS ONE, 6, e17658 10.1371/journal.pone.0017658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton, B. J. , Kotanen, P. M. , Cheptou, P. O. , Adolphe, C. , & Shykoff, J. A. (2005). Enemy release but no evolutionary loss of defence in a plant invasion: An inter‐continental reciprocal transplant experiment. Oecologia, 146, 404–414. 10.1007/s00442-005-0234-x [DOI] [PubMed] [Google Scholar]
- Gerber, E. , Schaffner, U. , Gassmann, A. , Hinz, H. , Seier, M. , & Müller‐schärer, H. (2011). Prospects for biological control of Ambrosia artemisiifolia in Europe: Learning from the past. Weed Research, 51, 559–573. 10.1111/j.1365-3180.2011.00879.x [DOI] [Google Scholar]
- Gladieux, P. , Giraud, T. , Kiss, L. , Genton, B. J. , Jonot, O. , & Shykoff, J. A. (2010). Distinct invasion sources of common ragweed (Ambrosia artemisiifolia) in eastern and western europe. Biological Invasions, 13, 933–944. [Google Scholar]
- Grime, J. P. (1977). Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist, 111, 1169–1194. 10.1086/283244 [DOI] [Google Scholar]
- Gu, X. , Siemann, E. , Zhu, L. , Gao, S. , Wang, Y. , & Ding, J. (2014). Invasive plant population and herbivore identity affect latex induction. Ecological Entomology, 39, 1–9. 10.1111/een.12054 [DOI] [Google Scholar]
- Hahn, P. G. , & Maron, J. L. (2016). A framework for predicting intraspecific variation in plant defense. Trends in Ecology & Evolution, 31, 646–656. 10.1016/j.tree.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Hauser, M.‐T. (2014). Molecular basis of natural variation and environmental control of trichome patterning. Frontiers in Plant Science, 5, 320 10.3389/fpls.2014.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W.‐M. , Thelen, G. C. , Ridenour, W. M. , & Callaway, R. M. (2010). Is there a risk to living large? Large size correlates with reduced growth when stressed for knapweed populations. Biological Invasions, 12, 3591–3598. 10.1007/s10530-010-9753-4 [DOI] [Google Scholar]
- Heredia, J. B. , & Cisneros‐Zevallos, L. (2009). The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biology and Technology, 51, 242–249. 10.1016/j.postharvbio.2008.07.001 [DOI] [Google Scholar]
- Hodgins, K. A. , Bock, D. G. , & Rieseberg, L. (2018). Trait evolution in invasive species. Annual Plant Reviews Online, 1, 1–37. [Google Scholar]
- Hodgins, K. A. , & Rieseberg, L. (2011). Genetic differentiation in life‐history traits of introduced and native common ragweed (Ambrosia artemisiifolia) populations. Journal of Evolutionary Biology, 24, 2731–2749. 10.1111/j.1420-9101.2011.02404.x [DOI] [PubMed] [Google Scholar]
- Ito, K. , & Sakai, S. (2009). Optimal defense strategy against herbivory in plants: Conditions selecting for induced defense, constitutive defense, and no‐defense. Journal of Theoretical Biology, 260, 453–459. 10.1016/j.jtbi.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Jordan, C. Y. , Ally, D. , & Hodgins, K. A. (2015). When can stress facilitate divergence by altering time to flowering? Ecology and Evolution, 5, 5962–5973. 10.1002/ece3.1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, J. , & Vrieling, K. (2005). The enemy release and eica hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecology Letters, 8, 704–714. 10.1111/j.1461-0248.2005.00769.x [DOI] [Google Scholar]
- Katabuchi, M. (2015). LeafArea: An R package for rapid digital image analysis of leaf area. Ecological Research, 30, 1073–1077. 10.1007/s11284-015-1307-x [DOI] [Google Scholar]
- Kazinczi, G. , Béres, I. , Novák, R. , Bíró, K. , & Pathy, Z. (2008). Common ragweed (Ambrosia artemisiifolia): A review with special regards to the results in Hungary. I. Taxonomy, origin and distribution, morphology, life cycle and reproduction strategy. Herbologia, 9, 55–91. [Google Scholar]
- Keinänen, M. , Oldham, N. J. , & Baldwin, I. T. (2001). Rapid HPLC screening of jasmonate‐induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata . Journal of Agricultural and Food Chemistry, 49, 3553–3558. [DOI] [PubMed] [Google Scholar]
- Kessler, A. , & Baldwin, I. T. (2002). Plant responses to insect herbivory: The emerging molecular analysis. Annual Review of Plant Biology, 53, 299–328. [DOI] [PubMed] [Google Scholar]
- Koricheva, J. , Nykänen, H. , & Gianoli, E. (2004). Meta‐analysis of trade‐offs among plant antiherbivore defenses: Are plants jacks‐of‐all‐trades, masters of all? The American Naturalist, 163, E64–E75. 10.1086/382601 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy, K. , & Lee, M. (2014). Improved tests for the equality of normal coefficients of variation. Computational Statistics, 29, 215–232. 10.1007/s00180-013-0445-2 [DOI] [Google Scholar]
- Kumschick, S. , Hufbauer, R. A. , Alba, C. , & Blumenthal, D. M. (2013). Evolution of fast‐growing and more resistant phenotypes in introduced common mullein (Verbascum thapsus). Journal of Ecology, 101, 378–387. [Google Scholar]
- Lachmuth, S. , Durka, W. , & Schurr, F. M. (2011). Differentiation of reproductive and competitive ability in the invaded range of Senecio inaequidens: The role of genetic allee effects, adaptive and nonadaptive evolution. New Phytologist, 192, 529–541. 10.1111/j.1469-8137.2011.03808.x [DOI] [PubMed] [Google Scholar]
- Lande, R. (2015). Evolution of phenotypic plasticity in colonizing species. Molecular Ecology, 24, 2038–2045. 10.1111/mec.13037 [DOI] [PubMed] [Google Scholar]
- Lee, C. E. (2002). Evolutionary genetics of invasive species. Trends in Ecology and Evolution, 17, 386–391. 10.1016/S0169-5347(02)02554-5 [DOI] [Google Scholar]
- Lee, J. , Vogt, T. , Schmidt, J. , Parthier, B. , & Löbler, M. (1997). Methyljasmonate‐induced accumulation of coumaroyl conjugates in barley leaf segments. Phytochemistry, 44, 589–592. 10.1016/S0031-9422(96)00562-6 [DOI] [Google Scholar]
- Li, Z.‐H. , Wang, Q. , Ruan, X. , Pan, C.‐D. , & Jiang, D.‐A. (2010). Phenolics and plant allelopathy. Molecules, 15, 8933–8952. 10.3390/molecules15128933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen, S. T. E. , Hallmann, C. A. , Jongejans, E. , Chauvel, B. , Leitsch‐Vitalos, M. , Aleksanyan, A. , … Müller‐Schärer, H. (2017). Explaining variability in the production of seed and allergenic pollen by invasive Ambrosia artemisiifolia across Europe. Biological Invasions, 20, 1475–1491. [Google Scholar]
- Macel, M. , Dostálek, T. , Esch, S. , Bucharová, A. , van Dam, N. M. , Tielbörger, K. , … Münzbergová, Z. (2017). Evolutionary responses to climate change in a range expanding plant. Oecologia, 184, 543–554. 10.1007/s00442-017-3864-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron, J. L. , & Vilà, M. (2001). When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos, 95, 361–373. 10.1034/j.1600-0706.2001.950301.x [DOI] [Google Scholar]
- Marwick, B. , & Krishnamoorth, K. (2018). Cvequality: Tests for the equality of coefficients of variation from multiple groups: R package. Retrieved from https://cran.r-project.org/web/packages/cvequality/cvequality.pdf [Google Scholar]
- Mole, S. (1994). Trade‐offs and constraints in plant‐herbivore defense theory: A life‐history perspective. Oikos, 71, 3–12. 10.2307/3546166 [DOI] [Google Scholar]
- Moles, A. T. , Bonser, S. P. , Poore, A. G. , Wallis, I. R. , & Foley, W. J. (2011). Assessing the evidence for latitudinal gradients in plant defence and herbivory. Functional Ecology, 25, 380–388. 10.1111/j.1365-2435.2010.01814.x [DOI] [Google Scholar]
- Moles, A. T. , Wallis, I. R. , Foley, W. J. , Warton, D. I. , Stegen, J. C. , Bisigato, A. J. , … Prior, L. D. (2011). Putting plant resistance traits on the map: A test of the idea that plants are better defended at lower latitudes. New Phytologist, 191, 777–788. 10.1111/j.1469-8137.2011.03732.x [DOI] [PubMed] [Google Scholar]
- Moreira, X. , Mooney, K. A. , Rasmann, S. , Petry, W. K. , Carrillo‐Gavilán, A. , Zas, R. , & Sampedro, L. (2014). Trade‐offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecology Letters, 17, 537–546. 10.1111/ele.12253 [DOI] [PubMed] [Google Scholar]
- Morris, W. F. , Traw, M. B. , & Bergelson, J. (2006). On testing for a tradeoff between constitutive and induced resistance. Oikos, 112, 102–110. 10.1111/j.0030-1299.2006.14253.x [DOI] [Google Scholar]
- Müller‐Schärer, H. , Lommen, S. T. , Rossinelli, M. , Bonini, M. , Boriani, M. , Bosio, G. , & Schaffner, U. (2014). Ophraella communa, the ragweed leaf beetle, has successfully landed in Europe: Fortunate coincidence or threat? Weed Research, 54, 109–119. [Google Scholar]
- Müller‐Schärer, H. , Schaffner, U. , & Steinger, T. (2004). Evolution in invasive plants: Implications for biological control. Trends in Ecology & Evolution, 19, 417–422. 10.1016/j.tree.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Neilson, E. H. , Goodger, J. Q. , Woodrow, I. E. , & Møller, B. L. (2013). Plant chemical defense: At what cost? Trends in Plant Science, 18, 250–258. 10.1016/j.tplants.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Orians, C. M. , & Ward, D. (2010). Evolution of plant defenses in nonindigenous environments. Annual Review of Entomology, 55, 439–459. 10.1146/annurev-ento-112408-085333 [DOI] [PubMed] [Google Scholar]
- Orrock, J. L. , Sih, A. , Ferrari, M. C. , Karban, R. , Preisser, E. L. , Sheriff, M. J. , & Thaler, J. S. (2015). Error management in plant allocation to herbivore defense. Trends in Ecology & Evolution, 30, 441–445. 10.1016/j.tree.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Oswalt, M. L. , & Marshall, G. D. (2008). Ragweed as an example of worldwide allergen expansion. Allergy, Asthma and Clinical Immunology, 4, 130–135. 10.1186/1710-1492-4-3-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, B. , & McFadyen, R. E. (2012). Ambrosia artemisiifolia l. – Annual ragweed In Julien M. H., McFadyen R. E., & Cullen J. (Eds.), Biological control of weeds in Australia (pp. 52–59). Collingwood, VIC, Australia: CSIRO. [Google Scholar]
- Parker, J. D. , Torchin, M. E. , Hufbauer, R. A. , Lemoine, N. P. , Alba, C. , Blumenthal, D. M. , … Wolfe, L. M. (2013). Do invasive species perform better in their new ranges? Ecology, 94, 985–994. 10.1890/12-1810.1 [DOI] [PubMed] [Google Scholar]
- Prentis, P. J. , Wilson, J. R. , Dormontt, E. E. , Richardson, D. M. , & Lowe, A. J. (2008). Adaptive evolution in invasive species. Trends in Plant Sciences, 13, 288–294. 10.1016/j.tplants.2008.03.004 [DOI] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing (v3.5.1 “feather spray“). Vienna, Austria: The R Foundation for Statistical Computing. [Google Scholar]
- Rasmann, S. , & Agrawal, A. A. (2011). Latitudinal patterns in plant defense: Evolution of cardenolides, their toxicity and induction following herbivory. Ecology Letters, 14, 476–483. 10.1111/j.1461-0248.2011.01609.x [DOI] [PubMed] [Google Scholar]
- Rasmann, S. , Agrawal, A. A. , Cook, S. C. , & Erwin, A. C. (2009). Cardenolides, induced responses, and interactions between above‐and belowground herbivores of milkweed (Asclepias spp.). Ecology, 90, 2393–2404. [DOI] [PubMed] [Google Scholar]
- Ricciardi, A. (2007). Are modern biological invasions an unprecedented form of global change? Conservation Biology, 21, 329–336. 10.1111/j.1523-1739.2006.00615.x [DOI] [PubMed] [Google Scholar]
- Rius, M. , & Darling, J. A. (2014). How important is intraspecific genetic admixture to the success of colonising populations? Trends in Ecology & Evolution, 29, 233–242. 10.1016/j.tree.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Sax, D. F. , & Brown, J. H. (2000). The paradox of invasion. Global Ecology and Biogeography, 9, 363–371. 10.1046/j.1365-2699.2000.00217.x [DOI] [Google Scholar]
- Scheiner, S. M. (2001). Multiple response variables and multi‐species interactions In Scheiner S. M., & Gurevitch J. (Eds.), Design and analysis of ecological experiments (pp. 99–133). New York, NY: Chapman & Hall. [Google Scholar]
- Schrieber, K. , Wolf, S. , Wypior, C. , Höhlig, D. , Hensen, I. , & Lachmuth, S. (2017). Adaptive and non‐adaptive evolution of trait means and genetic trait correlations for herbivory resistance and performance in an invasive plant. Oikos, 126, 572–582. 10.1111/oik.03781 [DOI] [Google Scholar]
- Sun, Y. , Brönnimann, O. , Roderick, G. K. , Poltavsky, A. , Lommen, S. T. , & Müller‐Schärer, H. (2017). Climatic suitability ranking of biological control candidates: A biogeographic approach for ragweed management in europe. Ecosphere, 8, e01731 10.1002/ecs2.1731 [DOI] [Google Scholar]
- Taramarcaz, P. , Lambelet, C. , Clot, B. , Keimer, C. , & Hauser, C. (2005). Ragweed (Ambrosia) progression and its health risks: Will Switzerland resist this invasion? Swiss Medical Weekly, 135, 538–548. [DOI] [PubMed] [Google Scholar]
- Thébaud and Simberloff (2001). Are plants really larger in their introduced ranges? The American Naturalist, 157, 231–236. 10.1086/318635 [DOI] [PubMed] [Google Scholar]
- Throop, H. L. (2005). Nitrogen deposition and herbivory affect biomass production and allocation in an annual plant. Oikos, 111, 91–100. 10.1111/j.0030-1299.2005.14026.x [DOI] [Google Scholar]
- Tian, D. , Tooker, J. , Peiffer, M. , Chung, S. H. , & Felton, G. W. (2012). Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta, 236, 1053–1066. 10.1007/s00425-012-1651-9 [DOI] [PubMed] [Google Scholar]
- Turner, K. G. , Fréville, H. , & Rieseberg, L. H. (2015). Adaptive plasticity and niche expansion in an invasive thistle. Ecology and Evolution, 5, 3183–3197. 10.1002/ece3.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, K. G. , Hufbauer, R. A. , & Rieseberg, L. H. (2014). Rapid evolution of an invasive weed. New Phytologist, 202, 309–321. 10.1111/nph.12634 [DOI] [PubMed] [Google Scholar]
- Uesugi, A. , Connallon, T. , Kessler, A. , & Monro, K. (2017). Relaxation of herbivore‐mediated selection drives the evolution of genetic covariances between plant competitive and defense traits. Evolution, 71, 1700–1709. 10.1111/evo.13247 [DOI] [PubMed] [Google Scholar]
- Uesugi, A. , & Kessler, A. (2016). Herbivore release drives parallel patterns of evolutionary divergence in invasive plant phenotypes. Journal of Ecology, 104, 876–886. 10.1111/1365-2745.12542 [DOI] [Google Scholar]
- van Boheemen, L. A. , Atwater, D. Z. , & Hodgins, K. A. (2018). Rapid and repeated local adaptation to climate in an invasive plant. New Phytologist, 222, 1 10.1101/420752 [DOI] [PubMed] [Google Scholar]
- van Boheemen, L. A. , Lombaert, E. , Nurkowski, K. A. , Gauffre, B. , Rieseberg, L. H. , & Hodgins, K. A. (2017). Multiple introductions, admixture and bridgehead invasion characterize the introduction history of Ambrosia artemisiifolia in Europe and Australia. Molecular Ecology, 26, 5421–5434. [DOI] [PubMed] [Google Scholar]
- Van Noordwijk, A. J. , & de Jong, G. (1986). Acquisition and allocation of resources: Their influence on variation in life history tactics. The American Naturalist, 128, 137–142. 10.1086/284547 [DOI] [Google Scholar]
- Wang, Y. , Carrillo, J. , Siemann, E. , Wheeler, G. S. , Zhu, L. , Gu, X. , & Ding, J. (2013). Specificity of extrafloral nectar induction by herbivores differs among native and invasive populations of tallow tree. Annals of Botany, 112, 751–756. 10.1093/aob/mct129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Siemann, E. , Wheeler, G. S. , Zhu, L. , Gu, X. , & Ding, J. (2012). Genetic variation in anti‐herbivore chemical defences in an invasive plant. Journal of Ecology, 100, 894–904. 10.1111/j.1365-2745.2012.01980.x [DOI] [Google Scholar]
- War, A. R. , Kumar Taggar, G. , Hussain, B. , Sachdeva Taggar, M. , Nair, R. M. , & Sharma, H. C. (2018). Plant defense against herbivory and insect adaptations. AoB Plants. [Google Scholar]
- War, A. R. , Paulraj, M. G. , Ahmad, T. , Buhroo, A. A. , Hussain, B. , Ignacimuthu, S. , & Sharma, H. C. (2012). Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior, 7, 1306–1320. 10.4161/psb.21663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1998). Global solar uv index. Global Solar UV Index. Retrieved from https://www.who.int/uv/publications/globalindex/en/ [Google Scholar]
- Willemsen, R. W. (1975). Dormancy and germination of common ragweed seeds in the field. American Journal of Botany, 62, 639–643. 10.1002/j.1537-2197.1975.tb14095.x [DOI] [PubMed] [Google Scholar]
- Woods, E. C. , Hastings, A. P. , Turley, N. E. , Heard, S. B. , & Agrawal, A. A. (2012). Adaptive geographical clines in the growth and defense of a native plant. Ecological Monographs, 82, 149–168. 10.1890/11-1446.1 [DOI] [Google Scholar]
- Zandt, P. A. V. (2007). Plant defense, growth, and habitat: A comparative assessment of constitutive and induced resistance. Ecology, 88, 1984–1993. 10.1890/06-1329.1 [DOI] [PubMed] [Google Scholar]
- Züst, T. , & Agrawal, A. A. (2017). Trade‐offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annual Review of Plant Biology, 68, 513–534. 10.1146/annurev-arplant-042916-040856 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive under Bioproject PRJNA449949. Scripts are available on https://github.com/lotteanna/defence_adaptation. Data are available on https://doi.org/10.6084/m9.figshare.8028875.v1.


