Abstract
Background
AGT (angiotensinogen) synthesis occurs in renal proximal tubular epithelial cells, independent from systemic AGT, as a component of the intrarenal renin–angiotensin system. We investigated urinary AGT, as a biomarker for renin–angiotensin system activation, and electrolyte concentrations, in relation to glomerular volume, as a proxy for glomerular endotheliosis in renal biopsy tissue from pregnant normotensive control and hypertensive women.
Methods and Results
Urine samples were collected from normotensive control (n=10), gestational hypertensive (n=6), and pre‐eclamptic (n=16) women at the time a renal biopsy was obtained. Samples were collected from Lund University Hospital between November 1999 and June 2001. Urinary AGT, potassium, and sodium were measured, normalized to urinary creatinine. Mean glomerular volume was estimated from biopsy sections. AGT protein expression and localization were assessed in renal biopsies by immunohistochemistry. Urinary AGT concentrations were higher in hypertensive pregnancies (median, gestational hypertension: 11.3 ng/mmol [interquartile range: 2.8–13.6]; preeclampsia: 8.4 ng/mmol [interquartile range: 4.2–29.1]; normotensive control: 0.6 ng/mmol [interquartile range: 0.4–0.8]; P<0.0001) and showed a positive relationship with estimated mean glomerular volume. Urinary potassium strongly correlated with urinary AGT (P<0.0001). Although numbers were small, AGT protein was found in both glomeruli and proximal tubules in normotensive control but was present only in proximal tubules in women with hypertensive pregnancy.
Conclusions
This study shows that pregnant women with gestational hypertension or preeclampsia have increased urinary AGT and potassium excretion associated with signs of glomerular swelling. Our data suggest that the kidneys of women with hypertensive pregnancies and endotheliosis have inappropriate intrarenal renin–angiotensin system activation, which may contribute toward the pathogenesis of hypertension and renal injury.
Keywords: angiotensinogen, glomerular, hypertension, kidney, pregnancy, urine
Subject Categories: ACE/Angiotension Receptors/Renin Angiotensin System, Basic Science Research, Physiology, Preeclampsia, Hypertension
Clinical Perspective
What Is New?
To the best of our knowledge, this study is the first to investigate, both functionally and histologically, intrarenal angiotensinogen in normal and hypertensive human pregnancies.
What Are the Clinical Implications?
Monitoring urinary angiotensinogen in preeclampsia as a marker of an inappropriately activated renin–angiotensin system might be a useful indicator of renal injury and its progression both pre‐ and postpartum.
Introduction
The renin‐angiotensin system (RAS) was originally described as a classical endocrine system, meaning that hormones are produced by glands in one organ and have remote effects elsewhere in the body after being transported by the circulatory system.1 However, it is now known that several tissues have autonomous or quasi‐autonomous RAS, among them the placenta and kidney.2, 3 In the proximal tubules of the kidney, all components of the RAS necessary for Ang II (angiotensin II) synthesis (ie, renin, AGT [angiotensinogen], and ACE [angiotensin‐converting enzyme]) are present, together with the downstream effectors of RAS activation, Ang II type 1 receptor (AT1R) and AT2R.4
During pregnancy, considerable homeorhetic responses are activated to enable the expansion of blood volume during early gestation that facilitates perfusion of the placenta and fetal growth.5 To allow for such dynamic changes in circulatory volume, concomitant adaptations are made in hormonal axes that regulate blood volume, including the RAS, which is activated in the first few weeks of pregnancy.6 The effects of continuing progesterone synthesis by the corpus luteum after a conception cycle, if unchecked, would result in sodium loss because of competitive binding to the structurally similar aldosterone receptor MR (mineralocorticoid receptor). However, early sodium loss is recognized by the macula densa and the RAS becomes activated, in turn stimulating adrenal cortical synthesis of aldosterone and improved sodium retention. Increased estrogen synthesis also stimulates hepatic AGT synthesis. In addition, during pregnancy, Ang II itself further enhances sodium retention through a direct action in the proximal tubule. There, Ang II is able to mediate through AT1R both increased uptake of circulating Ang II and augmentation of AGT expression, which can lead to local formation of Ang II and spillover of AGT excretion into the urine, where it can be measured as a marker of intrarenal RAS activity. In addition, renin secretion from principal cells of the collecting ducts can be increased by AT1R activation, and the activity of renin and/or prorenin can be enhanced by binding to the (pro)renin receptor either on intercalated cells or in soluble form. All described feed‐forward mechanisms can, together, augment intratubular and renal interstitial Ang II concentrations, contributing to further renal vasoconstriction and sodium reabsorption.4 Consequently, maternal plasma volume increases significantly from around 6 weeks’ gestation.
Normally, such extensive sodium retention would be expected to be accompanied by a reciprocal increase in the secretion of potassium, and a relative kaliuresis.7 However, pregnant women cumulatively retain some 300 to 350 mEq of potassium during normal pregnancy.8 There is a paucity of data on potassium homeostasis during normal pregnancy, let alone during pregnancies complicated by gestational hypertension (GH). One early study suggested that the kidneys of pregnant women become refractory to the kaliuretic effects of increased RAS and estrogen, perhaps due to an increased sensitivity to progesterone.9 To our knowledge, no clinical studies have reported associations between potassium homeostasis during pregnancy in women with GH alone and/or in women with proteinuric GH (preeclampsia).
Preeclampsia affects 2% to 8% of pregnant women worldwide and is one of the top 3 causes of maternal death.10 Perturbations of activity of the RAS in preeclampsia have been noted for >50 years,11 but research into the role of a dysfunctional RAS in preeclampsia has been limited. ACE inhibitors are well‐known teratogens in animal models12 and in humans13, 14 and are contraindicated in later pregnancy because of their serious adverse effects on the fetus.15 Nevertheless, Zhou et al recently showed that oxidation of circulating AGT is associated with increased generation of Ang I,16 and the proportion of circulating oxidized AGT is increased in pre‐eclamptic pregnancies.16
A number of other mechanisms exist by which not only circulating and/or local placental RAS but also an inappropriately activated intrarenal RAS could initiate or contribute to the pathology of preeclampsia through, for instance, angiogenic factors,17 the immune18 and coagulatory systems, and the complement system.19
To date, no correlation between plasma and urinary AGT in pregnant women has been shown, suggesting that autonomous regulation of the renal RAS occurs during pregnancy as it does in the nonpregnant state. Urinary AGT has been shown to reflect the rate of intrarenal synthesis and can be regarded as a biomarker of intrarenal RAS activity20; levels of the marker may also predict acute kidney injury and failure.21
In this study, for the first time, we examined urinary AGT, sodium, and potassium in normotensive and hypertensive pregnancies as a potential biomarker of intrarenal RAS activity, related to glomerular swelling as a sign of endotheliosis. In a limited number of kidney biopsies from this cohort,22 we confirmed the presence of intrarenal AGT by immunohistochemistry. Evidence of an important role for the inappropriate activation of intrarenal RAS in the pathophysiology of preeclampsia could indicate novel management and postnatal therapeutic strategies and targets to prevent short‐term and long‐term renal injury.
Methods
The data and analytic methods, but not study materials, will be available to other researchers for purposes of reproducing the results or replicating the procedure. Material is not freely available because of the limited amounts and potential degradation.
Sample Collection
Fully informed, signed consent for participation was obtained from all women, as previously described,22 following the ethics committee approval of the study by the University of Lund, and all procedures involving the participants were in accordance with the Helsinki Declaration of 1975. GH was defined as diastolic blood pressure >90 mm Hg, determined on >2 occasions >4 hours apart and arising after 20 weeks of gestation. Preeclampsia was defined as for GH but also including proteinuria (urine protein concentration >3000 mg/L in 2 random clean‐catch midstream specimens collected ≥4 hours apart). No woman had any known underlying renal or hypertensive disease before 20 weeks’ gestation.
Overnight urine samples were taken 1 to 3 days before renal biopsy in women with newly diagnosed GH admitted to Lund University Hospital from November 1999 to June 2001. All samples were collected before beginning antihypertensive treatment. Healthy pregnant women with comparable gestational age who were recruited from maternal healthcare centers in the catchment area of Lund University Hospital provided overnight urine samples as controls. Samples with evidence of urinary tract infection on urinalysis were excluded from all analyses. All samples were stored at −80°C in aliquots until analysis. Urine samples were available from 32 participants (10 normotensive controls, 6 women with GH, and 16 pre‐eclamptic women).
Renal biopsies were obtained as described previously.23 Briefly, biopsies were collected by an experienced radiologist according to a standard procedure. The sample was taken with a 1.2‐mm (outside diameter) needle using a Bard biopsy device under ultrasound guidance. Each biopsy, ≈20 mm in length, was immediately submerged in 0.9% sodium chloride solution and processed, with 1 part fixed in neutral formaldehyde 4% for immunohistochemical analysis.23
Biochemical Assays
Urinary AGT concentrations were measured in duplicate by ELISA, following the manufacturer's protocol. Urine samples were diluted 1:10 using assay buffer. Intra‐ and interassay coefficients of variation were 4.4% and 5.6%, respectively. The mean urinary AGT concentration for each woman was normalized to urinary creatinine. Urinary volume and creatinine concentrations were measured in the Clinical Biochemistry Laboratory at Lund University by standard clinically validated methods. Urinary potassium and sodium concentrations were measured by inductively coupled plasma mass spectroscopy, as described previously, with specific standards.24, 25 All analyses were conducted as blinded to pregnancy outcome.
Glomerular Volume
Mean glomerular volume had been estimated previously in biopsies containing at least 6 glomerular sections by a computer‐assisted stereological algorithm, as detailed previously.22
Immunohistochemical Analysis From Matched Renal Biopsies
Only a limited amount of renal tissue remained from the biopsies after previous evaluations and analyses,23 and samples were used opportunistically for a preliminary evaluation of the immunohistochemical distribution of AGT within the nephron. Three biopsies contained glomeruli (2–4), but no biopsies contained sufficient vasculature for meaningful analysis. Protein expression in the kidney was analyzed by immunohistochemistry using the Dako Envision staining kits, as described previously,26 with rabbit anti‐AGT (0.6 mg/mL; HPA0031557 [Sigma Prestige]). For immunohistochemistry, a semiquantitative scoring system was applied, as described by Roy‐Chaudhury et al.27 In brief, the scale was as follows: 0=no specific staining, 0.5=very weakly positive, 1=weakly positive, 2=moderately strongly positive, 3=strongly positive. Scoring was generally influenced by the extent rather than the intensity of staining (ie, higher scores were given for widespread staining as opposed to evidence of localized intense staining).
Statistical Analysis
Data were first assessed for distribution before analysis using SPSS (v24; IBM Corp). Urinary data were not normally distributed when all 3 groups (controls, GH, pre‐eclamptic pregnancies) were initially combined and thus are presented as median (interquartile range [IQR]; 25–75% quartiles] unless otherwise stated. Kruskal–Wallis nonparametric ANOVA was used throughout the study, with the null hypothesis being rejected at P<0.05. Linear correlations were computed using Spearman ρ. The association of urine potassium with AGT concentration was evaluated after log10 normalization. Curve characteristics were determined by regression analysis (GraphPad Prism, v6).
Results
Demographics of the Study Population
Table summarizes the basic demographic and pregnancy outcome data.
Table 1.
Patient Demographics, BP, and Proteinuria and Urinary Sodium, Potassium, and Albumin Concentrations in NCs and Women With GH or Preeclampsia
| Parameter | NC (n=10) | GH (n=6) | Preeclampsia (n=16) |
|---|---|---|---|
| Maternal age, y | 29 (27–34) | 28 (28–30) | 30 (26–36) |
| BMI at booking, kg/m2 | 22.1 (21.3–22.3)* , † | 25.9 (25.4–26.5)* | 25.7 (24.3–30.3)† |
| After clinical diagnosis | |||
| Highest systolic BP | 130 (120–130)* , † | 145 (140–150)* , ‡ | 160 (160–180)† , ‡ |
| Highest diastolic BP | 75 (75–85)* , † | 110 (95–110)* | 110 (107–118)† |
| Proteinuria, g/L | ··· | 146 (84–251)‡ | 931 (701–1814)‡ |
| PCR, mg/mmol | ··· | 13.2 (5.8–22.5)‡ | 151.5 (77.3–270.9)‡ |
| Gestation at delivery, d | 281 (274–288)* , † | 257 (254–265)‡ | 244 (233–256)† |
| Birth weight, g | 3655 (3430–3890)* , † | 2935 (2865–3205)* | 2630 (2030–2840)† |
| Sodium, mg/mmol | 33.3 (30.3–65.4)* , † | 325.1 (194.1–429.5)* | 344.2 (168.6–471.4)† |
| Potassium, mg/mmol | 18.9 (15.2–32.8)* , † | 216.8 (194–292.2)* | 222.9 (165.9–288.5)† |
| Albumin, mg/mmol | 0.1 (0.02–0.2)* , † | 10.1 (7.5–13.4)* , ‡ | 59.7 (29.3–176.8)† , ‡ |
Data are presented as median (interquartile range) for continuous variables. Urinary parameters are normalized to creatinine levels. Groups were compared by Kruskall–Wallis. BMI indicates body mass index; BP, blood pressure; GH, gestational hypertension; NC, normotensive control; PCR indicates protein:creatinine ratio.
P<0.05 between NC and GH.
P<0.05 between NC and preeclampsia.
P<0.05 between GH and preeclampsia.
Urinary AGT Assay
Urinary AGT was significantly elevated in hypertensive pregnancies compared with normotensive controls (in nanograms of AGT per millimole of creatinine; normotensive control: 0.6 [IQR: 0.4–0.8]; GH: 11.3 [IQR: 2.8–13.6]; preeclampsia: 8.4 [IQR: 4.2–29.1]; Kruskal–Wallis, P<0.0001; Figure 1). Post hoc Mann–Whitney tests showed highly significant differences between normotensive controls and women with either GH (P=0.002) and preeclampsia (P<0.0001). The difference between women with GH and preeclampsia was not significant (P>0.3).
Figure 1.
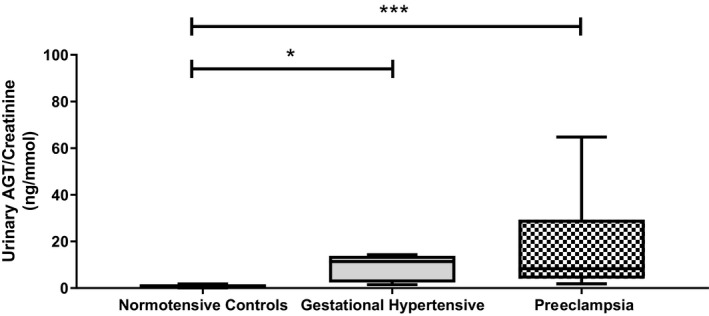
Urinary AGT (angiotensinogen) concentrations in normotensive control, gestational hypertensive, and pre‐eclamptic women. Data normalized to creatinine levels and presented as median (interquartile range). *P<0.05; ***P<0.0001.
The patients’ blood pressures were measured at varying times throughout pregnancy. We considered only the highest recorded systolic and diastolic pressures for each patient in relation to urinary AGT output at the time and during the days of sampling. Regression analysis revealed highly significant associations between log10 urinary AGT/creatinine and maximum systolic (r=0.623; P<0.0001) and diastolic (r=0.787; P<0.0001) pressures. Furthermore, including log10 urinary potassium/creatinine output in the analysis with diastolic pressure significantly (P<0.02) improved r to 0.829, whereas adding in urinary sodium/creatinine output was without effect.
Severity of preeclampsia is partly defined by the level of proteinuria/albuminuria.28, 29 Figure 2 shows a strong linear association between albuminuria and urinary AGT (r=0.76; P<0.0001) across the groups.
Figure 2.
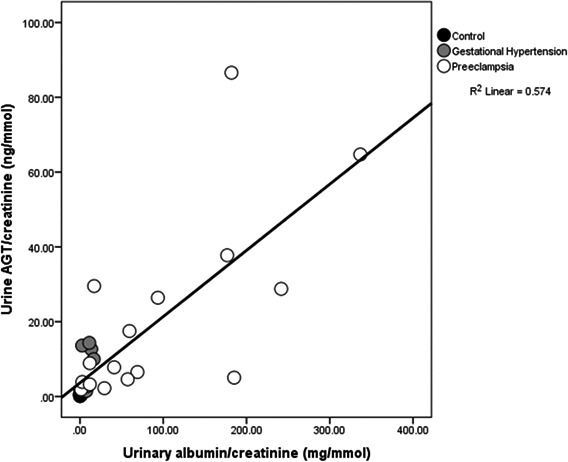
Scatterplot illustrating a strong positive relationship between urinary AGT (angiotensinogen) concentrations and albuminuria (r=0.76; P<0.0001).
Urine Composition
Urinary sodium and potassium concentrations were significantly higher in both hypertensive groups compared with the normotensive controls (P<0.001 for all; Table). Figure 3 shows a highly significant positive relationship between urinary potassium concentration and AGT in all groups (r=0.86; P<0.0001). No such association was identified between urinary sodium concentration and AGT (P>0.1).
Figure 3.
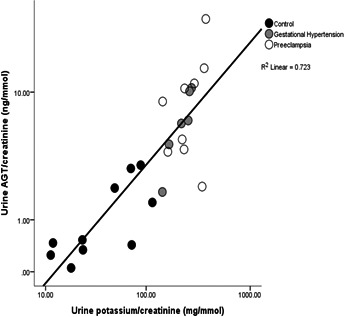
Scatterplot illustrating a clear positive relationship between urinary AGT (angiotensinogen) and potassium in all groups collectively (r=0.86; P<0.0001). Data normalized to creatinine levels and presented in log scale.
Glomerular Volume Calculations
Glomerular volume estimation was available only for women with GH (n=3) and preeclampsia (n=10). Figure 4 shows that the higher glomerular volumes were associated with higher urinary AGT, but the sample size was too small to allow statistical analysis.
Figure 4.
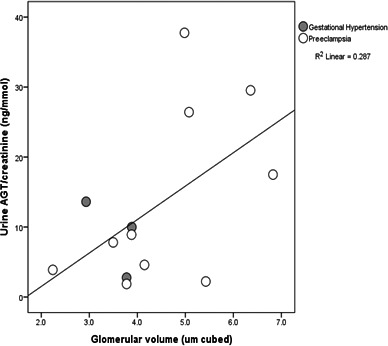
Scatterplot illustrating the positive relationship between urinary AGT (angiotensinogen) concentrations and estimated mean glomerular volume (μm3) in biopsies containing at least 6 glomerular sections, previously measured by a computer‐assisted stereological algorithm.22 Sample size was too small for statistical analysis.
Immunohistochemical Analysis
Inevitably, only limited tissue blocks were available from the previous study,23 and glomeruli were identifiable only in sections from 3 patients: fortuitously, one was a normotensive control, one had GH, and one had severe early onset preeclampsia. As Figure 5A shows, AGT protein was widely distributed throughout the glomerulus and in the proximal tubules but not in the distal tubules in the normotensive control. In contrast, Figure 5B2 and 5C2 show the absence of AGT protein in glomeruli from the GH and preeclampsia patients, although Figure 5B1 and 5C1 demonstrate AGT in the proximal tubules. Proximal and distal tubules were identifiable in sections from 9 normotensive controls, 6 patients with GH (Figure 5B1), and 16 pre‐eclamptic participants (Figure 5B2). Staining at level 2 or 3 was present in at least half of the sections in which proximal tubules were identified in all groups; no staining was observed in distal tubules from any patient.
Figure 5.
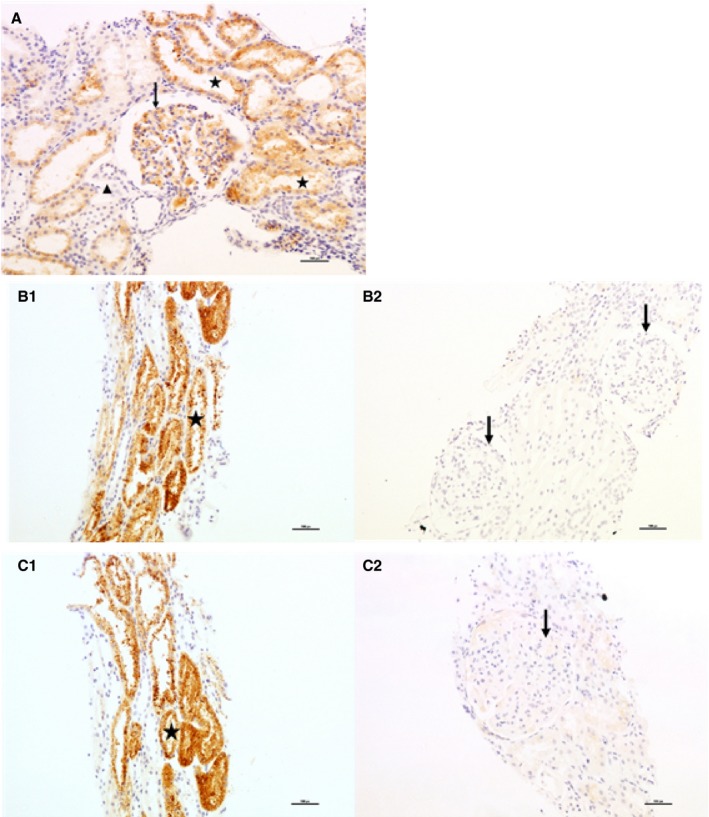
Immunohistochemical staining (×200 magnification) analysis of AGT (angiotensinogen) in kidney biopsies. A, Healthy control. Strong cytoplasmic staining (score: 3) is seen in >50% of proximal tubuli (asterisks in lumen) and, similarly, strong cytoplasmic staining (score: 3) in glomerular mesangium (arrow). A small distal tubule/collecting duct shows no staining (score: 0; above arrow head). B, Patient with gestational hypertension. (B1) Strong cytoplasmic staining (score: 3) is seen in >70% of proximal tubuli (asterisk in lumen). (B2) Two glomeruli are present showing no expression (arrows). C, Patient with severe preeclampsia. (C1) Strong cytoplasmic staining (score: 3) is seen in >70% of proximal tubuli (asterisk in lumen). (C2) One glomerulus is present showing very weak cytoplasmic staining (score: 0.50) in the mesangial area (arrow).
Discussion
We have shown, for the first time, that urinary AGT—a marker of activated intrarenal RAS—is significantly increased in women with both GH and preeclampsia. Although levels did not significantly differ by type of hypertension, we found a positive relationship with glomerular volume, indicating glomerular endotheliosis. There were also strong associations between urinary AGT and blood pressure across the study group as a whole. Ang II is a potent systemic vasoconstrictor; possible mechanisms linking intrarenal generation of Ang II and blood pressure have been reviewed previously.30 A strong association between urinary AGT and albumin excretion (Figure 2) was also demonstrated, although a causative relationship is impossible to determine from an observational study. However, it has been shown that the infusion of Ang II significantly increases albumin excretion in late‐pregnant spontaneously hypertensive rats.31 In nonpregnant humans, increased RAS activity is associated with increased proteinuria.32 Glomerular basement membrane “leakiness” might allow the passage of both albumin (molecular weight: 66.5 kDa) and AGT (molecular weight: 62 kDa). However, kidney podocytes, which form a filtration barrier against protein loss, are functionally disorganized in glomerular disease and in vitro by Ang II. Blockade of Ang II receptors in nonpregnant animal models of nephropathy results in improved function through reduced methylation of the nephrin promoter and decreased proteinuria.33 Human studies, both in vitro and observational, also suggest an intrarenal role for Ang II in proteinuria.34
Renin was first identified as an enzyme synthesized in the kidney, which was thought to be the only site of production until other autonomous and tissue‐specific RASs were discovered >50 years ago.35 AGT, the substrate for renin, is primarily synthesized in the liver, but mRNA for AGT has been identified at other sites, including the renal tubule.36 In the kidney, renin is released in response to physiological stimuli that normally activate the RAS, such as a decrease in tubular delivery of sodium chloride to the macula densa. Ordinarily, although the reaction of renin with AGT is rate limited by renin concentration, following first‐order kinetics, sufficient circulating concentrations of AGT (which are close to the Michelis–Menten constant for the reaction37) prevent any such limitation being reached. However, under circumstances that result in longer term RAS activation, such as treatment with estrogen‐based contraceptives or during pregnancy, AGT may become rate limiting.38 By 11 weeks’ gestation, an additional high‐molecular‐weight form of AGT is identifiable in the plasma of pregnant women.39 Moreover, the proportion of high‐molecular‐weight AGT to normal AGT is also increased in women who develop pregnancy‐associated hypertension,40 suggesting a means by which continued RAS activation might underpin GH.
AGT formed locally in the kidney in proximal tubular cells can spill over into the tubular lumen and be excreted in urine, where it can be measured as an indicator of internal RAS activity.41 ACE, which is responsible for the conversion of Ang I to Ang II, exists both bound to vascular endothelial cells and in a circulating form, allowing for both local and systemic generation of Ang II. ACE is also present in renal proximal tubules,4 confirming the plausibility of a renal RAS tailoring local generation of Ang II and capable of fine‐tuning intrarenal sodium reabsorption independent of the classical systemic RAS. This concept is supported by the observation that sodium depletion in Wistar Kyoto rats enhances renal AGT mRNA expression without a parallel change in renin expression.42 Intrarenally generated Ang II, by binding to AT1R, can cause direct renal vasoconstriction, altering tubuloglomerular feedback and increasing proximal tubular sodium reabsorption.43, 44 AT1R‐mediated uptake of circulating Ang II and augmentation of AGT expression in proximal tubular cells further augments intrarenal Ang II concentration and function, as does the AT1R‐mediated renin secretion from collecting ducts.41
Investigations of a role for the intrarenal RAS and, in particular, for AGT during pregnancy are in their infancy,3 although many roles both in pregnancy and preeclampsia have been suggested for the placental and circulating RAS.45
Ang II has a variety of roles in the body; not only is it one of the most potent circulating vasoconstrictors known, it also, paradoxically, stimulates synthesis and/or release of the main tonic vasodilator nitric oxide. Ang II promotes sodium resorption, both directly across the proximal tubule and indirectly by activating aldosterone, and stimulates vasculogenesis and angiogenesis, among other effects.46 The role of circulating RAS in preeclampsia remains controversial, however, and its suppression has been demonstrated in established hypertensive diseases of pregnancy (eg47). Sensitivity to exogenous Ang II is reduced in normotensive pregnancy but remains high in pre‐eclamptic women,48, 49 whereas sensitivity to other vasoconstrictors remains unchanged. This difference is apparent before the onset of preeclampsia.50
The finding of a strong association between urinary potassium and AGT excretion is shown in Figure 3. Ang II stimulates aldosterone synthesis and thus distal tubular sodium retention in exchange for potassium; therefore, potassium excretion rises. In normal pregnancy, the RAS is activated early; plasma aldosterone rises and some 950 mEq of sodium are retained,8 much of which is associated with the increase in plasma volume needed for the normal progress of pregnancy. However, the pregnant woman also retains some 450 mEq of potassium, although the mechanism for this has not yet been identified. In hypertensive pregnancy, plasma volume expansion is reduced.51 Global overexpression of AGT in transgenic mice was associated with a fall in plasma volume from midpregnancy.52 Although data from animal models must be viewed with caution, these data suggest the intriguing possibility that there may be prepregnancy or early pregnancy differences in the RAS that predispose a woman to developing preeclampsia. It follows that the increased renal synthesis of AGT that we report here in hypertensive pregnancy could be a response to relative hypovolemia in these women, although this was not measured in our study. Increasing dietary potassium intake in healthy volunteers has been associated with a fall in urinary AGT excretion.53 Due to limitations in sample size we were not able to compare fractional clearance of sodium and potassium in women with preeclampsia in this material, although it has been reported that renal hemodynamic measurements are on average 25% to 30% lower in women with preeclampsia than in gestation age–matched, healthy, normotensive pregnant women.54 The urinary renin concentration is known to be low.20, 55 Stored volumes of urine were too small to allow renin to be measured in this study.
In the limited amount of renal tissue that remained from biopsies after previous investigations,23 we opportunistically chose to demonstrate the presence of renal AGT expression by immunohistochemistry. We showed that in human pregnancy, AGT expression seems to be confined to the proximal tubule; no staining was demonstrated in any distal tubular segment. This result was also shown in biopsy material from nonpregnant patients,56 mice,52 and rats.43 The complete absence of glomerular AGT staining in hypertensive pregnancy, in contrast to normotensive control, is of interest and worthy of further study in appropriate animal models.
Measures of mean glomerular volume were available for 13 hypertensive women, having been estimated stereologically in renal tissue. Larger glomeruli were found in women with higher urinary AGT excretion, as shown in Figure 4. Glomerular volume is known to be increased in proportion to the severity of preeclampsia.57 The glomerular endotheliosis of preeclampsia has been ascribed to a decrease in circulating VEGF (vascular endothelial growth factor), itself a consequence of increased soluble Flt‐1 (fms‐like tyrosine kinase 1).58 Ang II stimulates soluble Flt in response to placental ischemia in rats.17 Ang II immunostaining in glomeruli has been shown in renal biopsies of patients with chronic kidney disease, in whom an association between urinary AGT and proteinuria also was noted.59 In these samples, we observed a strong positive correlation between urinary AGT and albuminuria (Figure 2), presumably due to podocyte and glomerular endothelial damage disrupting the filtration barrier of both.
There are a number of mechanisms by which an inappropriately activated intrarenal RAS could initiate or contribute to the pathology of preeclampsia, for instance through angiogenic and antiangiogenic factors,45 the immune system,18 and coagulation systems and the complement system.19 An activated complement system has been implicated in the pathophysiology of preeclampsia by an increasing body of evidence and can play a major role in causing renal injury also in other complement‐mediated renal diseases.19, 60 Renin can cleave C3 into C3a and C3b, thus activating the complement system by this alternative pathway.60 Targeting excessive complement activation has been suggested as a possible therapeutic strategy in prevention or treatment of preeclampsia19 and might also prevent long‐lasting damage to the kidney. Nevertheless, the risk of disrupting homeostatic functions and host defense cannot be disregarded.
Women with a history of preeclampsia have not only a 2‐fold increased risk of developing long‐term cardiovascular disease but also a 5‐ to 12‐fold increased risk of developing end‐stage renal disease,61 and there is need for renoprotective strategies to reduce late disease burden. In a paper highlighting the fact that World Kidney Day and International Women's Day coincided in 2018,62 the World Kidney Day Steering Committee emphasized that chronic kidney disease affects 10% of the world's adult population and takes place among the top 10 causes of death worldwide. With the knowledge that pregnancy is the most common cause of acute kidney injury in women of childbearing age,60 the urgency to monitor disease progression in preeclampsia, possibly by urinary AGT as a marker of inappropriately activated intrarenal RAS, is underscored. A timely delivery, also for renoprotective reasons, followed by possible therapeutic measures to prevent further renal injury could decrease women's risk for future renal and cardiovascular disease.
To the best of our knowledge, this study is the first to investigate, both functionally and histologically, intrarenal AGT in normal and hypertensive human pregnancies. While accepting the limitations on availability of renal biopsy specimens, similar samples are unlikely ever to be obtained again. The renal biopsy study in which these samples were procured showed no renal disease as an underlying cause of preeclampsia. They also showed the typical lesion of “endotheliosis” to be present in some normal pregnant controls, thus no clinical indication remains for performing a renal biopsy to diagnose renal disease or preeclampsia in pregnancy in either a clinical or research setting.
These results indicate the presence of intrarenal AGT in renal tissue from women with GH and preeclampsia and increased urinary AGT and potassium excretion in our unique set of matched urine samples. The findings suggest inappropriately increased intrarenal RAS activation, seemingly associated with glomerular swelling as an indication of endotheliosis. These changes are likely to be independent of the systemic renin‐angiotensin status. An inappropriately activated RAS can lead to renal injury through renal vasoconstriction and numerous other mechanisms, including activation of the complement system. Apart from a timely delivery, other therapeutic measures should be considered to limit damage to the maternal kidneys. Even if treatment were available only in the immediate postpartum period, it might attenuate the risk of future renal disease.
Sources of Funding
This work was produced by Mistry under the terms of a British Heart Foundation Basic Science Intermediate Basic Science Fellowship (FS/15/32/31604) and an European Renal Association‐European Dialysis and Transplant Association Long‐Term Fellowship (LTF 137‐2013). Mistry, Kurlak, and Broughton Pipkin received an International Collaboration Grant from the University of Nottingham to travel to Lund during this study. Strevens received a travel research grant from the Faculty of Medicine, Lund University (Carl Swartz Minnesfond).
Disclosures
None.
Acknowledgments
We thank all the women who participated in the study.
(J Am Heart Assoc. 2019;8:e012611 DOI: 10.1161/JAHA.119.012611.)
References
- 1. Broughton Pipkin F. The renin‐angiotensin system in pre‐eclampsia In: Lyall F, Belfort M, eds. Pre‐Eclampsia: Etiology and Clinical Practice. Cambridge: Cambridge University Press; 2007:78–91. [Google Scholar]
- 2. Mistry HD, Kurlak LO, Broughton Pipkin F. The placental renin‐angiotensin system and oxidative stress in pre‐eclampsia. Placenta. 2013;34:182–186. [DOI] [PubMed] [Google Scholar]
- 3. Pringle KG, de Meaultsart CC, Sykes SD, Weatherall LJ, Keogh L, Clausen DC, Dekker GA, Smith R, Roberts CT, Rae KM, Lumbers ER. Urinary angiotensinogen excretion in Australian indigenous and non‐indigenous pregnant women. Pregnancy Hypertens. 2018;12:110–117. [DOI] [PubMed] [Google Scholar]
- 4. Li XC, Zhuo JL. Recent updates on the proximal tubule renin‐angiotensin system in angiotensin II‐dependent hypertension. Curr Hypertens Rep. 2016;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chesley LC. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol. 1972;112:440–450. [DOI] [PubMed] [Google Scholar]
- 6. Al Kadi H, Nasrat H, Broughton Pipkin F. A prospective, longitudinal study of the renin‐angiotensin system, prostacyclin and thromboxane in the first trimester of normal human pregnancy: association with birthweight. Hum Reprod. 2005;20:3157–3162. [DOI] [PubMed] [Google Scholar]
- 7. Ellison DH, Terker AS, Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol. 2016;27:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindheimer MD, Katz AI. Kidney Function and Disease in Pregnancy. Philadelphia: Lea & Febiger; 1977. [Google Scholar]
- 9. Ehrlich EN, Lindheimer MD. Effect of administered mineralocorticoids or ACTH in pregnant women. Attenuation of kaliuretic influence of mineralocorticoids during pregnancy. J Clin Invest. 1972;51:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre‐eclampsia. Lancet. 2010;376:631–644. [DOI] [PubMed] [Google Scholar]
- 11. Brown JJ, Davies DL, Doak PB, Lever AF, Robertson JI, Trust P. Plasma‐renin concentration in hypertensive disease of pregnancy. Lancet. 1965;2:1219. [DOI] [PubMed] [Google Scholar]
- 12. Barr M. Teratogen update—angiotensin‐converting enzyme‐inhibitors. Teratology. 1994;50:399–409. [DOI] [PubMed] [Google Scholar]
- 13. Broughton Pipkin F, Sharif J, Lal S. Angiotensin and asymmetric fetal growth. Lancet. 1995;346:844–845. [DOI] [PubMed] [Google Scholar]
- 14. Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NICE . Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. 2010;107. [PubMed]
- 16. Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy SR, Cockrell K. Regulation of soluble fms‐like tyrosine kinase‐1 production in response to placental ischemia/hypoxia: role of angiotensin II. Physiol Rep. 2015;3:e12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramseyer VD, Garvin JL. Tumor necrosis factor‐alpha: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304:F1231–F1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Regal JF, Laule CF, McCutcheon L, Root KM, Lund H, Hashmat S, Mattson DL. The complement system in hypertension and renal damage in the Dahl SS rat. Physiol Rep. 2018;6:e13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pringle KG, Sykes SD, Lumbers ER. Circulating and intrarenal renin‐angiotensin systems in healthy men and nonpregnant women. Physiol Rep. 2015;3:e12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Chen C, Tian J, Zha Y, Xiong Y, Sun Z, Chen P, Li J, Yang T, Ma C, Liu H, Wang X, Hou FF. Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: a prospective, two‐stage study. J Am Soc Nephrol. 2015;26:2032–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strevens H, Wide‐Swensson D, Grubb A, Hansen A, Horn T, Ingemarsson I, Larsen S, Nyengaard JR, Torffvit O, Willner J, Olsen S. Serum cystatin C reflects glomerular endotheliosis in normal, hypertensive and pre‐eclamptic pregnancies. BJOG. 2003;110:825–830. [PubMed] [Google Scholar]
- 23. Strevens H, Wide‐Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S, Willner J, Olsen S. Glomerular endotheliosis in normal pregnancy and pre‐eclampsia. BJOG. 2003;110:831–836. [PubMed] [Google Scholar]
- 24. Mistry HD, Gill CA, Kurlak LO, Seed PT, Hesketh JE, Meplan C, Schomburg L, Chappell LC, Morgan L, Poston L; SCOPE Consortium . Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks gestation in nulliparous women who subsequently develop preeclampsia. Free Radic Biol Med. 2015;78:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gray C, Al‐Dujaili EA, Sparrow AJ, Gardiner SM, Craigon J, Welham SJ, Gardner DS. Excess maternal salt intake produces sex‐specific hypertension in offspring: putative roles for kidney and gastrointestinal sodium handling. PLoS One. 2013;8:e72682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mistry HD, McCallum LA, Kurlak LO, Greenwood IA, Broughton Pipkin F, Tribe RM. Novel expression and regulation of voltage‐dependent potassium channels in placentas from women with preeclampsia. Hypertension. 2011;58:497–504. [DOI] [PubMed] [Google Scholar]
- 27. Roy‐Chaudhury P, Wu B, King G, Campbell M, Macleod AM, Haites NE, Simpson JG, Power DA. Adhesion molecule interactions in human glomerulonephritis: importance of the tubulointerstitium. Kidney Int. 1996;49:127–134. [DOI] [PubMed] [Google Scholar]
- 28. Bramham K, Poli‐de‐Figueiredo CE, Seed PT, Briley AL, Poston L, Shennan AH, Chappell LC. Association of proteinuria threshold in pre‐eclampsia with maternal and perinatal outcomes: a nested case control cohort of high risk women. PLoS One. 2013;8:e76083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elia EG, Robb AO, Hemming K, Price MJ, Riley RD, French‐Constant A, Denison FC, Kilby MD, Morris RK, Stock SJ. Is the first urinary albumin/creatinine ratio (ACR) in women with suspected preeclampsia a prognostic factor for maternal and neonatal adverse outcome? A retrospective cohort study. Acta Obstet Gynecol Scand. 2017;96:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramkumar N, Kohan DE. Proximal tubule angiotensinogen modulation of arterial pressure. Curr Opin Nephrol Hypertens. 2013;22:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morgan H, Butler E, Beattie E, McBride M, Graham D. Establishment of a model of superimposed pre‐eclampsia in the pregnant stroke prone spontaneously hypertensive rat by exposure to angiotensin II. Placenta. 2017;57:248. [Google Scholar]
- 32. Nicholl DD, Hemmelgarn BR, Turin TC, MacRae JM, Muruve DA, Sola DY, Ahmed SB. Increased urinary protein excretion in the “normal” range is associated with increased renin‐angiotensin system activity. Am J Physiol Renal Physiol. 2012;302:F526–F532. [DOI] [PubMed] [Google Scholar]
- 33. Hayashi K, Sasamura H, Ishiguro K, Sakamaki Y, Azegami T, Itoh H. Regression of glomerulosclerosis in response to transient treatment with angiotensin II blockers is attenuated by blockade of matrix metalloproteinase‐2. Kidney Int. 2010;78:69–78. [DOI] [PubMed] [Google Scholar]
- 34. Hayashi K, Sasamura H, Nakamura M, Sakamaki Y, Azegami T, Oguchi H, Tokuyama H, Wakino S, Hayashi K, Itoh H. Renin‐angiotensin blockade resets podocyte epigenome through kruppel‐like factor 4 and attenuates proteinuria. Kidney Int. 2015;88:745–753. [DOI] [PubMed] [Google Scholar]
- 35. Skinner SL, Lumbers ER, Symonds EM. Renin concentration in human fetal and maternal tissues. Am J Obstet Gynecol. 1968;101:529–533. [DOI] [PubMed] [Google Scholar]
- 36. Paul M, Wagner J, Dzau VJ. Gene expression of the renin‐angiotensin system in human tissues. Quantitative analysis by the polymerase chain reaction. J Clin Invest. 1993;91:2058–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. [DOI] [PubMed] [Google Scholar]
- 38. Skinner SL, Lumbers ER, Symonds EM. Analysis of changes in the renin‐angiotensin system during pregnancy. Clin Sci. 1972;42:479–488. [DOI] [PubMed] [Google Scholar]
- 39. Tetlow HJ, Broughton Pipkin F. Changing renin substrates in human pregnancy. J Endocrinol. 1986;109:257–262. [DOI] [PubMed] [Google Scholar]
- 40. Tewksbury DA, Kaiser SJ, Burrill RE. A study of the temporal relationship between plasma high molecular weight angiotensinogen and the development of pregnancy‐induced hypertension. Am J Hypertens. 2001;14:794–797. [DOI] [PubMed] [Google Scholar]
- 41. Navar LG. Translational studies on augmentation of intratubular renin‐angiotensin system in hypertension. Kidney Int Suppl (2011). 2013;3:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pratt RE, Zou WM, Naftilan AJ, Ingelfinger JR, Dzau VJ. Altered sodium regulation of renal angiotensinogen mRNA in the spontaneously hypertensive rat. Am J Physiol. 1989;256:F469–F474. [DOI] [PubMed] [Google Scholar]
- 43. Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 44. Satou R, Shao W, Navar LG. Role of stimulated intrarenal angiotensinogen in hypertension. Ther Adv Cardiovasc Dis. 2015;9:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Irani RA, Xia Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin Nephrol. 2011;31:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Farag E, Maheshwari K, Morgan J, Sakr Esa WA, Doyle DJ. An update of the role of renin angiotensin in cardiovascular homeostasis. Anesth Analg. 2015;120:275–292. [DOI] [PubMed] [Google Scholar]
- 47. Symonds EM, Broughton Pipkin F, Craven DJ. Changes in the renin‐angiotensin system in primigravidae with hypertensive disease of pregnancy. Br J Obstet Gynaecol. 1975;82:643–650. [DOI] [PubMed] [Google Scholar]
- 48. Gant NF, Chand S, Whalley PJ, MacDonald PC. The nature of pressor responsiveness to angiotensin II in human pregnancy. Obstet Gynecol. 1974;43:854. [PubMed] [Google Scholar]
- 49. Gant NF, Whalley PJ, Everett RB, Worley RJ, MacDonald PC. Control of vascular reactivity in pregnancy. Am J Kidney Dis. 1987;9:303–307. [DOI] [PubMed] [Google Scholar]
- 50. Baker PN, Broughton Pipkin F, Symonds EM. Comparative study of platelet angiotensin II binding and the angiotensin II sensitivity test as predictors of pregnancy‐induced hypertension. Clin Sci (Lond). 1992;83:89–95. [DOI] [PubMed] [Google Scholar]
- 51. de Haas S, Ghossein‐Doha C, van Kuijk SM, van Drongelen J, Spaanderman ME. Physiological adaptation of maternal plasma volume during pregnancy: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2017;49:177–187. [DOI] [PubMed] [Google Scholar]
- 52. Morgan TK, Rohrwasser A, Zhao L, Hillas E, Cheng T, Ward KJ, Lalouel JM. Hypervolemia of pregnancy is not maintained in mice chronically overexpressing angiotensinogen. Am J Obstet Gynecol. 2006;195:1700–1706. [DOI] [PubMed] [Google Scholar]
- 53. Rebholz CM, Chen J, Zhao Q, Chen JC, Li J, Cao J, Gabriel Navar L, Lee Hamm L, Gu D, He J; GenSalt Collaborative Research G . Urine angiotensinogen and salt‐sensitivity and potassium‐sensitivity of blood pressure. J Hypertens. 2015;33:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindheimer MD, Cunningham FG, Roberts JM, Chesley LC. Chesley's Hypertensive Disorders in Pregnancy. Stamford: CT: Appleton & Lange; 1999. [Google Scholar]
- 55. Lumbers ER, Skinner SL. The occurrence and assay of renin in human urine. Aust J Exp Biol Med Sci. 1969;47:251–262. [DOI] [PubMed] [Google Scholar]
- 56. Cao W, Jin L, Zhou Z, Yang M, Wu C, Wu L, Cui S. Overexpression of intrarenal renin‐angiotensin system in human acute tubular necrosis. Kidney Blood Press Res. 2016;41:746–756. [DOI] [PubMed] [Google Scholar]
- 57. Nochy D, Birembaut P, Hinglais N, Freund M, Idatte JM, Jacquot C, Chartier M, Bariety J. Renal lesions in the hypertensive syndromes of pregnancy: immunomorphological and ultrastructural studies in 114 cases. Clin Nephrol. 1980;13:155–162. [PubMed] [Google Scholar]
- 58. Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. [DOI] [PubMed] [Google Scholar]
- 59. Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. [DOI] [PubMed] [Google Scholar]
- 60. Bekassy ZD, Kristoffersson AC, Rebetz J, Tati R, Olin AI, Karpman D. Aliskiren inhibits renin‐mediated complement activation. Kidney Int. 2018;94:689–700. [DOI] [PubMed] [Google Scholar]
- 61. Paauw ND, Luijken K, Franx A, Verhaar MC, Lely AT. Long‐term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin Sci (Lond). 2016;130:239–246. [DOI] [PubMed] [Google Scholar]
- 62. Piccoli GB, Alrukhaimi M, Liu ZH, Zakharova E, Levin A; World Kidney Day Steering Committee . What we do and do not know about women and kidney diseases; questions unanswered and answers unquestioned: reflection on World Kidney Day and International Woman's Day. BMC Nephrol. 2018;19:66. [DOI] [PubMed] [Google Scholar]


