Abstract
Background
Although it is traditionally regarded as a single entity, perioperative stroke comprises 2 separate phenomena (early/intraoperative and delayed/postoperative stroke). We aimed to systematically evaluate incidence, risk factors, and clinical outcome of early and delayed stroke after cardiac surgery.
Methods and Results
A systematic review (MEDLINE, EMBASE, Cochrane Library) was performed to identify all articles reporting early (on awakening from anesthesia) and delayed (after normal awakening from anesthesia) stroke after cardiac surgery. End points were pooled event rates of stroke and operative mortality and incident rate of late mortality. Thirty‐six articles were included (174 969 patients). The pooled event rate for early stroke was 0.98% (95% CI 0.79% to 1.23%) and was 0.93% for delayed stoke (95% CI 0.77% to 1.11%; P=0.68). The pooled event rate of operative mortality was 28.8% (95% CI 17.6% to 43.4%) for early and 17.9% (95% CI 14.0% to 22.7%) for delayed stroke, compared with 2.4% (95% CI 1.9% to 3.1%) for patients without stroke (P<0.001 for early versus delayed, and for perioperative stroke, early stroke, and delayed stroke versus no stroke). At a mean follow‐up of 8.25 years, the incident rate of late mortality was 11.7% (95% CI 7.5% to 18.3%) for early and 9.4% (95% CI 5.9% to 14.9%) for delayed stroke, compared with 3.4% (95% CI 2.4% to 4.8%) in patients with no stroke. Meta‐regression demonstrated that off‐pump was inversely associated with early stroke (β=−0.009, P=0.01), whereas previous stroke (β=0.02, P<0.001) was associated with delayed stroke.
Conclusions
Early and delayed stroke after cardiac surgery have different risk factors and impacts on operative mortality as well as on long‐term survival.
Keywords: cardiac surgery, delayed stroke, early stroke, stroke
Subject Categories: Cardiovascular Surgery, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
This is the first systematic review and meta‐analysis to examine the incidence of early and delayed stroke after cardiac operations.
What Are the Clinical Implications?
Early and delayed stroke after cardiac surgery have different risk factors and impacts on operative mortality as well as on long‐term survival.
Perioperative stroke is a devastating complication after cardiac surgery, and the incidence has remained largely unchanged despite advances in surgical techniques.1 Data from administrative databases and observational registries suggest that the incidence of perioperative stroke after cardiac surgery ranges from 0.8% to 5.2%.2 In landmark trials comparing outcomes of coronary artery bypass graft (CABG) and percutaneous coronary intervention, including SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) and FREEDOM (Future REvascularization Evaluation in patients with Diabetes Mellitus), the burden of stroke has been a major limitation for surgery.3, 4, 5
Stroke may occur intraoperatively (usually detected when patients initially awaken from anesthesia) or thereafter. The 2 types of strokes have different pathophysiologic mechanisms: early/intraoperative stroke occurs primarily from aortic manipulation and atheroembolism, whereas delayed/postoperative stroke is usually related to postoperative atrial fibrillation or cerebral vascular disease.6 The conceptual framework of early and delayed stroke is important because it facilitates implementation and evaluation of tailored preventative strategies for both. Greater understanding of the incidence, risk factors, and sequelae of early and delayed stroke will facilitate the continued improvement in the safety of surgical intervention.
Here we performed a systematic review and meta‐analysis to give an objective and weighted estimate of the incidence and risk of early and delayed stroke following cardiac surgery and their impact on operative and long‐term patient survival.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. This systematic review and meta‐analysis were performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement7 and the MOOSE (Meta‐Analysis of Observational Studies in Epidemiology) guidelines (Table S1).8
Search Strategy
A medical librarian (M.D.) performed comprehensive systematic searches to identify studies that evaluated perioperative stroke after cardiac surgery. Searches were run in April 2018 on the following databases: Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE 1946 to Present); Ovid EMBASE (1974 to present); and The Cochrane Library (Wiley, Hoboken, NJ). The search strategy included all appropriate controlled vocabulary and keywords for identified “cardiac surgical procedures” and “intra‐ and postoperative stroke.” Full details regarding the search strategy for Ovid MEDLINE are provided in Table S2.
Study Selection and Inclusion Criteria
Database searches were conducted and deduplicated by a qualified librarian (M.D.). Three preliminary reviewers screened the searched database for inclusion. A fourth independent reviewer confirmed the adequacy of studies based on predefined inclusion criteria for titles and abstracts. Inclusion criteria were full‐text English articles on adult patients who had undergone cardiac surgery and reported perioperative strokes and classified them as early or delayed. A full text of preliminary screened studies was then retrieved for a second round of eligibility screening. Reference lists of the included articles were also searched, and additional studies included (ie, backward snowballing). The full PRISMA flow chart outlining the study selection process is available in Figure S1. The Newcastle‐Ottawa Scale for quality assessment was used for the critical appraisal of included studies (Table S3).9 Studies with scores of 6 or more were included.
Clinical Outcomes/Definitions
The primary outcome was the rate of early and delayed stroke. Secondary outcomes were (1) rate of perioperative (early+delayed) stroke; (2) operative mortality among patients with perioperative stroke, early stroke, delayed stroke, and among patients without stroke; and (3) late mortality for the above groups of patients.
We used the original articles’ definitions for early and delayed stroke. The most common definitions used were stroke observed “on awakening” or “after extubation” for early stroke and stroke occurring after a symptom‐free interval for delayed stroke (Table S4).
Data Extraction and Statistical Analysis
Extracted variables included study name, publication year, study design, type of surgery, total sample size, number of patients with perioperative, early, and delayed stroke, mean age (years), percentages of women, diabetes mellitus, preoperative atrial fibrillation (AF), preoperative carotid disease, previous history of stroke or urgent or emergency surgery, peripheral vascular disease, chronic renal failure, redo surgery, in‐hospital mortality in the whole sample and in different subgroups, long‐term mortality, and mean follow‐up.
Measurement data were reported as mean±SD. Pooled event rates with 95% CI were calculated for binary outcomes. For late outcomes the incidence rate with an underlying Poisson process with a constant event rate was used to account for different follow‐up periods in different studies with the total number of events observed within a treatment group out of the total person‐time of follow‐up for that treatment group calculated from the study follow‐up. Additionally, for long‐term survival, individual patient survival data were reconstructed using an iterative algorithm that was applied to solve the Kaplan‐Meier equations originally used to produce the published graphs. This algorithm uses digitalized Kaplan‐Meier curve data obtained by the Graph Grabber software package (Quintessa, Oxfordshire, UK) to find numerical solutions to the inverted Kaplan‐Meier equation. Based on the published data in each included study, 4 different levels of information might be available (“all information,” “no numbers at risk,” “no total events,” and “neither”). The censoring pattern varied based on the numbers at risk published intervals as in Williamson.10 For the “no number at risk” case, the censoring pattern is assumed constant over the interval, and for the “neither” case, no censoring is assumed.11
The reconstructed patient survival data were then aggregated to obtain combined survival curves.
Subgroup analysis was used to compare early and delayed stroke for primary and secondary outcomes. Meta‐regression was used to assess the effect of age, sex, diabetes mellitus, preoperative AF, preoperative carotid disease, previous stroke, urgency or emergency surgery, off‐pump CABG, single or multiple aortic clamping, ascending aorta atheroma or calcification, cardiopulmonary bypass time, and aortic clamp time on the rate of early and delayed stroke.
Study heterogeneity was assessed using the Cochran Q statistic and the I2 test. For the primary outcomes, if heterogeneity was significant (I2 >75%), a leave‐one‐out sensitivity analysis was performed. Potential publication bias was assessed using a funnel plot and the Egger regression test.
A random‐effect model (inverse variance method) was used. In addition, prediction interval was calculated as described by Riley et al.12 Supplementary analyses using a fixed‐effect model were also performed, and τ2 was provided as an inference on between‐study variability; we then used meta‐regression, which used covariates to explain some of this variability. A restricted maximum‐likelihood model was used for meta‐regression because it estimates parameters that maximize the likelihood of the error distribution while imposing restrictions to avoid overfitting, which makes it possible to obtain a better balance between the fractions of the variability captured by the fixed part versus the random part of the statistical model.13 Hypothesis testing for equivalence was set at the 2‐tailed P<0.05. Analyses were performed using R (version 3.3.3, R Project for Statistical Computing, Vienna, Austria) with the statistical packages “meta” and “metafor” within RStudio (0.99.489, http://www.rstudio.com).
Results
Characteristics of Eligible Studies
We retrieved 5212 articles and 3784 articles after deduplication. Thirty‐six articles met our inclusion criteria (list of the included studies provided in the supplemental references). The PRISMA flowchart is shown in Figure S1. The mean sample size for each study was 4860 patients (range: 245‐45 432), and the mean follow‐up time was 8.25 years (range 1.0‐11.0 years). The mean age was 65.5 years (range: 54.0‐74.0 years) (Table S5). Women represented 15% to 83% of the included patients, and diabetes mellitus, AF, carotid disease, and urgent/emergent procedures were reported in 5% to 47%, 1% to 19%, 6% to 32%, and 1% to 70% of patients, respectively. Sixty‐three percent of procedures were isolated CABG (Table S5). A total of 174 969 patients were included in the analysis, of whom 2.0% (3421 of 174 969 patients) had perioperative stroke, 1.0% (1767/174 969) had early stroke, and 1.0% (1654/174 969) had delayed stroke (P=0.68) (Table 1).14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49
Table 1.
Details of Outcomes in the Included Studies
| Study/Year | Study Type | Cohort Size | Perioperative Stroke | Early Stroke | Delayed Stroke |
|---|---|---|---|---|---|
| Blossom 199214 | R | 3428 | 46 | 16 | 30 |
| Boivie 200515 | R | 2641 | 98 | 76 | 22 |
| Borger 200116 | R | 6682 | 98 | 90 | 8 |
| Bull 199317 | P | 245 | 5 | 4 | 1 |
| Calafiore 200218 | R | 4875 | 49 | 24 | 25 |
| Cao 201119 | R | 430 | 32 | 4 | 28 |
| Carrascal 201420 | R | 844 | 32 | 23 | 9 |
| Chen 201521 | R | 1010 | 11 | 5 | 6 |
| Doi 201022 | R | 611 | 8 | 0 | 8 |
| Fessatidis 199123 | R | 1487 | 15 | 12 | 3 |
| Filsoufi.A 200824 | R | 2808 | 63 | 35 | 28 |
| Filsoufi.B 200825 | R | 2985 | 48 | 25 | 23 |
| Gaudino 199926 | R | 2987 | 31 | 25 | 6 |
| Goto 200327 | P | 463 | 18 | 13 | 5 |
| Hedberg 200528 | R | 2641 | 77 | 58 | 19 |
| Hedberg 201129 | R | 9122 | 245 | 146 | 99 |
| Hedberg 201330 | R | 10 809 | 339 | 223 | 116 |
| Hogue 199931 | P | 2972 | 48 | 17 | 31 |
| Imasaka 201832 | R | 1134 | 20 | 8 | 12 |
| Karhausen 201733 | R | 6130 | 110 | 35 | 75 |
| Karkouti 200534 | R | 10 949 | 160 | 110 | 50 |
| Kinnunen 201535 | R | 1314 | 23 | 7 | 16 |
| Lahtinen 200436 | R | 2630 | 52 | 20 | 32 |
| Lee 201137 | P | 1367 | 33 | 15 | 18 |
| Lisle 200838 | R | 7201 | 202 | 46 | 156 |
| Martin 198239 | R | 253 | 8 | 4 | 4 |
| Marui 201240 | R | 2446 | 45 | 20 | 25 |
| Murdock 200341 | R | 2104 | 68 | 18 | 50 |
| Nishiyama 200942 | P | 2516 | 46 | 17 | 29 |
| Peel 200443 | R | 10 573 | 211 | 57 | 154 |
| Ridderstolpe 200244 | R | 3282 | 64 | 47 | 17 |
| Salazar 200145 | R | 5971 | 214 | 158 | 56 |
| Tarakji 201146 | P | 45 432 | 688 | 279 | 409 |
| Toumpoulis 200847 | R | 4140 | 138 | 102 | 36 |
| Weinstein 200148 | P | 2217 | 51 | 24 | 27 |
| Wijdicks 199649 | R | 8270 | 25 | 4 | 21 |
P indicates prospective; R, retrospective.
The majority of stroke patients suffered from an ischemic event (88%). Transient ischemic attacks were reported in 1.38% of cases.
Mean bypass time was 81.6 minutes, and mean cross‐clamp time was 82.2 minutes.
Meta‐Analysis
Rate of Stroke
The pooled event rate of perioperative stroke was 2.03% (CI 1.75% to 2.35%) (Figure S2), early stroke was 0.98% (CI 0.79% to 1.23%; Figure 1), and delayed stroke was 0.93% (CI 0.77% to 1.11%; P=0.68 Figure 2; Table 2; a summary of the outcomes as analyzed by means of a fixed‐effect model is reported in Table S6).
Figure 1.
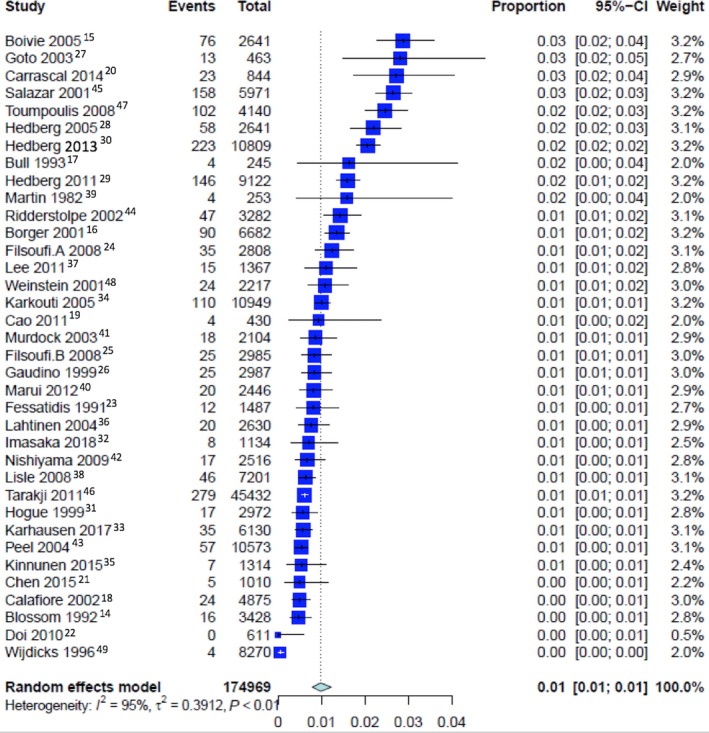
Pooled event rate for early stroke.
Figure 2.
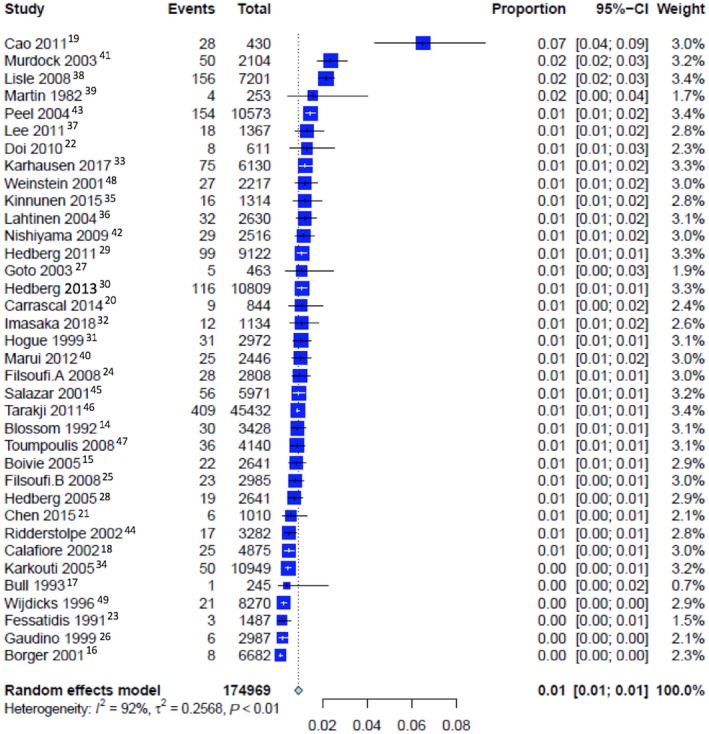
Pooled event rate for delayed stroke.
Table 2.
Summary of the Outcomes (Random‐Effect Model)
| Outcomes | No. of Studies | Proportion [CI] | Heterogeneity (I2, P Value) | τ2 | Perioperative Stroke vs No Strokea | Early vs Delayed Stroke Differencea |
|---|---|---|---|---|---|---|
| Random‐effect model | ||||||
| Pooled rate of perioperative stroke | 36 | 2.03% [1.75; 2.35]; PI=0.85‐4.74 | 94.1%, P<0.0001 | 0.1804 | ··· | ··· |
| Pooled rate of early stroke | 36 | 0.98% [0.79; 1.23]; PI=0.27‐3.49 | 94.7%, P<0.0001 | 0.3912 | ··· | 0.6774 |
| Pooled rate of delayed stroke | 36 | 0.93% [0.77; 1.11]; PI=0.33‐2.59 | 91.5%, P<0.0001 | 0.2568 | ··· | 0.6774 |
| Pooled rate of operative mortality in the whole group | 20 | 2.2% [1.8; 2.8] | 96.9%, P<0.0001 | 0.2543 | ··· | ··· |
| Pooled rate of operative mortality for patients with perioperative stroke | 22 | 21.3% [18.3; 24.5] | 58.8%, P=0.0003 | 0.0935 | <0.0001 | ··· |
| Pooled rate of operative mortality for patients without stroke | 16 | 2.4% [1.9; 3.1] | 96.9%, P<0.0001 | 0.2419 | <0.0001 | ··· |
| Pooled rate of operative mortality for patients with early stroke | 12 | 28.8% [17.6; 43.4] | 84.2%, P<0.0001 | 0.9440 | ··· | <0.0001 |
| Pooled rate of operative mortality for patients with late stroke | 13 | 17.9% [14.0; 22.7] | 20.1%, P=0.2407 | 0.0550 | ··· | <0.0001 |
| Incidence rate of late mortality in the “all” group | 5 | 3.4% [2.3; 5.2] | 99.3%, P<0.0001 | 0.2150 | ··· | ··· |
| Incidence rate of late mortality in patients with perioperative stroke | 5 | 10.9% [7.3; 16.2] | 84.8%, P<0.0001 | 0.1600 | <0.0001 | ··· |
| Incidence rate of late mortality in patients without stroke | 8 | 3.4% [2.4; 4.8] | 99.7%, P=0 | 0.2426 | <0.0001 | ··· |
| Incidence rate of late mortality in patients with early stroke | 5 | 11.7% [7.5; 18.3] | 87.6%, P<0.0001 | 0.2194 | ··· | 0.5063 |
| Incidence rate of late mortality in patients with delayed stroke | 5 | 9.4% [5.9; 14.9] | 71.2%, P=0.008 | 0.1771 | ··· | 0.5063 |
PI indicates prediction interval.
P value for subgroup difference.
Operative Mortality
Overall pooled event rates for operative mortality was 2.2% (CI: 1.8% to 2.8%). For patients with perioperative stroke, the operative mortality was 21.3% (CI: 18.3% to 24.5%), 28.8% (CI: 17.6% to 43.3%) for patients with early stroke and 17.9% (CI: 14.0% to 22.7%) for patients with delayed stroke (P<0.001 for early versus delayed stroke; Figures 3 and 4). The pooled event rate for operative mortality without stroke was 2.4% (CI 1.9% to 3.1%) (P<0.001 compared with patients with perioperative stroke, early stroke, and delayed stroke; Figure 4).
Figure 3.
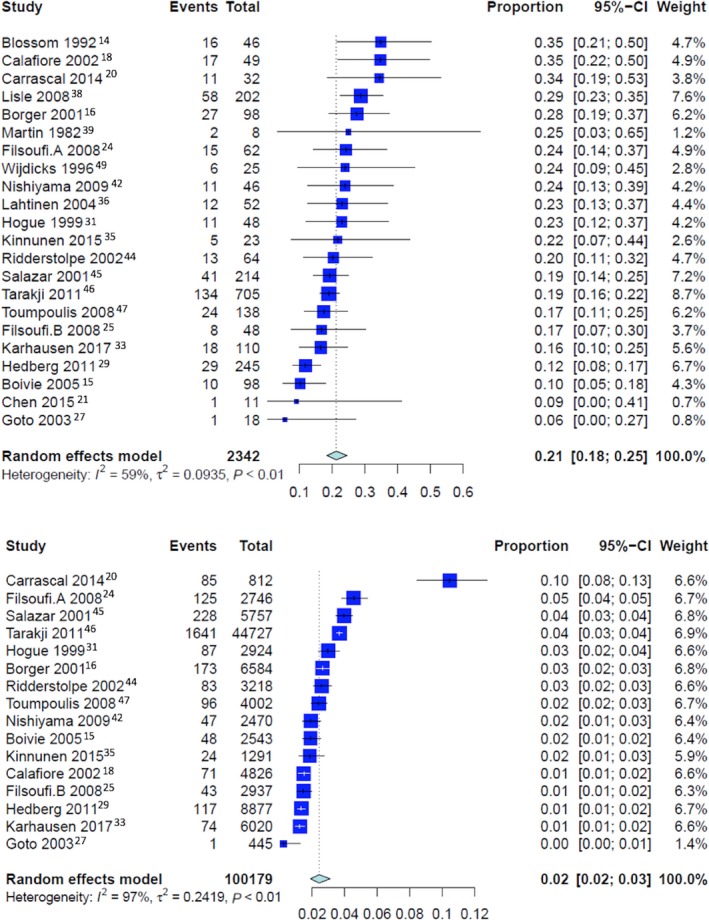
Pooled event rate for operative mortality in patients with (top) and without perioperative stroke (bottom).
Figure 4.
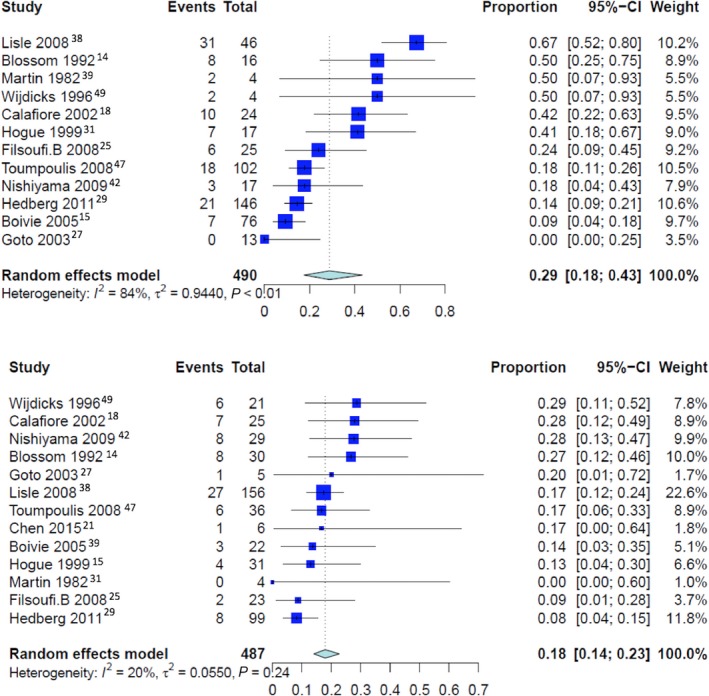
Pooled event rate for operative mortality for patients with early stroke (top) and late stroke (bottom).
Late Mortality
The weighted mean follow‐up was 8.25 years. Overall incidence rate for late mortality for the entire cohort was 3.4% (CI 2.3% to 5.2%). The incidence rate for late mortality was 10.9% (CI 7.3% to 16.2%) for patients with perioperative stroke, 11.7% (CI 7.5% to 18.3%) for early stroke, and 9.4% (CI 5.9% to 14.9%) for delayed stroke (P=0.50). The incidence rate for late mortality for patients without stroke was 3.4% (CI 2.4% to 4.8%; P<0.0001 compared with patients with perioperative, early, and delayed stroke; Table 2; a summary of the outcomes as analyzed by means of a fixed‐effect model is reported in Table S6).
Reconstructed individual patient survival data from Kaplan‐Meier survival curves showed 1‐, 3‐, 5‐, and 10‐year survival of 80.2%, 73.0%, 63.3%, and 40.7%, respectively, in the early‐stroke group and 88.1%, 85.2%, 71.3%, and 30.2%, respectively, in the delayed‐stroke group (Figure S3). For patients who did not experience stroke, 1‐, 3‐, 5‐, and 10‐year survivals were 99.5%, 99.2%, 99.1%, and 97.1%, respectively.
The funnel plot of observed and imputed studies (trim‐and‐fill method) and leave‐one‐out analysis for the primary outcomes revealed absence of publication bias (the Egger intercept is −2.25±1.33 [P=0.10] for early and −1.47±1.04 [P=0.17] for delayed stroke; Figure S4). The cumulative analyses for the primary outcomes are shown in Figure 5.
Figure 5.
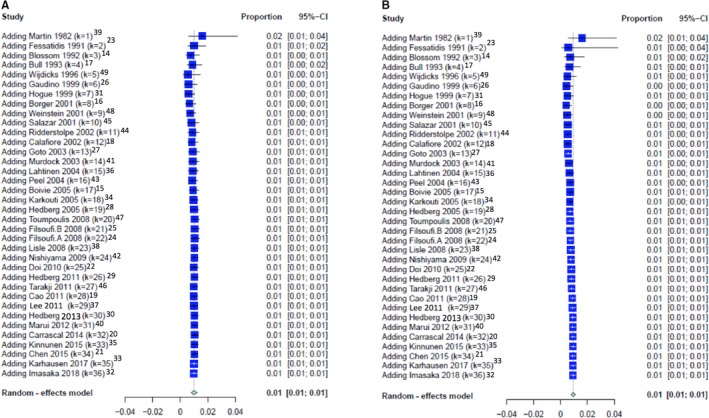
Cumulative analysis of incidence of (A) early stroke and (B) delayed stroke.
Meta‐Regression
Off‐pump surgery was inversely associated with early stroke (β=−0.009, P=0.01). Previous stroke (β=0.02, P<0.001) was associated with delayed stroke. Single versus multiple aortic clamping, ascending aortic atheroma or calcification, use of circulatory arrest, cardiopulmonary bypass, and aortic cross‐clamp times were not associated with either early or delayed stroke (Table S7).
Discussion
To our knowledge the present work is the first systematic review and meta‐analysis to examine the incidence, risk factors, and impact on clinical outcomes of early and delayed stroke after cardiac surgery.
Previous evidence was based on single‐center cohorts with variable sample size, incidence of events, and follow‐up duration so that a general and objective estimate of the incidence of the 2 types of stroke was difficult to ascertain. For this reason, the results of this meta‐analysis are of substantial relevance for patient counseling, clinical decision making, and planning of research for preventive interventions.
The main findings were as follows: (1) the rates of early and late stroke were similar at ≈1% each, (2) both early and delayed stroke were associated with a significant increase in operative as well as late mortality, (3) the impact on operative mortality was significantly higher for early versus delayed stroke, (4) a prior history of stroke was associated with delayed stroke, whereas (5) off‐pump CABG was inversely associated with early stroke.
Early stroke (defined as detected “on awakening” or “after extubation”) is directly linked to intraoperative events. Early strokes were inversely associated with off‐pump CABG but not with any patient characteristics, suggesting the technical/surgical nature of their etiology. Cerebral embolization is known to occur mainly due to aortic manipulation (cannulation, cross‐clamping, and performance of proximal aortic anastomoses during CABG).50, 51, 52 Early stroke has been reported to be usually located in the right hemisphere, consistent with the jet of the flow from the aortic cannula.15, 28
Although large randomized controlled trials have reported similar neurological outcomes after on‐ and off‐pump CABG (30‐day stroke incidence for on‐ versus off‐pump, respectively; 0.7% versus 1.3% [P=0.28] in the ROOBY [Randomized On/Off Bypass] trial53; 2.7% versus 2.2% [P=0.47] in the GOPCABE (German Off‐Pump Coronary Artery Bypass Grafting in Elderly Patients) trial54; 1.1% versus 1.0% [P‐value not reported] in the CORONARY (CABG Off or On Pump Revascularization Study) trial55), in our analysis off‐pump surgery was significantly and adversely associated with early stroke. Differences in sample size, treatment allocation, and surgeon expertise are the possible reasons for these differences.
Hedberg et al in a series of 10 809 patients reported that early strokes were predominantly located in the right hemisphere (P=0.009), whereas delayed stroke had a uniform spatial distribution. Authors suggested that the preponderance for right‐hemispheric lesions might suggest an embolic etiology via the brachiocephalic trunk.30 Higher stroke‐related mortality (odds ratio 9.16; P<0.0001) and greater rehabilitation needs for early versus delayed stroke were reported in a review of 7201 patients.38
Significant efforts have been aimed at intraoperative stroke reduction including minimizing or eliminating aortic manipulation, eliminating cardiopulmonary bypass, and using preoperative CT scan of the ascending aorta and duplex scanning of the carotid arteries as well as epiaortic ultrasound.56, 57, 58, 59, 60
Motallebzadeh et al randomized a total of 212 patients to receive on‐pump versus off‐pump coronary artery bypass and demonstrated reduced cerebral embolism with a better neurocognitive score at discharge in those undergoing off‐pump surgery (P<0.001 and P=0.01, respectively); there were 3 nonfatal strokes in the on‐pump group and 1 in the off‐pump group within 30 days of surgery.61
In a large series including more than 12 000 patients, the use an aortic facilitating device to perform the proximal anastomosis significantly reduced the postoperative stroke rate but was inferior to no–aortic touch technique (stroke rates 0.6%, 1.2%, and 1.5% in the no‐touch, clampless facilitating device, and the clamp group, respectively).62 Consistent with these results, Vallely reported that anaortic off‐pump coronary artery bypass resulted in 0.25% of neurological adverse events as compared with 1.1% in the groups with side‐clamping for proximal anastomoses.63
A multicenter randomized trial enrolling 383 patients undergoing surgical aortic valve replacement recently evaluated the potential neuroprotective role of 2 cannulation systems designed to capture aortic microemboli (Embol‐X Embolic Protection Device, Edwards Life Science, Irvine, CA; and CardioGard Cannula, CardioGard Medical Ltd, Or‐Yehuda, Israel). The rate of freedom from cerebral infarction at 7 days was 32.0% with suction‐based extraction versus 33.3% with control (ie, standard aortic cannula) (between‐group difference, −1.3%; 95% CI −13.8% to 11.2%) and 25.6% with intra‐aortic filtration versus 32.4% with control (between‐group difference −6.9%; 95% CI −17.9% to 4.2%); no significant differences in mortality (3.4% for suction‐based extraction versus 1.7% for control; and 2.3% for intra‐aortic filtration versus 1.5% for control) or clinical stroke (5.1% for suction‐based extraction versus 5.8% for control; and 8.3% for intra‐aortic filtration versus 6.1% for control) were detected.64
The effectiveness of early stroke reduction strategies was recently demonstrated by the EXCEL (Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trial, in which surgeons were encouraged to use intraoperative adjunctive techniques for stroke reduction including epiaortic ultrasound and transesophageal echocardiography for assessment of ascending aortic calcification.65 The result was an overall incidence of stroke that did not differ between CABG and percutaneous coronary intervention (2.9% versus 2.3%, P=0.37).
In our study early stroke was associated with a 12‐fold increase in operative mortality (29% versus 2% without stroke) as well as much higher increases in the risk of late death (12% versus 3% without stroke), suggesting that addressing this potential complication can significantly improve the outcomes of cardiac surgery. Of note, the impact on operative mortality was significantly higher for early versus delayed stroke.
Delayed stroke (defined as stroke occurring after a normal awakening from anesthesia) is probably mostly related to postoperative AF or to cerebrovascular disease.66, 67, 68, 69, 70, 71, 72, 73, 74 In our analysis delayed stroke was associated with a 7‐ and 3‐fold increase in operative and late mortality, respectively. Late stroke was also associated with history of stroke, suggesting a greater influence of patient‐related factors such as vascular disease compared with early stroke. Indeed, contemporary cardiac surgical patients are older and have greater numbers of cardiovascular comorbidities including hypertension, diabetes mellitus, advanced age, kidney disease, peripheral artery disease, and cerebrovascular disease.75 Validated stroke risk prediction tools such as the CHA2DS2–VASC (congestive heart failure, hypertension, age, diabetes [mellitus], and stroke/TIA–vascular disease and female gender) scoring schema indicate that a substantial portion of cardiac surgical patients are at high risk for AF‐related stroke.76
The main strategies for delayed stroke prevention are (1) pharmacological or nonpharmacological AF prophylaxis,77 (2) anticoagulation for prevention and treatment of clot formation,78 and (3) elimination of the left atrial appendage.1 AF prophylaxis includes amiodarone, β‐blockers, magnesium, atrial pacing, and posterior pericardiotomy.77 Regarding left atrial appendage isolation, LAAOS III (the Left Atrial Appendage Occlusion Study) is an ongoing prospective, double‐blind, randomized trial comparing concomitant surgical left atrial appendage occlusion and no‐occlusion in patients with AF or flutter who are undergoing cardiac surgery (ClinicalTrials.gov Identifier: NCT01561651). Again, continued efforts to evaluate interventions to lower the risk of delayed stroke in prospective surgical trials are needed.
This study shares the common limitations of analyses of aggregate data. First, this analysis included a range of cardiac surgical procedures, although isolated CABG was the most common type of procedure (Table S5). There was heterogeneity in the definitions used by the different studies, in the surgical and postoperative protocols (Table S4), as well as in the follow‐up approaches, in the involvement of a neurologist in the diagnosis of stroke events, and in the documentation of these events by cerebral‐imaging studies. Moreover, postdischarge stroke might have been missed in some studies. Finally, most of the studies did not use continuous monitoring of postoperative cardiac rhythm, and thus, we have no solid information on the occurrence of postoperative AF, and we were unable to include this variable in our meta‐regression analysis. As in all meta‐analyses, ecological fallacy is a concern. Finally, it was not possible to determine whether early or late deaths were directly related to strokes.
Summary
This is the first systematic review and meta‐analysis to examine the incidence of early and delayed stroke after cardiac operations. There is a 1% risk for both early and delayed stroke after cardiac surgery. Early stroke is not associated with any patient‐level risk factors, suggesting a technical cause, and is associated with a significant increase in operative mortality as well as reduction in long‐term survival. The impact of early stroke on operative mortality is significantly higher than that of delayed stroke. Delayed stroke is associated with previous stroke and also negatively impacts survival. Continued targeted interventions to reduce the burden of both early and delayed strokes are imperative to improve overall surgical outcomes.
Disclosures
None.
Supporting information
Table S1. MOOSE Checklist for Meta‐Analyses of Observational Studies
Table S2. The Search Strategy for Ovid MEDLINE
Table S3. Critical Appraisal of Included Studies Using the Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies
Table S4. Stroke Definitions in the Included Studies
Table S5. Demographics of the Included Studies
Table S6. Summary of the Outcomes (Fixed‐Effect Model)
Table S7. Meta‐Regression for Early and Delayed Stroke (Restricted Maximum‐Likelihood Model)
Figure S1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flowchart.
Figure S2. Pooled event rate for perioperative stroke.
Figure S3. Reconstructed Kaplan‐Meier survival curves from derived individual patient data (IPD) for (A) no stroke vs perioperative stroke and (B) no stroke vs early and delayed stroke. Solid/dotted line represents aggregation of all available Kaplan‐Meier curves with 95% CI.
Figure S4. Leave‐one‐out analysis (top) and funnel plot (bottom) for incidence of (A) early stroke and (B) delayed stroke.
(J Am Heart Assoc. 2019;8:e012447 DOI: 10.1161/JAHA.119.012447.)
References
- 1. Whitlock R, Healey JS, Connolly SJ, Wang J, Danter MR, Tu JV, Novick R, Fremes S, Teoh K, Khera V, Yusuf S. Predictors of early and late stroke following cardiac surgery. CMAJ. 2014;186:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selnes OA, Goldsborough MA, Borowicz LM, McKhann GM. Neurobehavioural sequelae of cardiopulmonary bypass. Lancet. 1999;353:1601–1606. [DOI] [PubMed] [Google Scholar]
- 3. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, Bertrand M, Fuster V; FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 4. Head SJ, Milojevic M, Daemen J, Ahn J‐M, Boersma E, Christiansen EH, Domanski MJ, Farkouh ME, Flather M, Fuster V, Hlatky MA, Holm NR, Hueb WA, Kamalesh M, Kim Y‐H, Mäkikallio T, Mohr FW, Papageorgiou G, Park S‐J, Rodriguez AE, Sabik JF, Stables RH, Stone GW, Serruys PW, Kappetein AP. Stroke rates following surgical versus percutaneous coronary revascularization. J Am Coll Cardiol. 2018;72:386–398. [DOI] [PubMed] [Google Scholar]
- 5. Serruys PW, Morice M‐C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; SYNTAX Investigators . Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 6. Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolm an R, Aggarwal A, Marschall K, Graham SH, Ley C. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335:1857–1863. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA‐P Group . Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 9. Ottawa Hospital Research Institute [Internet]. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 16, 2018.
- 10. Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Stat Med. 2002;21:3337–3351. [DOI] [PubMed] [Google Scholar]
- 11. Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riley R, Higgins J, Deeks J. Interpretation of random effects meta‐analyses. BMJ. 2011;10:d549. [DOI] [PubMed] [Google Scholar]
- 13. Meta‐Regression | Columbia University Mailman School of Public Health [Internet]. Available at: https://www.mailman.columbia.edu/research/population-health-methods/meta-regression. Accessed May 1, 2019.
- 14. Blossom GB, Fietsam R, Bassett JS, Glover JL, Bendick PJ. Characteristics of cerebrovascular accidents after coronary artery bypass grafting. Am Surg. 1992;58:584–589; discussion 589. [PubMed] [Google Scholar]
- 15. Boivie P, Edström C, Engström KG. Side differences in cerebrovascular accidents after cardiac surgery: a statistical analysis of neurologic symptoms and possible implications for anatomic mechanisms of aortic particle embolization. J Thorac Cardiovasc Surg. 2005;129:591–598. [DOI] [PubMed] [Google Scholar]
- 16. Borger MA, Ivanov J, Weisel RD, Rao V, Peniston CM. Stroke during coronary bypass surgery: principal role of cerebral macroemboli. Eur J Cardiothorac Surg. 2001;19:627–632. [DOI] [PubMed] [Google Scholar]
- 17. Bull DA, Neumayer LA, Hunter GC, Keksz J, Sethi GK, McIntyre KE, Bernhard VM. Risk factors for stroke in patients undergoing coronary artery bypass grafting. Cardiovasc Surg. 1993;1:182–185. [PubMed] [Google Scholar]
- 18. Calafiore AM, Di Mauro M, Teodori G, Di Giammarco G, Cirmeni S, Contini M, Iacò AL, Pano M. Impact of aortic manipulation on incidence of cerebrovascular accidents after surgical myocardial revascularization. Ann Thorac Surg. 2002;73:1387–1393. [DOI] [PubMed] [Google Scholar]
- 19. Cao L, Li Q, Bi Q, Yu Q‐J. Risk factors for recurrent stroke after coronary artery bypass grafting. J Cardiothorac Surg. 2011;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carrascal Y, Guerrero AL, Blanco M, Valenzuela H, Pareja P, Laguna G. Postoperative stroke related to cardiac surgery in octogenarians. Interact Cardiovasc Thorac Surg. 2014;18:596–601. [DOI] [PubMed] [Google Scholar]
- 21. Chen J‐W, Lin C‐H, Hsu R‐B. Mechanisms of early and delayed stroke after systematic off‐pump coronary artery bypass. J Formos Med Assoc. 2015;114:988–994. [DOI] [PubMed] [Google Scholar]
- 22. Doi K, Yaku H. Importance of cerebral artery risk evaluation before off‐pump coronary artery bypass grafting to avoid perioperative stroke. Eur J Cardiothorac Surg. 2010;38:568–572. [DOI] [PubMed] [Google Scholar]
- 23. Fessatidis I, Prapas S, Hevas A, Didilis V, Alotzeilat A, Missias G, Asteri T, Spyrou P. Prevention of perioperative neurological dysfunction. A six year perspective of cardiac surgery. J Cardiovasc Surg (Torino). 1991;32:570–574. [PubMed] [Google Scholar]
- 24. Filsoufi F, Rahmanian PB, Castillo JG, Bronster D, Adams DH. Incidence, topography, predictors and long‐term survival after stroke in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2008;85:862–870. [DOI] [PubMed] [Google Scholar]
- 25. Filsoufi F, Rahmanian PB, Castillo JG, Bronster D, Adams DH. Incidence, imaging analysis, and early and late outcomes of stroke after cardiac valve operation. Am J Cardiol. 2008;101:1472–1478. [DOI] [PubMed] [Google Scholar]
- 26. Gaudino M, Martinelli L, Di Lella G, Glieca F, Marano P, Schiavello R, Possati G. Superior extension of intraoperative brain damage in case of normothermic systemic perfusion during coronary artery bypass operations. J Thorac Cardiovasc Surg. 1999;118:432–437. [DOI] [PubMed] [Google Scholar]
- 27. Goto T, Baba T, Matsuyama K, Honma K, Ura M, Koshiji T. Aortic atherosclerosis and postoperative neurological dysfunction in elderly coronary surgical patients. Ann Thorac Surg. 2003;75:1912–1918. [DOI] [PubMed] [Google Scholar]
- 28. Hedberg M, Boivie P, Edström C, Engström KG. Cerebrovascular accidents after cardiac surgery: an analysis of CT scans in relation to clinical symptoms. Scand Cardiovasc J. 2005;39:299–305. [DOI] [PubMed] [Google Scholar]
- 29. Hedberg M, Boivie P, Engström KG. Early and delayed stroke after coronary surgery—an analysis of risk factors and the impact on short‐ and long‐term survival. Eur J Cardiothorac Surg. 2011;40:379–387. [DOI] [PubMed] [Google Scholar]
- 30. Hedberg M, Engström KG. Stroke after cardiac surgery—hemispheric distribution and survival. Scand Cardiovasc J. 2013;47:136–144. [DOI] [PubMed] [Google Scholar]
- 31. Hogue CW, Murphy SF, Schechtman KB, Dávila‐Román VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100:642–647. [DOI] [PubMed] [Google Scholar]
- 32. Imasaka K‐I, Tayama E, Morita S, Tomita Y. Neurological outcome and efficacy of intensive craniocervical screening for elective cardiac surgery. Interact Cardiovasc Thorac Surg. 2018;26:216–223. [DOI] [PubMed] [Google Scholar]
- 33. Karhausen JA, Smeltz AM, Akushevich I, Cooter M, Podgoreanu MV, Stafford‐Smith M, Martinelli SM, Fontes ML, Kertai MD. Platelet counts and postoperative stroke after coronary artery bypass grafting surgery. Anesth Analg. 2017;125:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karkouti K, Djaiani G, Borger MA, Beattie WS, Fedorko L, Wijeysundera D, Ivanov J, Karski J. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–1387. [DOI] [PubMed] [Google Scholar]
- 35. Kinnunen E‐M, Juvonen T, Biancari F. Use of blood products and diseased ascending aorta are determinants of stroke after off‐pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2015;29:1180–1186. [DOI] [PubMed] [Google Scholar]
- 36. Lahtinen J, Biancari F, Salmela E, Mosorin M, Satta J, Rainio P, Rimpiläinen J, Lepojärvi M, Juvonen T. Postoperative atrial fibrillation is a major cause of stroke after on‐pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:1241–1244. [DOI] [PubMed] [Google Scholar]
- 37. Lee E‐J, Choi K‐H, Ryu J‐S, Jeon S‐B, Lee S‐W, Park S‐W, Park S‐J, Lee J‐W, Choo S‐J, Chung C‐H, Jung S‐H, Kang D‐W, Kim JS, Kwon SU. Stroke risk after coronary artery bypass graft surgery and extent of cerebral artery atherosclerosis. J Am Coll Cardiol. 2011;57:1811–1818. [DOI] [PubMed] [Google Scholar]
- 38. Lisle TC, Barrett KM, Gazoni LM, Swenson BR, Scott CD, Kazemi A, Kern JA, Peeler BB, Kron IL, Johnston KC. Timing of stroke after cardiopulmonary bypass determines mortality. Ann Thorac Surg. 2008;85:1556–1562. discussion 1562–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin WR, Hashimoto SA. Stroke in coronary bypass surgery. Can J Neurol Sci. 1982;9:21–26. [DOI] [PubMed] [Google Scholar]
- 40. Marui A, Kimura T, Tanaka S, Okabayashi H, Komiya T, Furukawa Y, Kita T, Sakata R; CREDO‐Kyoto Investigators . Comparison of frequency of postoperative stroke in off‐pump coronary artery bypass grafting versus on‐pump coronary artery bypass grafting versus percutaneous coronary intervention. Am J Cardiol. 2012;110:1773–1778. [DOI] [PubMed] [Google Scholar]
- 41. Murdock DK, Rengel LR, Schlund A, Olson KJ, Kaliebe JW, Johnkoski JA, Riveron FA. Stroke and atrial fibrillation following cardiac surgery. WMJ. 2003;102:26–30. [PubMed] [Google Scholar]
- 42. Nishiyama K, Horiguchi M, Shizuta S, Doi T, Ehara N, Tanuguchi R, Haruna Y, Nakagawa Y, Furukawa Y, Fukushima M, Kita T, Kimura T. Temporal pattern of strokes after on‐pump and off‐pump coronary artery bypass graft surgery. Ann Thorac Surg. 2009;87:1839–1844. [DOI] [PubMed] [Google Scholar]
- 43. Peel GK, Stamou SC, Dullum MKC, Hill PC, Jablonski KA, Bafi AS, Boyce SW, Petro KR, Corso PJ. Chronologic distribution of stroke after minimally invasive versus conventional coronary artery bypass. J Am Coll Cardiol. 2004;43:752–756. [DOI] [PubMed] [Google Scholar]
- 44. Ridderstolpe L, Ahlgren E, Gill H, Rutberg H. Risk factor analysis of early and delayed cerebral complications after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:278–285. [DOI] [PubMed] [Google Scholar]
- 45. Salazar JD, Wityk RJ, Grega MA, Borowicz LM, Doty JR, Petrofski JA, Baumgartner WA. Stroke after cardiac surgery: short‐ and long‐term outcomes. Ann Thorac Surg. 2001;72:1195–1201; discussion 1201–1202. [DOI] [PubMed] [Google Scholar]
- 46. Tarakji KG, Sabik JF, Bhudia SK, Batizy LH, Blackstone EH. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011;305:381–390. [DOI] [PubMed] [Google Scholar]
- 47. Toumpoulis IK, Anagnostopoulos CE, Chamogeorgakis TP, Angouras DC, Kariou MA, Swistel DG, Rokkas CK. Impact of early and delayed stroke on in‐hospital and long‐term mortality after isolated coronary artery bypass grafting. Am J Cardiol. 2008;102:411–417. [DOI] [PubMed] [Google Scholar]
- 48. Weinstein GS. Left hemispheric strokes in coronary surgery: implications for end‐hole aortic cannulas. Ann Thorac Surg. 2001;71:128–132. [DOI] [PubMed] [Google Scholar]
- 49. Wijdicks EF, Jack CR. Coronary artery bypass grafting‐associated ischemic stroke. A clinical and neuroradiological study. J Neuroimaging. 1996;6:20–22. [DOI] [PubMed] [Google Scholar]
- 50. Zhao DF, Edelman JJ, Seco M, Bannon PG, Wilson MK, Byrom MJ, Thourani V, Lamy A, Taggart DP, Puskas JD, Vallely MP. Coronary artery bypass grafting with and without manipulation of the ascending aorta: a network meta‐analysis. J Am Coll Cardiol. 2017;69:924–936. [DOI] [PubMed] [Google Scholar]
- 51. Baufreton C, Allain P, Chevailler A, Etcharry‐Bouyx F, Corbeau JJ, Legall D, de Brux JL. Brain injury and neuropsychological outcome after coronary artery surgery are affected by complement activation. Ann Thorac Surg. 2005;79:1597–1605. [DOI] [PubMed] [Google Scholar]
- 52. Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–1399. [DOI] [PubMed] [Google Scholar]
- 53. Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D; Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group . On‐pump versus off‐pump coronary‐artery bypass surgery. N Engl J Med. 2009;361:1827–1837.19890125 [Google Scholar]
- 54. Diegeler A, Börgermann J, Kappert U, Breuer M, Böning A, Ursulescu A, Rastan A, Holzhey D, Treede H, Rieß F‐C, Veeckmann P, Asfoor A, Reents W, Zacher M, Hilker M; GOPCABE Study Group . Off‐pump versus on‐pump coronary‐artery bypass grafting in elderly patients. N Engl J Med. 2013;368:1189–1198. [DOI] [PubMed] [Google Scholar]
- 55. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes J‐C, Novick RJ, Vaijyanath P, Reddy S, Tao L, Olavegogeascoechea PA, Airan B, Sulling T‐A, Whitlock RP, Ou Y, Ng J, Chrolavicius S, Yusuf S; CORONARY Investigators . Off‐pump or on‐pump coronary‐artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. [DOI] [PubMed] [Google Scholar]
- 56. Rosenberger P, Shernan SK, Löffler M, Shekar PS, Fox JA, Tuli JK, Nowak M, Eltzschig HK. The influence of epiaortic ultrasonography on intraoperative surgical management in 6051 cardiac surgical patients. Ann Thorac Surg. 2008;85:548–553. [DOI] [PubMed] [Google Scholar]
- 57. Emmert MY, Seifert B, Wilhelm M, Grünenfelder J, Falk V, Salzberg SP. Aortic no‐touch technique makes the difference in off‐pump coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;142:1499–1506. [DOI] [PubMed] [Google Scholar]
- 58. Daniel WT, Kilgo P, Puskas JD, Thourani VH, Lattouf OM, Guyton RA, Halkos ME. Trends in aortic clamp use during coronary artery bypass surgery: effect of aortic clamping strategies on neurologic outcomes. J Thorac Cardiovasc Surg. 2014;147:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Patel NC, Deodhar AP, Grayson AD, Pullan DM, Keenan DJM, Hasan R, Fabri BM. Neurological outcomes in coronary surgery: independent effect of avoiding cardiopulmonary bypass. Ann Thorac Surg. 2002;74:400–405; discussion 405–406. [DOI] [PubMed] [Google Scholar]
- 60. Albert A, Ennker J, Hegazy Y, Ullrich S, Petrov G, Akhyari P, Bauer S, Ürer E, Ennker IC, Lichtenberg A, Priss H, Assmann A. Implementation of the aortic no‐touch technique to reduce stroke after off‐pump coronary surgery. J Thorac Cardiovasc Surg. 2018;156:544–554.e4. [DOI] [PubMed] [Google Scholar]
- 61. Motallebzadeh R, Bland JM, Markus HS, Kaski JC, Jahangiri M. Neurocognitive function and cerebral emboli: randomized study of on‐pump versus off‐pump coronary artery bypass surgery. Ann Thorac Surg. 2007;83:475–482. [DOI] [PubMed] [Google Scholar]
- 62. Moss E, Puskas JD, Thourani VH, Kilgo P, Chen EP, Leshnower BG, Lattouf OM, Guyton RA, Glas KE, Halkos ME. Avoiding aortic clamping during coronary artery bypass grafting reduces postoperative stroke. J Thorac Cardiovasc Surg. 2015;149:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vallely MP, Potger K, McMillan D, Hemli JM, Brady PW, Brereton RJL, Marshman D, Mathur MN, Ross DE. Anaortic techniques reduce neurological morbidity after off‐pump coronary artery bypass surgery. Heart Lung Circ. 2008;17:299–304. [DOI] [PubMed] [Google Scholar]
- 64. Mack MJ, Acker MA, Gelijns AC, Overbey JR, Parides MK, Browndyke JN, Groh MA, Moskowitz AJ, Jeffries NO, Ailawadi G, Thourani VH, Moquete EG, Iribarne A, Voisine P, Perrault LP, Bowdish ME, Bilello M, Davatzikos C, Mangusan RF, Winkle RA, Smith PK, Michler RE, Miller MA, O'Sullivan KL, Taddei‐Peters WC, Rose EA, Weisel RD, Furie KL, Bagiella E, Moy CS, O'Gara PT, Messé SR; Cardiothoracic Surgical Trials Network (CTSN) . Effect of cerebral embolic protection devices on CNS infarction in surgical aortic valve replacement: a randomized clinical trial. JAMA. 2017;318:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, Kandzari DE, Morice M‐C, Lembo N, Brown WM, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogáts G, Mansour S, Noiseux N, Sabaté M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Pagé P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP; EXCEL Trial Investigators . Everolimus‐eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 66. Kosmidou I, Chen S, Kappetein AP, Serruys PW, Gersh BJ, Puskas JD, Kandzari DE, Taggart DP, Morice M‐C, Buszman PE, Bochenek A, Schampaert E, Pagé P, Sabik JF, McAndrew T, Redfors B, Ben‐Yehuda O, Stone GW. New‐onset atrial fibrillation after PCI or CABG for left main disease: the EXCEL trial. J Am Coll Cardiol. 2018;71:739–748. [DOI] [PubMed] [Google Scholar]
- 67. Villareal RP, Hariharan R, Liu BC, Kar B, Lee V‐V, Elayda M, Lopez JA, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. [DOI] [PubMed] [Google Scholar]
- 68. Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–549. [DOI] [PubMed] [Google Scholar]
- 69. Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Circulation. 1996;94:390–397. [DOI] [PubMed] [Google Scholar]
- 70. Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, Tarazi R, Shroyer AL, Sethi GK, Grover FL, Hammermeister KE. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–511; discussion 511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT; Investigators of the Ischemia Research and Education Foundation, Multicenter Study of Perioperative Ischemia Research Group . A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. [DOI] [PubMed] [Google Scholar]
- 72. Banach M, Rysz J, Drozdz JA, Okonski P, Misztal M, Barylski M, Irzmanski R, Zaslonka J. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006;70:438–441. [DOI] [PubMed] [Google Scholar]
- 73. Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37:1353–1359. [DOI] [PubMed] [Google Scholar]
- 74. Filardo G, Damiano RJ, Ailawadi G, Thourani VH, Pollock BD, Sass DM, Phan TK, Nguyen H, da Graca B. Epidemiology of new‐onset atrial fibrillation following coronary artery bypass graft surgery. Heart. 2018;104:985–992. [DOI] [PubMed] [Google Scholar]
- 75. Pettersson GB, Martino D, Blackstone EH, Nowicki ER, Houghtaling PL, Sabik JF, Lytle BW. Advising complex patients who require complex heart operations. J Thorac Cardiovasc Surg. 2013;145:1159–1169.e3. [DOI] [PubMed] [Google Scholar]
- 76. Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long‐term risk of ischemic stroke. JAMA. 2014;312:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, Whitlock RP. Interventions for preventing post‐operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;1:CD003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. MOOSE Checklist for Meta‐Analyses of Observational Studies
Table S2. The Search Strategy for Ovid MEDLINE
Table S3. Critical Appraisal of Included Studies Using the Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies
Table S4. Stroke Definitions in the Included Studies
Table S5. Demographics of the Included Studies
Table S6. Summary of the Outcomes (Fixed‐Effect Model)
Table S7. Meta‐Regression for Early and Delayed Stroke (Restricted Maximum‐Likelihood Model)
Figure S1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flowchart.
Figure S2. Pooled event rate for perioperative stroke.
Figure S3. Reconstructed Kaplan‐Meier survival curves from derived individual patient data (IPD) for (A) no stroke vs perioperative stroke and (B) no stroke vs early and delayed stroke. Solid/dotted line represents aggregation of all available Kaplan‐Meier curves with 95% CI.
Figure S4. Leave‐one‐out analysis (top) and funnel plot (bottom) for incidence of (A) early stroke and (B) delayed stroke.


