Abstract
Background
The efficacy of nifekalant in preexcited atrial fibrillation (AF) has not been assessed.
Methods and Results
The study populations consisted of patients with sustained preexcited AF (n=51), paroxysmal supraventricular tachycardia (n=201), and persistent AF (n=87). Effects of intravenous infusion of nifekalant were assessed on electrophysiological and clinical parameters. Nifekalant prolonged the shortest preexcited R‐R, the average preexcited R‐R, and the average R‐R intervals from 290±35 to 333±44 ms, 353±49 to 443±64 ms, and 356±53 to 467±75 ms, respectively, in patients with preexcited AF (all P<0.001). Nifekalant also decreased the percentage of preexcited QRS complexes, heart rate, and increased systolic pressure (all P<0.001). Nifekalant terminated AF in 33 of 51 patients (65%). Similar effects were also observed in a subgroup of 12 patients with preexcited AF and impaired left ventricular function. In patients with paroxysmal supraventricular tachycardia, nifekalant significantly prolonged the effective refractory period, the block cycle length of the antegrade accessory pathway, and the atrial effective refractory period (all P<0.001). Nifekalant had no effect on the effective refractory period of the antegrade atrioventricular node. Finally, in patients with persistent AF without an accessory pathway, nifekalant did not significantly decrease the ventricular rate of AF. One patient developed Torsades de Pointes. No other adverse effects were observed.
Conclusions
Nifekalant prolongs the effective refractory period of the antegrade accessory pathway and atrium without blocking antegrade conduction through the atrioventricular node, leading to slowing and/or to termination of preexcited AF. Thus, nifekalant might be an effective and a relatively safe drug in patients with preexcited AF.
Keywords: atrial fibrillation, efficacy, nifekalant, Wolff‐Parkinson‐White syndrome
Subject Categories: Arrhythmias, Atrial Fibrillation, Treatment, Electrophysiology
Clinical Perspective
What Is New?
Nifekalant significantly decreased ventricular response rate and improved heart rate and blood pressure in patients with preexcited AF by prolonging the preexcited R‐R interval and decreasing the percentage of preexcited QRS complexes.
Nifekalant was equally effective in a subgroup of patients with impaired left ventricular function and preexcited AF.
Nifekalant significantly prolonged the effective refractory period and block cycle length of antegrade accessory pathway but had no effect on the antegrade conduction through the atrioventricular node (AVN).
What Are the Clinical Implications?
Nifekalant seems to be an effective and a relatively safe drug for treatment of patients with preexcited AF with or without impaired left ventricular function.
Intravenous nifekalant might serve as an alternative drug to treat patients with preexcited AF and a preferred drug for preexcited AF with impaired left ventricular function.
Introduction
Patients with Wolff‐Parkinson‐White syndrome and atrial fibrillation (AF), also known as preexcited AF, typically presents with a rapid ventricular response rate and wide QRS complexes. The fast conduction of atrial high‐frequency impulses occurs preferentially via the accessory pathway (AP), which has a shorter refractory period than the atrioventricular node (AVN). Consequently, patients are at a higher risk of hemodynamic instability, spontaneous ventricular fibrillation (VF), and cardiac arrest.1, 2, 3 Patients with preexcited AF who present with hemodynamic instability typically undergo prompt electrical cardioversion. Those with stable hemodynamics are commonly treated with infusion of an antiarrhythmic drug to decrease the preexcited ventricular rate and prevent hemodynamic instability. The 2017 European Heart Rhythm Association Consensus4 recommends procainamide, propafenone, flecainide, or ibutilide, whereas the 2014 American Heart Association Guidelines recommend5 procainamide or ibutilide for intravenous use to acutely slow ventricular rate of preexcited AF. The above 4 agents, however, are not recommended for the treatment of patients with preexcited AF and impaired left ventricular function (ILVF).6, 7, 8, 9 Intravenous amiodarone, a multichannel blocker, was widely used in the treatment of patients with preexcited AF and ILVF.10 However, the recent reports of accelerated preexcited ventricular responses and VF during amiodarone infusion have raised concerns about its utility.11, 12, 13, 14 Therefore, intravenous amiodarone is no longer considered the preferred drug of choice for treatment of preexcited AF.4, 5 Thus, there is a need for a new agent for treatment of preexcited AF, particularly in patients with ILVF.
Nifekalant, initially named MS‐551 in Japan, is a pure class 3 antiarrhythmic drug that is highly selective for blocking the rapid component of the delayed‐rectifier potassium currents without affecting the inward sodium and calcium currents or β‐adrenergic activity.15, 16 Nifekalant prolongs the effective refractory period (ERP) of ventricular and atrial myocytes, exerts antiarrhythmic effects,16 and decreases the defibrillation threshold.17 Increasing clinical data indicate that nifekalant is an effective and safe drug in treatment of refractory ventricular arrhythmias, especially in the patients with ILVF.18, 19, 20, 21 Nifekalant is also recommended by the European Resuscitation Council Guidelines and International Consensus on Cardiopulmonary Resuscitation for treatment of ventricular arrhythmias.22, 23 More recently, nifekalant has been used in treatment of atrial arrhythmia and cardioversion of AF and atrial flutter.24, 25 However, the potential utility of nifekalant in the treatment of preexcited AF has not been studied. The purpose of this study was to assess the electrophysiological and clinical effects of nifekalant in patients with preexcited AF.
Methods
The authors declare that all supporting data are available within the article.
We conducted 3‐part prospective noninvasive and invasive electrophysiology (EP) studies to determine the efficacy of nifekalant in preexcited AF and to assess its mechanisms of action (Figure 1). All patients provided written informed consent, and the study protocol was approved by the Committee on Human Research at the Second Affiliated Hospital of NanChang University, NanChang of JiangXi Province. All the antiarrhythmic medications were discontinued for at least 5 drug half‐lives before the study.
Figure 1.
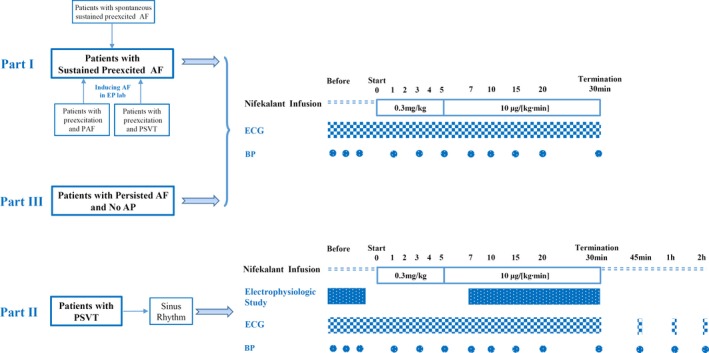
Protocol design. AF indicates atrial fibrillation; AP, accessory pathway; BP, blood pressure; EP, electrophysiological; PAF, paroxysmal AF; PSVT, paroxysmal supraventricular tachycardia.
Electrophysiological and Clinical Evaluation of Nifekalant in Patients With Preexcited AF
Fifty‐one patients (mean age 53±12 years, 33 males) with sustained preexcited AF and stable hemodynamics were treated with nifekalant. Twenty patients (39%) had spontaneous sustained preexcited AF (at least 1 hour before admission). Twelve (24%) had preexcitation and paroxysmal AF. These patients had sinus rhythm and preexcitation on ECG on admission. Nineteen patients (37%) had preexcitation and paroxysmal supraventricular tachycardia (PSVT) but no documented AF. They had sinus rhythm and preexcitation on ECG on admission. The latter 2 groups (31 patients) were induced to generate sustained AF during EP studies. Nifekalant was administered if induced AF was sustained for 10 minutes.
All patients undergoing electrophysiological stimulation for induction of AF were under the same anesthetic regimen as those who came in with spontaneous sustained preexcited AF. Both groups were given local anesthesia at the puncture sites before puncture. No other anesthetic was used during the EP studies and drug infusion. Twelve of 51 patients (24%) had structural heart disease and a low mean left ventricular ejection fraction of 36±6%. Clinical characteristics of the 51 patients, before infusion of nifekalant, are presented in Table 1.
Table 1.
Clinical Characteristics of the Patients Before Nifekalant Infusion
| Characteristics | |
|---|---|
| Patients with preexcited AF (n=51) | |
| Male sex, n (%) | 33 (65) |
| Age, y | 53±12 |
| History of cardiac disease, n (%) | 40 (78) |
| Spontaneous sustained preexcited AF, n (%) | 20 (39) |
| Induced sustained preexcited AF, n (%) | 31 (61) |
| Preexcitation and paroxysmal AF, n (%) | 12 (24) |
| Preexcitation and PSVT (no documented AF), n (%) | 19 (37) |
| LVEF (nonstructural heart disease), % | 61±5 |
| Structural heart disease, n (%) | 12 (24) |
| Rheumatic heart disease, n (%) | 4 (8) |
| Primary dilated cardiomyopathy, n (%) | 3 (6) |
| Ischemic cardiomyopathy, n (%) | 2 (4) |
| Hypertrophic cardiomyopathy, n (%) | 3 (6) |
| LVEF, % | 36±6 |
| Patients with PSVT (n=201) | |
| Male sex, n (%) | 128 (64) |
| Age, y | 38±13 |
| Preexcitation, n (%) | 87 (43) |
| Concealed AP and dual AVN pathway | 114 (57) |
| Structural heart disease, n (%) | 1 (0.5) |
| Ebstein anomaly, n (%) | 1 (0.5) |
| Patients with persistent AF and no AP (n=87) | |
| Male sex, n (%) | 52 (60) |
| Age, y | 53±11 |
| Structural heart disease, n (%) | 23 (26) |
| Ischemic cardiomyopathy, n (%) | 6 (7) |
| Rheumatic heart disease, n (%) | 4 (5) |
| Primary dilated cardiomyopathy, n (%) | 3 (3) |
| Hypertensive heart disease, n (%) | 7 (8) |
| Hypertrophic cardiomyopathy, n (%) | 3 (3) |
| LVEF, % | 39±5 |
AF indicates atrial fibrillation; AP, accessory pathway; AVN, atrioventricular node; LVEF, left ventricular ejection fraction; PSVT, paroxysmal supraventricular tachycardia.
EP studies were performed using a WorkMate Claris System (St. Jude Medical Inc, Little Canada, MN). Surface ECG and bipolar intracardiac electrograms were simultaneously recorded from high right atrium, coronary sinus, His bundle region, right ventricular apex, and left atrium (where appropriate). AF was routinely induced by rapid atrial decremental pacing (cycle length 200 to 50 ms; pulse width 2 ms). Isoproterenol and other adrenergic drugs were not used during EP studies, including induction of AF, in order to avoid potential confounding effects of adrenergic stimulation on the shortest preexcited R‐R (SPRR) interval and other electric parameters. AF was considered sustained if it lasted >10 minutes. Nifekalant infusion was started following 10 minutes of successful AF induction at an initial dose of 0.3 mg/kg for 5 minutes followed by the continuous infusion at 10 μg/kg per minute for 25 minutes. The infusion was discontinued when AF terminated during the infusion period.
The entire process of EP studies in preexcited AF was recorded and analyzed. Consecutive R‐R intervals for 1 minute were measured before and 1, 3, 5, 7, 10, 15, 20, and 30 minutes from onset of infusion. In addition, the R‐R interval was recorded and analyzed 1 minute before termination of AF. The EP parameters included the SPRR, average preexcited R‐R interval (APRR), average R‐R interval (ARR), the ratio of preexcited QRS complexes to total ventricular complexes (percentage preexcitation, PP). Clinical outcomes included heart rate (HR) and systolic blood pressure (SBP). The EP parameters and clinical outcomes were compared during AF at the baseline before the drug infusion (baseline state) and after nifekalant infusion (at infusion time of 7‐10 minutes, or 1 minute before termination of AF whenever cardioversion occurred before 7 minutes). AF cardioversion, acceleration of preexcited ventricular responses, deterioration to syncope/cardiac arrest, and Torsades de Pointes (TdP) were also investigated.
Electrophysiological Evaluation of Nifekalant in Patients With PSVT
A total of 201 patients with PSVT (mean age 38±13 years, 128 males) underwent EP studies (Table 1). All presented with sinus rhythm; 87 of 201 patients (43%) had preexcitation on ECG, and 114 (57%) had no evidence of preexcitation on ECG (concealed AP or dual AVN pathways). One patient had Ebstein anomaly, but the remainder had no structural heart disease. No patients had electrolyte abnormalities or a long QT interval (corrected QT interval [QTc] ≥470 ms).
Nifekalant was infused as described above. EP studies were performed at the baseline before the drug infusion (baseline state) and periodically during the infusion time (7‐30 minutes) as described above. Seven‐beat stimuli at a basic drive cycle length (S1) of 450 or 600 ms, delivered at −10‐ms decrements, were applied. The following indices were measured: antegrade AP ERP and block cycle length (BCL), antegrade AVN ERP, ERP of right atrium, ERP of right ventricle (RV); AH, HV, PR, QRS, and QT interval, as well as HR and blood pressure. Antegrade AP and AVN ERP were defined as the longest A1‐A2 interval that failed to conduct over the AP or AVN. The antegrade BCL over the AP was defined as the shortest S1 cycle length that maintained 1:1 conduction at a decremental paced interval of 10 ms. The right atrium and RV ERPs were defined as the longest S1‐S2 interval at which A2 (or V2) failed to produce a response. The antegrade AP ERP and BCL were measured in patients with preexcitation. The antegrade AVN ERP was recorded mainly in patients with no antegrade AP because it was generally longer than the ERP of the antegrade AP. QTc was calculated by the Bazett formula. The QT, PR, QRS, AH, and HV were measured only in patients with no antegrade AP to avoid interference of the preexcited wave. TdP and other arrhythmias were also identified and recorded.
Evaluation of Nifekalant in Patients With AF and No AP
Eighty‐seven patients (mean age 53±11 years, 52 males) with persistent AF and no AP were studied (Table 1). The participants did not have abnormal electrolytes, prolonged QT interval (QTc ≥470 ms), AV conduction disorders, or left atrial thrombus. Twenty‐three of the 87 patients (26%) had structural heart disease and a low left ventricular ejection fraction (39±5%).
Nifekalant was infused as described above, and the following parameters were assessed: ventricular rate of AF before, 1, 3, 5, 7, 10, 15, 20, and 30 minutes from onset of infusion, AF cardioversion, BP, TdP and other arrhythmias.
Statistical Analysis
Continuous variables with normal distribution were presented as mean values±SD, and the Student paired t test was used to determine the significance between baseline and drug infusion. The variable PP with skew distribution was presented as median (percentiles 25‐75), and Wilcoxon signed‐rank test was used. A 2‐sided P value of <0.05 was considered to indicate statistical significance.
Results
Effects of Nifekalant in Patients With Preexcited AF
The SPRR, APRR, ARR, and PP during AF were compared before and after nifekalant in 51 patients with sustained preexcited AF. As shown in Figure 2 and Table 2, nifekalant infusion was associated with prolongation of SPRR, APRR, and ARR, decreased PP and HR, and increased SBP (all P<0.001). In addition, nifekalant significantly decreased the proportion of patients with SPRR <260 ms and 260≤SPRR<300 ms from 22% to 6% and 37% to 16%, respectively. Likewise, nifekalant significantly increased the proportion of patients with 300≤SPRR<350 ms and SPRR ≥350 ms from 33% to 45% and from 8% to 33%, respectively. With the exception of 3 patients, nifekalant prolonged SPRR in all patients. Likewise, APRR and ARR were prolonged, and HR was decreased in all patients. Nifekalant infusion also reduced the PP in all except for 9 of 51 patients. It increased SBP in all but 3 patients. Figure 3 shows the characteristic responses to nifekalant infusion in 2 patients with preexcited AF (1 with normal left ventricular function and the other with ILVF).
Figure 2.
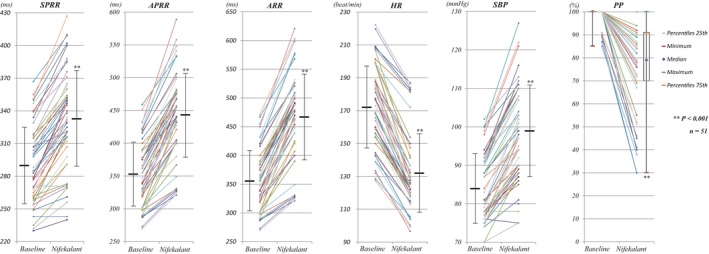
Electrophysiological and clinical effects of nifekalant in patients with preexcited AF (n=51). The lines with a dot at each end indicate the changes of parameters in every patient with preexcited AF. The horizontal bars and vertical lines represent mean values and standard deviations (for PP they represent median and percentiles 25‐75). AF indicates atrial fibrillation; APRR, average preexcited R‐R interval; ARR, average R‐R interval; HR, heart rate; PP, percentage preexcitation, the ratio of preexcited QRS complexes to total ventricular complexes during AF; SBP, systolic blood pressure; SPRR, shortest preexcited R‐R interval.
Table 2.
Effects of Nifekalant on the Electrophysiological and Clinical Indicators in Patients With Preexcited AF
| SPRR (ms) | APRR (ms) | ARR (ms) | PP (%) | HR (beats/min) | BP (mm Hg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ILVF Subgroup | Total | ILVF Subgroup | Total | ILVF Subgroup | Total | ILVF Subgroup | Total | ILVF Subgroup | Total | ILVF Subgroup | |
| Baseline | 290±35 | 281±30 | 353±49 | 350±41 | 356±53 | 354±45 | 100 (100‐100) | 100 (100‐100) | 172±25 | 172±24 | 84±9 | 76±3 |
| Nifekalant | 333±44a | 327±37a | 443±64a | 445±47a | 467±75a | 471±55a | 79 (70‐91)a | 74 (52‐90)a | 132±24a | 129±19a | 99±12a | 88±6a |
PP presented as median (25th‐75th percentiles). AF indicates atrial fibrillation; APRR, average preexcited R‐R interval; ARR, average R‐R interval; BP, blood pressure; HR, heart rate; ILVF, impaired left ventricular function; PP, percentage preexcitation, the ratio of preexcited QRS complexes to total ventricular complexes; SPRR, shortest preexcited R‐R interval.
P<0.001.
Figure 3.
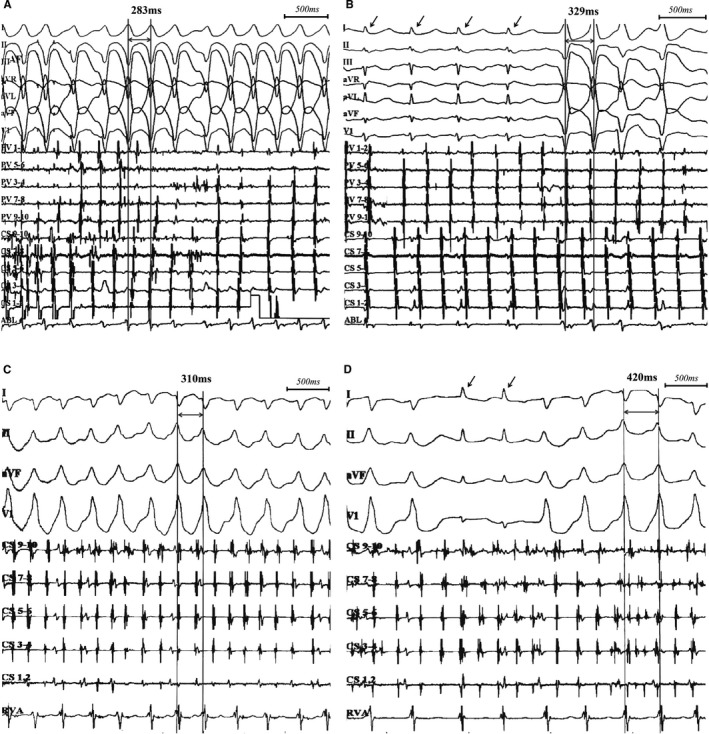
Characteristic response of nifekalant in 2 patients with preexcited AF. The first patient was a 47‐year‐old man with preexcitation and paroxysmal AF but no structural heart disease. Before nifekalant infusion, the SPRR was 283 ms, and there was no narrow QRS (A). At 7 minutes of nifekalant infusion, SPRR prolonged to 329 ms with an intermittent narrow QRS (B). This patient underwent the ablation of both AP and AF. The second patient was a 69‐year‐old woman with spontaneous sustained preexcited AF who had rheumatic heart disease with left ventricular ejection fraction 35%. Before nifekalant infusion, the SPRR was 310 ms and there was no narrow QRS (C). At 7 minutes of nifekalant infusion, SPRR prolonged to 420 ms with an intermittent narrow QRS (D). Arrows showed the narrow QRS and no preexcitation. This patient underwent the ablation of AP only. AF indicates atrial fibrillation; AP, accessory pathway; SPRR, shortest preexcited R‐R interval.
Nifekalant infusion led to termination of AF in 33 of 51 patients (65%), comprised of 27 patients with induced AF and 6 patients with spontaneous sustained AF. Cardioversion rate in patients with induced AF was 27/31 (87%), whereas it was 30% (6/20) in those with spontaneous sustained AF. Cardioversion of AF to sinus rhythm occurred with a mean time of 11±5 minutes (range from 4 to 26 minutes) from onset of infusion. The left atrial diameter in the spontaneous sustained AF patients was larger (41.9±6.6 mm) than that in the induced AF patients (34.1±3.7 mm) (P<0.001). Structural heart disease was more common in patients with spontaneous sustained AF (8/20, 40.0%) than in those with induced AF (4/31, 12.9%) (P=0.041). AF duration, determined from the onset of the patient's symptoms, such as palpitation, chest distress, or dyspnea to the start of nifekalant infusion, was 4.5 hours (2.5‐5 hours) (median [percentiles 25‐75]) in 6 patients with spontaneous sustained AF whose AF was terminated on nifekalant infusion. In contrast, AF duration was 9 hours (8‐18 hours) in 14 patients whose AF did not terminate on nifekalant infusion (P<0.001). The trend in ventricular response at different infusion time of nifekalant in patients with preexcited AF was analyzed, and no acceleration of preexcited ventricular responses was observed (Figure 4). The maximal therapeutic effects of SPRR, APRR, and ARR prolongation of nifekalant were observed at 7 to 10 minutes of infusion.
Figure 4.
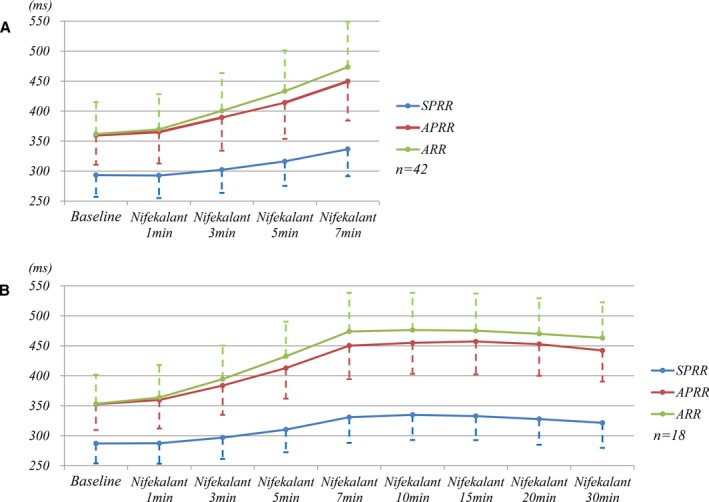
Trend of ventricular response at different infusion times of nifekalant during preexcited AF. A, The trend of preexcited ventricular response in 42 patients with AF not terminated before 7 minutes. B, The trend of 18 patients with AF not terminated before 30 minutes. Both panels showed no acceleration of ventricular response in patients with preexcited AF during the nifekalant infusion. The maximal therapeutic effects of nifekalant was at 7 to 10 minutes of infusion. AF indicates atrial fibrillation; APRR, average preexcited R‐R interval; ARR, average R‐R interval; SPRR, shortest preexcited R‐R interval.
One patient developed TdP after nifekalant infusion. This patient was a 58‐year old female who had spontaneous sustained preexcited AF. Twelve minutes after infusion, AF converted to sinus bradycardia (HR at 40 beats/min) and then TdP developed, which was immediately terminated by pacing the RV at 90 beats/min and a bolus intravenous injection of 2.5 g magnesium sulfate. The patient had a low blood potassium level of 3.0 mmol/L. AF changed to AFL in 2 patients. No patients developed VF, cardiac arrest, or syncope during nifekalant infusion. There was no transient loss of δ wave (defined as its complete disappearance on the ECG for at least 1 minute) in any of the patients during the infusion.
Effects on Preexcited AF With Structural Heart Disease
Twelve patients with preexcited AF had structural heart diseases and a reduced left ventricular ejection fraction (36±6%). Electrophysiological and clinical data before and after nifekalant infusion are shown in Table 2. Nifekalant infusion was associated with prolongation of SPRR, APRR, and ARR, decreased PP and HR, and increased SBP in patients with ILVF (all the P<0.001). Additionally, SPRR, APRR, and ARR were prolonged and the HR was decreased in all patients with ILVF after nifekalant infusion. Moreover, the PP was decreased and SBP was increased in all but 1 patient on nifekalant infusion.
Nifekalant infusion terminated AF in 4 of 12 patients (33%) with preexcited AF and ILVF. Of 4 patients who cardioverted, 3 had induced AF and 1 had spontaneous sustained AF. The mean time to AF cardioversion was 15±4 minutes (ranges: 10‐19 minutes).
No TdP occurred during nifekalant infusion in patients with preexcited AF and ILVF. Likewise, no patient developed acceleration of the preexcited ventricular responses, VF, cardiac arrest, or syncope. The δ wave persisted during nifekalant infusion.
Effects of Nifekalant on Antegrade AP and AVN Conduction in Patients With PSVT
The effects of nifekalant on antegrade conduction of AP were assessed in 78 of 87 patients with preexcitation. In the remaining 9 patients ERP of antegrade AP could not be determined because the atrium became refractory while AV conduction was still occurring over AP. As shown in Figure 5, nifekalant infusion was associated with prolongation of ERP and BCL of antegrade AP from 272±52 to 309±59 ms and from 323±51 to 381±63 ms, respectively (all the P<0.001). In addition, nifekalant infusion significantly decreased the proportion of patients with short ERP of antegrade AP (ERP <300 ms), and increased the proportion of patients with long ERP (ERP ≥300 ms) (Figure 5B). The δ wave persisted during nifekalant infusion in all patients with preexcitation.
Figure 5.
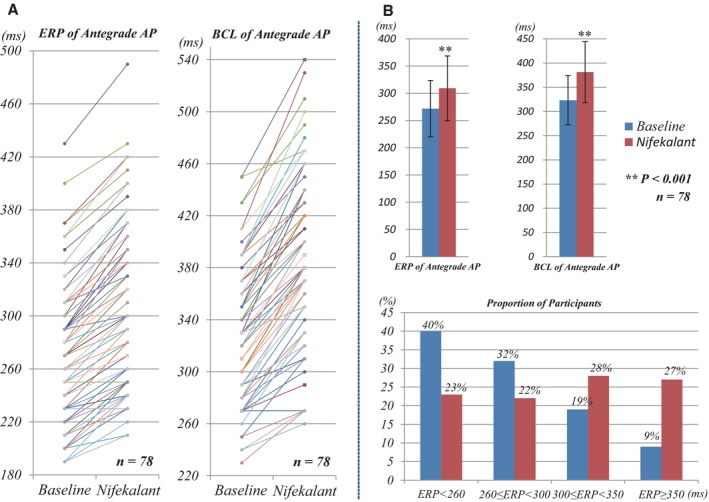
Effects of nifekalant on antegrade AP (n=78). A, The changes of ERP and BCL of antegrade AP in every patient in the baseline and nifekalant infusion state. B, Nifekalant was associated with the prolongation of ERP and BCL of antegrade AP from 272±52 to 309±59 ms and from 323±51 to 381±63 ms, respectively (all P<0.001). Nifekalant significantly decreased the proportion of patients with ERP <260 ms and 260≤ERP<300 ms from 40% to 23% and from 32% to 22%, respectively, increased the proportion of patients with 300≤ERP<350 ms and ERP≥350 ms from 19% to 28% and 9% to 27%, respectively. AP indicates accessory pathway; BCL, block cycle length; ERP, effective refractory period.
The effects of nifekalant on antegrade AVN conduction were assessed in 102 patients with PSVT (92 patients with concealed pathway or dual AVN pathway, 10 patients with preexcitation). ERP of the antegrade AVN was 315±69 ms at the baseline and 321±77 ms after nifekalant infusion (P=0.06; Figure 6). The presence of dual AVN pathways did not affect the effects on the AVN EP properties. There were no episodes of AV block during nifekalant infusion.
Figure 6.
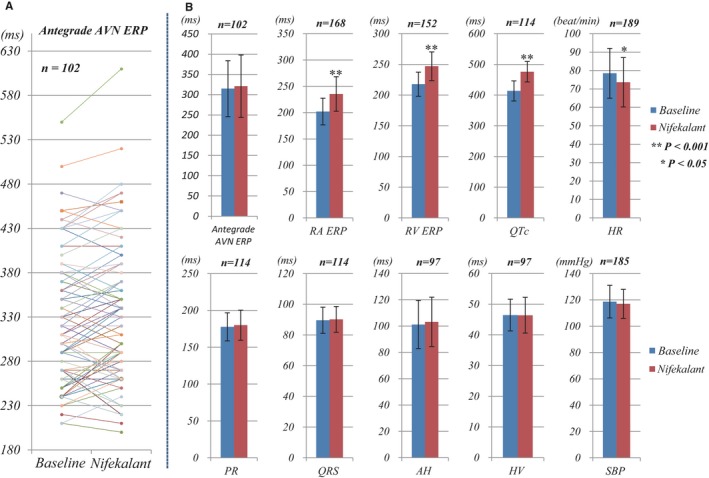
Effects of nifekalant on antegrade AVN and other parameters. A, The changes of antegrade AVN ERP in every patient in the baseline and nifekalant infusion state. B, The changes of AVN ERP and other parameters with nifekalant. The changes of antegrade AVN ERP with nifekalant were from 315±69 to 321±77 ms (n=102, P=0.06). Nifekalant significantly prolonged the ERP of RA, RV, and the QTc from 202±25 to 235±33 ms (n=168), 218±20 to 247±23 ms (n=152), and 413±33 to 476±33 ms (n=114), respectively (all P<0.001). Nifekalant slightly decreased the sinus rate from 79±14 to 74±13 beats/min (n=189, P=0.04). The changes of PR, QRS, AH, HV, and SBP with nifekalant were from 178±19 to 180±20 ms (n=114, P=0.41), 90±9 to 90±8 ms (n=114, P=0.53), 101±18 to 103±19 ms (n=97, P=0.26), 46±5 to 46±5 ms (n=97, P=0.75), and 119±12 to 117±11 mm Hg (n=185, P=0.30), respectively. AVN indicates atrioventricular node; ERP, effective refractory period; HR, heart rate; RA, right atrium; RV, right ventricle; SBP, systolic blood pressure.
The effects of nifekalant on ERP of right atrium and RV, interval of AH, HV, PR, QRS, and QT, sinus HR and SBP were also determined. As shown in Figure 6B, nifekalant infusion significantly prolonged the ERP of right atrium and RV as well as the QTc (all the P<0.001). It modestly decreased the sinus HR (from 79±14 to 74±13 beats/min, P=0.04). It did not change the PR, QRS, AH, or HV intervals or the SBP (P>0.05). All the above parameters were measured only during the sinus rhythm and not during PSVT. Patients with dual AVN pathways exhibited fast pathway conduction, and none had preferred slow pathway conduction when in sinus rhythm. Thus, the presence of dual AVN pathways did not affect measurement of the PR, AH, or HV interval.
The effects of nifekalant infusion on QTc before, during, and after infusion were assessed (n=114, Figure 7). Nifekalant increased QTc progressively, reaching the maximum effects at 10 minutes (Figure 7A). The prolonged QTc gradually recovered to its normal value at 1.5 hours after termination of infusion. Figure 7B shows the proportion of patients with different QTc in different infusion time points. No patient had a QTc ≥500 ms before infusion. At 10 minutes of infusion, 38 of 114 patients (33%) developed a QTc ≥500 ms but <550 ms. One patient (1%) had a QTc ≥550 ms. The QTc interval recovered to the baseline level 1.5 hours after termination of nifekalant infusion. No TdP or other arrhythmias occurred during and after nifekalant infusion.
Figure 7.
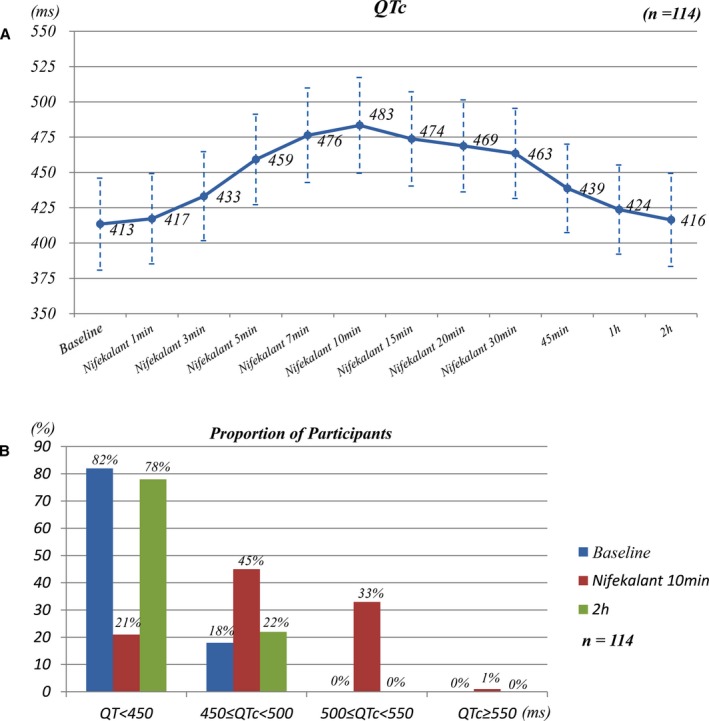
Effects of nifekalant on QTc at different times (n=114). A, The trend of QTc during and after nifekalant infusion. Nifekalant gradually increased the QTc along with the infusion time and reached its maximal effects at 10 minutes. The prolonged QTc gradually recovered to normal level at 2 hours (1.5 hours after infusion termination). B, The proportion of patients with different QTc durations at different times (before administration of nifekalant and after 10 minutes and 2 hours of infusion).
Effects of Nifekalant on AVN Conduction in Patients With AF and No AP
Electrophysiological effects of nifekalant on AVN conduction in patients with AF but no AP were determined. Nifekalant infusion terminated AF in 33 of 87 (38%) patients. Fifty‐four of 87 (62%) patients continued to have AF after 30 minutes of nifekalant treatment. The mean age of this group without AF termination was 58±13 years (range 18‐74 years), and 32 were male. Twenty of 54 patients (37%) had structural heart diseases and a low left ventricular ejection fraction (37±5%). The ventricular rate was 97±17, 97±16, 99±18, 101±20, 103±22, 104±24, 102±21, 96±19, and 95±15 beats/min at baseline and after 1, 3, 5, 7, 10, 15, 20, and 30 minutes of infusion, respectively. Nifekalant did not significantly decrease the mean ventricular rate of AF in the absence of an AP (97±17 versus 95±15 beats/min, n=54, P=0.22). A trend toward an increased ventricular rate was noted at infusion time 0 to 10 minutes, and a trend toward a decrease at 10 to 30 minutes of the infusion period. In addition, the mean SBP in patients with AF but no AP did not change with nifekalant infusion (106±16 versus 109±17 mm Hg, n=54, P=0.30). No TdP occurred in these patients during nifekalant infusion.
Discussion
We report the first prospective study examining electrophysiological and clinical effects of nifekalant in patients with preexcited AF. Nifekalant decreased ventricular responses in patients with preexcited AF through prolongation of the preexcited R‐R intervals (SPRR, APRR, and ARR) and decreasing the PP during AF. Nifekalant also significantly decreased the proportion of patients with SPRR <260 ms (high risk for VF) from 22% to 6%, suggesting a reduced risk of VF. Prolongation of ventricular ERP on nifekalant infusion is expected to provide additional protection against the risk of VF in those with a short ERP of the antegrade AP. Furthermore, nifekalant infusion was associated with improved hemodynamics in patients with preexcited AF, likely by decreasing the rapid ventricular rate resulting in alleviation of hypotension. Collectively, the data indicate nifekalant imparts desirable EP and clinical effects in patients with preexcited AF.
Nifekalant infusion was also associated with conversion of AF to sinus rhythm in 33 of 51 patients (65%), which occurred within 11±5 minutes of infusion. The cardioversion rate was higher in those with induced AF (87%) as compared with spontaneous sustained AF (30%). The sample size of the population in each group was rather small to allow firm conclusions. However, population characteristics seemed to differ between the 2 groups, as discussed earlier. For example, the subgroup of induced AF in the preexcitation and PSVT group had no history of documented AF. This subgroup had the highest cardioversion rate (17/19, 89.5%). These patients might have fewer triggers and substrates for AF than patients with sustained AF. In addition, structural heart disease was more common in the sustained group than in patients with induced AF. Moreover, the left atrial diameter was larger in the sustained AF patients. These differences in the characteristics of the 2 populations might affect the success rate of cardioversion with nifekalant. It seemed that a longer duration of AF was associated with a lower cardioversion rate in the group of spontaneous sustained preexcited AF, although the number of such patients was too small to permit any firm conclusions.
Nifekalant is a pure class 3 antiarrhythmic drug that is highly selective for blocking the delayed‐rectifier potassium currents and has no effect on the inward sodium and calcium currents.15, 16 Therefore, it has the advantage of not depressing ventricular contractility in patients with ILVF.18, 19, 20, 21 In the present study nifekalant was well tolerated and was effective in patients with preexcited AF and ILVF, supported by the significant prolongation of SPRR, APRR, and ARR, the decreases of PP and rapid ventricular rate, and the alleviation of hypotension. The findings suggest that nifekalant is a potentially safe and effective therapy for patients with preexcited AF with ILVF.
Nifekalant significantly prolonged the ERP of antegrade AP and the BCL of antegrade AP, resulting in the prolongation of the preexcited R‐R intervals. In contrast, nifekalant had no significant effect on AVN antegrade conduction, whether or not an AP was present and in those with AF but no AP. Our observations are consistent with the findings by Isomoto et al,26 who reported 11 patients with paroxysmal AF who received nifekalant infusion and had no significant change in the AVN ERP. Similarly, dofetilide, another highly selective blocker of delayed‐rectifier potassium currents, has no effect on the AVN antegrade conduction.27 The data suggest that the main mechanism of the effect of nifekalant in preexcited AF is through blockage of antegrade AP conduction (prolongation of the AP ERP) without affecting the AVN conduction. The effects lead to decreased preexcited ventricular responses, prolongation of the preexcited R‐R intervals, decrement of the PP, and increase in the percentage of narrow QRS complexes in preexcited AF. Nifekalant did not completely block the AP in patients with preexcited AF or PSVT. Therefore, the use of nifekalant is not expected to interfere with subsequent ablation of the AP. Furthermore, prolongation of atrial ERP with nifekalant infusion provides a mechanism for AF cardioversion similar to other Class 3 antiarrhythmic drugs.6, 28
The effects of procainamide, propafenone, flecainide, and ibutilide, recommended by the guideline or consensus,4, 5 on preexcited AF have been reported by several investigators. Sellers et al29 evaluated procainamide in 21 patients with preexcited AF. Boahene et al30 evaluated procainamide in 30 patients and propafenone in 25 patients. Ludmer et al31 evaluated propafenone in 10 patients, O'Nunain et al32 evaluated propafenone and flecainide in 16 patients, and Glatter et al33 evaluated ibutilide in 14 patients. All showed the effectiveness of the 4 agents in preexcited AF, albeit with different magnitudes of efficacy. For example, Boahene et al showed propafenone prolonged SPRR from 215±40 to 415±198 ms (ΔSPRR 200 ms), Ludmer et al31 showed propafenone ΔSPRR to be 75 ms, whereas O'Nunain et al showed propafenone ΔSPRR to be 37 ms, which indicated the presence of considerable heterogeneity among the studies. Factors contributing to the heterogeneity might include drug dose, infusion speed and time, intrinsic ERP of antegrade AP, hemodynamic status, sympathetic and parasympathetic tone, and others. The heterogeneity was more evident in the small‐sample‐size studies. Therefore, it is difficult to compare the effectiveness of nifekalant and other agents in treatment of preexcited AF. Randomized controlled studies with large samples would be valuable to determine the comparative effectiveness of these agents. One notable difference is that propafenone, flecainide, and ibutilide could completely block the AP, whereas nifekalant did not completely block AP in the present study and, hence, did not interfere with subsequent AP ablation. It is also important to note that procainamide, propafenone, flecainide, and ibutilide are not generally used in patients with ILVF, but nifekalant does not seem to have any deleterious effect in patients with ILVF. Thus, intravenous nifekalant might be considered as an alternative drug to treat preexcited AF in patients without left ventricular dysfunction and as a preferred drug in patients with preexcited AF and ILVF.
The primary concern with the use of nifekalant is the development of TdP. Katoh et al reported a TdP rate of 3.9% in 1402 emergency patients with ventricular arrhythmias who were treated with nifekalant,34 which was much lower than the corresponding rate of 8.3% for ibutilide.8 In the present study only 1 of 339 patients with preexcited AF, PSVT, or AF alone (0.3%) developed TdP during nifekalant infusion, which was associated with a low serum potassium level (3.0 mmol/L) and preceded by sinus bradycardia (40 beats/min). Nifekalant significantly prolonged the QT interval, which was transient and returned to normal within 1.5 hours after nifekalant termination. The latter likely reflects the short half‐life of nifekalant (1.5‐2.0 hours). QT prolongation by nifekalant was not associated with an arrhythmic event with the exception of 1 patient who developed TdP. Nifekalant slightly decreased the sinus rate but did not affect AH, HV, PR, QRS interval or SBP during sinus rhythm. In addition, nifekalant did not accelerate the preexcited ventricular responses or lead to VF or cardiac arrest.
AF induced in the EP lab often terminates spontaneously within 20 minutes. Our study did not use a randomized approach with a placebo group. This limitation of the study makes it difficult to interpret the efficacy of nifekalant in terminating AF in these patients. Hence, a randomized placebo‐controlled design with large samples would be expected to address this problem.
In conclusion, the findings of the present study show that nifekalant is an effective and a relatively safe drug for the treatment of patients with preexcited AF with or without ILVF. Nifekalant mainly exerts its effects by prolonging the ERP of antegrade AP without blocking the AVN conduction. Therefore, intravenous nifekalant might be a desirable agent for treatment of patients with preexcited AF, particularly in a subset of patients with ILVF.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (NSFC, 81860070).
Disclosures
None.
Acknowledgments
We are grateful to Chunhua Fu (Central Hospital of Xinyu Steel Company, China) and Huilin Xu (Jingdezhen First People's Hospital, China) for the partial data collection, and Xiaojing Lai (Second Affiliated Hospital of Nanchang University, China) for the statistics analyses.
(J Am Heart Assoc. 2019;8:e012511 DOI: 10.1161/JAHA.119.012511.)
Contributor Information
Juxiang Li, Email: lijuxiang1276@126.com.
Kui Hong, Email: hongkui88@163.com.
References
- 1. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff‐Parkinson‐White syndrome. N Engl J Med. 1979;301:1080–1085. [DOI] [PubMed] [Google Scholar]
- 2. Dreifus LS, Haiat R, Watanabe Y, Arriaga J, Reitman N. Ventricular fibrillation. A possible mechanism of sudden death in patients and Wolff‐Parkinson‐White syndrome. Circulation. 1971;43:520–527. [DOI] [PubMed] [Google Scholar]
- 3. Castellanos A Jr, Myerburg RJ, Craparo K, Befeler B, Agha AS. Factors regulating ventricular rates during atrial flutter and fibrillation in pre‐excitation (Wolff‐Parkinson‐White) syndrome. Br Heart J. 1973;35:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katritsis DG, Boriani G, Cosio FG, Hindricks G, Jaïs P, Josephson ME, Keegan R, Kim YH, Knight BP, Kuck KH, Lane DA, Lip GY, Malmborg H, Oral H, Pappone C, Themistoclakis S, Wood KA, Blomström‐Lundqvist C, Gorenek B, Dagres N, Dan GA, Vos MA, Kudaiberdieva G, Crijns H, Roberts‐Thomson K, Lin YJ, Vanegas D, Caorsi WR, Cronin E, Rickard J. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017;19:465–511. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 6. Dan GA, Martinez‐Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita F, van Gelder I, Gorenek B, Kaski JC, Kjeldsen K, Lip GYH, Merkely B, Okumura K, Piccini JP, Potpara T, Poulsen BK, Saba M, Savelieva I, Tamargo JL, Wolpert C; ESC Scientific Document Group . Antiarrhythmic drugs—clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace. 2018;20:731–732an. [DOI] [PubMed] [Google Scholar]
- 7. Blomström‐Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW Jr, Stevenson WG, Tomaselli GF, Antman EM, Smith SC Jr, Alpert JS, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines. Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias . ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108:1871–1909. [DOI] [PubMed] [Google Scholar]
- 8. Stambler BS, Wood MA, Ellenbogen KA, Perry KT, Wakefield LK, VanderLugt JT. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation. 1996;94:1613–1621. [DOI] [PubMed] [Google Scholar]
- 9. Oral H, Souza JJ, Michaud GF, Knight BP, Goyal R, Strickberger SA, Morady F. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med. 1999;340:1849–1854. [DOI] [PubMed] [Google Scholar]
- 10. Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: advanced cardiovascular life support: section 7: algorithm approach to ACLS emergencies: section 7A: principles and practice of ACLS. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102:I136–I139. [PubMed] [Google Scholar]
- 11. Boriani G, Biffi M, Frabetti L, Azzolini U, Sabbatani P, Bronzetti G, Capucci A, Magnani B. Ventricular fibrillation after intravenous amiodarone in Wolff‐Parkinson‐White syndrome with atrial fibrillation. Am Heart J. 1996;131:1214–1216. [DOI] [PubMed] [Google Scholar]
- 12. Sheinman BD, Evans T. Acceleration of ventricular rate by fibrillation associated with the Wolff‐Parkinson‐White syndrome. Br Med J (Clin Res Ed). 1982;285:999–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simonian SM, Lotfipour S, Wall C, Langdorf MI. Challenging the superiority of amiodarone for rate control in Wolff‐Parkinson‐White and atrial fibrillation. Intern Emerg Med. 2010;5:421–426. [DOI] [PubMed] [Google Scholar]
- 14. Tijunelis MA, Myth Herbert ME. Intravenous amiodarone is safe in patients with atrial fibrillation and Wolff‐Parkinson‐White syndrome in the emergency department. CJEM. 2005;7:262–265. [DOI] [PubMed] [Google Scholar]
- 15. Nakaya H, Tohse N, Takeda Y, Kanno M. Effects of MS‐551, a new class III antiarrhythmic drug, on action potential and membrane currents in rabbit ventricular myocytes. Br J Pharmacol. 1993;109:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakaya H, Uemura H. Electropharmacology of nifekalant, a new class III antiarrythmic drug. Cardiovasc Drug Rev. 1998;16:133–144. [Google Scholar]
- 17. Murakawa Y, Yamashita T, Kanese Y, Omata M. Can a class III antiarrhythmic drug improve electrical defibrillation efficacy during ventricular fibrillation? J Am Coll Cardiol. 1997;29:688–692. [DOI] [PubMed] [Google Scholar]
- 18. Amino M, Yoshioka K, Iwata O, Fujikura H, Deguchi Y, Ban K, Shiina Y, Goto S, Handa S, Tanabe T, Nakagawa Y, Morita S, Iwase H, Yamamoto I, Inokuchi S, Marutani Y. Efficacy of nifekalant hydrochloride for life‐threatening ventricular tachyarrhythmias in patients with resistance to lidocaine: a study of patients with out‐of‐hospital cardiac arrest. J Cardiol. 2003;41:127–134. [PubMed] [Google Scholar]
- 19. Ando J, Kakishita M, Sakai K, Komura Y, Nishiyama K, Iwabuchi M, Yokoi H, Yasumoto H, Nosaka H, Nobuyoshi M. Efficacy of nifekalant hydrochloride in the treatment of fatal ventricular arrhythmia in patients with ischemic heart disease. Int Heart J. 2005;46:647–656. [DOI] [PubMed] [Google Scholar]
- 20. Shiga T, Tanaka K, Kato R, Amino M, Matsudo Y, Honda T, Sagara K, Takahashi A, Katoh T, Urashima M, Ogawa S, Takano T, Kasanuki H; Refractory VT/VF, Prospective Evaluation to Differentiate Lidocaine Efficacy from Nifekalant (RELIEF) Study Investigators . Nifekalant versus lidocaine for in‐hospital shock‐resistant ventricular fibrillation or tachycardia. Resuscitation. 2010;81:47–52. [DOI] [PubMed] [Google Scholar]
- 21. Tagami T, Matsui H, Ishinokami S, Oyanagi M, Kitahashi A, Fukuda R, Unemoto K, Fushimi K, Yasunaga H. Amiodarone or nifekalant upon hospital arrival for refractory ventricular fibrillation after out‐of‐hospital cardiac arrest. Resuscitation. 2016;109:127–132. [DOI] [PubMed] [Google Scholar]
- 22. Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, Deakin C, Koster RW, Wyllie J, Böttiger B; ERC Guidelines Writing Group . European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81:1219–1276. [DOI] [PubMed] [Google Scholar]
- 23. Morrison LJ, Deakin CD, Morley PT, Callaway CW, Kerber RE, Kronick SL, Lavonas EJ, Link MS, Neumar RW, Otto CW, Parr M, Shuster M, Sunde K, Peberdy MA, Tang W, Hoek TL, Böttiger BW, Drajer S, Lim SH, Nolan JP; Advanced Life Support Chapter Collaborators . Part 8: advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S345–S421. [DOI] [PubMed] [Google Scholar]
- 24. Morita N, Tanaka K, Yodogawa K, Hayashi M, Akutsu K, Yamamoto T, Satoh N, Kobayashi Y, Katoh T, Takano T. Effect of nifekalant for acute conversion of atrial flutter: the possible termination mechanism of typical atrial flutter. Pacing Clin Electrophysiol. 2007;30:1242–1253. [DOI] [PubMed] [Google Scholar]
- 25. Kumagai K, Toyama H. Usefulness of ablation of complex fractionated atrial electrograms using nifekalant in persistent atrial fibrillation. J Cardiol. 2013;61:44–48. [DOI] [PubMed] [Google Scholar]
- 26. Isomoto S, Konoe A, Centurion OA, Hayano M, Kaibara M, Hirata T, Yano K. Electrophysiological effects of MS‐551 in humans: a class III antiarrhythmic agent. Pacing Clin Electrophysiol. 1995;18:2022–2027. [DOI] [PubMed] [Google Scholar]
- 27. Falk RH, Pollak A, Singh SN, Friedrich T. Intravenous dofetilide, a class III antiarrhythmic agent, for the termination of sustained atrial fibrillation or flutter. Intravenous Dofetilide Investigators. J Am Coll Cardiol. 1997;29:385–390. [DOI] [PubMed] [Google Scholar]
- 28. Lei M, Wu L, Terrar DA, Huang CL. Modernized classification of cardiac antiarrhythmic drugs. Circulation. 2018;138:1879–1896. [DOI] [PubMed] [Google Scholar]
- 29. Sellers TD Jr, Campbell RW, Bashore TM, Gallagher JJ. Effects of procainamide and quinidine sulfate in the Wolff‐Parkinson‐White syndrome. Circulation. 1977;55:15–22. [DOI] [PubMed] [Google Scholar]
- 30. Boahene KA, Klein GJ, Yee R, Sharma AD, Fujimura O. Termination of acute atrial fibrillation in the Wolff‐Parkinson‐White syndrome by procainamide and propafenone: importance of atrial fibrillatory cycle length. J Am Coll Cardiol. 1990;16:1408–1414. [DOI] [PubMed] [Google Scholar]
- 31. Ludmer PL, McGowan NE, Antman EM, Friedman PL. Efficacy of propafenone in Wolff‐Parkinson‐White syndrome: electrophysiologic findings and long‐term follow‐up. J Am Coll Cardiol. 1987;9:1357–1363. [DOI] [PubMed] [Google Scholar]
- 32. O'Nunain S, Garratt CJ, Linker NJ, Gill J, Ward DE, Camm AJ. A comparison of intravenous propafenone and flecainide in the treatment of tachycardias associated with the Wolff‐Parkinson‐White syndrome. Pacing Clin Electrophysiol. 1991;14:2028–2034. [DOI] [PubMed] [Google Scholar]
- 33. Glatter KA, Dorostkar PC, Yang Y, Lee RJ, Van Hare GF, Keung E, Modin G, Scheinman MM. Electrophysiological effects of ibutilide in patients with accessory pathways. Circulation. 2001;104:1933–1939. [DOI] [PubMed] [Google Scholar]
- 34. Katoh T, Mitamura H, Matsuda N, Takano T, Ogawa S, Kasanuki H. Emergency treatment with nifekalant, a novel class III anti‐arrhythmic agent, for life‐threatening refractory ventricular tachyarrhythmias: post‐marketing special investigation. Circ J. 2005;69:1237–1243. [DOI] [PubMed] [Google Scholar]


