Abstract
Atlantic salmon is characterized by a high degree of population genetic structure throughout its native range. However, while populations inhabiting rivers in Norway and Russia make up a significant proportion of salmon in the Atlantic, thus far, genetic studies in this region have only encompassed low to modest numbers of populations. Here, we provide the first “in‐depth” investigation of population genetic structuring in the species in this region. Analysis of 18 microsatellites on >9,000 fish from 115 rivers revealed highly significant population genetic structure throughout, following a hierarchical pattern. The highest and clearest level of division separated populations north and south of the Lofoten region in northern Norway. In this region, only a few populations displayed intermediate genetic profiles, strongly indicating a geographically limited transition zone. This was further supported by a dedicated cline analysis. Population genetic structure was also characterized by a pattern of isolation by distance. A decline in overall genetic diversity was observed from the south to the north, and two of the microsatellites showed a clear decrease in number of alleles across the observed transition zone. Together, these analyses support results from previous studies, that salmon in Norway originate from two main genetic lineages, one from the Barents–White Sea refugium that recolonized northern Norwegian and adjacent Russian rivers, and one from the eastern Atlantic that recolonized the rest of Norway. Furthermore, our results indicate that local conditions in the limited geographic transition zone between the two observed lineages, characterized by open coastline with no obvious barriers to gene flow, are strong enough to maintain the genetic differentiation between them.
Keywords: adaptation, isolation by distance, microsatellites, phylogenetics, Salmon
1. INTRODUCTION
Sustainable management of biodiversity in exploited species requires among other things, an understanding of their structuring into distinct breeding populations, as well as the nature and extent of population connectivity and adaptive population differentiation. Elucidating connectivity among populations, and identifying the underlying mechanisms that shape observed patterns, represents an ongoing challenge. Given the ever‐increasing pressure on much of the world's biota and ecosystems, this is increasingly urgent. For the Atlantic salmon (Salmo salar L.), an iconic and economically important anadromous fish that has and continues to be subjected to a diverse array of anthropogenic challenges (Forseth et al., 2017; Glover et al., 2017; Parrish, Behnke, Gephard, McCormick, & Reeves, 1998; Taranger et al., 2015), it has never been more important to map populations, and quantify their evolutionary and contemporary relatedness and connectivity.
Atlantic salmon inhabit cold‐water rivers on both sides of the north Atlantic. In anadromous populations, the quintessential form, fertilized eggs are deposited in well‐oxygenated gravel areas, and after hatching, juveniles spend 1–5 + years in freshwater before migrating to the sea (Klemetsen et al., 2003; Metcalfe & Thorpe, 1990). After 1–3 + years of oceanic feeding, they mature and return to freshwater to reproduce, completing the life cycle. The species' anadromous life history involves long‐distance migrations from individual spawning rivers and tributaries to shared oceanic feeding areas where fish from multiple populations and regions meet (Bradbury et al., 2016; Gilbey et al., 2017; Olafsson et al., 2016; Sheehan, Legault, King, & Spidle, 2010), with all but a very small fraction of returning salmon, homing back to their natal rivers (Jonsson, Jonsson, & Hansen, 2003; Stabell, 1984). Accurate homing and fidelity to natal river provides the isolating mechanism through which genetically distinct populations have been able to establish in this species throughout its native range (Bourret et al., 2013; King, Kalinowski, Schill, Spidle, & Lubinski, 2001; Ståhl, 1987; Verspoor et al., 2005). In turn, this has also provided the basis for the evolution of genetic differences in life‐history traits among populations, some of which may be adaptive (Garcia de Leaniz et al., 2007; Taylor, 1991).
Atlantic salmon genetic population structure has been widely studied. Beyond the general conclusion that there is a high level of fine scale structuring, often to the tributary level (King, Eackles, & Letcher, 2005), in general, the genetic relationship among populations follows a hierarchical pattern. The largest genetic differences have been observed between populations inhabiting rivers on the east and west sides of the Atlantic (Gilbey, Knox, O'Sullivan, & Verspoor, 2005; Rougemont & Bernatchez, 2018; Taggart, Verspoor, Galvin, Moran, & Ferguson, 1995) and the smallest within rivers (King et al., 2005). At the extreme, salmon native to the American and European continents, show differences in chromosome number (Brenna‐Hansen et al., 2012; Lubieniecki et al., 2010). In general, within continents, population genetic structure is further divided into smaller geographical regions (Bourret et al., 2013; Cauwelier et al., 2018; Olafsson, Pampoulie, Hjorleifsdottir, Gudjonsson, & Hreggvidsson, 2014), and thereafter, among populations inhabiting rivers within regions (Perrier, Guyomard, Bagliniere, & Evanno, 2011; Tonteri, Veselov, Zubchenko, Lumme, & Primmer, 2009; Wennevik, Skaala, Titov, Studyonov, & Nævdal, 2004). Detailed accounts of structuring exist for some parts of the species range (King et al., 2007), including extensive recent accounts of microsatellite variation for southern Europe (Griffiths et al., 2010; Perrier et al., 2011), Iceland (Olafsson et al., 2014), Canada (Bradbury et al., 2015), and more recently, Scotland (Cauwelier et al., 2018). At the finest end of the scale, genetic differences have even been observed among tributaries within larger river systems (Dillane et al., 2007, 2008; Dionne, Caron, Dodson, & Bernatchez, 2009; Vaha, Erkinaro, Niemela, & Primmer, 2007).
Population genetic structure in Atlantic salmon is often, but not always, associated with isolation by distance (Dillane et al., 2007; Glover et al., 2012; Perrier et al., 2011; Primmer et al., 2006). To some extent, this will be because the level of contemporary straying among populations is a function of distance. However, other factors such as landscape features (Dillane et al., 2008), association with climate clines through local adaptation (Gilbey, Verspoor, & Summers, 1999; Jeffery et al., 2017; Verspoor, Fraser, & Youngson, 1991), and colonization history in connection with ice‐cap retreat patterns (Cauwelier et al., 2018; Olafsson et al., 2014; Rougemont & Bernatchez, 2018), play an important role in shaping population genetic structure in this species. Other factors may well be involved and all are likely to be of variable importance in defining levels of within and among river population differentiation.
Norway and Russia have approximately 400 and 110 rivers containing Atlantic salmon populations, respectively (http://www.nasco.int/RiversDatabase.aspx) and populations in this region represent a large proportion of the wild Atlantic salmon resources globally. Yet, despite the significance of this region for Atlantic salmon, a detailed picture of population genetic structure in Norway is lacking, with the literature on Norwegian rivers confined largely to scattered population samples within broader scale assessments (Bourret et al., 2013; Verspoor, 1997; Wennevik et al., 2004), although some Norway‐specific population genetic studies have also been published (Glover et al., 2013, 2012). Russian populations have been more extensively studied. Early studies using allozyme (Kazakov & Titov, 1991) and mitochondrial DNA markers (Makhrov, Verspoor, Artamonova, & O'Sullivan, 2005) described some of the major structuring of Atlantic salmon populations of the Russian north. However, several more recent studies, applying different classes of markers, have extended understanding of the population structure and the recolonization history of these northern populations since the last glaciation. Asplund et al. (2004), looked at mtDNA haplotype variation in 30 rivers from the eastern Barents Sea to the river Tana in Finnmark and suggested grouping the populations into three major clusters; one western group including the Barents Sea coast, one group including rivers from Kola Peninsula draining to the White Sea and an eastern group. In a study of Atlantic salmon populations from the Baltic, White and Barents Seas, Tonteri et al. (2005) concluded that it was most likely that the populations from the White and Barents Seas were colonized from multiple refugia, one possibly located in the eastern Barents Sea. In a follow‐up study with populations from the White and Barents Seas, Tonteri et al. (2009) found evidence of four distinct population clusters; Atlantic Ocean and western Barents Sea, Kola Peninsula, western White Sea and eastern Barents Sea. More recently, Ozerov et al. (2017) developed a high‐density genetic baseline for northern Atlantic salmon populations, and also briefly described population structure, identifying seven major population complexes, largely consistent with the results from the above‐mentioned studies.
The primary objective of the present study it is to provide the first detailed analysis of the population genetic structure of salmon stocks across the whole of Norway and western Russia. This analysis encompasses data for 9,165 salmon from 115 rivers analyzed for a panel of 18 microsatellite DNA markers. The secondary objective of this study is to place the data set in the public domain to facilitate comparative and integrated analyses of structuring patterns across the species’ range.
2. MATERIAL AND METHODS
2.1. Sampling
In total, 9,165 individuals were sampled in 115 rivers from the Komi Republic in Russia to the Østfold region in southern Norway (Figure 1). This included samples of individuals from different stages of their life cycle (parr, fry, smolt, and adult), although most were of juveniles (fry & parr) collected by electrofishing at 2–4 locations within each river. In all cases, sampling encompassed individuals representing all juvenile year classes present at that particular sampling location. Fish were euthanized using an overdose of benzocaine, and fin clips were taken and transferred to tubes with 96% ethanol. Permits for collection of the samples were issued by County Governors in Norway, and by the Federal Agency for Fisheries in Russia. For simplicity, river samples are referred to as “population samples.”
Figure 1.
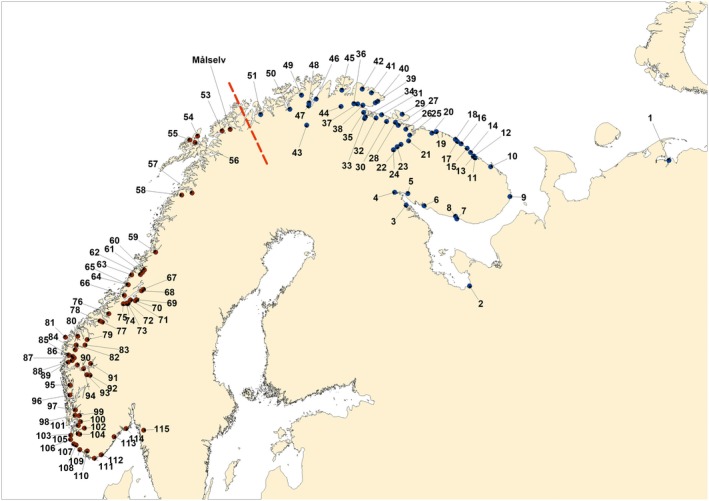
Map showing the location of the rivers sampled in Russia and Norway. Numbers refer to river names in Table A1. The major genetic division of the populations into two groups are indicated with a dashed line
2.2. Genotyping
DNA extraction was performed in 96‐well plates using the Qiagen DNeasyH96 Blood & Tissue Kit; each of which contained two or more negative controls. Eighteen loci were amplified in three multiplex reactions (full genotyping conditions available from authors upon request): SSsp3016 (GenBank no. AY372820), SSsp2210, SSspG7, SSsp2201, SSsp1605, SSsp2216 (Paterson, Piertney, Knox, Gilbey, & Verspoor, 2004), Ssa197, Ssa171, Ssa202 (O'Reilly, Hamilton, McConnell, & Wright, 1996), SsaD157, SsaD486, SsaD144 (King et al., 2005), Ssa289, Ssa14 (McConnell, O'Reilly, Hamilton, Wright, & Bentzen, 1995), SsaF43 (Sanchez et al., 1996), SsaOsl85 (Slettan, Olsaker, & Lie, 1995), MHC I (Grimholt, Drabløs, Jørgensen, Høyheim, & Stet, 2002), and MHC II (Stet et al., 2002). PCR products were analyzed on an ABI 3,730 Genetic Analyser and sized by a 500LIZ™ size standard. Automatically binned alleles were manually checked by two researchers prior to exporting data for statistical analysis. These markers have been extensively used in this laboratory for large‐scale pedigree reconstruction (Harvey, Glover, Taylor, Creer, & Carvalho, 2016; Solberg, Glover, Nilsen, & Skaala, 2013), forensic analysis (Glover, 2010; Glover, Skilbrei, & Skaala, 2008), ploidy validation (Glover et al., 2015; Jorgensen et al., 2018), and population analysis (Glover et al., 2012; Madhun et al., 2017). Thus, the data set is regarded as highly robust.
Data were screened using the software COLONY ver. 2.0.5.1 (Jones & Wang, 2010), which implements full‐pedigree likelihood methods to simultaneously infer sibship and parentage among individuals using multilocus genotype data, to purge the data set from full siblings that would lead to bias in allele frequency estimates as suggested by Allendorf and Phelps (1981), but see work by Waples and Anderson (2017). Analyses were run with no information on parental genotypes, assuming both male and female polygamy as well as possible inbreeding. The full‐likelihood model was chosen together with run length and precision set to medium. A total of 1,007 individuals were removed (Table A1).
2.3. Statistical analysis
Screening for outlier loci was performed using two methods. First, with the approach implemented in ARLEQUIN v.3.5.1.2 (Excoffier, Laval, & Schneider, 2005), which accounts for historical meta‐population structure with a hierarchical island model (H) (Excoffier, Hofer, & Foll, 2009) thus aiming to reduce the number of false positive F ST outlier loci. The underlying assumptions are that the average migration rate between populations on different islands is lower than that between demes on the same island and that the heterozygosity between populations can be inferred using the heterozygosity within a population (Excoffier & Lischer, 2010). Significance of outliers was assessed by running 50,000 simulations, 100 demes, and 20 groups. Second, with the Fdist approach (Beaumont & Nichols, 1996) implemented in LOSITAN (Antao, Lopes, Lopes, Beja‐Pereira, & Luikart, 2008) in which loci with an unusually high F ST are considered to be putatively under directional selection. We simulated the neutral distribution of F ST with 1,000,000 iterations at a significance level of 0.001 under a stepwise mutation model. This method also implements a multi‐test correction based on false discovery rates (FDR) to avoid high overestimation of the percentage of outliers (e.g., 1% of false positive with a threshold of 99%). Due to the impossibility of handling data sets exceeding 100 populations, LOSITAN was conducted separately for each of the regional divisions obtained from STRUCTURE.
Total number of alleles and allelic richness (A r) were calculated with MSA (Dieringer & Schlötterer, 2003), whereas observed (H o) and unbiased expected heterozygosity (uH e) were computed with GenAlEx (Peakall & Smouse, 2006). The genotype distribution of each locus per year class and its direction (heterozygote deficit or excess) was compared with the expected Hardy–Weinberg distribution using the program GENEPOP 7 (Rousset, 2008) as was the linkage disequilibrium. Both were examined using the following Markov chain parameters: 10,000 steps of dememorization, 1,000 batches and 10,000 iterations per batch. Significance was assessed after applying sequential Bonferroni correction (Holm, 1979). Effective population size (Ne) based on linkage disequilibrium was estimated using LDNe v1.31 (Waples & Do, 2008) using the random mating option and the Pcrit = 0.02 criterion for screening out rare alleles, and with 95% confidence intervals derived from a jack‐knife approach.
Allelic richness and heterozygosity were tested for latitudinal trends using the nonparametric Kendall measure of rank correlation (Kendall & Gibbons, 1976), which measures the similarity of the orderings of the data when ranked by north‐south gradient or by the value of the variable tested (Valz & Thompson, 1994), and implemented in the R Package “Kendall” (R Core Team, 2016). Besides, conservation limits (i.e., the number of spawning salmon needed for fully exploiting the rivers potential for production of juveniles) expressed as kg of female fish were tested for correlation with three different variables: N e, H o, and A r. River‐specific conservation limits information was only available for the Norwegian rivers.
Potential recent declines in effective population size were assessed using the software BOTTLENECK v1.2.02 (Piry, Luikart, & Cornuet, 1999) based on allele frequencies. As the data set was genotyped at <20 microsatellites, Wilcoxon's test and the graphical mode shift indicator were chosen (Piry et al., 1999). Likewise, loci were assumed to evolve under the two‐phase mutation model (Di Rienzo et al., 1994) with 5% of the mutations involving multiple steps with a variance of 12 (see Tonteri et al., 2009). Statistical significance of the Wilcoxon's test was assessed by 2,000 replications followed by the sequential Bonferroni correction for multiple significance tests.
Hierarchical population structure was explored using STRUCTURE (Pritchard, Stephens, & Donnelly, 2000) and traditional F ST (Weir & Cockerham, 1984). STRUCTURE v. 2.3.4 was used to identify genetic groups under a model assuming admixture and correlated allele frequencies using population information to assist the analysis. STRUCTURE was analyzed following a hierarchical approach (Gilbey et al., 2017) using the program ParallelStructure (Besnier & Glover, 2013) that distributes jobs between parallel processors in order to significantly speed up the analysis time. Ten runs with a burn‐in period consisting of 250,000 replications and a run length of 750,000 MCMC iterations were performed for K = 1 to K = 20 clusters for the total data set. To determine the number of clusters in which samples could be divided into, the STRUCTURE output was analyzed by combining the visual inspection of the barplots with the ad hoc summary statistic ΔK of Evanno, Regnaut, and Goudet (2005), which is based on the rate of change of the “estimated likelihood” between successive K values and allows the determination of the uppermost hierarchical level of structure in the data. The data set was split into smaller units based upon this analysis until coherence in the clusters were lost, or until single rivers appeared as independent entities. Finally, runs for the selected Ks were averaged with CLUMPP v.1.1.1 (Jakobsson & Rosenberg, 2007) using the LargeK Greedy algorithm and the G’ pairwise matrix similarity statistic and were graphically displayed using barplots. STRUCTURE allowed the partitioning of the data set into subsets of geographic regions that were analyzed in a hierarchical manner.
A Principal component analysis (PCA) was conducted using the program GenoDive, version 2.0b (Meirmans & Van Tienderen, 2004). The analysis was performed on populations (i.e., merged river samples) using a covariance matrix with 10,000 permutations. The results from the analysis were visualized as plots constructed in Microsoft Excel. The relationships among genetic distance and geographical distances were examined via a simple Mantel (1967) test between the matrices of pairwise F ST and geographical distance. Mantel tests were conducted with PASSaGE (Rosenberg & Anderson, 2011), and significance was tested after 10,000 permutations. The program PGDSpider 2.1.1.3 (Lischer & Excoffier, 2012) was used to conduct the file conversion to the software used for the different analyses when required.
In order to further investigate the geographically limited transition zone identified by STRUCTURE (see results), we conducted a cline analysis to estimate the shape, center, and width of the cline generated by our molecular data (Gay, Crochet, Bell, & Lenormand, 2008). Geographic cline analysis over a 3,600 km transect starting in Unya in the Komi Republic in Russia to Enningdalselva in the Østfold region in the Norwegian–Swedish border were conducted with the R package HZAR (Derryberry, Derryberry, Maley, & Brumfield, 2014). The 15 models implemented in HZAR were fitted to the normalized loading of the first principal component analysis (PCA) axis based both on the panel of 18 microsatellites as well as on each locus independently to determine the position, width, and shape of clines over the total geographic distance. The reference cline was built using STRUCTURE Q‐score for the total data set and, in both cases, the best cline model was decided upon AIC scores. Clines were considered significantly displaced if the two log‐likelihood unit support limits of the cline center did not overlap with the STRUCTURE Q‐score (Qb = 1−Qs).
3. RESULTS
The raw genetic data for all of the individuals included in the present study are deposited in Appendix S1.
3.1. Genetic variation within populations
ARLEQUIN reported two outlier loci (Ssa289 and MHC2) in the full data set, whereas LOSITAN suggested that MHC2 was the only locus under directional selection in the two main clusters resulting after the first hierarchical division of the 115 samples. Thus, using a combined approach, MHC2 remained the only candidate for directional selection. The influence of this locus was tested by conducting STRUCTURE with and without it (Appendix S2). As inclusion/exclusion of this locus had no influence on the resulting genetic structure, MHC2 was retained in all the subsequent analyses.
Hardy–Weinberg deviations were reported in ~10% of the tests performed across populations for every locus, but they were reduced to 2.9% after sequential Bonferroni correction. Likewise, the percentage of deviations from LD decreased from 16.5% to 5% after correction. In both cases, the departures from expectations were distributed across populations and loci, therefore, no loci were dropped from the data set based on the results from these analyses.
Over the 18 microsatellites, a total of 413 alleles were observed, ranging from 5 to 7 alleles in SsaD486 and Ssa14, respectively, to 41 in SsaD144 and SsaD157. The total number of alleles per population ranged from 78 to 259, with a mean of 217 (Table A1). The Kovda(3) river showed an extremely low number of alleles: 78 in 26 individuals whereas, for example, 190 alleles were reported from 24 individuals sampled in the river Soknedalselva(109). The average number of alleles per locus within a population ranged from 4.3 in Kovda(3) to 14.4 in Eidselva(82), whereas allelic richness ranged from 4.3 in Kovda(3) to 10.76 in Otra(112).
The level of genetic variation showed a significantly increasing latitudinal N‐S trend following the coastline from Russia to southern Norway when measured either as: average number of alleles per locus within population (τ = −0.177, p = 0.005), Ho (τ = −0.255, p < 0.0001), uHe (τ = −0.36, p < 0.0001) or overall allelic richness (τ = −0.281, p < 0.0001) (Figure 2). The same pattern of A r was statistically significant for 10 out of the 18 loci screened (i.e., SsaF43, MHC1, SsaD486, SSspG7, Ssa14, Ssa289, MHC2, SsaD157, SSsp2210, and Ssa197) whereas for locus Sp1605, the trend was reverse (τ = 0.3, p < 0.0001).
Figure 2.
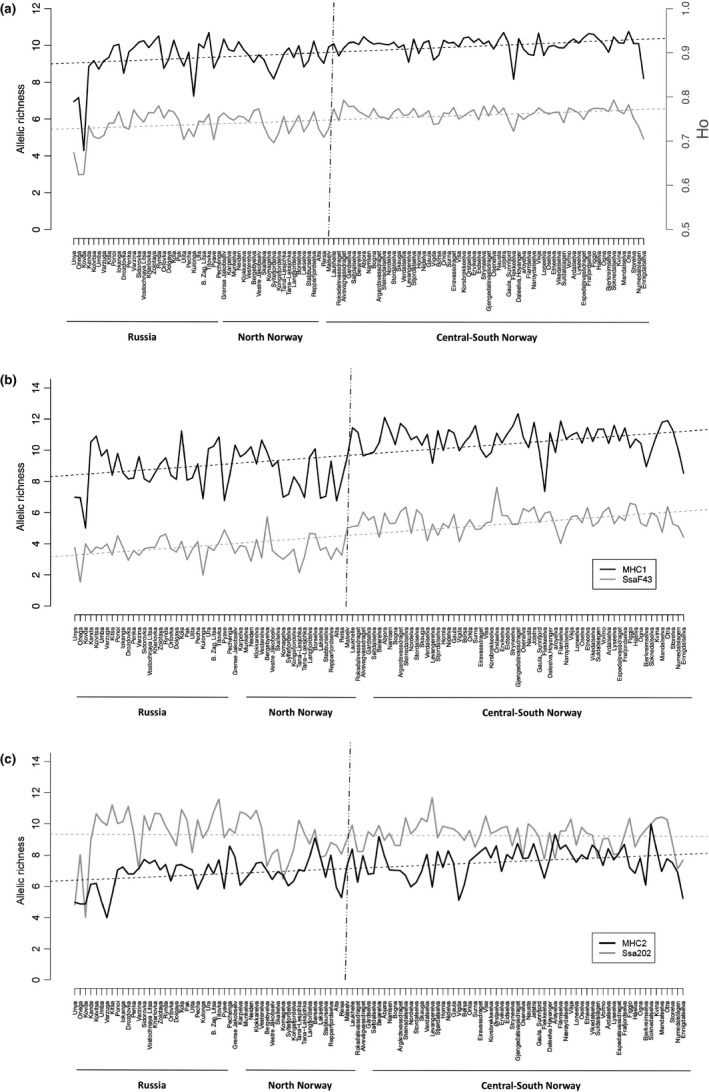
Patterns of genetic diversity assessed along the coastline from Russia to southern Norway. Genetic diversity was measured as: (a) Ho and overall allelic richness (Ar) per population; and (b) Ar for two out of the eleven loci (Sp1605, SsaF43, MHC1, SsaD486, SSspG7, Ssa14, Ssa289, MHC2, SsaD157, SSsp2210, Ssa197) showing significant trends. (c) Ar for MHC2 and SS202 (the second one showing no significant trend, for the sake of the comparison). Both the overall Ar for the 18 loci (τ = −0.281, p = 9.37e−06) and Ho (τ = −0.255, p < 0.001) experienced a significant decline from south to north. The vertical dashed line shows the first level of STRUCTURE division of the data set
In Norway, the conservation limits expressed as kg of female fish per river were significantly correlated with Ne (r 2 = 0.2085, p < 0.001), but not with Ho (r 2 = −0.01, p = 0.68) nor with Ar (r 2 = 0.004, p = 0.25). After performing Bonferroni correction for multiple comparisons, the Wilcoxon test did not reveal any population displaying evidence of having experienced recent bottlenecks. Likewise, no mode shift in allele frequencies was detected in any of them, all showing L‐shaped allele frequency distributions; that is, the number of alleles in the low‐frequency classes (<0.1) exceeded the number of alleles in the higher frequency ones.
3.2. Among‐population genetic structure
The first hierarchical level of division detected by ΔK test of STRUCTURE results showed two clusters (ΔK = 176.5) that divided the data set in a northernmost group ranging from the rivers Unya(1) to Reisa(51) (i.e., 51 sampled rivers), and a southern cluster from the rivers Laukhelle(53) to Enningdalselva(115) (63 rivers) (Figure 1). The ancestry of the population in the river Målselva(52) was almost evenly split between both clusters. At the second hierarchical level of division, further structure was revealed among populations (Figure 3). In the northern group, the eastern populations from Unya(1) to Kitsa(8) formed a distinct cluster in the plots from the structure analysis, different from the populations draining to the Barents Sea coast on the northern side of the Kola Penisula. The river Ponoi(9) appears as a transitional river. This genetic division also corresponds to a change in life history as the eastern populations and the White Sea populations are mainly “autumn‐run” salmon, which ascend the river the more than a year before spawning, while the Barents Sea rivers are dominated by “summer‐run” salmon spawning in the same year they return to the river. On the northern coast of the Kola Peninsula, there seems to be a genetic shift between the rivers east (10–20) and west 26–36 of the Kola Bay (Figure 3b). The rivers draining into the freshwater Tuloma lake (21–24) form a distinct cluster. Another genetic shift can be observed between rivers Bergebyelva(36) and Vestre Jakobselv(37) in the inner part of the Varanger Fjord. The two Tana tributaries Iesjohka(43) and Laksjohka(44) also appear different and distinct from neighboring rivers (Figure 3c). In the southern group, the rivers from Laukhelle(53) to Surna(76) appear fairly similar at K = 3 in the structure plot; however, the island rivers Roksdalsvassdraget(54), Alvsvågvassdraget(55), and Gårdselva(56) appear different from the rivers on the mainland. This was revealed more clearly at structure runs at higher values of K (Figure 3d). In the Trondheimsfjord, similarities can be seen between the larger salmon populations (67, 69, 71, 72 and 75) while the smaller rivers appear different (Figure 3d). Further south, a genetic division was observed between the rivers from Eiravassdraget(77) to Frafjordselva(104) and the more southern/eastern rivers. The rivers Numedalslågen (114) and Enningsdalselva(115) were distinct and different from other rivers in this southernmost region, while the rivers Figgjo(105) and Håelva(106), both draining directly into the ocean, show similarities.
Figure 3.
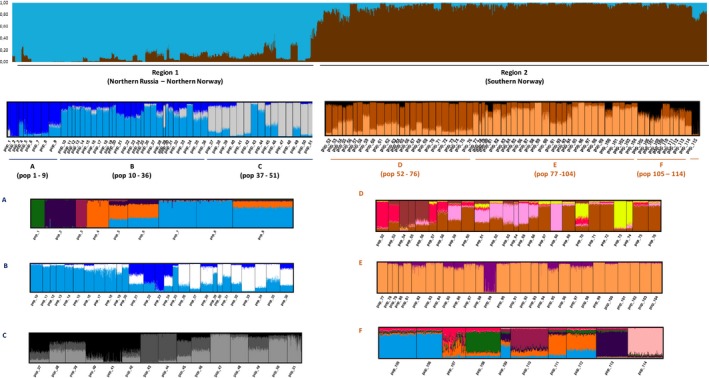
Hierarchical Bayesian clustering of the 115 populations using locprior information in structure
Results of the PCA analysis (Figure 4) were consistent with the geographical defined genetic clusters resolved by structure (Figure 3). The first PC described 26% of the variation along a mainly north‐south gradient and separated the two main clusters clearly, with Målselva(52) appearing as a transitional population between the two main groups. The second PC described 7% of the variation and separated the three main clusters within the northern group, with the Kovda(3) population appearing as an outlier. The three main clusters within the southern group were less clearly separated by this analysis.
Figure 4.
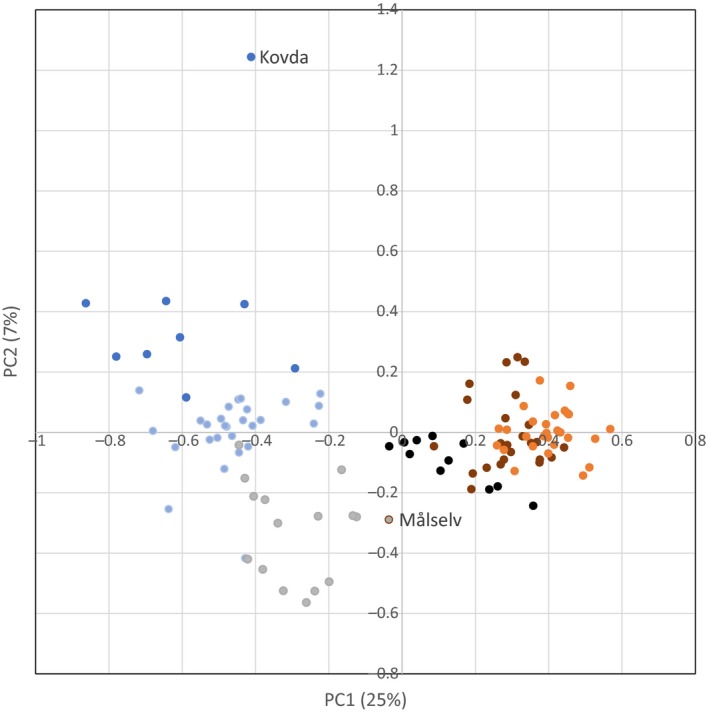
PCA plot of all 115 Atlantic salmon populations included in the analysis. The color coding corresponds to the major clusters detected in the STRUCTURE analysis, where dark blue is region 1.1 (Unya‐Ponoi), light blue is region 1.2 (Iokanga‐Vesterelva), gray is region 1.3 (Bergebyelva‐Reisa), brown is region 2.1 (Målselv‐Surna), orange is region 2.2 (Eira‐Frafjordelvaelva), and black is region 2.3–2.4 (Figgjo‐Enningdalselva)
All global single‐locus estimates for F ST were statistically different from zero (p < 0.0001), ranging between 0.012 (SsaD486) and 0.079 (Ssa289), with the global estimate over the 18 loci being 0.037 (p < 0.0001). The highest pairwise F ST (0.202) was identified between the two Russian rivers Unya(1) and Kovda(3) (see Appendix S2 for complete matrix), located 1,236 km apart. The lowest pairwise F ST values (<0.001) were recorded between five pairs of rivers within a range of 19 to 430 km of distance from each other. Almost all the pairwise comparisons except for 14 (0.2%) were significantly different from zero (p < 0.05). The nonsignificant values ranged from 0.0007 to 0.0041 in a range of geographic distances of 19–534 km.
A Mantel test revealed a positive association between genetic distance measured as F ST and geographic distance, demonstrating an overall pattern of genetic isolation by distance (IBD) among the 115 populations (r = 0.562, p < 0.0001, Figure 5a). The upper cluster of points in this graph corresponds mainly to the pairwise comparisons between samples from the Russian river Kovda(3) and other samples (F ST values from 0.1321 to 0.20). The removal of the aberrant Kodva(3) sample increased the strength of the IBD pattern (r = 0.618, p < 0.0001, Figure 5b).
Figure 5.
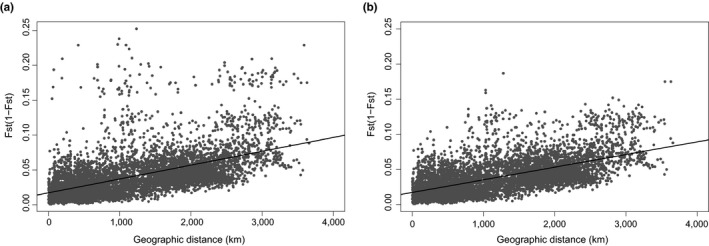
Relationship between geographic distance (km) and genetic distance measured as F ST/(1−F ST). Mantel test revealed a significant pattern of Isolation by Distance when using the full data set (a: r 2 = 0.295, p < 2.2e−16) that became stronger when removing Kovda from the analyses (b: r = 0.618, p < 0.0001)
3.3. Investigation of the transition zone by cline analysis
The PCA cline based on the total 18 microsatellites fitted a fixB model, with the center situated at 1,621 km from the Unya(1) and with a width of 296 km (Figure 6, Table S1—Appendix S2). Both the center and the width of this cline were geographically located between the rivers Reisa(51) and Målselva(52), in very close agreement with the results from STRUCTURE (Figure 3). The PCA cline overlapped with the STRUCTURE Q‐score cline, which also met a fixB model, with the center located at 1,600.6 km (also between rivers Reisa(51) and Målselva(52)) and 336.4 km of width. The clines generated by the microsatellite loci SSsp2210, SSspG7, SsaD144, MHC1, Ssa197, Sp2216, MHC2, SsaF43, and Ssa202 presented their centers within the width of the reference cline based on the STRUCTURE Q‐score. Loci SsaD486 and SSsp2201 showed clines centered further south, unlike loci Ssa289, SSsp3016, SsaD157, Ssa14, Sp1605, SsOsl85, and Ssa171, which were centered between the rivers Kovda(3) and Tana‐Iesjohka(43). The graphical representation of the clines computed for each marker separately is shown in Appendix S3—Figure S1.
Figure 6.
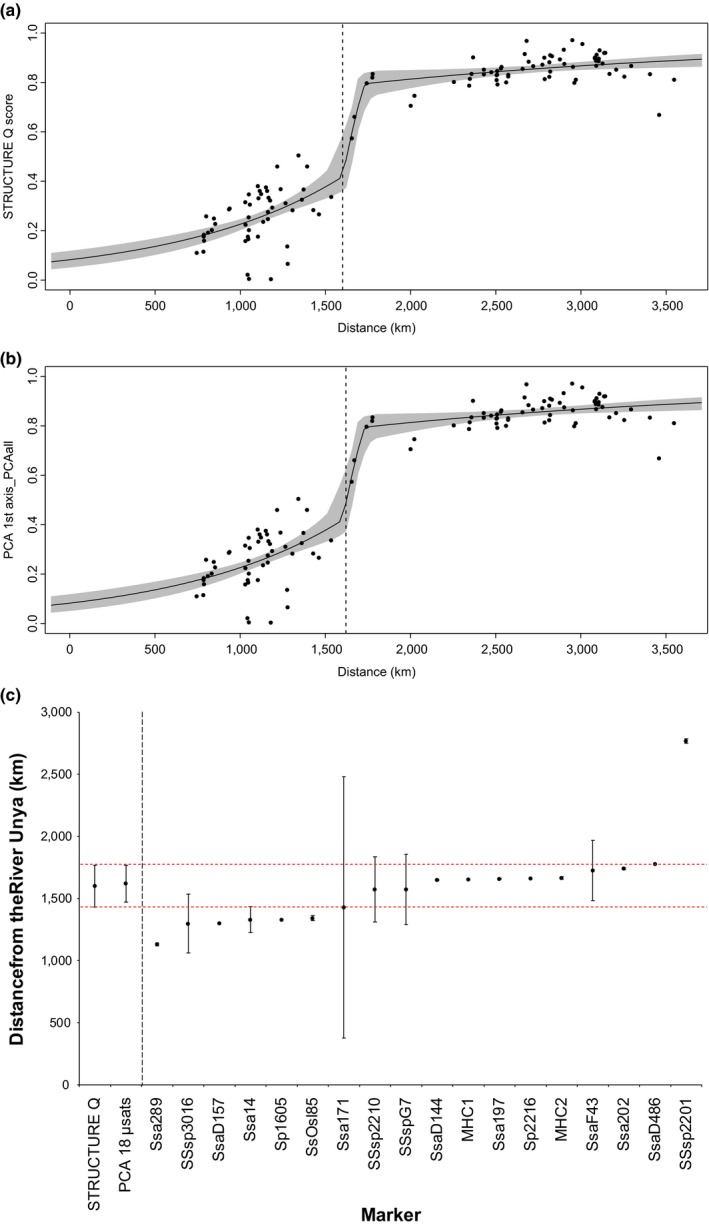
Geographical cline analysis for Atlantic salmon across a 3,600 km transect ranging from the Komi Republic in Russia to the Østfold region in the Norwegian‐Swedish border. Shape of the cline for the (a) STRUCTURE Q‐score and (b) the normalized loading on the first PCA axis based on the panel of 18 microsatellites with the narrow 95% credible cline region shaded in gray, and center of the cline depicted by the vertical dashed line. Furthermore, (c) position of the clines (center and width) for the STRUCTURE Q‐score, the normalized loading on the first PCA axis based on the panel of 18 microsatellites and on each locus separately. Red dashed lines depict the width of the STRUCTURE reference cline
4. DISCUSSION
This study, based on the analysis of >9,000 individuals sampled in 115 rivers, represents the first extensive investigation of genetic structure within and among Norwegian and northwest Russian Atlantic salmon populations. Our most important results are summarized as follows. We observed (a) highly significant population genetic structuring in all regions, following a hierarchical geographic pattern, (b) a clear genetic division in the north of Norway with a geographically limited transition zone (Figures 3 and 6), (c) population genetic structure further influenced by a pattern of isolation by distance across the entire study area, and (d) a decline in genetic variation within populations from the south to the north, with two of the microsatellites showing a clear decrease in number of alleles across the identified transition zone.
Based on the main observations detailed above, we conclude that Atlantic salmon in Norway originate mainly from two genetic lineages, one from the Barents–White Sea refugium that recolonized northern Norwegian and adjacent Russian rivers, and one from the eastern Atlantic that recolonized the rest of Norway. We also conclude that local conditions in the geographically limited transition zone between these two lineages in northern Norway, characterized by a relatively open coastline with no obvious barriers to straying nor gene flow, are strong enough to maintain its character since its post‐last glacial maximum establishment. Whether or not selection, restricted straying and gene flow, or other mechanisms are responsible for its maintenance, remains to be elucidated.
4.1. Phylogeographic patterns in northern Norway and northwest Russia
The distinctiveness of salmon in northern Norway and northwest Russia, as compared to other European regions, was first noted in a study of 15 rivers across the species’ range using allozyme markers (Bourke, Coughlan, Jansson, Galvin, & Cross, 1997). This has also been observed in subsequent studies applying different classes of molecular markers (Bourret et al., 2013; Gilbey et al., 2017; Ozerov et al., 2017; Rougemont & Bernatchez, 2018; Skaala et al., 1998; Tonteri et al., 2009). However, the number of rivers included in some of these studies was limited. The first study to report a more precise geographic location of the clear genetic break in Norway, potentially reflecting the recolonization ranges from different lineages, was a study of microsatellite genetic variation in 21 Norwegian populations (Glover et al., 2012). These authors identified a genetic division in the geographic region between Målselva and Roksdalsvassdraget (populations 52 and 54 in the present study) which is consistent with the division revealed from the analysis here (Figure 3). Subsequent studies with SNPs, primarily aimed at investigating introgression of domesticated Atlantic salmon escapees in Norwegian populations, have also detected a distinct genetic change in this region (Glover et al., 2013; Karlsson, Diserud, Fiske, & Hindar, 2016).
The existence of a clear genetic divide in northern Norway is most likely to reflect the postglacial colonization history of this region, and the influence of mechanisms maintaining this divide over time. As mentioned above and in the introduction, several studies have suggested that the northern areas of Russia and Norway were colonized by different lineages, originating from different refugia. The eastern part of the Barents Sea was not entirely covered by ice during the last glacial maximum (Hughes, Gyllencreutz, Lohne, Mangerud, & Svendsen, 2016), and this area has been suggested as the location of a refugium from which the northeastern part of the distribution range of Atlantic salmon was colonized (Asplund et al., 2004; Kazakov & Titov, 1991; Nilsson et al., 2001; Rougemont & Bernatchez, 2018; Tonteri et al., 2005, 2009). Asplund et al. (2004) suggested that populations east of the genetic divide, observed in the eastern part of the Kola peninsula (this divide also shown by Tonteri et al., 2009 and present in our data set), primarily originated from this eastern refugium, while populations on the northern side of the peninsula and westwards into northern Norway mainly originated from other Atlantic lineages. Our data are consistent with this.
Several studies have demonstrated the presence of North American alleles/haplotypes in populations along the Barents Sea coast (Asplund et al., 2004; Bourke et al., 1997; Makhrov et al., 2005; Nilsson et al., 2001; Rougemont & Bernatchez, 2018) suggesting a contribution from both eastern and western Atlantic lineages. Based on a joint analysis of both Esterase‐D* and mtDNA, Mahkrov, and colleagues (Makhrov et al., 2005) first proposed that the genetic affinities of the region's salmon populations to those in North America arose from the unique postglacial recolonization of the area by salmon from both Europe and North America. In combination with other observations (Asplund et al., 2004; Tonteri et al., 2009), a geographical cline in western Atlantic genetic types suggests that the western Barents Sea/northern Kola Peninsula rivers may represent a further zone of secondary contact between eastern and western Atlantic lineages colonizing this area, in addition to the transition zone between this area and southern Norway. This possibility needs to be explored by a more in‐depth genetic analysis as recently reported for the zone of secondary contact between European and North American salmon in eastern Canada (Lehnert et al., 2018).
4.2. The geographically sharp transition zone between the eastern Atlantic and Barents–White Sea lineages
The continued existence of a geographically limited transition zone in northern Norway between two highly divergent regional salmon lineages raises both evolutionary and ecological questions. From an ecological perspective, do the evolved differences in the two regional groups encompass significant differences in their biologies, and what mechanisms maintain this geographically sharp divide? We suggest that there are potentially two mechanisms that interlink: (a) restricted straying and/or gene flow, (b) divergent selective forces.
There are still many unknowns regarding straying rates among salmon populations, though they clearly vary in time and space (Jonsson et al., 2003; Pedersen, Rasmussen, Nielsen, Karlsson, & Nyberg, 2007; Skilbrei & Holm, 1998; Stabell, 1984), and while some knowledge has been gained in recent years on their marine migration behavior (Chittenden, Adlandsvik, Pedersen, Righton, & Rikardsen, 2013; Gilbey et al., 2017; Gudjonsson, Einarsson, Jonsson, & Gudbrandsson, 2015; Strøm, Thorstad, Hedger, & Rikardsen, 2018), a large number of questions remain with respect to their oceanic migration routes and offshore feeding areas. Nevertheless, a lack of synchrony in marine growth of salmon populations from northern versus western Norway suggest that salmon originating from these two regions may utilize different oceanic feeding areas (Jensen et al., 2011). If this is the case, then fish retuning to the coastline in the region just north and south of the geographically limited transition zone identified here may come from different directions/oceanic areas, and act to reduce straying between the two regions. In turn, this could limit gene flow. However, a study of straying from two populations north of this transition zone found that fish strayed into rivers south of it (Ulvan et al., 2018), suggesting that the occurrence of some genetic mixing cannot be ruled out.
We observed a decrease in several estimators of genetic diversity with an increase in latitude (Figure 2a) and a clear “shift” in allelic variation at two of the genetic markers in the transition zone where the aforementioned lineages meet (Figure 2b). In Canadian Atlantic salmon populations, a gradient in genetic diversity and allelic variation at the MHC2 locus has been reported (Dionne, Miller, Dodson, Caron, & Bernatchez, 2007), and a relationship between allele frequencies and latitude was observed for immune‐related genes among European Atlantic salmon populations (Tonteri, Vasemägi, Lumme, & Primmer, 2010). Furthermore, allelic gradients with latitude and temperature have also been observed in respect of allelic variation at the MEP‐2* locus on both sides of the Atlantic both within and among rivers (Verspoor & Jordan, 1989). A recent study using whole genome resequencing identified functional genetic differences between salmon populations from the north and the rest of Norway (Kjaerner‐Semb et al., 2016), with evidence of islands of divergence on chromosomes 5, 10, 11, 13–15, 21, 24, and 25, possibly resulting from divergent selection regimes. This divergence included 59 known genes, 15 of which displayed one or more differentiated missense mutations. The strongest of these islands of divergence, located on chromosomes 25 and 5, respectively, contained genes involved in anti‐viral and pathogen control. It is not possible to conclude the functional significance of the clear general decrease in genetic diversity as revealed in the present study, or specifically for two of the markers across the observed transition zone. While clearly further work is needed, what evidence there is points to the possibility of functional genetic differences between populations in these two regions, possibly arising from a combination of differences relating to phylogenetic background and lineage recolonization, and divergent selection regimes. If as suggested, divergent selection regimes between these areas exist, even if some interbreeding does occur due to straying across the transition zone, reduced survival of the “nonlocal” type, as observed across watercourses in Ireland (McGinnity et al., 2004), may strongly constrain effective gene flow and help maintain geographically restricted transition zone.
4.3. Patterns of population genetic connectivity
A hierarchical pattern in genetic structure as revealed here, that is, within and among‐regional levels of differentiation (Figures 3 and 4), also characterized by an overall pattern of isolation by distance (IBD) (Figure 5), is a typical feature of Atlantic salmon populations (Glover et al., 2012; Tonteri et al., 2009; Vaha, Erkinaro, Falkegard, Orell, & Niemela, 2017). In addition to the highest level regional differentiation in northern Norway, a further less marked splitting of the Barents–White Sea and eastern Atlantic lineages and several other genetic sub‐groups was resolved (Figure 2). A second order division in population structure was reported in the Kola Peninsula of Russia between samples from the Ponoi and Iokanga (populations 9 and 10 in Figure 3). This corresponds to the division reported in earlier studies (Ozerov et al., 2017; Saisa et al., 2005; Tonteri et al., 2009) and to changes in the life‐history pattern of populations (Berg, 1948). Other genetic divisions were also revealed (Figure 3), illustrating the existence of both long‐distance and regional levels of genetic structure.
Population size differences (as evaluated from catch statistics or conservation limits), and potentially life history or other adaptive characteristics, appear linked with some of the patterns of genetic structure observed here. For example, in the relatively isolated Trondheimsfjord in mid‐Norway, the ten rivers sampled show a clear pattern of genetic divergence between the rivers with demographically small populations (populations 66, 68, 70, 73, 74) and those with demographically large or very large populations (populations 67, 69, 71, 72, 75) (Figure 3). This effect is also apparent in respect of the rivers Gaula and Orkla, which are genetically very similar to each other (populations 72 and 75), yet very distinct from the two small populations located between them, Vigda and Børsa, that are also similar to each other (populations 73 and 74) (Figure 3). It is thus striking that the two very large rivers have not dominated or overridden the genetic characteristics of these two much smaller populations, something observed in studies in other regions (Verspoor, 2005; Verspoor, Knox, & Marshall, 2016), once again suggesting a role for adaptive divergence even on a local scale.
Landscape features are known to influence population genetic structure in Atlantic salmon (Dillane et al., 2008; Ozerov, Veselov, Lumme, & Primmer, 2012). Although beyond the scope of this study, obvious landscape features also appeared to be linked with some of the population genetic structure revealed here. For example, on the coastline of Jæren on southwestern Norway, a genetic divide was revealed among populations in the Boknafjord region (populations 100–104) versus the immediately neighboring open coastline stretch of Jæren (populations 105–109) (Figure 3). Also, the rivers located in the relatively isolated Trondheimsfjord area showed differentiation to rivers on the outside of this fjord area, which overlays the significant observations within the fjord as discussed above (Figure 3). Thus, there is considerable evidence that the evolutionary relationships among populations and their genetic differentiation is driven by more than just historical and contemporary gene flow conditioned by IBD.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
VW, ØS, SP, and KAG conceived the study. VW and SP conducted/organized sampling of rivers. MQ and VW conducted statistical analyses and drafted part of the text. VW and SP provided background material to the study. EV assisted in data interpretation and finalizing the manuscript. VW and KAG led the process of data interpretation and development of the final version of the manuscript, with scientific contributions from all other authors. All authors read and approved the final manuscript.
ETHICAL APPROVAL
Samples (fin clippings) were obtained from juvenile fish caught by electrofishing, or in some cases, scale samples collected in rod fisheries. Juvenile fish captured by electrofishing were euthanized before samples were collected. The permits required to obtain samples were issued by the Federal Agency for Fisheries (Russian Federation), and different County Governors of Norway.
Supporting information
ACKNOWLEDGMENTS
The authors wish to thank the numerous people who provided samples for genetic analysis, especially Rådgivende Biologer AS, who provided many of the samples from rivers in western Norway, the Norwegian Institute of Nature research, and the staff at the Freshwater Laboratory of PINRO‐ Russia, who collected samples from the rivers in Kola Peninsula. We are also grateful to Laila Unneland, Bjørghild Breistein Seliussen, and Anne Grete Sørvik Eide, who genotyped most of the samples.
APPENDIX A.
Table A1.
Summary statistics per sample: Geographic coordinates per river; type of sample; total number of individuals, and number of individuals once full siblings have been removed; total number of alleles; allelic richness (AR, based on a sample of minimum 24 diploid individuals), number of private alleles; observed heterozygosity, Ho (mean ± SE); unbiased expected heterozygosity, uHe (mean ± SE); inbreeding coefficient, F IS (mean ± SE); number (and percentage) of deviations from Hardy–Weinberg equilibrium (HWE) at α = 0.05; number (and percentage) of deviations from Linkage Disequilibrium (LD) at α = 0.05 and effective population size (Ne) with 95% confidence interval obtained by jack‐knife method (in brackets) calculated using the random mating option and the Pcrit = 0.02 criterion for screening out rare alleles
| No | River | Latitude | Longitude | Sample type | No samples | No samples after fullsib removal | No total alleles | Ar | No private alleles | Ho | uHe | F IS | No (%) dev LD at p < 0.05 | No (%) dev HWE at p < 0.05 | Ne (CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Unya | 68.2122 | 54.2189 | Parr/fry | 48 | 32 | 131 | 6.95 | 0 | 0.67 ± 0.06 | 0.66 ± 0.06 | −0.040 ± 0.027 | 11 (7.2) | 1 (5.6) | 48.4 (35.8, 71.3) |
| 2 | Onega | 63.9167 | 38.0000 | Smolt | 88 | 72 | 157 | 7.16 | 0 | 0.62 ± 0.06 | 0.65 ± 0.06 | 0.031 ± 0.017 | 19 (12.4) | 3 (16.7) | 84.4 (66.8, 111.1) |
| 3 | Kovda | 66.6833 | 32.8500 | Parr/fry | 47 | 26 | 78 | 4.29 | 0 | 0.63 ± 0.04 | 0.60 ± 0.04 | −0.047 ± 0.027 | 19 (12.4) | 2 (11.1) | 23.3 (16.2, 36.4) |
| 4 | Kanda | 67.1292 | 31.9053 | Parr/fry | 55 | 52 | 186 | 8.85 | 0 | 0.74 ± 0.06 | 0.72 ± 0.05 | −0.027 ± 0.015 | 10 (6.5) | 2 (11.1) | 207.7 (139.7, 384.6) |
| 5 | Kolvitsa | 67.0833 | 32.9833 | Parr/fry | 45 | 44 | 186 | 9.17 | 0 | 0.71 ± 0.06 | 0.73 ± 0.06 | 0.011 ± 0.012 | 11 (7.2) | 0 | 236.8 (151.4, 508.6) |
| 6 | Umba | 66.6667 | 34.3000 | Parr/fry | 89 | 72 | 192 | 8.71 | 0 | 0.71 ± 0.06 | 0.71 ± 0.06 | 0.001 ± 0.015 | 8 (5.2) | 3 (16.7) | 256.9 (174.1, 465.4) |
| 7 | Varzuga | 66.2167 | 36.9667 | Parr/fry | 91 | 88 | 213 | 9.16 | 2 | 0.71 ± 0.06 | 0.72 ± 0.06 | −0.006 ± 0.014 | 10 (6.5) | 2 (11.1) | 1,172.7 (488.1, Infinite) |
| 8 | Kitsa | 66.3020 | 36.8620 | Parr/fry | 96 | 85 | 211 | 9.34 | 1 | 0.74 ± 0.06 | 0.74 ± 0.06 | −0.014 ± 0.012 | 7 (4.6) | 0 | 298.8 (221.9, 446.7) |
| 9 | Ponoi | 66.9833 | 41.2833 | Parr/fry | 141 | 140 | 251 | 9.98 | 1 | 0.74 ± 0.06 | 0.75 ± 0.06 | 0.012 ± 0.010 | 10 (6.5) | 1 (5.6) | 1,336.8 (715, 7,956.6) |
| 10 | Iokanga | 68.0000 | 39.7167 | Parr/fry | 78 | 77 | 230 | 10.06 | 0 | 0.77 ± 0.06 | 0.75 ± 0.06 | −0.028 ± 0.016 | 12 (7.8) | 1 (5.6) | 1,244.1 (562, Infinite) |
| 11 | Drozdovka | 68.3000 | 38.4500 | Parr/fry | 63 | 49 | 174 | 8.48 | 0 | 0.73 ± 0.06 | 0.73 ± 0.06 | −0.008 ± 0.013 | 7 (4.6) | 0 | 96.4 (78.3, 123.4) |
| 12 | Penka | 68.3500 | 38.3000 | Parr/fry | 42 | 38 | 195 | 9.64 | 0 | 0.73 ± 0.06 | 0.74 ± 0.06 | −0.005 ± 0.018 | 5 (3.3) | 1 (5.6) | 393.2 (180.4, Infinite) |
| 13 | Varzina | 68.3667 | 38.3500 | Parr/fry | 61 | 55 | 214 | 9.88 | 0 | 0.77 ± 0.06 | 0.76 ± 0.06 | −0.029 ± 0.019 | 4 (2.6) | 0 | 1,198.9 (448.7, Infinite) |
| 14 | Sidorovka | 68.4833 | 38.0833 | Parr/fry | 74 | 55 | 217 | 10.17 | 0 | 0.75 ± 0.05 | 0.78 ± 0.05 | 0.005 ± 0.012 | 46 (30.1) | 2 (11.1) | 1,227.1 (406.2, Infinite) |
| 15 | Vostochnaya Litsa | 68.6333 | 37.8000 | Parr/fry | 87 | 70 | 233 | 10.25 | 0 | 0.74 ± 0.06 | 0.76 ± 0.06 | 0.009 ± 0.018 | 9 (5.9) | 2 (11.1) | 279.8 (207.4, 420.8) |
| 16 | Kharlovka | 68.7833 | 37.3167 | Parr/fry | 76 | 63 | 221 | 9.88 | 0 | 0.77 ± 0.06 | 0.76 ± 0.06 | −0.011 ± 0.012 | 11 (7.2) | 0 | 140.8 (111.2, 188) |
| 17 | Zolotaya | 68.8663 | 37.0166 | Parr/fry | 89 | 70 | 228 | 10.21 | 0 | 0.76 ± 0.06 | 0.77 ± 0.06 | −0.005 ± 0.012 | 12 (7.8) | 2 (11.1) | 192.1 (149.2, 264.8) |
| 18 | Rynda | 68.9333 | 36.8500 | Parr/fry | 79 | 75 | 242 | 10.51 | 1 | 0.78 ± 0.06 | 0.77 ± 0.06 | −0.023 ± 0.006 | 19 (12.4) | 1 (5.6) | 447.5 (308.1, 793.4) |
| 19 | Orlovka | 69.2043 | 35.2920 | Parr/fry | 69 | 35 | 171 | 8.75 | 0 | 0.75 ± 0.06 | 0.74 ± 0.06 | −0.027 ± 0.017 | 15 (9.8) | 1 (5.6) | 31.2 (27.1, 36.4) |
| 20 | Dolgaya | 69.1500 | 34.9333 | Parr/fry | 76 | 44 | 191 | 9.25 | 0 | 0.77 ± 0.06 | 0.75 ± 0.06 | −0.033 ± 0.02 | 14 (9.2) | 3 (16.7) | 47.3 (41.1, 55.3) |
| 21 | Kola | 68.8833 | 33.0333 | Parr/fry | 97 | 94 | 244 | 10.28 | 0 | 0.77 ± 0.06 | 0.76 ± 0.06 | −0.009 ± 0.009 | 10 (6.5) | 3 (16.7) | 296.8 (229.7, 412.3) |
| 22 | Pak | 68.7667 | 32.4167 | Parr/fry | 92 | 73 | 206 | 9.46 | 0 | 0.75 ± 0.06 | 0.75 ± 0.06 | −0.002 ± 0.011 | 23 (15.0) | 5 (27.8) | 258.6 (197.4, 368) |
| 23 | Ulita | 68.6833 | 32.1000 | Parr/fry | 74 | 60 | 197 | 8.88 | 0 | 0.70 ± 0.05 | 0.73 ± 0.05 | 0.030 ± 0.016 | 4 (2.6) | 3 (16.7) | 74.5 (63.7, 88.6) |
| 24 | Pecha | 68.5833 | 31.8000 | Parr/fry | 66 | 56 | 206 | 9.63 | 1 | 0.73 ± 0.06 | 0.75 ± 0.06 | 0.019 ± 0.013 | 7 (4.6) | 1 (5.6) | 471.2 (256, 2,362.9) |
| 25 | Kulonga | 69.0785 | 33.1292 | Parr/fry | 47 | 36 | 143 | 7.24 | 0 | 0.71 ± 0.06 | 0.70 ± 0.06 | −0.039 ± 0.021 | 10 (6.5) | 1 (5.6) | 67.8 (50.9, 97.5) |
| 26 | Ura | 69.2833 | 32.8167 | Parr/fry | 103 | 73 | 228 | 10.09 | 0 | 0.75 ± 0.05 | 0.75 ± 0.06 | −0.002 ± 0.018 | 18 (11.8) | 2 (11.1) | 112.9 (98.3, 131.6) |
| 27 | B. Zap. Litsa | 69.4171 | 32.2021 | Parr/fry | 99 | 69 | 216 | 9.86 | 0 | 0.74 ± 0.06 | 0.76 ± 0.06 | 0.010 ± 0.009 | 13 (8.5) | 1 (5.6) | 168.8 (138.2, 214.4) |
| 28 | Titovka | 69.5167 | 31.9667 | Parr/fry | 91 | 80 | 250 | 10.70 | 0 | 0.76 ± 0.06 | 0.77 ± 0.06 | 0.007 ± 0.014 | 6 (3.9) | 4 (22.2) | 151.3 (125.9, 187.2) |
| 29 | Pyave | 69.7939 | 32.5119 | Parr/fry | 38 | 28 | 167 | 8.76 | 0 | 0.70 ± 0.05 | 0.72 ± 0.05 | 0.002 ± 0.019 | 4 (2.6) | 1 (5.6) | 61.8 (44, 98.7) |
| 30 | Pechenga | 69.5500 | 31.2500 | Parr/fry | 44 | 33 | 182 | 9.34 | 1 | 0.75 ± 0.06 | 0.75 ± 0.06 | −0.014 ± 0.018 | 9 (5.9) | 0 | 151.4 (100.6, 288.9) |
| 31 | Grense Jakobselv | 69.7782 | 30.8383 | Parr | 80 | 55 | 222 | 10.34 | 0 | 0.76 ± 0.06 | 0.76 ± 0.06 | −0.021 ± 0.011 | 10 (6.5) | 2 (11.1) | 139.3 (112.7, 179.8) |
| 32 | Karpelva | 69.6672 | 30.3849 | Parr | 92 | 68 | 214 | 9.76 | 0 | 0.75 ± 0.06 | 0.75 ± 0.06 | −0.017 ± 0.018 | 9 (5.9) | 2 (11.1) | 192.6 (151.3, 260.7) |
| 33 | Munkelva | 69.6489 | 29.4597 | Parr | 93 | 86 | 220 | 9.68 | 0 | 0.75 ± 0.06 | 0.75 ± 0.06 | −0.001 ± 0.018 | 5 (3.3) | 1 (5.6) | 315.4 (228.8, 493.2) |
| 34 | Neiden | 69.7006 | 29.5208 | Parr | 94 | 72 | 227 | 10.20 | 0 | 0.76 ± 0.06 | 0.75 ± 0.06 | −0.017 ± 0.013 | 17 (11.1) | 3 (16.7) | 255.7 (196.6, 359.3) |
| 35 | Klokkarelva | 69.8580 | 29.3867 | Parr | 94 | 88 | 223 | 9.75 | 0 | 0.75 ± 0.06 | 0.75 ± 0.06 | 0.000 ± 0.018 | 10 (6.5) | 1 (5.6) | 514.4 (331, 1,093.4) |
| 36 | Vesterelva | 70.1587 | 28.5804 | Parr | 93 | 83 | 220 | 9.47 | 0 | 0.74 ± 0.06 | 0.74 ± 0.06 | −0.009 ± 0.013 | 5 (3.3) | 1 (5.6) | 126.7 (105.5, 156.4) |
| 37 | Bergebyelva | 70.1503 | 28.8985 | Parr | 107 | 87 | 206 | 9.07 | 0 | 0.77 ± 0.05 | 0.76 ± 0.05 | −0.028 ± 0.013 | 6 (3.9) | 0 | 185 (152.4, 232.4) |
| 38 | Vestre Jakobselv | 70.1092 | 29.3285 | Parr | 78 | 72 | 215 | 9.49 | 0 | 0.77 ± 0.05 | 0.75 ± 0.05 | −0.041 ± 0.014 | 8 (5.2) | 2 (11.1) | 149.7 (118.6, 199.2) |
| 39 | Skallelva | 70.1856 | 30.3284 | Parr | 94 | 90 | 214 | 9.24 | 1 | 0.73 ± 0.06 | 0.75 ± 0.06 | 0.011 ± 0.010 | 11 (7.2) | 2 (11.1) | 258.1 (197.6, 364.7) |
| 40 | Komagelva | 70.2422 | 30.5232 | Parr | 94 | 78 | 192 | 8.61 | 0 | 0.71 ± 0.06 | 0.70 ± 0.06 | −0.016 ± 0.013 | 10 (6.5) | 0 | 248.8 (170.3, 437.5) |
| 41 | Syltefjordelva | 70.5311 | 30.0115 | Parr | 93 | 83 | 193 | 8.18 | 0 | 0.70 ± 0.06 | 0.71 ± 0.05 | 0.021 ± 0.018 | 15 (9.8) | 3 (16.7) | 167.8 (129.4, 232.8) |
| 42 | Kongsfjordelva | 70.6570 | 29.2569 | Parr | 91 | 82 | 204 | 8.88 | 0 | 0.72 ± 0.06 | 0.73 ± 0.06 | 0.011 ± 0.017 | 15 (9.8) | 1 (5.6) | 280.2 (193.1, 486.8) |
| 43 | Tana‐Iesjohka | 69.4200 | 24.7499 | Parr | 94 | 78 | 217 | 9.52 | 0 | 0.76 ± 0.07 | 0.73 ± 0.07 | −0.021 ± 0.018 | 10 (6.5) | 2 (11.1) | 291 (205.2, 482.2) |
| 44 | Tana‐Laksjohka | 70.0651 | 27.5524 | Parr | 92 | 83 | 235 | 9.86 | 0 | 0.72 ± 0.06 | 0.73 ± 0.06 | 0.008 ± 0.010 | 15 (9.8) | 3 (16.7) | 667.9 (389.4, 2,119.1) |
| 45 | Langfjordelva | 70.6179 | 27.6089 | Parr | 93 | 65 | 217 | 9.34 | 0 | 0.74 ± 0.05 | 0.75 ± 0.05 | 0.007 ± 0.013 | 13 (8.5) | 4 (22.2) | 79.2 (66.7, 96.1) |
| 46 | Børselva | 70.3121 | 25.5208 | Parr | 92 | 83 | 236 | 9.98 | 0 | 0.76 ± 0.06 | 0.75 ± 0.06 | −0.014 ± 0.019 | 15 (9.8) | 5 (27.8) | 174.6 (141.9, 223.7) |
| 47 | Lakselva | 70.0825 | 24.9202 | Parr | 94 | 90 | 211 | 8.83 | 0 | 0.72 ± 0.06 | 0.71 ± 0.06 | −0.012 ± 0.013 | 10 (6.5) | 3 (16.7) | 1,155.9 (461.1, Infinite) |
| 48 | Stabburselva | 70.1846 | 24.9336 | Parr | 89 | 79 | 207 | 9.18 | 0 | 0.74 ± 0.06 | 0.72 ± 0.06 | −0.032 ± 0.014 | 13 (8.5) | 1 (5.6) | 185.7 (137.1, 278.1) |
| 49 | Repparfjordselva | 70.4480 | 24.3223 | Parr | 92 | 90 | 247 | 10.23 | 2 | 0.76 ± 0.06 | 0.75 ± 0.06 | −0.028 ± 0.015 | 11 (7.2) | 3 (16.7) | 417.8 (292.5, 708.1) |
| 50 | Alta | 69.9691 | 23.3752 | Parr | 85 | 84 | 220 | 9.40 | 1 | 0.79 ± 0.06 | 0.73 ± 0.06 | −0.002 ± 0.011 | 7 (4.6) | 1 (5.6) | 2,941.8 (659.4, Infinite) |
| 51 | Reisa | 69.7837 | 21.0095 | Parr | 64 | 61 | 196 | 9.02 | 0 | 0.71 ± 0.06 | 0.72 ± 0.06 | 0.001 ± 0.015 | 10 (6.5) | 2 (11.1) | 843.9 (293.9, Infinite) |
| 52 | Målselv | 69.2744 | 18.5146 | Parr | 82 | 77 | 227 | 9.92 | 1 | 0.73 ± 0.06 | 0.76 ± 0.06 | 0.032 ± 0.017 | 5 (3.3) | 1 (5.6) | 3,147.2 (469.8, Infinite) |
| 53 | Laukhelle | 69.2287 | 17.8531 | Parr | 87 | 72 | 232 | 10.09 | 0 | 0.77 ± 0.06 | 0.76 ± 0.06 | −0.026 ± 0.012 | 10 (6.5) | 1 (5.6) | 432.6 (289.5, 823.8) |
| 54 | Roksdalsvassdraget | 69.0502 | 15.8692 | Parr | 60 | 58 | 207 | 9.43 | 0 | 0.75 ± 0.05 | 0.75 ± 0.05 | −0.006 ± 0.014 | 5 (3.3) | 1 (5.6) | 140.6 (110, 190.9) |
| 55 | Alvsvågvassdraget | 68.9168 | 15.2343 | Parr | 57 | 45 | 206 | 9.85 | 0 | 0.79 ± 0.05 | 0.77 ± 0.05 | −0.040 ± 0.019 | 11 (7.2) | 3 (16.7) | 92.1 (75.8, 115.9) |
| 56 | Gårdselva | 68.8300 | 15.6572 | Parr | 104 | 88 | 241 | 10.14 | 0 | 0.78 ± 0.05 | 0.77 ± 0.05 | −0.017 ± 0.011 | 7 (4.6) | 2 (11.1) | 315.5 (228.6, 494.8) |
| 57 | Saltdalselva | 67.1023 | 15.4224 | Parr | 71 | 52 | 218 | 10.18 | 0 | 0.78 ± 0.05 | 0.77 ± 0.05 | −0.033 ± 0.014 | 9 (5.9) | 1 (5.6) | 291.2 (190.3, 590) |
| 58 | Beiarelva | 67.0302 | 14.5743 | Parr | 70 | 69 | 222 | 10.05 | 1 | 0.77 ± 0.06 | 0.76 ± 0.06 | −0.013 ± 0.014 | 9 (5.9) | 1 (5.6) | 139.2 (194.8, Infinite) |
| 59 | Åbjøra | 65.0784 | 12.4556 | Parr | 44 | 88 | 248 | 10.47 | 0 | 0.76 ± 0.05 | 0.78 ± 0.05 | 0.007 ± 0.017 | 13 (8.5) | 1 (5.6) | 101.2 (124.1, Infinite) |
| 60 | Namsen | 64.4657 | 11.5448 | Parr | 91 | 85 | 244 | 10.22 | 0 | 0.75 ± 0.05 | 0.76 ± 0.05 | 0.001 ± 0.009 | 10 (6.5) | 2 (11.1) | 583 (327.8, 2,229.2) |
| 61 | Bogna | 64.3880 | 11.3947 | Parr | 104 | 97 | 240 | 10.07 | 0 | 0.77 ± 0.05 | 0.77 ± 0.05 | −0.011 ± 0.011 | 13 (8.5) | 3 (16.7) | 696.6 (423.6, 1816.6) |
| 62 | Årgårdsvassdraget | 64.3127 | 11.2237 | Parr | 94 | 87 | 236 | 10.12 | 2 | 0.76 ± 0.05 | 0.77 ± 0.05 | 0.012 ± 0.014 | 10 (6.5) | 6 (33.3) | 959.8 (429.7, Infinite) |
| 63 | Steinsdalselva | 64.2977 | 10.5034 | Parr | 93 | 70 | 228 | 10.07 | 0 | 0.75 ± 0.06 | 0.76 ± 0.05 | 0.008 ± 0.017 | 6 (3.9) | 1 (5.6) | 298.2 (213, 483.1) |
| 64 | Nordelva | 63.9615 | 10.2221 | Parr | 34 | 33 | 199 | 10.03 | 0 | 0.75 ± 0.06 | 0.75 ± 0.05 | −0.019 ± 0.018 | 3 (2.0) | 1 (5.6) | 719.5 (759.9, Infinite) |
| 65 | Stordalselva | 63.9594 | 10.2269 | Parr | 68 | 65 | 226 | 10.17 | 0 | 0.77 ± 0.06 | 0.76 ± 0.06 | −0.023 ± 0.014 | 9 (5.9) | 2 (11.1) | 2,494.4 (655, Infinite) |
| 66 | Skauga | 63.5931 | 9.9195 | Parr | 71 | 63 | 219 | 9.87 | 0 | 0.77 ± 0.06 | 0.77 ± 0.05 | −0.008 ± 0.016 | 13 (8.5) | 1 (5.6) | 403 (251.2, 950.1) |
| 67 | Verdalselva | 63.8033 | 11.4591 | Parr | 95 | 79 | 233 | 10.03 | 1 | 0.76 ± 0.06 | 0.75 ± 0.06 | −0.015 ± 0.010 | 11 (7.2) | 1 (5.6) | 400.2 (267, 764) |
| 68 | Levangerelva | 63.7530 | 11.2990 | Parr | 83 | 75 | 202 | 9.08 | 0 | 0.76 ± 0.05 | 0.76 ± 0.05 | −0.013 ± 0.011 | 8 (5.2) | 4 (22.2) | 301.5 (220.8, 463.9) |
| 69 | Stjørdalselva | 63.4489 | 10.9039 | Parr | 92 | 84 | 239 | 10.33 | 0 | 0.77 ± 0.05 | 0.77 ± 0.05 | −0.013 ± 0.008 | 13 (8.5) | 1 (5.6) | 594.6 (364.4, 1504.6) |
| 70 | Homla | 63.4145 | 10.8023 | Parr | 93 | 87 | 222 | 9.56 | 0 | 0.75 ± 0.05 | 0.75 ± 0.05 | −0.012 ± 0.012 | 10 (6.5) | 0 | 314.1 (230.6, 479.4) |
| 71 | Nidelva | 63.4431 | 10.4146 | Parr | 82 | 73 | 234 | 10.14 | 0 | 0.76 ± 0.06 | 0.75 ± 0.06 | −0.021 ± 0.017 | 8 (5.2) | 1 (5.6) | 330.2 (231, 560.4) |
| 72 | Gaula | 63.3429 | 10.2286 | Parr | 93 | 90 | 244 | 10.25 | 0 | 0.77 ± 0.06 | 0.77 ± 0.05 | −0.010 ± 0.014 | 6 (3.9) | 1 (5.6) | 2,508.1 (672.2, Infinite) |
| 73 | Vigda | 63.3124 | 10.1824 | Parr | 85 | 82 | 213 | 9.20 | 0 | 0.74 ± 0.06 | 0.75 ± 0.06 | 0.014 ± 0.014 | 7 (4.6) | 2 (11.1) | 1,142.1 (494.8, Infinite) |
| 74 | Børsa | 63.3252 | 10.0759 | Parr | 46 | 40 | 191 | 9.47 | 0 | 0.74 ± 0.06 | 0.75 ± 0.05 | 0.008 ± 0.017 | 12 (7.8) | 1 (5.6) | 416.4 (206.1, 13,513.3) |
| 75 | Orkla | 63.3101 | 9.8303 | Parr | 104 | 101 | 253 | 10.27 | 0 | 0.76 ± 0.06 | 0.76 ± 0.05 | −0.003 ± 0.010 | 13 (8.5) | 2 (11.1) | 1,328.8 (633, Infinite) |
| 76 | Surna | 62.9706 | 8.6501 | Parr | 79 | 79 | 236 | 10.13 | 0 | 0.76 ± 0.06 | 0.77 ± 0.05 | 0.002 ± 0.010 | 18 (11.8) | 3 (16.7) | 2,769 (648.7, Infinite) |
| 77 | Eiravassdraget | 62.6851 | 8.1306 | Parr | 81 | 73 | 230 | 10.16 | 0 | 0.78 ± 0.06 | 0.77 ± 0.06 | −0.021 ± 0.009 | 17 (11.1) | 1 (5.6) | 162.5 (133.9, 204.3) |
| 78 | Visa | 62.7229 | 7.9266 | Parr | 36 | 35 | 194 | 9.92 | 0 | 0.75 ± 0.05 | 0.77 ± 0.05 | 0.008 ± 0.016 | 10 (6.5) | 0 | 128.6 (87.7, 229.2) |
| 79 | Korsbrekkeelva | 62.0818 | 6.8758 | Parr | 45 | 44 | 220 | 10.40 | 0 | 0.76 ± 0.06 | 0.77 ± 0.06 | 0.014 ± 0.016 | 5 (3.3) | 3 (16.7) | 234.8 (162.6, 409.1) |
| 80 | Ørstaelva | 62.1959 | 6.1241 | Adult | 36 | 34 | 207 | 10.45 | 0 | 0.77 ± 0.05 | 0.78 ± 0.05 | −0.009 ± 0.019 | 14 (9.2) | 0 | 3,016.6 (371, Infinite) |
| 81 | Ervikelva | 62.1648 | 5.1103 | Parr | 61 | 60 | 218 | 10.17 | 0 | 0.77 ± 0.05 | 0.79 ± 0.05 | 0.010 ± 0.010 | 11 (7.2) | 1 (5.6) | 573 (296.7, 5,108.4) |
| 82 | Eidselva | 61.9020 | 5.9846 | Adult | 104 | 103 | 259 | 10.35 | 0 | 0.75 ± 0.05 | 0.77 ± 0.05 | 0.014 ± 0.008 | 9 (5.9) | 5 (27.8) | 670.3 (414.9, 1626.9) |
| 83 | Stryneelva | 61.9018 | 6.7172 | Parr | 73 | 68 | 225 | 10.08 | 1 | 0.78 ± 0.05 | 0.78 ± 0.05 | −0.013 ± 0.017 | 5 (3.3) | 0 | 800.1 (401.5, 16,315) |
| 84 | Gjengedalsvassdraget | 61.7328 | 5.9173 | Parr | 63 | 58 | 225 | 10.23 | 0 | 0.76 ± 0.05 | 0.77 ± 0.05 | 0.011 ± 0.014 | 16 (10.5) | 3 (16.7) | 449.2 (10,713.6, Infinite) |
| 85 | Osenelva | 61.5512 | 5.3997 | Parr | 94 | 77 | 222 | 9.80 | 0 | 0.78 ± 0.05 | 0.77 ± 0.05 | −0.026 ± 0.015 | 14 (9.2) | 1 (5.6) | 296 (217, 454.6) |
| 86 | Nausta | 61.5061 | 5.7198 | Parr | 74 | 73 | 231 | 10.29 | 0 | 0.76 ± 0.06 | 0.78 ± 0.05 | 0.018 ± 0.017 | 11 (7.2) | 3 (16.7) | 1,315.7 (434.2, Infinite) |
| 87 | Jølstra | 61.4581 | 5.8306 | Parr | 91 | 84 | 255 | 10.70 | 0 | 0.78 ± 0.05 | 0.78 ± 0.05 | −0.002 ± 0.010 | 7 (4.6) | 1 (5.6) | 373 (260.8, 632.5) |
| 88 | Gaula, Sunnfjord | 61.3681 | 5.6739 | Parr | 63 | 60 | 216 | 10.09 | 0 | 0.75 ± 0.05 | 0.76 ± 0.05 | 0.010 ± 0.009 | 16 (10.5) | 1 (5.6) | 565.5 (1503.7, Infinite) |
| 89 | Flekkeelva | 61.3112 | 5.3452 | Parr | 93 | 90 | 188 | 8.16 | 0 | 0.72 ± 0.05 | 0.72 ± 0.05 | −0.002 ± 0.011 | 12 (7.8) | 4 (22.2) | 577.9 (319.6, 2,422.4) |
| 90 | Daleelva,Høyanger | 61.2158 | 6.0731 | Adult | 104 | 98 | 252 | 10.36 | 0 | 0.76 ± 0.05 | 0.78 ± 0.05 | 0.013 ± 0.007 | 9 (5.9) | 4 (22.2) | 504 (338.8, 944.3) |
| 91 | Årøyelva | 61.2685 | 7.1664 | Adult | 102 | 81 | 213 | 9.78 | 1 | 0.75 ± 0.06 | 0.76 ± 0.06 | 0.006 ± 0.014 | 14 (9.2) | 4 (22.2) | 170.9 (620, Infinite) |
| 92 | Flåmselva | 60.8649 | 7.1191 | Parr | 81 | 74 | 217 | 9.52 | 0 | 0.76 ± 0.05 | 0.75 ± 0.05 | −0.025 ± 0.012 | 19 (12.4) | 1 (5.6) | 269.7 (167.7, 626.5) |
| 93 | Nærøydalselva | 60.8808 | 6.8430 | Adult | 68 | 57 | 200 | 9.46 | 0 | 0.76 ± 0.06 | 0.76 ± 0.05 | −0.017 ± 0.016 | 5 (3.3) | 2 (11.1) | 162.1 (120.8, 239.4) |
| 94 | Vikja | 61.0897 | 6.5857 | Parr | 64 | 64 | 244 | 10.67 | 0 | 0.78 ± 0.05 | 0.78 ± 0.05 | 0.001 ± 0.010 | 4 (2.6) | 1 (5.6) | 3,062.8 (630.4, Infinite) |
| 95 | Loneelva | 60.5257 | 5.4895 | Parr | 85 | 79 | 213 | 9.45 | 1 | 0.77 ± 0.05 | 0.77 ± 0.05 | −0.008 ± 0.017 | 7 (4.6) | 2 (11.1) | 576 (330.9, 1965.6) |
| 96 | Oselva | 60.1836 | 5.4707 | Parr | 92 | 84 | 227 | 9.93 | 1 | 0.76 ± 0.05 | 0.77 ± 0.05 | 0.003 ± 0.017 | 21 (13.7) | 1 (5.6) | 206.2 (164, 273.1) |
| 97 | Etneelva | 59.6730 | 5.9342 | Parr | 93 | 83 | 233 | 10.00 | 0 | 0.77 ± 0.06 | 0.76 ± 0.06 | −0.012 ± 0.010 | 11 (7.2) | 2 (11.1) | 267.8 (192.2, 426.3) |
| 98 | Vikedalselva | 59.4969 | 5.8971 | Parr | 92 | 82 | 222 | 9.87 | 0 | 0.77 ± 0.06 | 0.76 ± 0.05 | −0.010 ± 0.011 | 14 (9.2) | 2 (11.1) | 189.6 (153.9, 243.4) |
| 99 | Suldalslågen | 59.4811 | 6.2488 | Parr | 91 | 86 | 227 | 9.86 | 1 | 0.74 ± 0.05 | 0.75 ± 0.05 | 0.000 ± 0.016 | 11 (7.2) | 1 (5.6) | 295.3 (223.2, 427.7) |
| 100 | Vormo | 59.2717 | 6.3326 | Parr | 94 | 94 | 238 | 10.16 | 0 | 0.78 ± 0.06 | 0.77 ± 0.05 | −0.014 ± 0.013 | 8 (5.2) | 5 (27.8) | 128.1 (111, 150.2) |
| 101 | Årdalselva | 59.1438 | 6.1694 | Parr | 93 | 89 | 240 | 10.34 | 0 | 0.77 ± 0.05 | 0.77 ± 0.05 | 0.004 ± 0.011 | 18 (11.8) | 0 | 395.7 (279.2, 658.1) |
| 102 | Lyseelva | 59.0517 | 6.6452 | Parr | 94 | 67 | 225 | 10.12 | 0 | 0.76 ± 0.05 | 0.76 ± 0.05 | −0.015 ± 0.009 | 13 (8.5) | 1 (5.6) | 130.9 (107.7, 164.6) |
| 103 | Espedalsvassdraget | 58.8602 | 6.1504 | Parr | 94 | 84 | 236 | 10.41 | 0 | 0.75 ± 0.05 | 0.77 ± 0.05 | 0.019 ± 0.010 | 8 (5.2) | 5 (27.8) | 325.5 (234.7, 515.8) |
| 104 | Frafjordselva | 58.8435 | 6.2799 | Parr | 87 | 76 | 245 | 10.64 | 0 | 0.77 ± 0.06 | 0.77 ± 0.05 | −0.001 ± 0.017 | 6 (3.9) | 0 | 466.8 (303.7, 961.1) |
| 105 | Figgjo | 58.8100 | 5.5468 | Parr | 93 | 89 | 247 | 10.60 | 0 | 0.77 ± 0.05 | 0.78 ± 0.05 | −0.004 ± 0.015 | 10 (6.5) | 2 (11.1) | 662.4 (401.5, 1756.5) |
| 106 | Håelva | 58.6695 | 5.5438 | Parr | 63 | 61 | 232 | 10.32 | 3 | 0.77 ± 0.06 | 0.77 ± 0.05 | −0.020 ± 0.009 | 7 (4.6) | 1 (5.6) | 544.9 (305.9, 2,162.8) |
| 107 | Ogna | 58.5169 | 5.7920 | Parr | 60 | 56 | 222 | 10.06 | 1 | 0.77 ± 0.05 | 0.77 ± 0.05 | −0.019 ± 0.014 | 12 (7.8) | 3 (16.7) | 147.2 (113.8, 204) |
| 108 | Bjerkreimselva | 58.4779 | 5.9949 | Parr | 83 | 83 | 219 | 9.62 | 0 | 0.77 ± 0.06 | 0.76 ± 0.05 | −0.016 ± 0.010 | 11 (7.2) | 3 (16.7) | 1917 (1,440.2, Infinite) |
| 109 | Soknedalselva | 58.3214 | 6.2857 | Parr | 24 | 24 | 190 | 10.46 | 1 | 0.79 ± 0.05 | 0.78 ± 0.05 | −0.044 ± 0.020 | 3 (2.0) | 679.8 (570.8, Infinite) | |
| 110 | Kvina | 58.2730 | 6.8910 | Parr | 91 | 90 | 240 | 10.14 | 1 | 0.77 ± 0.05 | 0.77 ± 0.05 | −0.005 ± 0.012 | 19 (12.4) | 2 (11.1) | 407.9 (289.6, 670.3) |
| 111 | Mandalselva | 58.0203 | 7.4563 | Parr | 45 | 43 | 211 | 10.12 | 0 | 0.76 ± 0.06 | 0.765 ± 0.06 | −0.006 ± 0.011 | 8 (5.2) | 1 (5.6) | 814.1 (321, Infinite) |
| 112 | Otra | 58.1441 | 8.0132 | Parr | 76 | 71 | 246 | 10.76 | 1 | 0.78 ± 0.06 | 0.78 ± 0.05 | −0.007 ± 0.017 | 8 (5.2) | 4 (22.2) | 958.5 (470.1, 4,113,951.3) |
| 113 | Storelva | 58.7607 | 9.0766 | Smolt | 79 | 75 | 229 | 10.10 | 0 | 0.75 ± 0.05 | 0.76 ± 0.06 | −0.006 ± 0.018 | 12 (7.8) | 4 (22.2) | 242.6 (181.4, 357.5) |
| 114 | Numedalslågen | 59.0378 | 10.0553 | Parr | 84 | 83 | 238 | 10.10 | 4 | 0.73 ± 0.05 | 0.75 ± 0.05 | 0.013 ± 0.020 | 10 (6.5) | 5 (27.8) | 3,001 (695.8, Infinite) |
| 115 | Ennigdalselva | 58.9818 | 11.4746 | Adult | 94 | 86 | 192 | 8.21 | 2 | 0.71 ± 0.05 | 0.72 ± 0.06 | 0.005 ± 0.020 | 13 (8.5) | 2 (11.1) | 368.4 (221.1, 973.4) |
Wennevik V, Quintela M, Skaala Ø, Verspoor E, Prusov S, Glover KA. Population genetic analysis reveals a geographically limited transition zone between two genetically distinct Atlantic salmon lineages in Norway. Ecol Evol. 2019;9:6901–6921. 10.1002/ece3.5258
Funding information
This work was funded by the Norwegian Directorate for Nature Management and the Ministry of Trade and Fisheries.
Data Availability Statement: Appendices S1–S3 with data and supporting information, including the entire raw data set of individual genetic profiles for >9,000 salmon can be downloaded via DRYAD entry https://doi.org/10.21335/NMDC-290855015.
DATA ACCESSIBILITY
Appendices S1–S3 with data and supporting information, including the entire raw data set of individual genetic profiles for >9,000 salmon can be downloaded from the Norwegian Marine Data Centre (NMDC): https://doi.org/10.21335/NMDC-290855015.
REFERENCES
- Allendorf, F. W. , & Phelps, S. R. (1981). Use of allelic frequencies to describe population structure. Canadian Journal of Fisheries and Aquatic Sciences, 38(12), 1507–1514. 10.1139/f81-203 [DOI] [Google Scholar]
- Antao, T. , Lopes, A. , Lopes, R. , Beja‐Pereira, A. , & Luikart, G. (2008). LOSITAN: A workbench to detect molecular adaptation based on a FST‐outlier method. BMC Bioinformatics, 9(1), 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund, T. , Veselov, A. , Primmer, C. R. , Bakhmet, I. , Potutkin, A. , Titov, S. , … Lumme, J. (2004). Geographical structure and postglacial history of mtDNA haplotype variation in Atlantic salmon (Salmo salar L.) among rivers of the White and Barents Sea basins. Annales Zoologici Fennici, 41(3), 465–475. [Google Scholar]
- Beaumont, M. A. , & Nichols, R. A. (1996). Evaluating loci for use in the genetic analysis of population structure. Proceedings of the Royal Society of London Series B. Biological Sciences, 263(1377), 1619–1626. [Google Scholar]
- Berg, L. S. (1948). Fishes of freshwaters in the USSR and neighbouring countries. Moscow, Russia: Academy of Sciences Press. [Google Scholar]
- Besnier, F. , & Glover, K. A. (2013). ParallelStructure: A R package to distribute parallel runs of the population genetics program Strcuture on multi‐core computers. PLoS ONE, 8(7), e70651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke, E. A. , Coughlan, J. , Jansson, H. , Galvin, P. , & Cross, T. F. (1997). Allozyme variation in populations of Atlantic salmon located throughout Europe: Diversity that could be compromised by introductions of reared fish. ICES Journal of Marine Science, 54(6), 974–985. 10.1016/S1054-3139(97)80002-1 [DOI] [Google Scholar]
- Bourret, V. , Kent, M. P. , Primmer, C. R. , Vasemagi, A. , Karlsson, S. , Hindar, K. , … Lien, S. (2013). SNP‐array reveals genome‐wide patterns of geographical and potential adaptive divergence across the natural range of Atlantic salmon (Salmo salar). Molecular Ecology, 22(3), 532–551. [DOI] [PubMed] [Google Scholar]
- Bradbury, I. R. , Hamilton, L. C. , Dempson, B. , Robertson, M. J. , Bourret, V. , Bernatchez, L. , & Verspoor, E. (2015). Transatlantic secondary contact in Atlantic Salmon, comparing microsatellites, a single nucleotide polymorphism array and restriction‐site associated DNA sequencing for the resolution of complex spatial structure. Molecular Ecology, 24(20), 5130–5144. 10.1111/mec.13395 [DOI] [PubMed] [Google Scholar]
- Bradbury, I. R. , Hamilton, L. C. , Sheehan, T. F. , Chaput, G. , Robertson, M. J. , Dempson, J. B. , … Bernatchez, L. (2016). Genetic mixed‐stock analysis disentangles spatial and temporal variation in composition of the West Greenland Atlantic Salmon fishery. ICES Journal of Marine Science, 73(9), 2311–2321. 10.1093/icesjms/fsw072 [DOI] [Google Scholar]
- Brenna‐Hansen, S. , Li, J. Y. , Kent, M. P. , Boulding, E. G. , Dominik, S. , Davidson, W. S. , & Lien, S. (2012). Chromosomal differences between European and North American Atlantic salmon discovered by linkage mapping and supported by fluorescence in situ hybridization analysis. BMC Genomics, 13, 432 10.1186/1471-2164-13-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauwelier, E. , Verspoor, E. , Coulson, M. W. , Armstrong, A. , Knox, D. , Stradmeyer, L. , … Gilbey, J. (2018). Ice sheets and genetics: Insights into the phylogeography of Scottish Atlantic salmon, Salmo salar L. Journal of Biogeography, 45(1), 51–63. [Google Scholar]
- Chittenden, C. M. , Adlandsvik, B. , Pedersen, O. P. , Righton, D. , & Rikardsen, A. H. (2013). Testing a model to track fish migrations in polar regions using pop‐up satellite archival tags. Fisheries Oceanography, 22(1), 6901–13. 10.1111/fog.12000 [DOI] [Google Scholar]
- Derryberry, E. P. , Derryberry, G. E. , Maley, J. M. , & Brumfield, R. T. (2014). HZAR: Hybrid zone analysis using an R software package. Molecular Ecology Resources, 14(3), 652–663. [DOI] [PubMed] [Google Scholar]
- Di Rienzo, A. , Peterson, A. C. , Garza, J. C. , Valdes, A. M. , Slatkin, M. , & Freimer, N. B. (1994). Mutational processes of simple‐sequence repeat loci in human populations. Proceedings of the National Academy of Sciences of the United States of America, 91(8), 3166–3170. 10.1073/pnas.91.8.3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieringer, D. , & Schlötterer, C. (2003). MICROSATELLITE ANALYSER (MSA): A platform independent analysis tool for large microsatellite data sets. Molecular Ecology Notes, 3(1), 167–169. [Google Scholar]
- Dillane, E. , Cross, M. C. , McGinnity, P. , Coughlan, J. P. , Galvin, P. T. , Wilkins, N. P. , & Cross, T. F. (2007). Spatial and temporal patterns in microsatellite DNA variation of wild Atlantic salmon, Salmo salar, in Irish Rivers. Fisheries Management and Ecology, 14(3), 209–219. 10.1111/j.1365-2400.2007.00544.x [DOI] [Google Scholar]
- Dillane, E. , McGinnity, P. , Coughlan, J. P. , Cross, M. C. , de Eyto, E. , Kenchington, E. , … Cross, T. F. (2008. ). Demographics and landscape features determine intrariver population structure in Atlantic salmon (Salmo salar L.): the case of the River Moy in Ireland. Molecular Ecology, 17(22), 4786–4800. [DOI] [PubMed] [Google Scholar]
- Dionne, M. , Caron, F. , Dodson, J. J. , & Bernatchez, L. (2009). Comparative survey of within‐river genetic structure in Atlantic salmon; relevance for management and conservation. Conservation Genetics, 10(4), 869–879. 10.1007/s10592-008-9647-5 [DOI] [Google Scholar]
- Dionne, M. , Miller, K. M. , Dodson, J. J. , Caron, F. , & Bernatchez, L. (2007). Clinal variation in MHC diversity with temperature: Evidence for the role of host‐pathogen interaction on local adaptation in Atlantic salmon. Evolution, 61(9), 2154–2164. 10.1111/j.1558-5646.2007.00178.x [DOI] [PubMed] [Google Scholar]
- Evanno, G. , Regnaut, S. , & Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Molecular Ecology, 14(8), 2611–2620. 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- Excoffier, L. , Hofer, T. , & Foll, M. (2009). Detecting loci under selection in a hierarchically structured population. Heredity, 103(4), 285–298. 10.1038/hdy.2009.74 [DOI] [PubMed] [Google Scholar]
- Excoffier, L. , Laval, G. , & Schneider, S. (2005). Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online, 1, 47–50. [PMC free article] [PubMed] [Google Scholar]
- Excoffier, L. , & Lischer, H. E. L. (2010). Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10(3), 564–567. [DOI] [PubMed] [Google Scholar]
- Forseth, T. , Barlaup, B. T. , Finstad, B. , Fiske, P. , Gjoaester, H. , Falkegard, M. , … Wennevik, V. (2017). The major threats to Atlantic salmon in Norway. ICES Journal of Marine Science, 74(6), 1496–1513. 10.1093/icesjms/fsx020 [DOI] [Google Scholar]
- Garcia de Leaniz, C. , Fleming, I. A. , Einum, S. , Verspoor, E. , Jordan, W. C. , Consuegra, S. , … Quinn, T. P. (2007). A critical review of adaptive genetic variation in Atlantic salmon: Implications for conservation. Biological Reviews, 82(2), 173–211. 10.1111/j.1469-185X.2006.00004.x [DOI] [PubMed] [Google Scholar]
- Gay, L. , Crochet, P. A. , Bell, D. A. , & Lenormand, T. (2008). Comparing clines on molecular and phenotypic traits in hybrid zones: A window on tension zone models. Evolution, 62(11), 2789–2806. 10.1111/j.1558-5646.2008.00491.x [DOI] [PubMed] [Google Scholar]
- Gilbey, J. , Knox, D. , O'Sullivan, M. , & Verspoor, E. (2005). Novel DNA markers for rapid, accurate, and cost‐effective discrimination of the continental origin of Atlantic salmon (Salmo salar L.). ICES Journal of Marine Science, 62(8), 1609–1616. [Google Scholar]
- Gilbey, J. , Verspoor, E. , & Summers, D. (1999). Size and MEP‐2* variation in juvenile Atlantic salmon (Salmo salar) in the River North Esk, Scotland. Aquatic Living Resources, 12(4), 295–299. 10.1016/S0990-7440(00)86641-3 [DOI] [Google Scholar]
- Gilbey, J. , Wennevik, V. , Bradbury, I. R. , Fiske, P. , Hansen, L. P. , Jacobsen, J. A. , & Potter, T. (2017). Genetic stock identification of Atlantic salmon caught in the Faroese fishery. Fisheries Research, 187, 110–119. 10.1016/j.fishres.2016.11.020 [DOI] [Google Scholar]
- Glover, K. A. (2010). Forensic identification of fish farm escapees: The Norwegian experience. Aquaculture Environment Interactions, 1, 6901–10. 10.3354/aei00002 [DOI] [Google Scholar]
- Glover, K. A. , Madhun, A. S. , Dahle, G. , Sorvik, A. G. E. , Wennevik, V. , Skaala, O. , … Fjelldal, P. G. (2015). The frequency of spontaneous triploidy in farmed Atlantic salmon produced in Norway during the period 2007–2014. BMC Genetics, 16, 37 10.1186/s12863-015-0193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, K. A. , Pertoldi, C. , Besnier, F. , Wennevik, V. , Kent, M. , & Skaala, Ø. (2013). Atlantic salmon populations invaded by farmed escapees: Quantifying genetic introgression with a Bayesian approach and SNPs. BMC Genetics, 14, 4 10.1186/1471-2156-14-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, K. A. , Quintela, M. , Wennevik, V. , Besnier, F. , Sørvik, A. G. E. , & Skaala, O. (2012). Three decades of farmed escapees in the wild: A spatio‐temporal analysis of population genetic structure throughout Norway. PLoS ONE, 7(8), e43129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, K. A. , Skilbrei, O. T. , & Skaala, O. (2008). Genetic assignment identifies farm of origin for Atlantic salmon Salmo salar escapees in a Norwegian fjord. ICES Journal of Marine Science, 65(6), 912–920. 10.1093/icesjms/fsn056 [DOI] [Google Scholar]
- Glover, K. A. , Solberg, M. F. , McGinnity, P. , Hindar, K. , Verspoor, E. , Coulson, M. W. , … Svåsand, T. (2017). Half a century of genetic interaction between farmed and wild Atlantic salmon: Status of knowledge and unanswered questions. Fish and Fisheries, 18(5), 890–927. 10.1111/faf.12214 [DOI] [Google Scholar]
- Griffiths, A. M. , Machado‐Schiaffino, G. , Dillane, E. , Coughlan, J. , Horreo, J. L. , Bowkett, A. E. , … Stevens, J. R. (2010). Genetic stock identification of Atlantic salmon (Salmo salar) populations in the southern part of the European range. BMC Genetics, 11(1), 31 10.1186/1471-2156-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimholt, U. , Drabløs, F. , Jørgensen, S. , Høyheim, B. , & Stet, R. J. (2002). The major histocompatibility class I locus in Atlantic salmon (Salmo salar L.): polymorphism, linkage analysis and protein modelling. Immunogenetics, 54(8), 570–581. [DOI] [PubMed] [Google Scholar]
- Gudjonsson, S. , Einarsson, S. M. , Jonsson, I. R. , & Gudbrandsson, J. (2015). Marine feeding areas and vertical movements of Atlantic salmon (Salmo salar) as inferred from recoveries of data storage tags. Canadian Journal of Fisheries and Aquatic Sciences, 72(7), 1087–1098. [Google Scholar]
- Harvey, A. C. , Glover, K. A. , Taylor, M. I. , Creer, S. , & Carvalho, G. R. (2016). A common garden design reveals population‐specific variability in potential impacts of hybridization between populations of farmed and wild Atlantic salmon, Salmo salar L. Evolutionary Applications, 9(3), 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70. [Google Scholar]
- Hughes, A. L. C. , Gyllencreutz, R. , Lohne, O. S. , Mangerud, J. , & Svendsen, J. I. (2016). The last Eurasian ice sheets ‐ a chronological database and time‐slice reconstruction, DATED‐1. Boreas, 45(1), 6901–45. [Google Scholar]
- Jakobsson, M. , & Rosenberg, N. A. (2007). CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics, 23(14), 1801–1806. 10.1093/bioinformatics/btm233 [DOI] [PubMed] [Google Scholar]
- Jeffery, N. W. , Stanley, R. R. E. , Wringe, B. F. , Guijarro‐Sabaniel, J. , Bourret, V. , Bernatchez, L. , … Bradbury, I. R. (2017). Range‐wide parallel climate‐associated genomic clines in Atlantic salmon. Royal Society Open Science, 4(11), 12 10.1098/rsos.171394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, A. J. , Fiske, P. , Hansen, L. P. , Johnsen, B. O. , Mork, K. A. , & Næsje, T. F. (2011). Synchrony in marine growth among Atlantic salmon (Salmo salar) populations. Canadian Journal of Fisheries and Aquatic Sciences, 68(3), 444–457. [Google Scholar]
- Jones, O. R. , & Wang, J. (2010). COLONY: A program for parentage and sibship inference from multilocus genotype data. Molecular Ecology Resources, 10(3), 551–555. 10.1111/j.1755-0998.2009.02787.x [DOI] [PubMed] [Google Scholar]
- Jonsson, B. , Jonsson, N. , & Hansen, L. P. (2003). Atlantic salmon straying from the River Imsa. Journal of Fish Biology, 62(3), 641–657. 10.1046/j.1095-8649.2003.00053.x [DOI] [Google Scholar]
- Jorgensen, K. M. , Wennevik, V. , Sorvik, A. G. E. , Unneland, L. , Prusov, S. , Ayllon, F. , & Glover, K. A. (2018). Investigating the frequency of triploid Atlantic salmon in wild Norwegian and Russian populations. BMC Genetics, 19, 9 10.1186/s12863-018-0676-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, S. , Diserud, O. H. , Fiske, P. , & Hindar, K. (2016). Widespread genetic introgression of escaped farmed Atlantic salmon in wild salmon populations. ICES Journal of Marine Science, 73(10), 2488–2498. 10.1093/icesjms/fsw121 [DOI] [Google Scholar]
- Kazakov, R. V. , & Titov, S. F. (1991). Geographical patterns in the population‐genetics of Atlantic salmon, Salmo salar L., on USSR terratory, as evidence of colonization routes. Journal of Fish Biology, 39(1), 6901–6. [Google Scholar]
- Kendall, M. , & Gibbons, J. D. (1976). Rank correlation methods, 5th ed. Charles Griffin Book Series. Oxford, UK: Oxford University Press. [Google Scholar]
- King, T. L. , Eackles, M. S. , & Letcher, B. H. (2005). Microsatellite DNA markers for the study of Atlantic salmon (Salmo salar) kinship, population structure, and mixed‐fishery analyses. Molecular Ecology Notes, 5(1), 130–132. 10.1111/j.1471-8286.2005.00860.x [DOI] [Google Scholar]
- King, T. L. , Kalinowski, S. T. , Schill, W. B. , Spidle, A. P. , & Lubinski, B. A. (2001). Population structure of Atlantic salmon (Salmo salar L.): a range‐wide perspective from microsatellite DNA variation. Molecular Ecology, 10(4), 807–821. [DOI] [PubMed] [Google Scholar]
- King, T. L. , Verspoor, E. , Spidle, A. P. , Gross, R. , Philips, R. B. , Koljonen, M. L. , … Morrison, C. L. (2007). Biodiversity and population structure In Verspoor E., Stradmeyer L., & Nielsen J. (Eds.), The Atlantic salmon: Genetics, conservation and management. Oxford, UK: Oxford Blackwell Publishing. [Google Scholar]
- Kjaerner‐Semb, E. , Ayllon, F. , Furmanek, T. , Wennevik, V. , Dahle, G. , Niemela, E. , … Edvardsen, R. B. (2016). Atlantic salmon populations reveal adaptive divergence of immune related genes – A duplicated genome under selection. BMC Genomics, 17, 12 10.1186/s12864-016-2867-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemetsen, A. , Amundsen, P. A. , Dempson, J. B. , Jonsson, B. , Jonsson, N. , O'Connell, M. F. , & Mortensen, E. (2003). Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecology of Freshwater Fish, 12(1), 6901–59. [Google Scholar]
- Lehnert, S. J. , Bentzen, P. , Kess, K. , Lien, S. , Horne, J. B. , Clement, M. , & Bradbury, I. R. (2018). Chromosome polymorphisms track trans‐Atlantic divergence, admixture and adaptive evolution in salmon. bioRxiv, 351338 10.1101/351338 [DOI] [PubMed] [Google Scholar]
- Lischer, H. E. L. , & Excoffier, L. (2012). PGDSpider: An automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics, 28(2), 298–299. 10.1093/bioinformatics/btr642 [DOI] [PubMed] [Google Scholar]
- Lubieniecki, K. P. , Jones, S. L. , Davidson, E. A. , Park, J. , Koop, B. F. , Walker, S. , & Davidson, W. S. (2010). Comparative genomic analysis of Atlantic salmon, Salmo salar, from Europe and North America. BMC Genetics, 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhun, A. S. , Wennevik, V. , Skilbrei, O. T. , Karlsbakk, E. , Skaala, O. , Fiksdal, I. U. , … Glover, K. A. (2017). The ecological profile of Atlantic salmon escapees entering a river throughout an entire season: Diverse in escape history and genetic background, but frequently virus‐infected. ICES Journal of Marine Science, 74(5), 1371–1381. 10.1093/icesjms/fsw243 [DOI] [Google Scholar]
- Makhrov, A. A. , Verspoor, E. , Artamonova, V. S. , & O'Sullivan, M. (2005). Atlantic salmon colonization of the Russian Arctic coast: Pioneers from North America. Journal of Fish Biology, 67, 68–79. 10.1111/j.0022-1112.2005.00840.x [DOI] [Google Scholar]
- Mantel, N. (1967). The detection of disease of clustering and a generalized regression approach. Cancer Research, 27(2), 209–220. [PubMed] [Google Scholar]
- McConnell, S. K. , O'Reilly, P. , Hamilton, L. , Wright, J. M. , & Bentzen, P. (1995). Polymorphic microsatellite loci from Atlantic salmon (Salmo salar): Genetic differentiation of North American and European populations. Canadian Journal of Fisheries and Aquatic Sciences, 52(9), 1863–1872. [Google Scholar]
- McGinnity, P. , Prodohl, P. , Maoileidigh, N. O. , Hynes, R. , Cotter, D. , Baker, N. , … Ferguson, A. (2004). Differential lifetime success and performance of native and non‐native Atlantic salmon examined under communal natural conditions. Journal of Fish Biology, 65, 173–187. 10.1111/j.0022-1112.2004.00557.x [DOI] [Google Scholar]
- Meirmans, P. G. , & Van Tienderen, P. H. (2004). GENOTYPE and GENODIVE: Two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes, 4(4), 792–794. 10.1111/j.1471-8286.2004.00770.x [DOI] [Google Scholar]
- Metcalfe, N. B. , & Thorpe, J. E. (1990). Determinants of geographical variation in the age of seaward migrating salmon, Salmo salar . Journal of Animal Ecology, 59(1), 135–145. 10.2307/5163 [DOI] [Google Scholar]
- Nilsson, J. , Gross, R. , Asplund, T. , Dove, O. , Jansson, H. , Kelloniemi, J. , … Lumme, J. (2001). Matrilinear phylogeography of Atlantic salmon (Salmo salar L.) in Europe and postglacial colonization of the Baltic Sea area. Molecular Ecology, 10(1), 89–102. [DOI] [PubMed] [Google Scholar]
- Olafsson, K. , Einarsson, S. M. , Gilbey, J. , Pampoulie, C. , Hreggvidsson, G. O. , Hjorleifsdottir, S. , & Gudjonsson, S. (2016). Origin of Atlantic salmon (Salmo salar) at sea in Icelandic waters. ICES Journal of Marine Science, 73(6), 1525–1532. [Google Scholar]
- Olafsson, K. , Pampoulie, C. , Hjorleifsdottir, S. , Gudjonsson, S. , & Hreggvidsson, G. O. (2014). Present‐day genetic structure of Atlantic Salmon (Salmo salar) in Icelandic Rivers and Ice‐Cap Retreat Models. PLoS ONE, 9(2), 12 10.1371/journal.pone.0086809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly, P. T. , Hamilton, L. C. , McConnell, S. K. , & Wright, J. M. (1996). Rapid analysis of genetic variation in Atlantic salmon (Salmo salar) by PCR multiplexing of dinucleotide and tetranucleotide microsatellites. Canadian Journal of Fisheries and Aquatic Sciences, 53(10), 2292–2298. [Google Scholar]
- Ozerov, M. , Vaha, J. P. , Wennevik, V. , Niemela, E. , Svenning, M. A. , Prusov, S. , & Christiansen, B. (2017). Comprehensive microsatellite baseline for genetic stock identification of Atlantic salmon (Salmo salar L.) in northernmost Europe. ICES Journal of Marine Science, 74(8), 2159–2169. [Google Scholar]
- Ozerov, M. Y. , Veselov, A. E. , Lumme, J. , & Primmer, C. R. (2012). “Riverscape” genetics: River characteristics influence the genetic structure and diversity of anadromous and freshwater Atlantic salmon (Salmo salar) populations in northwest Russia. Canadian Journal of Fisheries and Aquatic Sciences, 69(12), 1947–1958. [Google Scholar]
- Parrish, D. L. , Behnke, R. J. , Gephard, S. R. , McCormick, S. D. , & Reeves, G. H. (1998). Why aren't there more Atlantic salmon (Salmo salar)? Canadian Journal of Fisheries and Aquatic Sciences, 55, 281–287. [Google Scholar]
- Paterson, S. , Piertney, S. B. , Knox, D. , Gilbey, J. , & Verspoor, E. (2004). Characterization and PCR multiplexing of novel highly variable tetranucleotide Atlantic salmon (Salmo salar L.) microsatellites. Molecular Ecology Notes, 4(2), 160–162. [Google Scholar]
- Peakall, R. , & Smouse, P. E. (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6(1), 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, S. , Rasmussen, G. , Nielsen, E. E. , Karlsson, L. , & Nyberg, P. (2007). Straying of Atlantic salmon, Salmo salar, from delayed and coastal releases in the Baltic Sea, with special focus on the Swedish west coast. Fisheries Management and Ecology, 14(1), 21–32. 10.1111/j.1365-2400.2006.00520.x [DOI] [Google Scholar]
- Perrier, C. , Guyomard, R. , Bagliniere, J. L. , & Evanno, G. (2011). Determinants of hierarchical genetic structure in Atlantic salmon populations: environmental factors vs. anthropogenic influences. Molecular Ecology, 20(20), 4231–4245. [DOI] [PubMed] [Google Scholar]
- Piry, S. , Luikart, G. , & Cornuet, J. M. (1999). BOTTLENECK: A computer program for detecting recent reductions in the effective size using allele frequency data. Journal of Heredity, 90(4), 502–503. [Google Scholar]
- Primmer, C. R. , Veselov, A. J. , Zubchenko, A. , Poututkin, A. , Bakhmet, I. , & Koskinen, M. T. (2006). Isolation by distance within a river system: Genetic population structuring of Atlantic salmon, Salmo salar, in tributaries of the Varzuga River in northwest Russia. Molecular Ecology, 15(3), 653–666. 10.1111/j.1365-294X.2005.02844.x [DOI] [PubMed] [Google Scholar]
- Pritchard, J. K. , Stephens, M. , & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155(2), 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016). A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rosenberg, M. S. , & Anderson, C. D. (2011). PASSaGE: Pattern analysis, spatial statistics and geographic exegesis. Version 2. Methods in Ecology and Evolution, 2(3), 229–232. [Google Scholar]
- Rougemont, Q. , & Bernatchez, L. (2018). The demographic history of Atlantic salmon (Salmo salar) across its distribution range reconstructed from approximate Bayesian computations. Evolution, 72(6), 1261–1277. [DOI] [PubMed] [Google Scholar]
- Rousset, F. (2008). GENEPOP'007: A complete re‐implementation of the genepop software for Windows and Linux. Molecular Ecology Resources, 8(1), 103–106. 10.1111/j.1471-8286.2007.01931.x [DOI] [PubMed] [Google Scholar]
- Saisa, M. , Koljonen, M. L. , Gross, R. , Nilsson, J. , Tahtinen, J. , Koskiniemi, J. , & Vasemagi, A. (2005). Population genetic structure and postglacial colonization of Atlantic salmon (Salmo salar) in the Baltic Sea area based on microsatellite DNA variation. Canadian Journal of Fisheries and Aquatic Sciences, 62(8), 1887–1904. [Google Scholar]
- Sanchez, J. A. , Clabby, C. , Ramos, D. , Blanco, G. , Flavin, F. , Vazquez, E. , & Powell, R. (1996). Protein and microsatellite single locus variability in Salmo salar L (Atlantic salmon). Heredity, 77, 423–432. 10.1038/hdy.1996.162 [DOI] [PubMed] [Google Scholar]
- Sheehan, T. F. , Legault, C. M. , King, T. L. , & Spidle, A. P. (2010). Probabilistic‐based genetic assignment model: Assignments to subcontinent of origin of the West Greenland Atlantic salmon harvest. ICES Journal of Marine Science, 67(3), 537–550. 10.1093/icesjms/fsp247 [DOI] [Google Scholar]
- Skaala, O. , Makhrov, A. A. , Karlsen, T. , Jorstad, K. E. , Altukhov, Y. P. , Politov, D. V. , … Novikov, G. G. (1998). Genetic comparison of salmon from the White Sea and north‐western Atlantic Ocean. Journal of Fish Biology, 53(3), 569–580. 10.1111/j.1095-8649.1998.tb01002.x [DOI] [Google Scholar]
- Skilbrei, O. T. , & Holm, M. (1998). Effects of long‐term exercise on survival, homing and straying of released Atlantic salmon smolts. Journal of Fish Biology, 52(5), 1083–1086. 10.1111/j.1095-8649.1998.tb00606.x [DOI] [Google Scholar]
- Slettan, A. , Olsaker, I. , & Lie, Ø. (1995). Atlantic salmon, Salmo salar, microsatellites at the SSOSL25, SSOSL85, SSOSL311, SSOSL417 loci. Animal Genetics, 26(4), 281–282. [DOI] [PubMed] [Google Scholar]
- Solberg, M. F. , Glover, K. A. , Nilsen, F. , & Skaala, Ø. (2013). Does domestication cause changes in growth reaction norms? A study of farmed, wild and hybrid Atlantic salmon families exposed to environmental stress. PLoS ONE, 8(1), e54469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabell, O. B. (1984). Homing and olfaction in salmonids ‐ a critical review with special reference to the Atlantic salmon. Biological Reviews of the Cambridge Philosophical Society, 59(3), 333–388. 10.1111/j.1469-185X.1984.tb00709.x [DOI] [Google Scholar]
- Ståhl, G. (1987). Genetic population structure of Atlantic salmon In Ryman N., & Utter F. (Eds.), Population genetics and fishery management. Seattle, WA: University of Washington Press. [Google Scholar]
- Stet, R. J. , de Vries, B. , Mudde, K. , Hermsen, T. , van Heerwaarden, J. , Shum, B. P. , & Grimholt, U. (2002). Unique haplotypes of co‐segregating major histocompatibility class II A and class II B alleles in Atlantic salmon (Salmo salar) give rise to diverse class II genotypes. Immunogenetics, 54(5), 320–331. 10.1007/s00251-002-0477-1 [DOI] [PubMed] [Google Scholar]
- Strøm, J. F. , Thorstad, E. B. , Hedger, R. D. , & Rikardsen, A. H. (2018). Revealing the full ocean migration of individual Atlantic salmon. Animal Biotelemetry, 6, 2. [Google Scholar]
- Taggart, J. B. , Verspoor, E. , Galvin, P. T. , Moran, P. , & Ferguson, A. (1995). A minisatellite DNA marker for discriminating between European and North American Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences, 52(11), 2305–2311. [Google Scholar]
- Taranger, G. L. , Karlsen, O. , Bannister, R. J. , Glover, K. A. , Husa, V. , Karlsbakk, E. , … Svasand, T. (2015). Risk assessment of the environmental impact of Norwegian Atlantic salmon farming. ICES Journal of Marine Science, 72(3), 997–1021. 10.1093/icesjms/fsu132 [DOI] [Google Scholar]
- Taylor, E. B. (1991). A review of local adaptation in salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture, 98(1–3), 185–207. [Google Scholar]
- Tonteri, A. , Titov, S. , Veselov, A. , Zubchenko, A. , Koskinen, M. T. , Lesbarreres, D. , … Primmer, C. R. (2005). Phylogeography of anadromous and non‐anadromous Atlantic salmon (Salmo salar) from northern Europe. Annales Zoologici Fennici, 42(1), 6901–22. [Google Scholar]
- Tonteri, A. , Vasemägi, A. , Lumme, J. , & Primmer, R. C. (2010). Beyond MHC: Signals of elevated selection pressure on Atlantic salmon (Salmo salar) immune‐relevant loci. Molecular Ecology, 19(7), 1273–1282. [DOI] [PubMed] [Google Scholar]
- Tonteri, A. , Veselov, A. J. , Zubchenko, A. V. , Lumme, J. , & Primmer, C. R. (2009). Microsatellites reveal clear genetic boundaries among Atlantic salmon (Salmo salar) populations from the Barents and White seas, northwest Russia. Canadian Journal of Fisheries and Aquatic Sciences, 66(5), 717–735. [Google Scholar]
- Ulvan, E. M. , Foldvik, A. , Jensen, A. J. , & Finstad, B. , Thorstad, E. B. , Rikardsen, A. H. , & Næsje, T. F. (2018). Return migration of adult Atlantic salmon (Salmo salar L.) to northern Norway. ICES Journal of Marine Science, 75(2), 653–661. [Google Scholar]
- Vaha, J. P. , Erkinaro, J. , Falkegard, M. , Orell, P. , & Niemela, E. (2017). Genetic stock identification of Atlantic salmon and its evaluation in a large population complex. Canadian Journal of Fisheries and Aquatic Sciences, 74(3), 327–338. 10.1139/cjfas-2015-0606 [DOI] [Google Scholar]
- Vaha, J. P. , Erkinaro, J. , Niemela, E. , & Primmer, C. R. (2007). Life‐history and habitat features influence the within‐river genetic structure of Atlantic salmon. Molecular Ecology, 16(13), 2638–2654. 10.1111/j.1365-294X.2007.03329.x [DOI] [PubMed] [Google Scholar]
- Valz, P. D. , & Thompson, M. E. (1994). Exact inference for Kendall's S and Spearman's rho. Journal of Computational and Graphical Statistics, 3, 459–472. [Google Scholar]
- Verspoor, E. (1997). Genetic diversity among Atlantic salmon (Salmo salar L.) populations. ICES Journal of Marine Science, 54(6), 965–973. [Google Scholar]
- Verspoor, E. (2005). Regional differentiation of North American Atlantic salmon at allozyme loci. Journal of Fish Biology, 67, 80–103. 10.1111/j.0022-1112.2005.00841.x [DOI] [Google Scholar]
- Verspoor, E. , Beardmore, J. A. , Consuegra, S. , De Leaniz, C. G. , Hindar, K. , Jordan, W. C. , … Cross, T. F. (2005). Population structure in the Atlantic salmon: Insights from 40 years of research into genetic protein variation. Journal of Fish Biology, 67, 3–54. 10.1111/j.0022-1112.2005.00838.x [DOI] [Google Scholar]
- Verspoor, E. , Fraser, N. H. C. , & Youngson, A. F. (1991). Protein polymorphism in Atlantic salmon within a Scottish river – Evidence for selection and estimates of gene flow between tributaries. Aquaculture, 98(1–3), 217–230. 10.1016/0044-8486(91)90385-K [DOI] [Google Scholar]
- Verspoor, E. , & Jordan, W. C. (1989). Geneetic variation at the ME‐2 locus in the Atlantic salmon within and between rivers – Evidence for its selective maintenance. Journal of Fish Biology, 35, 205–213. [Google Scholar]
- Verspoor, E. , Knox, D. , & Marshall, S. (2016). Assessment of interbreeding and introgression of farm genes into a small Scottish Atlantic salmon Salmo salar stock: Ad hoc samples–ad hoc results? Journal of Fish Biology, 89, 2680–2696. [DOI] [PubMed] [Google Scholar]
- Waples, R. S. , & Anderson, E. C. (2017). Purging putative siblings from population genetic data sets: A cautionary view. Molecular Ecology, 26(5), 1211–1224. 10.1111/mec.14022 [DOI] [PubMed] [Google Scholar]
- Waples, R. S. , & Do, C. H. I. (2008). LDNE: A program for estimating effective population size from data on linkage disequilibrium. Molecular Ecology Resources, 8(4), 753–756. [DOI] [PubMed] [Google Scholar]
- Weir, B. S. , & Cockerham, C. C. (1984). Estimating F‐statistics for the analysis of population structure. Evolution, 38(6), 1358–1370. [DOI] [PubMed] [Google Scholar]
- Wennevik, V. , Skaala, Ø. , Titov, S. F. , Studyonov, I. , & Nævdal, G. (2004). Microsatellite variation in populations of Atlantic salmon from North Europe. Environmental Biology of Fishes, 69(1–4), 143–152. 10.1023/B:EBFI.0000022890.15512.29 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Appendices S1–S3 with data and supporting information, including the entire raw data set of individual genetic profiles for >9,000 salmon can be downloaded from the Norwegian Marine Data Centre (NMDC): https://doi.org/10.21335/NMDC-290855015.


