Introduction
Since the first percutaneous coronary intervention (PCI) in 1977,1 interventional cardiology has undergone tremendous evolution in device technology and pharmacotherapy, which has made successful treatment of the most complex lesions possible. Despite the steady progress in nearly all facets of the field, the mechanical aspects of PCI—manipulation of coronary guidewires, balloons, and stents—and the occupational hazards for operators and catheterization laboratory staff remain largely unchanged. The interventional cardiologist works under the guidance of direct fluoroscopy to manipulate intravascular devices, and this requires donning heavy protective garments. Over the course of a career in interventional cardiology, operators are subject to the adverse consequences of cumulative radiation exposure and an increased prevalence of orthopedic injuries.2, 3
Robotic Assisted PCI: Evolution and Potential Advantages
In 2006, Beyar and colleagues developed a remote‐controlled robotic system to address the occupational hazards of interventional cardiology and the specific procedural challenges of PCI.4 With this platform, which was the basis for the CorPath 200 system (Corindus Vascular Robotics), operators could remotely control intravascular devices loaded onto a robotic cassette while sitting in a shielded interventional cockpit (Figure).5 Arterial access, diagnostic coronary angiography, and engagement of the guiding catheter were still achieved by the traditional manual method, with the primary operator standing at the catheterization laboratory table. Once the guiding catheter was engaged, operators could remove lead aprons and position themselves in the interventional cockpit to advance, retract, and rotate a guidewire with a joystick and touch‐screen interface. After achieving a satisfactory guidewire position distal to the lesion of interest, rapid‐exchange angioplasty balloons and stents could be deployed using the remote‐controlled platform.
Figure 1.
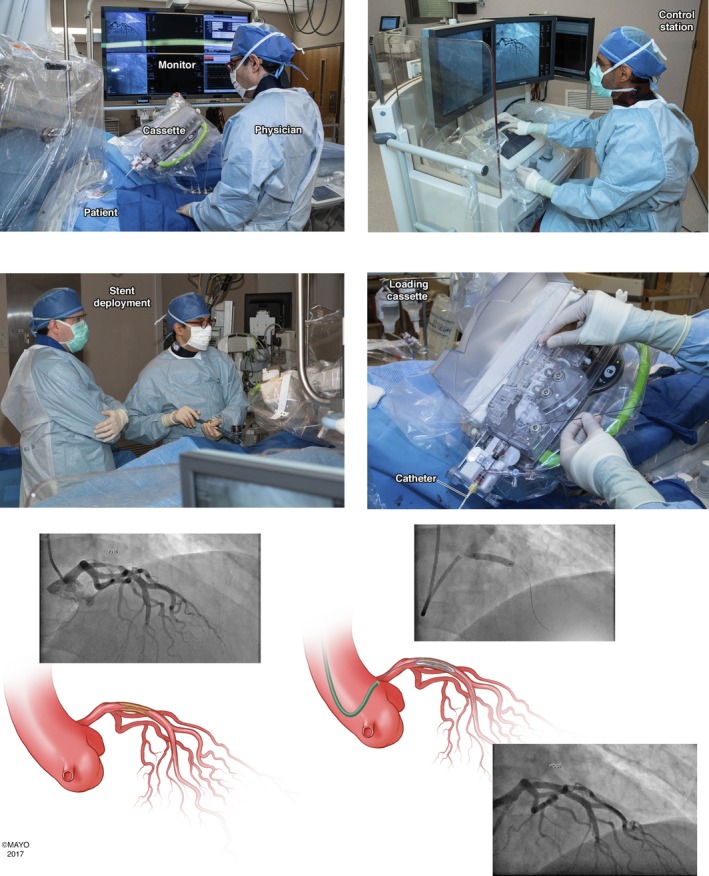
Use of the CorPath 200 system for robotic assisted percutaneous coronary intervention of the proximal left anterior descending artery (LAD). The procedure was done with transradial arterial access (top left). The interventional cardiologist manipulates the coronary guidewire, angioplasty balloons, and stents from the shielded interventional cockpit (top right). Stents are deployed in the usual fashion (bottom left). The coronary guidewire, angioplasty balloons, and stents are loaded onto the robotic cassette, which is attached to the guiding catheter (bottom right). Cineangiographic images and 2 illustrations show the lesion in the LAD (top left), stent deployment (top right), and the final result (bottom). Reproduced from Maor et al,5 which is an open access article published by Wiley under the terms of the Creative Commons Attribution‐NonCommercial License.
The safety of robotic assisted PCI (R‐PCI) with the CorPath 200 system was initially evaluated in the PRECISE (Percutaneous Robotically Enhanced Coronary Intervention) trial,6 which was a nonrandomized multicenter registry that enrolled 164 patients with at least 50% stenosis in a coronary artery 2.5 to 4.0 mm in size that could be covered with a single stent. Key exclusion criteria were presence of a previous stent within 5 mm of the planned stent deployment, planned atherectomy, intraluminal thrombus, severe tortuosity or calcification proximal to the lesion, ostial location, bifurcation lesion, and unprotected left main lesions. In all, 112 of 164 patients (68.3%) had type A or B1 lesions. The remainder of the patients had type B2 (18.9%) or type C (12.8%) lesions. Procedural success (without conversion to manual operation) was achieved in 162 of the 164 patients. There were no deaths, strokes, Q‐wave myocardial infarctions, or target lesion revascularization after 30 days of follow‐up. Finally, the median radiation exposure for the primary operator during the time in the interventional cockpit was 95.2% less than during the time spent at the procedure table. The results of this initial study represented a resounding success for the robotic platform in treating coronary lesions of low to moderate complexity.
But how would R‐PCI fare in treating increasingly complex lesions? With the lower profile and improved deliverability of the newest generation of intracoronary devices, there is the potential for wider application of R‐PCI. Initially, there were case reports of successful complex PCI, including treatment of diffusely diseased vessels requiring multiple stents, saphenous vein graft lesions with distal protection, unprotected left main stenosis, and acutely thrombosed vessels in the context of ST‐segment–elevation myocardial infarction (STEMI).7, 8 The CORA‐PCI (Complex Robotically Assisted Percutaneous Coronary Intervention) study was the first to systematically evaluate the role of R‐PCI in complex coronary interventions.9 This study was a nonrandomized single‐center comparison of patients undergoing R‐PCI as part of the PRECISION (Post‐Market CorPath Registry on the CorPath 200 System in Percutaneous Coronary Interventions) registry versus those undergoing manual PCI (M‐PCI) in the CathPCI registry. It is worth noting that a single operator performed all of the R‐PCI cases, whereas multiple operators performed the M‐PCI cases. Type C lesions constituted 69.4% of the R‐PCI cohort compared with 66.4% of the M‐PCI group. Important exclusions were STEMI cases requiring primary PCI, requirement for any over‐the‐wire devices, or planned bifurcation stenting. In the R‐PCI cohort, 81.5% of cases were completed fully robotically, with partial manual assistance required in 11.1% and full manual conversion required in 7.4% of cases. After propensity matching, there was no difference in contrast volume or dose area product between the 2 groups. Total procedure time was 43 minutes in the R‐PCI group versus 34 minutes in the M‐PCI group (P=0.007). There was no difference between in‐hospital or 12‐month major adverse cardiac events in the R‐PCI and M‐PCI groups.10 Despite the limitations of these data, namely, nonrandomized groups and a single center and single operator for the R‐PCI group, it is compelling to think that a majority of relatively complex PCI cases can be done with full robotic assistance. The CorPath GRX system, the second generation of the Corindus robotic platform, which is currently in use, has new features that further facilitate R‐PCI in complex and tortuous coronary anatomy, including remote manipulation of the guide catheter to help augment support after engagement and incorporation of wiring algorithms such as “rotate on retract,” which automatically rotates the wire up to 270° after pulling it back. These advances could potentially improve the success rates of complex R‐PCI, but as of yet, data are not available to test this hypothesis. R‐One (Robocath) is a new R‐PCI platform that has just received regulatory approval for usage in Europe. Although it has functional capabilities similar to those of the CorPath 200 system (eg, manipulation of coronary guidewire and 1 balloon or stent catheter), competition among R‐PCI systems may foster innovation that expands capabilities in the future.
In addition to mitigating occupational hazards for interventional cardiologists, R‐PCI offers the potential advantages of more precise measurements of lesion length and more stable deployment of angioplasty balloons and stents. In nonrandomized case series, R‐PCI was associated with reduced incidence of longitudinal geographic miss compared with M‐PCI.11 If combined with intravascular imaging guidance, it is possible that robotic platforms could further advance the precision of modern PCI technique, but this concept needs to be tested prospectively.
Perhaps the most innovative application of R‐PCI is the possibility of fully remote PCI procedures, or telestenting. Madder and colleagues reported the first case series of R‐PCI in which the primary operator and the interventional cockpit were located in a physically separate location from the patients (an adjacent catheterization laboratory).12 Madder subsequently led a team that performed successful PCI in a porcine subject from a distance of >100 miles.13 If made widely available, this technology could break down geographic constraints to achieve prompt primary PCI for STEMI in underserved areas; however, the current limitations of robotic platforms, as discussed next, present a significant barrier.
Current Limitations of R‐PCI
R‐PCI has a number of limitations that need to be recognized before it can achieve its full potential. Many of these limitations are technical and may be addressed through iteration and innovation. As mentioned, even with the current generation of robotic platforms, vascular access and engagement of the coronary artery with the guide catheter must still be done by the operator. In addition, a major impediment to widespread adoption of R‐PCI is the incompatibility of robotic platforms with a large number of devices and strategies in the standard interventional cardiology toolkit. Both generations of the CorPath device allow for manipulation of only 1 coronary guidewire at a time and positioning of only 1 balloon or stent simultaneously. Anatomic or lesion characteristics requiring planned use of any over‐the‐wire device including over‐the‐wire balloons or microcatheters (eg, chronic total occlusions) also preclude a complete robotic approach. Cases with tortuous or otherwise challenging anatomy requiring robust guide support may be very difficult to complete with current robotic platforms because guide catheter extensions are incompatible with these devices. Although the CorPath GRX system allows for robotically controlled guide catheter manipulation, this still may provide insufficient support for crossing lesions and delivering angioplasty balloons and stents in more complex cases. Heavily calcified lesions also pose significant obstacles for R‐PCI, given incompatibility with atherectomy devices and aforementioned difficulties with guide catheter support. Finally, R‐PCI systems cannot be used to manipulate some intravascular imaging catheters, so planned use of imaging would require manual assistance. The limitations of robotic platforms prevent them from fully alleviating the occupational hazards of interventional cardiology. The most complicated subset of cases (eg, bifurcations, chronic total occlusions, heavily calcified lesions, tortuous anatomy) are typically associated with the highest radiation exposure and case length; because many of these procedures cannot be completed with only robotic assistance, the putative occupational risk reduction of R‐PCI is diminished.
Although R‐PCI has been used successfully in STEMI cases, the lead time required to set up the robotic system following diagnostic angiography may be problematic in centers early in their R‐PCI learning curve. Similarly, unintended complications arising during R‐PCI, such as dissection, abrupt vessel closure, and perforation, can suddenly make a patient unstable during a procedure and necessitate conversion to manual operation for more rapid management of the underlying complication. It is important to note that, based on the current evidence, R‐PCI does not increase the likelihood of these complications, but managing such complications requires conversion to M‐PCI. This also challenges the potential promise of telestenting, especially to achieve more rapid reperfusion in primary PCI for STEMI. Vascular access, diagnostic coronary angiography, and placement of the coronary guide catheter must be done manually at the remote site. In addition, there must be an interventional cardiologist on site to deal with any PCI complications requiring conversion to M‐PCI. This limitation is a clear practical constraint for the possibilities of fully remote PCI because the availability of these bedside operators and appropriate catheterization facilities may present logistical and financial barriers to performing telestenting in underserved areas.
Summary
R‐PCI is an emerging technology with significant potential for transforming PCI. When used as indicated, R‐PCI appears to provide the interventional cardiologist with protection from radiation exposure and orthopedic injuries. The ability to manipulate the guide catheter and the implementation of wiring algorithms are significant advances in robotic technology; however, to reach its full potential, the next generations of R‐PCI systems must address the limitations of the current generation of devices. These include lack of compatibility with over‐the‐wire devices and ability to manipulate multiple devices (wires, balloons, stents) simultaneously so that more complex PCI cases can be completed without manual conversion. Until the issues regarding diagnostic angiography and handling of PCI complications are addressed, there will be significant barriers to robotic telestenting. Further iterations of the toolkit to deal with increasingly complex lesions and PCI complications, as are currently under way, will be necessary for widespread adoption of this technology by the interventional cardiology community.
Disclosures
None.
J Am Heart Assoc. 2019;8:e012743 DOI: 10.1161/JAHA.119.012743.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Gruntzig A, Senning A, Siegenthaler W. Nonoperative dilatation of coronary‐artery stenosis. N Engl J Med. 1979;301:61–68. [DOI] [PubMed] [Google Scholar]
- 2. Goldstein JA, Balter S, Cowley M, Hodgson J, Klein LW; Interventional Committee of the Society of Cardiovascular I . Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004;63:407–411. [DOI] [PubMed] [Google Scholar]
- 3. Klein LW, Tra Y, Garratt KN, Powell W, Lopez‐Cruz G, Chambers C, Goldstein JA; Society for Cardiovascular A and Interventions . Occupational health hazards of interventional cardiologists in the current decade: results of the 2014 SCAI membership survey. Catheter Cardiovasc Interv. 2015;86:913–924. [DOI] [PubMed] [Google Scholar]
- 4. Beyar R, Gruberg L, Deleanu D, Roguin A, Almagor Y, Cohen S, Kumar G, Wenderow T. Remote‐control percutaneous coronary interventions: concept, validation, and first‐in‐humans pilot clinical trial. J Am Coll Cardiol. 2006;47:296–300. [DOI] [PubMed] [Google Scholar]
- 5. Maor E, Eleid MF, Gulati R, Lerman A, Sandhu GS. Current and future use of robotic devices to perform percutaneous coronary interventions: a review. J Am Heart Assoc. 2017;6:e006239 DOI: 10.1161/JAHA.117.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisz G, Metzger DC, Caputo RP, Delgado JA, Marshall JJ, Vetrovec GW, Reisman M, Waksman R, Granada JF, Novack V, Moses JW, Carrozza JP. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically‐Enhanced Coronary Intervention) Study. J Am Coll Cardiol. 2013;61:1596–1600. [DOI] [PubMed] [Google Scholar]
- 7. Kapur V, Smilowitz NR, Weisz G. Complex robotic‐enhanced percutaneous coronary intervention. Catheter Cardiovasc Interv. 2014;83:915–921. [DOI] [PubMed] [Google Scholar]
- 8. Mahmud E, Dominguez A, Bahadorani J. First‐in‐human robotic percutaneous coronary intervention for unprotected left main stenosis. Catheter Cardiovasc Interv. 2016;88:565–570. [DOI] [PubMed] [Google Scholar]
- 9. Mahmud E, Naghi J, Ang L, Harrison J, Behnamfar O, Pourdjabbar A, Reeves R, Patel M. Demonstration of the safety and feasibility of robotically assisted percutaneous coronary intervention in complex coronary lesions: results of the CORA‐PCI study (Complex Robotically Assisted Percutaneous Coronary Intervention). JACC Cardiovasc Interv. 2017;10:1320–1327. [DOI] [PubMed] [Google Scholar]
- 10. Walters D, Reeves RR, Patel M, Naghi J, Ang L, Mahmud E. Complex robotic compared to manual coronary interventions: 6‐ and 12‐month outcomes. Catheter Cardiovasc Interv. 2019;93:613–617. [DOI] [PubMed] [Google Scholar]
- 11. Bezerra HG, Mehanna E, W Vetrovec G, A Costa M, Weisz G. Longitudinal geographic miss (LGM) in robotic assisted versus manual percutaneous coronary interventions. J Interv Cardiol. 2015;28:449–455. [DOI] [PubMed] [Google Scholar]
- 12. Madder RD, VanOosterhout SM, Jacoby ME, Collins JS, Borgman AS, Mulder AN, Elmore MA, Campbell JL, McNamara RF, Wohns DH. Percutaneous coronary intervention using a combination of robotics and telecommunications by an operator in a separate physical location from the patient: an early exploration into the feasibility of telestenting (the REMOTE‐PCI study). EuroIntervention. 2017;12:1569–1576. [DOI] [PubMed] [Google Scholar]
- 13. Madder RD, VanOosterhout S, Mulder A, Bush J, Martin S, Rash A, Tan JM, Parker J, Li Y, Kottenstette N, Bergman P, Nowak B. Feasibility of robotic telestenting over long geographic distances a pre‐clinical ex vivo and in vivo study. EuroIntervention. 2019; DOI: 10.4244/EIJ-D-19-00106. [DOI] [PubMed] [Google Scholar]


