Abstract
Background
The angiotensin‐receptor neprilysin inhibitor (ARNI) sacubitril/valsartan was shown to be superior to the angiotensin‐converting enzyme inhibitor enalapril in terms of reducing cardiovascular mortality in the PARADIGM‐HF (Prospective Comparison of ARNI with angiotensin‐converting enzyme inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) study. However, the impact of ARNI on cardiac reverse remodeling (CRR) has not been established.
Methods and Results
We conducted a meta‐analysis to compare the effects of ARNI versus angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers on CRR indices. We searched databases for studies published between 2010 and 2019 that reported CRR indices following ARNI administration. Effect size was expressed as mean difference (MD) with 95% CIs. Twenty studies enrolling 10 175 patients were included. ARNI improved functional capacity in patients with heart failure (HF) and a reduced ejection fraction (EF), including increasing New York Heart Association functional class (MD −0.79, 95% CI −0.86, −0.71) and 6‐minute walking distance (MD 27.62 m, 95% CI 15.76, 39.48). ARNI outperformed angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers in terms of CRR indices, with striking changes in left ventricular EF, diameter, and volume. However, there were no significant improvements in indices except left ventricular mass index (MD −3.25 g/m2, 95% CI −3.78, −2.72) and left atrial volume (MD −7.20 mL, 95% CI −14.11, −0.29) in HF patients with preserved EF treated with ARNI. Improvements in CRR indices were observed at 3 months and became more significant with longer follow‐up to 12 months. The regression equation for the relationship between left ventricular EF and end‐diastolic dimension was y=0.041+0.071x+0.045x2+0.006x3.
Conclusions
ARNI distinctly improved left ventricular size and hypertrophy compared with angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers in HF with reduced EF patients, even after short‐term follow‐up. Patients appeared to benefit more in terms of CRR treated with ARNI as early as possible and for at least 3 months. Further large sample trials are required to determine the effects of ARNI on CRR in HF with preserved EF patients.
Keywords: angiotensin‐receptor neprilysin inhibitor, cardiac reverse remodeling, end‐diastolic dimension, heart failure with a reduced ejection fraction, meta‐analysis
Subject Categories: Heart Failure, Remodeling, Echocardiography, Treatment, Meta Analysis
Clinical Perspective
What Is New?
To our knowledge this is the first meta‐analysis directly assessing the effects of the first angiotensin‐receptor neprilysin inhibitor, sacubitril/valsartan, on cardiac reverse remodeling.
What Are the Clinical Implications?
The current results suggest that an angiotensin‐receptor neprilysin inhibitor can improve functional capacity and cardiac reverse remodeling in heart failure patients with reduced ejection fraction versus angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, with more prominent changes occurring over time.
The results of our meta‐analysis suggest that patients with heart failure may receive greater cardiac reverse remodeling benefit if they are treated with an angiotensin‐receptor neprilysin inhibitor as early as possible.
Introduction
Ventricular and/or atrial remodeling occurs in many cardiovascular diseases, mainly as a result of abnormal neurohumoral regulation culminating in heart failure (HF) with high morbidity and mortality.1, 2 HF patients can be classified as either HF with preserved ejection fraction (HFpEF; typically left ventricle [LV]EF ≥50%) or HF with reduced ejection fraction (HFrEF; typically LVEF <40%), based on LVEF values.3 Cardiac reverse remodeling (CRR) generally refers to improvements in damaged ventricular/atrial volume, dimension, and shape.4 Previous studies reported that inhibition of renin‐angiotensin‐aldosterone system improved LVEF and antagonized cardiac remodeling,5, 6, 7 as well as reducing the risk of cardiovascular death in HFrEF patients.5, 6, 7, 8, 9 However, the effects of the anti–renin‐angiotensin‐aldosterone system in HFpEF patients remain controversial.10
The PARADIGM‐HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial revealed that a combined angiotensin receptor blocker (ARB, valsartan)‐neprilysin inhibitor (ARNI) sacubitril/valsartan markedly decreased cardiovascular and all‐cause mortality in patients with HFrEF compared with the angiotensin‐converting enzyme inhibitor (ACEI) enalapril.11 This breakthrough promoted ARNI use in HFrEF patients,3, 12 and the findings of preclinical trials have suggested that ARNI may improve the prognoses of HFrEF patients in terms of cardiac fibrosis and cardiomyocyte hypertrophy.13, 14 ARNI augmented the inhibitory effects of valsartan alone by increasing the systemic exposure to valsartan by 40%, suggesting that ARNI would have greater antiremodeling effects than either valsartan or neprilysin inhibitor alone.13, 14
Improvements in CRR have been used to evaluate the effects of ARNI in several randomized controlled trials (RCTs) and observational studies.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 The results of some of these studies support the superior effects of ARNI over ACEIs/ARBs on remodeling.17, 18 However, the PRIME (Pharmacological Reduction of Functional, Ischemic Mitral Regurgitation) study found that, among all the CRR indices, only ARNI resulted in a significant decrease in end‐diastolic volume (EDV) compared with valsartan.19 This inconsistency may affect the judgment of ARNI effects. Furthermore, the results in terms of different doses and follow‐up periods remain inconclusive. Most studies have demonstrated a dose‐dependent effect of ARNI on CRR indices, with higher doses resulting in greater CRR.15, 16, 17, 26, 27 However, other studies have produced different conclusions.18, 25 Martens and colleagues found that LVEF was enhanced after longer treatment with ARNI.25 This coincided with no significant short‐term impacts on CRR in some RCTs,16 compared with other studies that demonstrated short‐term effectiveness.18, 19, 25, 28, 30, 31 These aspects therefore remain controversial.
We addressed these questions by conducting a meta‐analysis to compare the effects of ARNI and ACEIs/ARBs on indices including functional capacity, CRR, and biomarkers to assess the effects of ARNI and these indices with respect to follow‐up periods, distinct control drugs, and baseline characteristics and to determine which CRR indices were correlated with changes in LVEF in patients taking ARNI.
Methods
This meta‐analysis was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines.34 The data supporting the findings are available from the corresponding author on reasonable request.
Search Strategy and Study Selection
A systematic literature search of studies published between 2010 and 2019 was conducted by 2 authors (Y.W. and R.Z.) using PubMed, EMBASE, the Cochrane Library, Web of Science, and Clinicaltrials.gov, with subjects including “angiotensin‐receptor neprilysin inhibitor,” “ventricular remodeling,” “atrial remodeling,” “clinical trials,” and random words (see Data S1 for full list). We also included the following terms: (1) adult patients (>18 years) with cardiac dysfunction; (2) patients assigned to ARNI treatment orally; (3) patients with baseline and follow‐up data for at least 1 CRR index, measured by echocardiography or cardiac magnetic resonance imaging; and (4) follow‐up for at least 3 months. All publications in English that met the above criteria were included. The search was limited to studies in humans. Studies reporting only 1 biomarker and unpublished studies were excluded. We also searched the reference lists of publications and conference abstracts for additional data. All titles, abstracts, and full articles were screened by 2 authors (Y.W. and R.Z.) to identify the final included studies. In the event of multiple articles reporting the same study, the article with the most complete data was used. Disagreements were resolved by consensus discussion. The search strategy and exclusion criteria are presented in Figure 1.
Figure 1.
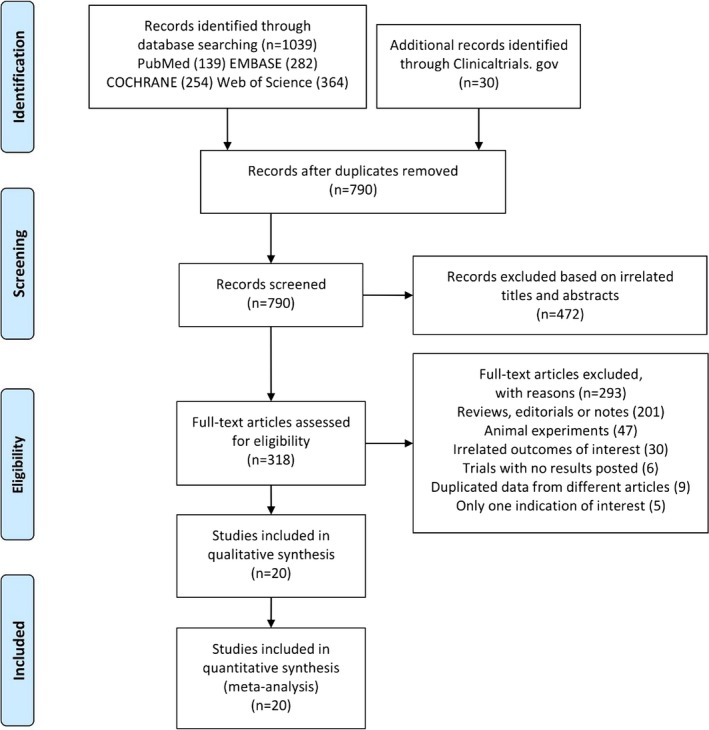
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram showing detailed study selection process.
Data Extraction
Data extraction was performed independently by 2 authors (Y.W. and R.Z.). The following data were extracted: first author's name, study publication year, design (RCT, cohort study, observational study), study location, patient characteristics (sex, age, previous medication), setting (HFrEF or HFpEF), sample size, treatments of control groups, follow‐up period, and methods of measurement. Three classification indices were then extracted (functional capacity, CRR, and biomarkers) comprising baseline and postintervention data.
Indices of functional capacity included New York Heart Association (NYHA) functional class and 6‐minute walking distance (6MWD). We also chose CRR indices that directly reflected changes in cardiac structure, including indices of LV volume and dimension (LVEF, end‐systolic volume [ESV], EDV, end‐systolic dimension [ESD], end‐diastolic dimension [EDD]) and hypertrophy (LV mass index [LVMI]), and indices of atrial remodeling (left atrial volume [LAV]). LV reverse remodeling was defined as an absolute improvement in LVEF of ≥10%, accompanied by a decrease in EDD of at least 10%, assessed over a period of time.35 Indices should be measured using the Simpson method.16 Biomarkers reflecting wall stress and fibrosis, namely N‐terminal pro–brain natriuretic peptide (NT‐proBNP) and soluble suppressor of tumorigenesis‐2 (sST2), were also chosen and monitored according to standard laboratory methods. Mean±SD or median±interquartile range needed to be provided for all parameters or to be calculable from the provided data.
Quality Assessment
The methodological qualities of the RCTs were assessed using the Cochrane Collaboration bias risk tools for random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Other studies were appraised using the Newcastle‐Ottawa scale. The quality was assessed by the scores for 9 questions related to study selection, comparability, and outcomes, namely the comparability of baseline characteristics across groups for confounding factors, the appropriateness of outcome evaluation, and missing data handling. Quality assessment was finalized independently by 2 authors (Y.W. and R.Z.).
Outcome Measures and Statistical Analyses
The primary study outcomes were changes in functional capacity (NYHA functional class, 6MWD), CRR indices (LVEF, ESV, EDV, ESD, EDD, LVMI, LAV), and biomarkers (NT‐proBNP, sST2) in both ARNI and ACEIs/ARBs groups. We used fixed‐effect meta‐analyses to compare the 2 groups directly. We initially performed meta‐analyses of the effects of ARNI on functional capacity, CRR, and biomarkers, including studies reporting data for both ARNI and ACEIs/ARBs and studies reporting data for ARNI use alone. We then excluded studies without control groups and conducted fixed‐effect head‐to‐head meta‐analyses to compare the effects of ARNI versus ACEIs/ARBs. All analyses were stratified according to HFrEF or HFpEF. Dichotomous variables were reported as proportions, and continuous variables were primarily expressed as mean±SD. The mean differences (MD) with 95% CIs for the indices were plotted as forest plots. Statistically significant results were identified as CIs excluding a null effect and a P<0.05. Heterogeneity between studies was assessed using the Q statistic, and its extent was calculated by the I2 test, to determine if variability between studies resulted from heterogeneity or chance, with an I2 value >50% indicating high heterogeneity. The effect of each study on the overall effect size was assessed by sensitivity analysis using the leave‐one‐out approach.
Secondary end points were the relationships between mean changes in LVEF and CRR indices. Pearson and Spearman correlations were used as appropriate according to the Shapiro‐Wilk test to detect if the data were normally distributed. If the data did not show a Gaussian distribution, the Spearman correlation was used. Regression analyses were used to select the best‐fitted model to explore the relationships between LVEF and other CRR values.
Subgroup analyses were conducted based on control drugs, follow‐up durations, and other covariates, including the proportion of patients reaching the target dosage of ARNI, baseline medication, comorbidities, and baseline blood pressure (BP). Publication bias risk was estimated by funnel plot and Egger test. Meta‐analyses were performed using Review Manager software (version 5.3; The Cochrane Collaboration, Software Update, Oxford, UK), and correlation analyses were conducted using SPSS (version 22; IBM, Armonk, NY).
Results
Search Results and Baseline Characteristics
The search identified 1039 articles and 30 completed studies registered at ClinicalTrials.gov that met the inclusion criteria. After study selection, 20 studies11, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 were finally eligible for analysis. Baseline and follow‐up LVEF scores were available in 9 studies,16, 18, 19, 20, 23, 25, 26, 27, 29 and NYHA functional class, 6MWD, EDV, and EDD were reported in 7 trials. Changes in ESV were available in 6 trials.16, 17, 18, 23, 25, 27 Other baseline and follow‐up echocardiography data included ESD, LVMI, and LAV in 3 studies. NT‐proBNP and sST2 scores were evaluated from data extracted from 611, 16, 19, 21, 22, 30 and 4 studies,11, 16, 21, 22 respectively.
The baseline characteristics are presented in Table 1. Of the 20 included studies, 16 were non‐RCTs and 4 were RCTs, all of which clearly stated that they used explicit allocation concealment, blinding, and randomization strategies. A total of 10 175 patients were finally included, of whom 5696 were assigned to ARNI and 4479 were assigned to ACEIs/ARBs. A total of 9760 patients in 18 trials had HFrEF, 114 patients in 1 RCT had essential hypertension, and 301 patients in another RCT had HFpEF. Among 7 controlled trials, 211, 19 and 315, 16, 17 studies used ACEIs and ARBs as controls, respectively, and 2 publications reported no specific control drugs.18, 20 The year of publication ranged from 2010 to 2019. The mean patient age ranged from 58.0 to 78.6 years, and 76.6% of subjects were male. The included studies were conducted worldwide, and the ARNI dose at baseline ranged from 50 mg to 200 mg twice a day. The follow‐up duration ranged from 3 to 27 months. Only 1 trial assessed indices using MRI,15 and the others used echocardiography.11, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33
Table 1.
Characteristics of Included Studies and Patients of the Meta‐Analysis
| First Author (Year)Refs. | Study Design | Interventions and Controls | Patients (n) | Setting | Age (y, mean±SD) | Men (%) | Imaging Modality | Indices | FU (mo) |
|---|---|---|---|---|---|---|---|---|---|
| Controlled trials | |||||||||
| McMurray (2014)11 | RCT |
ARNI Enalapril |
8399 | HFrEF (LVEF ≤35%) | 63.8±11.39 | 78.1 | ECHO | Biomarkers | 27 |
| Schmieder (2016)15 | RCT |
ARNI Olmesartan |
114 | EH | 59.8±10.7 | 67.5 | MRI | CRR indices | 13 |
| Solomon (2012)16 | RCT |
ARNI Valsartan |
301 | HFpEF (LVEF≥45%) | 71.0±9.15 | 43.5 | ECHO | Functional capacity, CRR indices and biomarkers | 9 |
| Kang (2018)19 | RCT |
ARNI Valsartan |
118 | HFrEF | 62.6±11 | 61 | ECHO | CRR indices | 12 |
| Almufleh (2017)17 | Cohort study |
ARNI ACEI/ARB |
48 | HFrEF | 70±11.1 | 79.2 | ECHO | Functional capacity, CRR indices | 3 |
| De Diego (2018)18 | Cohort study |
ARNI Ramipril |
250 | HFrEF (LVEF ≤40%) | 69±8 | 76 | ECHO | Functional capacity, CRR indices and biomarkers | 6 |
| Nazzari (2017)20 | Cohort study |
ARNI ACEI/ARB |
43 | HFrEF | 58.0±12.9 | NR | ECHO | Functional capacity, CRR indices | 6 |
| Uncontrolled trials | |||||||||
| Barrett (2018)21 | Observational study | ARNI | 61 | HFrEF | 68 | 65 | ECHO | Biomarkers | 3.4 |
| Murray (2017)22 | Observational study | ARNI | 112 | HFrEF | NR | NR | ECHO | Biomarkers | 18 |
| Maurin (2017)23 | Cohort study | ARNI | 80 | Systolic HF | 59 | 76 | ECHO | Functional capacity and CRR indices | 3 |
| Canu (2017)24 | Observational study | ARNI | 200 | Systolic HF | 59 | 81 | ECHO | Functional capacity indices | 6 |
| Martens (2018)25 | Cohort study | ARNI | 125 | HFrEF (LVEF <35%) | 66±10 | 81 | ECHO | Functional capacity CRR indices | 4 |
| Groba‐Marco (2018)26 | Observational study | ARNI | 17 | Symptomatic HFrEF | 60.6±10.93 | 76 | ECHO | Functional capacity, CRR indices | 4.9 |
| Kalantari (2018)27 | Observational study | ARNI | 40 | HFrEF | NR | NR | ECHO | Functional capacity, CRR indices | 3 |
| Mercedes Faraudo (2017)28 | Observational study | ARNI | 23 | HFrEF | 71 | 91 | ECHO | Functional capacity indices | 3 |
| Rafael Bravo Marques (2017)29 | Observational study | ARNI | 57 | HFrEF | 69.1±10.1 | 80.7 | ECHO | Functional capacity, CRR indices | 12 |
| Hlavata (2018)30 | Observational study | ARNI | 12 | HFrEF | NR | 91.7 | ECHO | Functional capacity indices and biomarkers | 3 |
| Beltrán (2018)31 | Observational study | ARNI | 58 | HFrEF | 70±11 | 72.4 | ECHO | Functional capacity indices | 3 |
| Rodil Fraile (2018)32 | Observational study | ARNI | 65 | HFrEF | 78.6±7.4 | 68 | ECHO | Functional capacity indices | 9.5 |
| Mantis (2018)33 | Observational study | ARNI | 52 | HFrEF | 64±11 | 69 | ECHO | Functional capacity indices | 6 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; CRR, cardiac reverse remodeling; ECHO, echocardiography; EH, essential hypertension; FU, follow‐up; HFpEF, heart failure with a preserved ejection fraction; HFrEF, heart failure with a reduced ejection fraction; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; NR, not reported; RCT, randomized controlled trials; Ref, reference.
Quality Assessment and Publication Bias
The quality of the 4 RCTs was assessed (Figure S1), and all were generally of good quality. The other 16 studies were assessed by Newcastle‐Ottawa quality assessment (Table S1), and all reported explicit inclusion criteria and previous medication of ACEIs/ARBs. Fifteen studies were at risk of bias because of limited reporting of participant allocation methods and unclear blinding strategies.18, 19, 20, 21, 22, 23, 24, 26, 27, 28, 29, 30, 31, 32, 33 Detailed methods of measurement using echocardiography or magnetic resonance imaging were reported in only 8 studies.15, 16, 17, 18, 19, 23, 25, 27 Among the 7 controlled trials analyzed in the head‐to‐head meta‐analyses, the comparability of subjects with ARNI versus ACEIs/ARBs was almost addressed, and adjustment for potential confounders was reported. No significant publication bias was indicated by the funnel plot (Figure S2) or Egger test (P=0.191).
Effects of ARNI on Functional Capacity
Significant improvements in NYHA functional class (MD −0.79, 95% CI −0.86, −0.71; Figure 2A and Table S2) and 6MWD (MD 27.62 m, 95% CI 15.76, 39.48; Figure 2B and Table S2) were observed in HFrEF patients and HFpEF patients (NYHA functional class, MD −0.20, 95% CI −0.31, −0.99; Figure 2A and Table S2). However, the I2 value for studies assessing changes in NYHA functional class was 90% in HFrEF patients, indicating significant heterogeneity across the studies. Subgrouping according to sex, publication year, age, and follow‐up duration had no pronounced effect on the I2 values, but I2 was reduced to 0 after exclusion of data for 2 studies with higher weightings (>50%).19, 32 The heterogeneity may have been partly attributed to the outcome assessment and dependence on the judgment of the physicians. The evaluation criteria for various assessment methods may also have varied among the studies. By excluding each study in turn, we achieved an I2 of 0 for 6MWD after exclusion of 1 publication with a high weighting (52.5%).33
Figure 2.
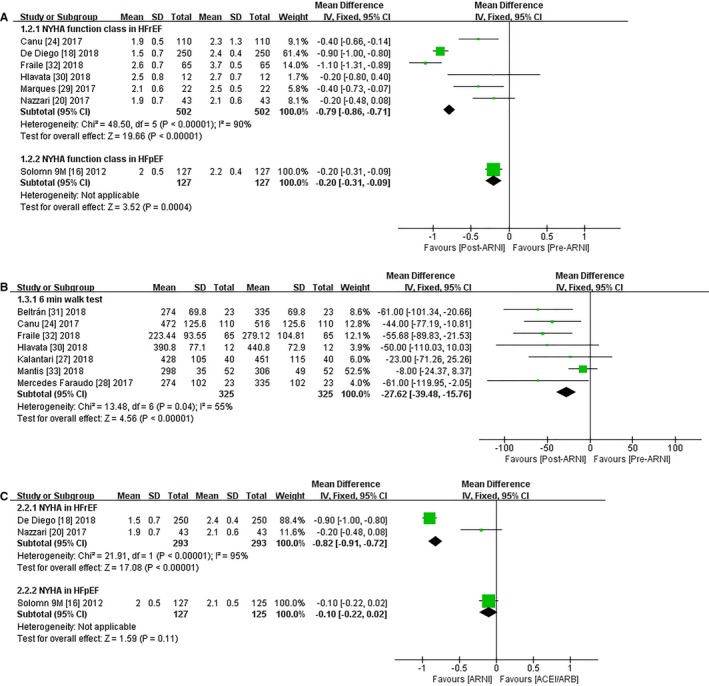
Forest plot showing changes in functional capacity including (A) NYHA functional class, (B) 6MWD following ARNI, and (C) changes of NYHA functional class comparing ARNI with ACEIs/ARBs. 6MWD indicates 6‐minute walking distance; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; df, degrees of freedom; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; NYHA, New York Heart Association.
In contrast to ACEIs/ARBs, NYHA functional class changed by 0.82 (95% CI −0.91, −0.72; Figure 2C) in HFrEF patients taking ARNI. The I2 value was decreased after exclusion of the data from the study with the highest weighting (>88%) due to its large sample size.19 No significant changes in NYHA functional class were observed (Figure 2C), and no data on 6MWD were available for patients with HFpEF.
Effects of ARNI on CRR Indices
The pooled data from 10 studies (Table 2 and Table S3) showed increases in LVEF (MD 4.64%, 95% CI 3.93, 5.35; Figure 3A). Subgroup analyses based on HFrEF or HFpEF showed a greater increment in LVEF (MD 4.89%, 95% CI 4.13, 5.65; Figure 3A) among HFrEF subjects, but improvements in LVEF were observed only after 9 months of treatment in patients with HFpEF (MD 2.70%, 95% CI 0.60, 4.80; Figure 3A). Relevant results were extracted from 7 publications, including 2 RCTs (Table 2), regarding baseline and follow‐up data for ESV and EDV.16, 19 Specifically, the mean ESV decreased by 18.23 mL compared with baseline after treatment with ARNI (95% CI −27.25, −9.20; Figure 3B), and mean EDV decreased by 21.60 mL (95% CI −24.32, −18.88; Figure 3B) in HFrEF patients. Likewise, ESD (MD −3.50 mm, 95% CI −5.56, −1.44; Figure 3B), EDD (MD −2.42 mm, 95% CI −3.06, −1.78; Figure 3B), LAV (MD −7.59 mL, 95% CI −14.03, −1.14; Figure 3B), and LVMI (MD −14.44 g/m2, 95% CI −22.61, −6.27; Figure 3B) were all significantly reduced in patients with HFrEF. ESV (MD −6.90 mL, 95% CI −11.35, −2.45; Figure 4A), EDV (MD −10.40 mL, 95% CI −17.86, −2.94; Figure 4A), and LVMI (MD −4.55 g/m2, 95% CI −8.92, −0.18) were significantly reduced in patients with HFpEF, but there was no significant change in LAV (MD −4.60 mL, 95% CI −10.91, 1.71).
Table 2.
Discrepancy of Indices Between ARNI and ACEIs/ARBs Groups With a Period Time of Follow‐Up
| Index | Intervention | Solomon 3 mo | Solomon 9 mo | Kang DH | Almufleh | De Diego | Nazzari | McMurray 1 mo | McMurray 8 mo | Schmieder 3 mo | Schmieder 13 mo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEF, % | ACEI/ARB | 59±8 | 61.2±8 | 115.4±50.8 | 25.33±7.8 | 31±6 | 27.4±6.9 | NR | NR | NR | NR |
| ARNI | 59.3±7 | 61.0±7 | 104.6±71.4 | 30.14±8 | 36.5±8 | 36.4±12.4 | NR | NR | NR | NR | |
| NYHA function class | ACEI/ARB | NR | 2.1±0.5 | NR | NR | 2.4±0.4 | 2.1±0.6 | NR | NR | NR | NR |
| ARNI | NR | 2±0.5 | NR | NR | 1.5±0.7 | 1.9±0.7 | NR | NR | NR | NR | |
| ESV, mL | ACEI/ARB | 45.8±19.1 | 40.1±11.2 | 125.6±58 | 165.0±91.5 | NR | NR | NR | NR | NR | NR |
| ARNI | 43.2±15 | 40±15.5 | 105.2±51.1 | 143.7±91.5 | NR | NR | NR | NR | NR | NR | |
| EDV, mL | ACEI/ARB | 109.8±29.8 | 101.6±30.7 | 193.3±71.3 | 221.4±3546 | 141±17 | NR | NR | NR | NR | NR |
| ARNI | 107±25.9 | 101±25.9 | 164.4±60 | 207.5±3546 | 119±15 | NR | NR | NR | NR | NR | |
| ESD, mm | ACEI/ARB | NR | NR | 53.9±11.3 | 56.3±6.5 | NR | NR | NR | NR | NR | NR |
| ARNI | NR | NR | 50.3±9.5 | 52.9±6.5 | NR | NR | NR | NR | NR | NR | |
| EDD, mm | ACEI/ARB | NR | NR | 66.6±9.5 | 65.8±3.4 | 62±6 | 67.6±4.2 | NR | NR | NR | NR |
| ARNI | NR | NR | 63.4±7.8 | 63.15±3.4 | 60±6 | 65.2±4.2 | NR | NR | NR | NR | |
| LVMI, g/m2 | ACEI/ARB | 74.6±20.6 | 77.6±21.9 | NR | 128.1±16.4 | 128.1±17 | NR | NR | NR | 69.8±12 | 68.6±12 |
| ARNI | 76.2±21.1 | 73.8±20.2 | NR | 113.66±16.4 | 113.66±14 | NR | NR | NR | 65.74±16 | 65.27±15.8 | |
| LAV, mL | ACEI/ARB | 66.8±27.8 | 67.9±28.7 | 115.4±50.8 | NR | NR | NR | NR | NR | NR | NR |
| ARNI | 63.8±22.6 | 60.7±22.1 | 104.6±71.4 | NR | NR | NR | NR | NR | NR | NR | |
| NT‐proBNP, pg/mL | ACEI/ARB | 835±200.74 | 607±204 | NR | NR | NR | NR | 1203±225 | 1102±243.8 | NR | NR |
| ARNI | 605±149.6 | 496±157 | NR | NR | NR | NR | 938±180.7 | 859±209.7 | NR | NR | |
| sST2, ng/mL | ACEI/ARB | 31±15.2 | 35.2±15.9 | NR | NR | NR | NR | 32.6±9.6 | 31.8±11.56 | NR | NR |
| ARNI | 29.8±16.7 | 31.4±19.9 | NR | NR | NR | NR | 31±9.6 | 30.2±10.07 | NR | NR |
All data were presented by mean±SD. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; EDD, end‐diastolic dimension; EDV, end‐diastolic volume; ESD, end‐systolic dimension; ESV, end‐systolic volume; LAV, left atrial volume; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; mo, months; NR, not reported; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; NYHA, New York Heart Association; sST2, soluble suppressor of tumorigenesis‐2.
Figure 3.
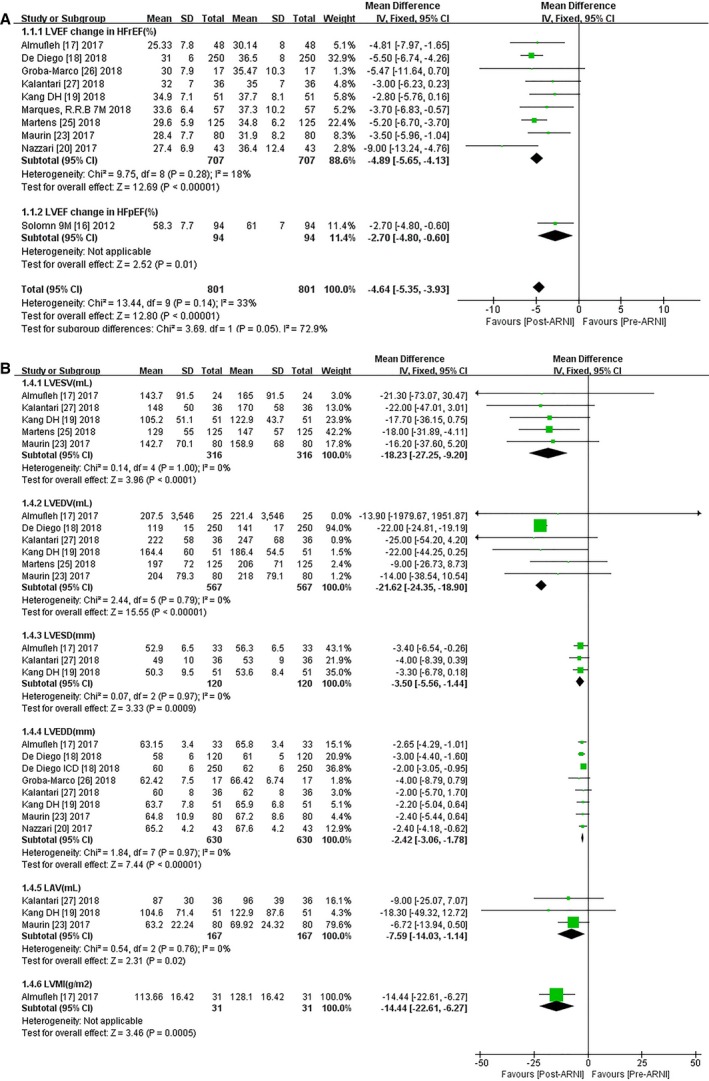
Forest plots for (A) effect of ARNI on LVEF and (B) other CRR indices of HFrEF patients. ARNI indicates angiotensin‐receptor neprilysin inhibitor; df, degrees of freedom; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; LAV, left atrial volume; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end‐diastolic dimension; LVEDV, left ventricular end‐diastolic volume; LVESD, left ventricular end‐systolic dimension; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index.
Figure 4.
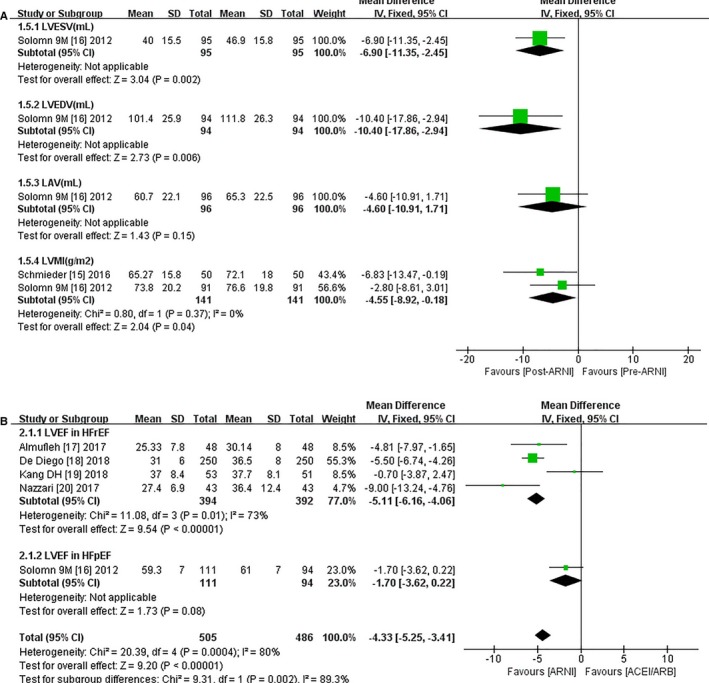
Forest plots for effect of ARNI on remodeling indexes (LVESV, LVEDV, LVESD, LVEDD, LAV, LVMI) (A) in HFpEF patients following ARNI and (B) effect of ARNI on LVEF compared with ACEIs/ARBs. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; df, degrees of freedom; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; LAV indicates left atrial volume; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index.
LVEF scores increased by 5.11% in HFrEF patients with ARNI compared with patients using ACEIs/ARBs (95% CI 4.06, 6.16; Figure 4B). Both ESV (MD −20.53 mL, 95% CI −39.98, −1.08; Figure 5A) and EDV were significantly decreased (MD −22.08 mL, 95% CI −24.88, −19.29; Figure 5A), and ESD showed a notable decline (MD −3.48 mm, 95% CI −5.95, −1.01; Figure 5A) in patients taking ARNI. EDD was significantly reduced (MD −2.45 mm, 95% CI −3.13, −1.78; Figure 5A) in 4 HFrEF studies.17, 18, 19, 20 ARNI outperformed ACEIs/ARBs in HFpEF patients in terms of LVMI and LAV (LVMI, MD −3.25 g/m2, 95% CI −3.78, −2.72; LAV, MD −7.20 mL, 95% CI −14.11, −0.29; Figure 5B), but there were no significant improvements in other CRR indices with ARNI treatment.
Figure 5.
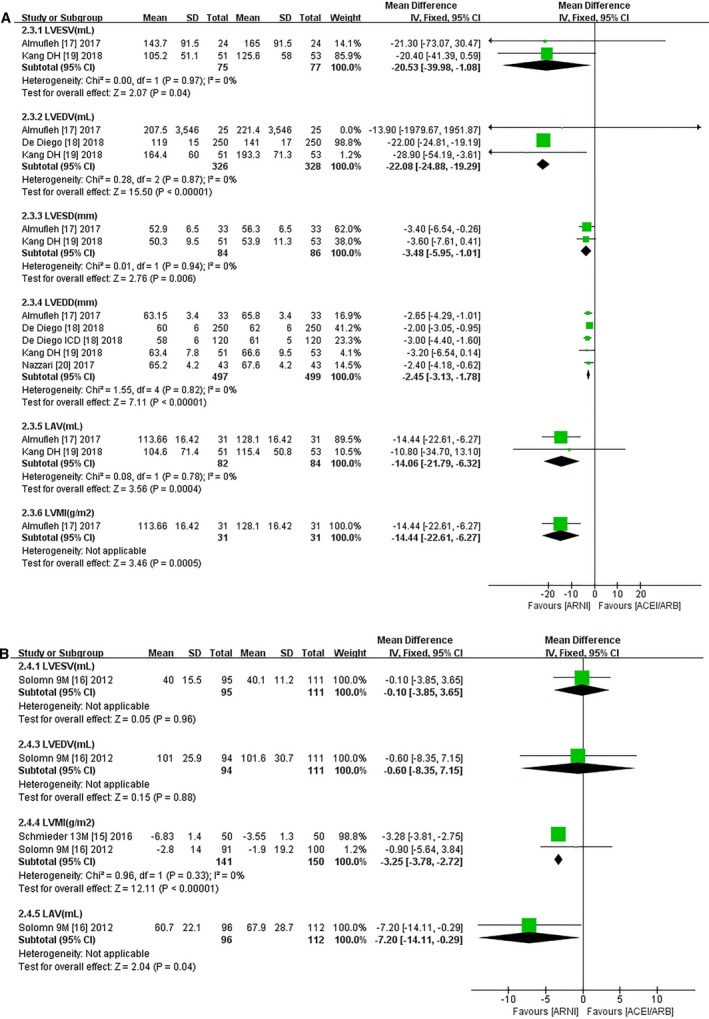
Forest plots for effect of ARNI on main remodeling indices (LVESV, LVEDV, LVESD, LVEDD, LAV, LVMI) (A) in HFrEF patients and (B) in HFpEF patients following ARNI compared with ACEIs/ARBs. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; df, degrees of freedom; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; LAV, left atrial volume; LVEDD, left ventricular end‐diastolic dimension; LVEDV, left ventricular end‐diastolic volume; LVESD, left ventricular end‐systolic dimension; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index.
Notably, ARNI markedly reduced LVMI compared with olmesartan in patients with essential hypertension (MD −4.04 g/m2, 95% CI −4.75, −3.33) after a short‐term follow‐up of 3 months, and the effects lasted for at least 13 months (MD −3.28 g/m2, 95% CI −3.81, −2.75; Table 3).
Table 3.
Subgroup Analysis of Effects of ARNI on LV Indices According to Characteristics
| Subgroup | No. of Studies | n | NYHA Function Classification | 6MWD, m | LVEF, % |
|---|---|---|---|---|---|
| Age, y | |||||
| <65 | 8 | 9023 | −0.31 (−0.49, −0.12) I2=6%, Z=3.17 (P=0.002) | 15.04 (0.36, 29.72) I2=72%, Z=2.01 (P=0.04) | 4.27 (2.60, 5.93) I2=52%, Z=5.03 (P<0.00001) |
| ≥65 | 9 | 988 | −0.63 (−0.70, −0.57) I2=97%, Z=18.15 (P<0.00001) | 58.41 (34.57, 82.25) I2=0, Z=4.8 (P<0.00001) | 4.26 (3.51, 5.00) I2=73%, Z=11.19 (P<0.00001) |
| Region | |||||
| Europe | 14 | 1226 | −0.84 (−0.92, −0.76) I2=86%, Z=20.09 (P<0.00001) | 27.92 (15.68, 40.16) I2=63%, Z=4.47 (P<0.00001) | 5.03 (4.18, 5.88) I2=0, Z=11.62 (P<0.00001) |
| North America | 3 | 131 | −0.2 (−0.48, 0.08)*, Z=1.42 (P=0.15) | 23 (−25.26, 71.26)*, Z=0.93 (P=0.35) | 5.05 (3.05, 7.04) I2=59%, Z=4.96 (P<0.00001) |
| Asia | 1 | 118 | NR | NR | 2.8 (−0.16, 5.76)*, Z=1.86 (P=0.06) |
| Global multiple centers | 2 | 8700 | −0.2 (−0.31, −0.09)*, Z=3.52 (P=0.0004) | NR | 1.82 (0.41, 3.23) I2=18%, Z=2.53 (P=0.01) |
| Follow‐up | |||||
| Intervention effect ≤6 mo | 13 | 1009 | −0.76 (−0.85, −0.67) I2=91%, Z=17.00 (P<0.00001) | 23.77 (11.12, 36.42) I2=53%, Z=3.68 (P=0.0002) | 4.51 (3.77, 5.25) I2=68%, Z=11.9 (P<0.00001) |
| Intervention effect ≥9 mo | 7 | 9166 | −0.40 (−0.49, −0.30) I2=96%, Z=8.31 (P<0.00001) | 55.68 (21.53, 89.83),* Z=3.20 (P=0.001) | 2.96 (1.45, 4.46) I2=0, Z=3.86 (P=0.0001) |
| Dosage of ARNI | |||||
| Medium/high dose ≤50% | 7 | 386 | −1.1 (−1.31, −0.89)*, Z=10.31 (P<0.00001) | 24.15 (10.65, 37.64) I2=74%, Z=3.51 (P=0.0005) | 5.38 (4.44, 6.32) I2=0, Z=11.17 (P<0.00001) |
| Medium/high dose >50% | 11 | 9671 | −0.24 (−0.33, −0.15) I2=0, Z=5.17 (P<0.00001) | 45.4 (16.36, 74.45) I2=0, Z=3.06 (P=0.002) | 3.76 (2.61, 4.9) I2=35%, Z=6.43 (P<0.00001) |
| Mean baseline SBP | |||||
| SBP ≤120 mm Hg | 4 | 423 | −0.4 (−0.6, −0.2) I2=0, Z=3.85 (P=0.0001) | 44 (10.81, 77.19)*, Z=2.6 (P=0.009) | 3.73 (1.95, 5.5) I2=0, Z=4.11 (P<0.0001) |
| SBP >120 mm Hg | 8 | 9324 | −0.64 (−0.71, −0.57) I2=97%, Z=18 (P<0.00001) | 55.65 (29.31, 82.16) I2=0, Z=4.11 (P<0.0001) | 4.92 (4.05, 5.79) I2=62%, Z=11.08 (P<0.00001) |
| Different control groups | |||||
| ACEIs | 2 | 8649 | NR | NR | NR |
| ARBs | 3 | 533 | NR | NR | 2.73 (1.02, 4.45) I2=0, Z=3.13 (P=0.002) |
| Etiology | |||||
| Ischemic heart disease ≤50% | 6 | 310 | −0.35 (−0.64, −0.07) I2=0, Z=2.42 (P=0.02) | 57.58 (24.09, 91.06) I2=0, Z=3.37 (P=0.0008) | 3.86 (2.15, 5.57) I2=0, Z=4.43 (P<0.00001) |
| Ischemic heart disease >50% | 8 | 9194 | −0.8 (−0.96, −0.79) I2=89%, Z=20.23 (P<0.00001) | 23.35 (10.20, 36.49) I2=70%, Z=3.48 (P=0.0005) | 5.13 (4.24, 6.02) I2=1%, Z=11.29 (P<0.00001) |
| Concomitant therapy | |||||
| MRA ≤50% | 3 | 484 | −0.4 (−0.5, −0.3) I2=98%, Z=7.95 (P<0.00001) | 55.68 (21.53, 89.83),* Z=3.20 (P=0.001) | 2.73 (1.02, 4.45) I2=0, Z=3.13 (P=0.002) |
| MRA >50% | 12 | 9341 | −0.73 (−0.82, −0.65) I2=90%, Z=17.04 (P<0.00001) | 23.77 (11.12, 36.42) I2=53%, Z=3.68 (P=0.0002) | 5.04 (4.25, 5.82) I2=9%, Z=12.64 (P<0.00001) |
| Subgroup | No. of Studies | n | LVESV, mL | LVEDV, mL | LVESD, mm | LVEDD, mm | LVMI, g/m2 | LAV, mL | NT‐proBNP, pg/mL | sST2, ng/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | ||||||||||
| <65 | 8 | 9023 | −17.06 (−31.04, −3.08) I2=0, Z=2.39 (P=0.02) | −18.39 (−34.87, −1.91) I2=0, Z=2.19 (P=0.03) | −3.30 (−6.78, 0.18)*, Z=1.86 (P=0.06) | −2.48 (−3.77, −1.18) I2=0, Z=3.74 (P=0.0002) | −6.59 (−11.23, −1.95) I2=0, Z=2.78 (P=0.005) | −7.32 (−14.35, −0.28) I2=0, Z=2.04 (P=0.04) | −474.47 (−538.92, −410.02) I2=0, Z=14.43 (P<0.00001) | −2.35 (−2.87, −1.83) I2=0, Z=8.86 (P<0.00001) |
| ≥65 | 9 | 988 | −5.52 (−8.42, −2.63) I2=40%, Z=3.73 (P=0.0002) | −18.07 (−20.50, −15.64) I2=87%, Z=14.60 (P<0.00001) | −3.40 (−6.54, −0.26)*, Z=2.12 (P=0.03) | −2.42 (−3.17, −1.67) I2=0, Z=6.35 (P<0.00001) | −4.35 (−7.93, −0.77)I2=73%, Z=2.38 (P=0.02) | −3.86 (−8.2, 0.48) I2=0, Z=1.75 (P=0.08) | −228.49 (−257.90, −199.09) I2=91%, Z=15.23 (P<0.00001) | −1.98 (−5.03, 1.08) I2=0, Z=1.27 (P=0.2) |
| Region | ||||||||||
| Europe | 14 | 1226 | −17.47 (−29.12, −5.82) I2=0, Z=2.94 (P=0.003) | −21.58 (−24.34, −18.83) I2=16%, Z=15.34 (P<0.00001) | NR | −2.41 (−3.21, −1.61) I2=0, Z=5.91 (P<0.00001) | NR | −6.72 (−13.94, 0.5)*, Z=1.82 (P=0.07) | −228.49 (−257.90, −199.09) I2=91%, Z=15.23 (P<0.00001) | −5.52 (−12.16, 1.12) I2=0, Z=1.63 (P=0.1) |
| North America | 3 | 131 | −21.87 (−44.39, 0.66) I2=0, Z=1.90 (P=0.06) | −22.17 (−47.37, 3.03) I2=0, Z=1.72 (P=0.08) | −3.60 (−6.16, −1.05) I2=0, Z=2.77 (P=0.006) | −2.48 (−3.63, −1.34) I2=0, Z=4.25 (P<0.0001) | NR | −9 (−25.07, 7.07)*, Z=1.1 (P=0.27) | NR | NR |
| Asia | 1 | 118 | −17.7 (−36.15, 0.75)*, Z=1.88 (P=0.06) | −22 (−44.25, 0.25)*, Z=1.94 (P=0.05) | −3.30 (−6.78, 0.18)*, Z=1.86 (P=0.06) | −2.20 (−5.04, 0.64)*, Z=1.52 (P=0.13) | NR | −18.30 (−49.32, 12.72)*, Z=1.16 (P=0.25) | NR | NR |
| Global multiple centers | 2 | 8700 | −6.9 (−11.35, −2.45)*, Z=3.04 (P=0.002) | −10.4 (−17.86, −2.94)*, Z=2.73 (P=0.006) | NR | NR | NR | NR | −305.95 (−346.51, −265.38) I2=94%, Z=14.78 (P<0.00001) | −2.46 (−3.19, −1.74) I2=0, Z=6.64 (P<0.00001) |
| Follow‐up | ||||||||||
| Intervention effect ≤6 mo | 13 | 1009 | −5.25 (−8.97, −1.53) I2=45%, Z=2.77 (P=0.006) | −18.97 (−21.51, −16.43) I2=82%, Z=14.63 (P<0.00001) | −3.60 (−6.16, −1.05) I2=0, Z=2.77 (P=0.006) | −2.43 (−3.09, −1.78) I2=0, Z=7.28 (P<0.00001) | −5.65 (−9.37, −1.93) I2=72%, Z=2.97 (P=0.003) | −7.1 (−13.69, −0.52) I2=0, Z=2.11 (P=0.03) | −244.40 (−279.97, −208.82) I2=93%, Z=13.46 (P<0.00001) | −2.22 (−2.94, −1.49) I2=0, Z=6.02 (P<0.00001) |
| Intervention effect ≥9 mo | 7 | 9166 | −7.49 (−11.82, −3.17) I2=20%, Z=3.39 (P=0.0007) | −11.57 (−18.65, −4.50) I2=0, Z=3.21 (P=0.001) | −3.30 (−6.78, 0.18)*, Z=1.86 (P=0.06) | −2.20 (−5.04, 0.64)*, Z=1.52 (P=0.13) | −4.55 (−8.92, −0.18) I2=0, Z=2.04 (P=0.04) | −18.30 (−49.32, 12.72)*, Z=1.16 (P=0.25) | −310.37 (−350.24, −270.50) I2=88%, Z=15.26 (P<0.00001) | −2.49 (−3.22, −1.77) I2=0, Z=6.75 (P<0.00001) |
| Dosage of ARNI | ||||||||||
| Medium/high dose ≤50% | 7 | 386 | −18 (−31.89, −4.11)*, Z=2.54 (P=0.01) | −21.68 (−24.46, −18.91), I2=50%, Z=15.31 (P<0.00001) | NR | −2.41 (−3.24, −1.58) I2=0, Z=5.71 (P<0.00001) | NR | NR | −691 (−892.88, −489.12)*, Z=6.71 (P<0.00001) | NR |
| Medium/high dose >50% | 11 | 9671 | −7.93 (−12.15, −3.7) I2=0, Z=3.68 (P=0.0002) | −11.8 (−18.53, −5.06) I2=0, Z=3.43 (P=0.0006) | −3.36 (−5.69, −1.03) I2=0, Z=2.82 (P=0.005) | −2.47 (−3.52, −1.43) I2=0, Z=4.65 (P<0.00001) | −5.81 (−10.51, −1.11) I2=0, Z=2.43 (P=0.02) | −6.75 (−10.6, −2.89), I2=61%, Z=3.43 (P=0.0006) | −308.8 (−349.28, −268.32) I2=85%, Z=14.95 (P<0.00001) | −2.47 (−3.2, −1.75) I2=0, Z=6.67 (P<0.00001) |
| Mean baseline SBP | ||||||||||
| SBP ≤120 mm Hg | 4 | 423 | −18.11 (−35.49, −0.72) I2=0, Z=2.04 (P=0.04) | −20.66 (−40.97, −0.34), I2=0, Z=1.99 (P=0.05) | −3.36 (−5.69, −1.03) I2=0, Z=2.82 (P=0.005) | −2.54 (−3.96, −1.12) I2=0, Z=3.50 (P=0.0005) | −14.44 (−22.61, −6.27)*, Z=3.46 (P=0.0005) | −18.30 (−49.32, 12.72)*, Z=1.16 (P=0.25) | NR | NR |
| SBP >120 mm Hg | 8 | 9324 | −7.93 (−12.17, −3.7) I2=55%, Z=3.67 (P=0.0002) | −20.31 (−22.91, −17.71), I2=79%, Z=15.3 (P<0.00001) | NR | −2.36 (−3.2, −1.52) I2=20%, Z=5.51 (P<0.00001) | −4.55 (−8.92, −0.18) I2=0, Z=2.04 (P=0.04) | −4.6 (−10.91, 1.71)*, Z=1.43 (P=0.15) | −321.46 (−361.22, −281.71) I2=90%, Z=15.85 (P<0.00001) | −2.46 (−3.19, −1.74) I2=0, Z=6.64 (P<0.00001) |
| Different control groups | ||||||||||
| ACEIs | 2 | 8649 | NR | −22 (−24.81, −19.19)*, Z=15.34 (P<0.00001) | NR | −3 (−4.4, −1.6)*, Z=4.21 (P<0.0001) | NR | NR | −479.3 (−574.02, −384.58)*, Z=9.92 (P<0.00001) | −2.5 (−3.24, −1.76)*, Z=6.66 (P<0.00001) |
| ARBs | 3 | 533 | −7.49 (−11.82, −3.17) I2=20%, Z=3.39 (P=0.0007) | −11.57 (−18.65, −4.5), I2=0, Z=3.21 (P=0.001) | −3.30 (−6.78, 0.18)*, Z=1.86 (P=0.06) | −2.20 (−5.04, 0.64)*, Z=1.52 (P=0.13) | −4.55 (−8.92, −0.18) I2=0, Z=2.04 (P=0.04) | −5.14 (−11.33, 1.04), I2=0, Z=1.63 (P=0.1) | −267 (−311.9, −222.1)*, Z=11.66 (P<0.00001) | −0.8 (−5.86, 4.26)*, Z=0.31 (P=0.76) |
| Etiology | ||||||||||
| Ischemic heart disease ≤50% | 6 | 310 | −18.11 (−35.49, −0.72) I2=0, Z=2.04 (P=0.04) | −20.66 (−40.97, −0.34), I2=0, Z=1.99 (P=0.05) | −3.36 (−5.69, −1.03) I2=0, Z=2.82 (P=0.005) | −2.66 (−4.02, −1.29) I2=0, Z=3.82 (P=0.0001) | NR | −18.30 (−49.32, 12.72)*, Z=1.16 (P=0.25) | −977.7 (−2324.81, 369.41)*, Z=1.42 (P=0.15) | NR |
| Ischemic heart disease >50% | 8 | 9194 | −17.47 (−29.12, −5.82) I2=0, Z=2.94 (P=0.003) | −21.58 (−24.34, −18.83), I2=16%,Z=15.34 (P<0.00001) | NR | −2.36 (−3.17, −1.55) I2=0, Z=5.72 (P<0.00001) | NR | −6.72 (−13.94, 0.5)*, Z=1.82 (P=0.07) | −517.5 (−603.25, −431.74) I2=71%, Z=11.83 (P<0.00001) | NR |
| Concomitant therapy | ||||||||||
| MRA ≤50% | 3 | 484 | −7.49 (−11.82, −3.17) I2=20%, Z=3.39 (P=0.0007) | −3.30 (−6.78, 0.18)*, Z=1.86 (P=0.06) | −2.20 (−5.04, 0.64)*, Z=1.52 (P=0.13) | −2.8 (−8.61, 3.01)*, Z=0.94 (P=0.35) | −5.14 (−11.33, 1.04), I2=0, Z=1.63 (P=0.1) | −267 (−311.9, −222.1)*, Z=11.66 (P<0.00001) | −0.8 (−5.86, 4.26)*, Z=0.31 (P=0.76) | |
| MRA >50% | 12 | 9341 | −18.4 (−28.74, −8.05) I2=0, Z=3.48 (P=0.0005) | −21.59 (−24.33, −18.85), I2=0, Z=15.43 (P<0.00001) | −3.60 (−6.16, −1.05) I2=0, Z=2.77 (P=0.006) | −2.43 (−3.09, −1.78) I2=0, Z=7.28 (P<0.00001) | −9.85 (−15.01, −4.7) I2=50%, Z=3.75 (P=0.0002) | −7.1 (−13.69, −0.52) I2=0, Z=2.11 (P=0.03) | −513.66 (−592.64, −434.67) I2=33%, Z=12.75 (P<0.00001) | −2.54 (−3.27, −1.81) I2=0, Z=6.8 (P<0.00001) |
Results at 3‐ to 6‐month follow‐up used unless otherwise stated. Mean differences are pooled estimates from meta‐analysis with 95% CIs. I2 values reported as measure of heterogeneity. Z scores with associated P values reported as test for overall effect. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; EDD, end‐diastolic dimension; EDV, end‐diastolic volume; ESD, end‐systolic dimension; ESV, end‐systolic volume; LAV, left atrial volume; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MRA, mineralocorticoid receptor antagonist; NR, not reported; NT‐proBNP, N‐terminal pro–brain‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; sST2, soluble suppressor of tumorigenesis‐2; 6MWD, 6‐minute walking distance.*Data was available in only one study. (This sentence should start on a new line.)
Effects of ARNI on Biomarkers
Compared with ACEIs/ARBs, ARNI reduced NT‐proBNP in both HFrEF patients11, 18, 21, 22, 30 and HFpEF patients16 (HFrEF, MD −243.00 pg/mL, 95% CI −264.26, −221.74; HFpEF, MD −111.00 pg/mL, 95% CI −157.92, −64.08). ARNI reduced sST2 in HFrEF (MD −1.60 ng/mL, 95% CI −2.61, −0.59) but not in HFpEF patients (MD −3.80 ng/mL, 95% CI −8.67, 1.07). The detailed data are provided in Figure S3 and in Table S4.
Subgroup Analyses
The results of subgroup analyses are shown in Table 3. Age >65 years, European studies, short‐term follow‐up (3‐6 months), baseline systolic BP >120 mm Hg, proportion of patients with ischemic heart disease >50%, and concomitant therapy with mineralocorticoid receptor antagonists (MRAs) >50% were associated with greater enhancements in NYHA functional class. The I2 statistic was reduced from 90% to 0%, without altering the significance of the pooled effect size, when studies were restricted to those in which >50% of patients achieved the target dose of ARNI. Two studies were excluded, 1 because the proportion of patients with a target dose of ARNI was ≤50%,32 and another because of a lack of information on the proportion of patients reaching the target dose of ARNI18 (Figure S4). An increase in 6MWD was related to older age, but there were no significant differences in 6MWD changes in relation to other baseline characteristics. Subgroup analysis failed to provide a consistent explanation for the moderate heterogeneity (I2=55%) between studies in terms of 6MWD, although the I2 value was decreased to 53% when studies were limited to patients with MRA use >50% and short‐term follow‐up (Figure S5).
In the analyses of CRR indices, age, region, baseline systolic BP, follow‐up, proportion of patients with ischemic heart disease, proportion of patients with target dose of ARNI, and MRA use were not associated with significant improvements in ESV, ESD, EDD, or LVMI. However, European studies and MRA use >50% were related to greater improvements in LVEF and I2 value decreased substantially. EDV seemed to decline with MRA use >50% and in studies with an ACEI as the control drug. In terms of biomarkers, sST2 was not related to any baseline characteristics, but NT‐proBNP decreased more with age <65 years, MRA use >50%, follow‐up longer than 9 months, and ACEI controls in European studies.
Correlation and Regression Analyses
Functional capacity and CRR indices followed normal distributions, and the potential relationships between LVEF and other CRR indices were therefore calculated using Pearson correlations. There was no significant correlation between improvements in LVEF and reductions in other CRR indices (LVEF and ESV, r=−0.423, P=0.404; LVEF and EDV, r=0.191, P=0.682; LVEF and ESD, r=−0.366, P=0.634; LVEF and EDD, r=−0.450, P=0.263; LVEF and LAV, r=0.261, P=0.739; LVEF and LVMI, r=−0.995, P=0.066; Figure S6), although sample sizes were limited. Scatterplots showed that the data for 1 study deviated from most of the other data.20 Analysis of the data after this study had been excluded showed a possible correlation between LVEF and EDD (r=−0.801, P=0.030).
Eleven models were selected, and the best model was chosen according to the statistical results. The results of curve fitting for the 11 models are shown in Table 4. All the regression models, except the inverse, quadratic, and S regression models, were statistically significant (P<0.050). However, the R 2 value was higher for the cubic regression model (R 2=0.948, P=0.020) than for the linear model (R 2=0.642, P=0.030). The regression equation was y=0.041+0.071x+0.045x2+0.006x3 (Figure 6).
Table 4.
Model Summary and Parameter Estimates In Analyzing Relation of LVEF and LVEDD (mm)
| Equation | Model Summary | Parameter Estimates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 | F | df1 | df2 | Sig. | Constant | b1 | b2 | b3 | |
| Linear | 0.642 | 8.962 | 1 | 5 | 0.030 | 0.019 | −0.009 | ||
| Inverse | 0.240 | 1.576 | 1 | 5 | 0.265 | 0.044 | 0.004 | ||
| Quadratic | 0.709 | 4.883 | 2 | 4 | 0.084 | 0.025 | −0.001 | 0.002 | |
| Cubic | 0.948 | 18.380 | 3 | 3 | 0.020 | 0.041 | 0.071 | 0.045 | 0.006 |
| Compound | 0.659 | 9.679 | 1 | 5 | 0.027 | 0.022 | 0.800 | ||
| Logarithmica | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ||
| Powera | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ||
| S | 0.271 | 1.860 | 1 | 5 | 0.231 | −3.165 | 0.110 | ||
| Growth | 0.659 | 9.679 | 1 | 5 | 0.027 | −3.796 | −0.223 | ||
| Exponential | 0.659 | 9.679 | 1 | 5 | 0.027 | 0.022 | −0.223 | ||
| Logistic | 0.659 | 9.679 | 1 | 5 | 0.027 | 44.526 | 1.250 | ||
Dependent Variable: ∆LVEF. The independent variable is ∆EDD. df indicates degreed of freedom; LVEDD, end‐diastolic dimension; LVEF, left ventricular ejection fraction.
The independent variable (∆EDD) contains nonpositive values. The minimum value is −4.00. The Logarithmic and Power models cannot be calculated.
Figure 6.
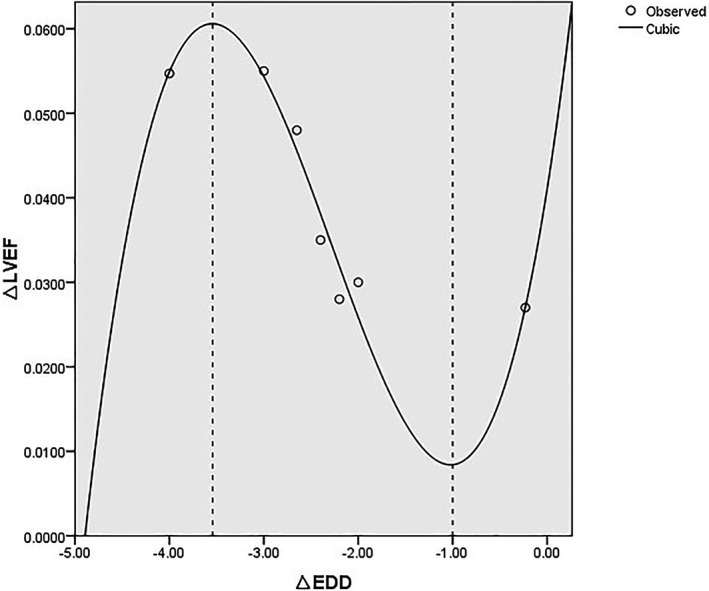
Fitting curve using cubic curve model to explore the relationship between LVEF and EDD changes. EDD indicates end‐diastolic dimension; LVEF, left ventricular ejection fraction.
We conducted correlation analyses to determine the effects of baseline characteristics on the results but found no significant correlations between CRR indices and the main factors (age, region, essential hypertension, diabetes mellitus, and concomitant treatments including β‐blockers and MRAs). The relationship between LVEF and EDD remained significant after adjusting the baseline information, but the result merely indicated a possible trend in the relationship between LVEF and EDD because of the small sample size.
Discussion
The present study provided the first meta‐analysis to evaluate the effects of ARNI on functional capacity, CRR indices, and biomarkers in HF patients based on all available studies to date. We distinguished between patients with HFrEF and those with HFpEF, and the pooled results showed significant improvements in all indices following ARNI treatment compared with ACEIs/ARBs in HFrEF patients, but they showed only marked changes in LVMI and LAV in HFpEF patients. The benefits of ARNI were manifest at 3 months and lasted for 12 months. Subgroup analyses were performed to address the heterogeneities in NYHA functional class, 6MWD, and LVEF, and a possible curvilinear relationship between LVEF and EDD was observed. ARNI had notable effects on CRR indices in HFrEF patients, including patients who failed to reach the target dose. Both ACEIs and ARBs are accepted drugs for improving the prognosis of patients with HF and myocardial infarction, with beneficial effects in terms of reducing cardiovascular mortality and reversing myocardial remodeling.5, 6, 7, 8, 9 It is therefore reasonable that ARNI, as a combination of an ARB and neprilysin inhibitor, would have a good effect on CRR. Improvements in CRR may be 1 of the mechanisms by which ARNI can reduce both cardiovascular and all‐cause mortality. The relationship between BP lowering and the effects of ARNI was evaluated previously, but no significant association was found, consistent with the current results based on BP.36 The current meta‐analysis showed robust results in terms of the remarkable improvements in CRR, regardless of the follow‐up period and region. Interestingly, however, use of an MRA was associated with changes in CRR indices. This may be related to the effects of MRAs on the renin‐angiotensin‐aldosterone system and their confirmed effects on CRR.37, 38 The more distinct improvements in CRR with MRA use may be associated with the effects of diuresis, BP lowering, and antifibrosis. Moderate to considerable heterogeneity was observed among studies in relation to NYHA functional class, 6MWD, and LVEF. However, because the target dosage of ARNI was an independent factor, the heterogeneity was removed after excluding studies with the few patients who reached the target dose of ARNI.18, 32
The effects of ARNI on most indices, except LVMI and LAV, were not significant in patients with HFpEF. To determine the effect of ARNI on LV diastolic function was one of the original aims of our analyses. LAV was used as an index reflecting the possible benefits of ARNI on diastolic function in HF patients, but data on other diastolic function indices were limited (Figure S7). It was difficult to judge the effects of ARNI on diastolic function in HFpEF patients, but we aim to update the results based on ongoing studies in HFpEF patients.39 We did not directly compare the effects of different doses of ARNI. Although we performed a subgroup analysis to roughly assess the effects of ARNI dose on CRR, no significant differences in CRR indices were observed between groups based on the proportion of patients who reached the target dose. This may have been because of our crude analyses and the fact that most studies included >50% of patients with the target dose. However, it may also have been related to the superior effects of lower doses of ARNI. The results should therefore be interpreted with caution given the loss of statistical power, and because indirect comparison tests failed to confirm any statistically significant differences. Further studies are needed to directly compare different doses of ARNI, especially in patients prone to hypotension.
We demonstrated a linear relation between LVEF and EDD, with a low r value. The r value seemed higher by curve estimation. Curve fitting inferred that LVEF improved in line with greater reductions in EDD, within a certain range. However, further decreases in EDD did not continue to improve diastole and LV filling, and insufficient filling volume affects the ejection process and the LVEF.40, 41 Furthermore, EDD is not the only determinant of LVEF, and LVEF can increase significantly only when both diastolic and systolic functions are improved reasonably. This may be the main reason for the nonlinear correlation between LVEF and EDD. However, as we warned above, the results need to be interpreted with caution because of the small sample size. Furthermore, we did not determine the correlation between LVEF and ESD, and although the nonlinear correlation between LVEF and EDD may indicate a trend whereby LVEF increased when EDD decreased within a certain range, the current study could not prove such a relationship.
Previous meta‐analyses focused on the effects of ARNI on BP and on the composite end point of death and HF hospitalization.42, 43, 44 Decreases in LVMI in patients with HFrEF and in patients with essential hypertension showed the potential of ARNI for treating cardiac hypertrophy. Although some studies showed close relationships between mortality and cardiac remodeling in patients taking ACEIs/ARBs, not all drugs that achieved short‐term CRR improved prognosis.5, 6, 45 More studies are needed to elucidate the relationship between CRR and reduced mortality after ARNI administration.
The results of the current meta‐analysis were more significant when only observational studies were included, compared with the results from only RCTs. This difference may be due to the different characteristics of the 2 types of study. The RCTs had strict inclusion criteria, and there was an observational phase to ensure patient tolerance before randomization. This could result in weaker patients being excluded from the RCTs, suggesting that the RCTs may include healthier patients than the observational studies. Furthermore, all RCT patients reached the target dosage of ARNI. The conclusions based on RCTs may thus be applicable to populations similar to the RCT population but may not extend to the population as a whole. In contrast, although more patients with different health states were included in the observational studies, the outcomes may have been affected by baseline confounding factors. However, comparisons stratified by baseline characteristics showed no significant differences or interstudy heterogeneity for most indices, except NYHA functional class, 6MWD, and LVEF. The results of the current meta‐analysis were therefore generally reliable. In addition, >71% of patients in noncontrolled studies received ACEIs/ARBs before transferring to ARNI at baseline, suggesting that ARNI further improved CRR indices.
Subgroup analysis according to follow‐up period showed striking effects of ARNI on CRR indices and functional capacity at 3 months, increasing over time. This suggested that ARNI had a rapid therapeutic effect within 3 months, but the maximal treatment effects were uncertain. Equally, patients with acute conditions often have high NT‐proBNP levels and severe fluid retention, and short‐term use of ARNI had significant effects in these patients, suggesting a possible mechanism why these patients benefit more with long‐term use according to the present results. The short‐term benefits of ARNI on CRR may relate to its long‐term effects on functional capacity and cardiovascular outcomes. It may be beneficial to administer ARNI to eligible patients as early as possible. The PIONEER‐HF (Comparison Of Sacubitril/valsartaN Versus Enalapril on Effect on nt‐pRo‐bnp in Patients Stabilized From an Acute Heart Failure Episode) study may help to clarify this issue.46 Future studies should assess the dose‐dependent and long‐term (>1 year) effects of ARNI on CRR. Previous studies on renin‐angiotensin‐aldosterone system inhibitors showed no significant effects in patients with HFpEF. The current meta‐analysis included only 1 HFpEF trial [PARAMOUNT study (Prospective Comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction)], and no conclusions could therefore be drawn regarding the benefits of ARNI in HFpEF patients. However, the ongoing PARAGON‐HF (Prospective Comparison of ARNI with ARB Global Outcomes in HF With Preserved Ejection Fraction) trial may help to elucidate the efficacy and safety of ARNI in relation to morbidity and mortality in HFpEF patients.39
Strength and Limitations
This was the first meta‐analysis to compare the effects of ARNI and ACEIs/ARBs on CRR indices, and the data supported the superiority of ARNI therapies. We also conducted subgroup analyses according to baseline characteristics to address the issue of heterogeneity, and determined a relationship between LVEF and EDD. The low level of heterogeneity between the data suggested that the observations were valid.
This study had several limitations. Some analyzed studies were conference abstracts with unrefined design methodologies, which affected the overall study quality. The results should therefore be interpreted with caution. Only 7 trials were included in the comparison of ARNI with ACEIs/ARBs, and the effects of ARNI in patients with HFpEF were assessed in only 1 trial; the results may therefore have been affected by unpredictable factors. In addition, some data from the control groups were incomplete (conference abstracts), but we chose studies with detailed information on sample sizes, changes of indices, and follow‐up periods.
Conclusions
This meta‐analysis confirmed that ARNI can improve functional capacity and CRR in patients with HFrEF. ARNI initially acts rapidly, with more prominent changes occurring over time. The relationship between LVEF and EDD defined by curve estimations may reflect a mechanism responsible for the effects of ARNI. The current results suggested that patients may benefit more in terms of CRR if they are treated with ARNI as early as possible and for at least 3 months. Further studies are needed to explore the long‐term effects of ARNI in patients with HFpEF and to clarify the relationship between short‐term CRR and long‐term clinical outcomes, to support the ability of physicians to make an early prognosis.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (81570326) and the Science and Technology Plan Projects of Xuzhou City (KC16SH099).
Disclosures
None.
Supporting information
Data S1. Supplemental Methods
Table S1. Study Population and Quality Assessment of Included Non‐RCT
Table S2. Functional Exercise Capacity Before and After Treatment With ARNI
Table S3. Remodeling Parameters After Taking ARNI From Baseline
Table S4. Changes of Biomarkers From Baseline With ARNI
Figure S1. A, Methodological quality graph: reviewer author's judgments about each methodological quality item presented as percentage across all included studies; B, Methodological quality summary: review authors’ judgments about each methodological quality.
Figure S2. Funnel plot estimating publication bias for changes of main parameters following ARNI. A, New York Heart Association (NYHA) functional class, (B) 6‐minute walking distance (6MWD), (C) left ventricular ejection fraction (LVEF), (D and E) remodeling indices in patients showing heart failure with reduced ejection fraction (HFrEF), (F) remodeling indices in patients showing heart failure with preserved ejection fraction (HFpEF), (G and H) biomarkers including NT‐proBNP and sST2. NT‐proBNP indicates N‐terminal pro–brain‐type natriuretic peptide; sST2, soluble suppressor of tumorigenesis‐2.
Figure S3. Forest plots for effect of ARNI on remodeling biomarkers (A) in contrast with ACEIs/ARBs (B). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor.
Figure S4. Subgroup analysis of ARNI effects on NYHA functional class according to different proportions of patients reaching target dosage of ARNI. ARNI indicates angiotensin‐receptor neprilysin inhibitor; NYHA, New York Heart Association.
Figure S5. Subgroup analysis of ARNI effects on 6MWD according to different (A) proportions of patients with MRA use and (B) follow‐up periods. ARNI indicates angiotensin‐receptor neprilysin inhibitor; 6MWD, 6‐minute walking distance; MRA, mineralocorticoid receptor antagonist.
Figure S6. Correlation analyses of LVEF and CRR indices, except LVEF, (A) LVESV, (B) LVEDV, (C) LVESD, (D) LVEDD, (E) LAV, (F) LVMI, respectively in patients following ARNI. LAV indicates left atrial volume; LVEDD, left ventricular end‐diastolic dimension; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index.
Figure S7. Forest plots for effects of ARNI on main left ventricular diastolic function indices. ARNI indicates angiotensin‐receptor neprilysin inhibitor.
Acknowledgments
We wish to thank the Clinical Librarians in the Library of Xuzhou Medical University for their advice regarding the selection of search terms and literature databases during search strategy development.
(J Am Heart Assoc. 2019;8:e012272 DOI: 10.1161/JAHA.119.012272.)
Contributor Information
Tongda Xu, Email: xutongda3004@163.com.
Dongye Li, Email: dongyeli@xzhmu.edu.cn.
References
- 1. Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. [DOI] [PubMed] [Google Scholar]
- 2. Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep. 2017;19:71. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 4. White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end‐systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. [DOI] [PubMed] [Google Scholar]
- 5. Mentz RJ, Bakris GL, Waeber B, McMurray JJ, Gheorghiade M, Ruilope LM, Maggioni AP, Swedberg K, Piña IL, Fiuzat M, O'Connor CM, Zannad F, Pitt B. The past, present and future of renin–angiotensin aldosterone system inhibition. Int J Cardiol. 2013;167:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, Shelton B. Effects of long‐term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573–2581. [DOI] [PubMed] [Google Scholar]
- 7. Konstam MA, Patten RD, Thomas I, Ramahi T, Bresh KL, Goldman S, Lewis W, Gradman A, Self KS, Bittner V, Rand W, Kinan D, Smith JJ, Ford T, Segal R, Udelson JE. Effects of losartan and captopril on left ventricular volumes in elderly patients with heart failure: results of the ELITE ventricular function substudy. Am Heart J. 2000;139:1081–1087. [DOI] [PubMed] [Google Scholar]
- 8. The CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S; CHARM Investigators and Committees . Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet. 2003;362:759–766. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Q, Chen Y, Liu Q, Shan Q. Effects of renin–angiotensin–aldosterone system inhibitors on mortality, hospitalization, and diastolic function in patients with HFpEF. Herz. 2016;41:76–86. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon S, Swedberg K, Zile MR, Kardos A; PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 12. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–e293. [DOI] [PubMed] [Google Scholar]
- 13. Von Lueder T, Wang B, Kompa A, Huang L, Webb R, Jordan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibition (ARNI) attenuates adverse cardiac remodeling in vitro and in vivo. Heart Lung Circ. 2013;22:S71. [Google Scholar]
- 14. Suematsu Y, Miura S, Goto M, Yahiro E, Uehara Y, Saku K. LCZ696, the angiotensin‐receptor neprilysin inhibitor, attenuates cardiac fibrosis and improves its function in the heart failure model of diabetes mellitus in mice. Eur Heart J. 2015;36:661. [Google Scholar]
- 15. Schmieder RE, Wagner F, Mayr M, Delles C, Ott C, Keicher C, Hrabak‐Paar M, Heye T, Aichner S, Khder Y, Yates D, Albrecht D, Langenickel T, Freyhardt P, Janka R, Bremerich J. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodeling in subjects with essential hypertension: the results of a randomized, double‐blind, active‐controlled study. Eur Heart J. 2017;38:3308–3317. [DOI] [PubMed] [Google Scholar]
- 16. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher‐Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double‐blind randomized controlled trial. Lancet. 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- 17. Almufleh A, Marbach J, Chih S, Stadnick E, Davies R, Liu P, Mielniczuk L. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis. 2017;7:108–113. [PMC free article] [PubMed] [Google Scholar]
- 18. De Diego C, González‐Torres L, Núñez JM, Centurión ER, De Lara G, Macias M. Angiotensin‐neprilysin inhibition further reverses cardiac remodeling as compared to angiotensin inhibition in reduced heart failure patients. Europace. 2018;20:i139. [Google Scholar]
- 19. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. [DOI] [PubMed] [Google Scholar]
- 20. Nazzari H, Yeung M, Marceau A, Luong M, Clark C, Ahuja S, Knoll J, Koscal M, Ignaszewski A, Virani S, Toma M. Left ventricular function improves in heart failure patients treated with sacubitril‐valsartan. Can J Cardiol. 2017;33:S163. [Google Scholar]
- 21. Barrett MJ, Hammond M, Zhou S, Hanlon RO, Campbell P, Mcdonald K. Effect of sacubitril/valsartan therapy on risk stratification biomarkers in a real‐world heart failure population. Eur J Heart Fail. 2018;19:585–586. [Google Scholar]
- 22. Murray G, Barrett M, Earls S, Hammond M, Campbell P, O'Hanlon R, McDonald K. The use of sacubitril/valsartan: a real world experience in a high volume specialist heart failure service. Heart. 2017;103:A8–A9. [Google Scholar]
- 23. Maurin V, Canu A, Bernard A, Lafitte S, Picard F. Early reverse remodeling and improvement of echo parameters after introduction of sacubitril/valsartan in 80 stable and well treated HFrEF patients. Eur J Heart Fail. 2017;19:296. [Google Scholar]
- 24. Canu A, Hebert M, Gachet A, Arabucki F, Maurin V, Picard F, Dos Santos P. Results of a single center experience on 110 consecutive patients treated with Entresto (sacubitril/valsartan). Arch Cardiovasc Dis Suppl. 2017;9:33. [Google Scholar]
- 25. Martens P, Beliën H, Dupont M, Vandervoort P, Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther. 2018;36:e12435. [DOI] [PubMed] [Google Scholar]
- 26. Groba‐Marco M, Singh M, Galvan Ruiz M, Fernandez‐De‐Sanmamed‐Giron M, Montiel Quintero R, Perez‐Nogales E, Medina Gil JM, Blanco‐Nuez M, Caballero Dorta E, Ortega Trujillo JR, Menduina Gallego I, Morales Gonzalez J, Quevedo Nelson V, Mendoza Lemes H, Antonio Garcia Quintana A. Early left ventricular reverse remodeling after sacubitril/valsartan treatment in clinical practice. Eur J Heart Fail. 2018;20:225. [Google Scholar]
- 27. Kalantari S, Medvedofsky DM, Grinstein JG, Tayazime ST, Kim GK, Sarswat NS, Raikelkhar JR, Smith BS, Maffessanti FM, Beiser DB, Ward PW, Uriel NU. Remodel: demonstration of reverse remodeling effects of sacubitril/valsartan. Eur J Heart Fail. 2018;20:36–37. [Google Scholar]
- 28. Mercedes Faraudo M, Beltran P, Freixa R, Guri O, Mena E, Contra A, Ceresuela L, Masip J. How does sacubitril/valsartan improve submaximal functional capacity measured through six‐minute walk test? Eur J Heart Fail. 2017;19:307.27891719 [Google Scholar]
- 29. Rafael Bravo Marques R, Torres Calvo F, Lopez Tejero S, Valle Alberca A, Corona Barrio C, Chinchurreta Capote PA, Siles Rubio JR, Mesa Prado FE, Milan Pinilla AC, Perez Cabeza AI, Moreno Sanjuan D, Ruiz Mateas F. Initial experience using LCZ696 in real life: tolerability and clinical evolution in a short term. Eur J Heart Fail. 2017;19:290–291.27647481 [Google Scholar]
- 30. Hlavata K, Hoskova L, Franekova J, Jabor A, Kautzner J, Melenovsky V, Benes J. Transition from angiotensin‐converting enzyme inhibitor/angiotensin‐II‐receptor‐blocker to sacubitril/valsartan in chronic heart failure patients: initial experiences in clinical practice. Cor Vasa. 2018;60:e209–e214. [Google Scholar]
- 31. Beltrán P, Palau P, Domínguez E, Faraudo M, Nunez E, Guri O, Mollar A, Sanchis J, Bayes‐Genis A, Nunez J. Sacubitril/valsartan and short‐term changes in the 6‐minute walk test: a pilot study. Int J Cardiol. 2018;252:136–139. [DOI] [PubMed] [Google Scholar]
- 32. Rodil Fraile R, Malafarina V, Tiberio Lopez G. Sacubitril‐valsartan in heart failure and multimorbidity patients. ESC Heart Fail. 2018;5:957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mantis C, Anadiotis A, Patsilinakos S. Impact of sacubitril/valsartan on functional exercise capacity and quality of life in patients with heart failure with reduced ejection fraction. Eur J Prev Cardiol. 2018;25:S73. [Google Scholar]
- 34. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA‐P Group . Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Lenarda AD, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–1476. [DOI] [PubMed] [Google Scholar]
- 36. Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood‐pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double‐blind, placebo‐controlled, active comparator study. Lancet. 2010;375:1255–1266. [DOI] [PubMed] [Google Scholar]
- 37. Chan AK, Sanderson JE, Wang T, Lam W, Yip G, Wang M, Lam YY, Zhang Y, Yeung L, Wu EB, Chan WW, Wong JT, So N, Yu CM. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;50:591–596. [DOI] [PubMed] [Google Scholar]
- 38. Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long term, dose‐dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304–310. [DOI] [PubMed] [Google Scholar]
- 39. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJV. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Heart Fail. 2017;5:471. [DOI] [PubMed] [Google Scholar]
- 40. Bijnens B, Cikes M, Butakoff C, Sitges M, Crispi F. Myocardial motion and deformation: what does it tell us and how does it relate to function? Fetal Diagn Ther. 2012;32:5–16. [DOI] [PubMed] [Google Scholar]
- 41. Feild BJ, Russell RO, Moraski RE, Soto B, Hood WP Jr, Burdeshaw JA, Smith M, Maurer BJ, Rackley CE. Left ventricular size and function and heart size in the year following myocardial infarction. Circulation. 1974;50:331–339. [DOI] [PubMed] [Google Scholar]
- 42. Solomon SD, Claggett B, Mcmurray JJ, Hernandez AF, Fonarow GC. Combined neprilysin and renin‐angiotensin system inhibition in heart failure with reduced ejection fraction: a meta‐analysis. Eur J Heart Fail. 2016;18:1238–1243. [DOI] [PubMed] [Google Scholar]
- 43. Ye L, Wang J, Chen Q, Yang X. LCZ696, a promising novel agent in treating hypertension (a meta‐analysis of randomized controlled trials). Oncotarget. 2017;8:107991–108005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao Y, Yu H, Zhao X, Ma R, Li N, Yu J. The effects of LCZ696 in patients with hypertension compared with angiotensin receptor blockers: a meta‐analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2017;22:447–457. [DOI] [PubMed] [Google Scholar]
- 45. Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta‐analytic approach. J Am Coll Cardiol. 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E; PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods
Table S1. Study Population and Quality Assessment of Included Non‐RCT
Table S2. Functional Exercise Capacity Before and After Treatment With ARNI
Table S3. Remodeling Parameters After Taking ARNI From Baseline
Table S4. Changes of Biomarkers From Baseline With ARNI
Figure S1. A, Methodological quality graph: reviewer author's judgments about each methodological quality item presented as percentage across all included studies; B, Methodological quality summary: review authors’ judgments about each methodological quality.
Figure S2. Funnel plot estimating publication bias for changes of main parameters following ARNI. A, New York Heart Association (NYHA) functional class, (B) 6‐minute walking distance (6MWD), (C) left ventricular ejection fraction (LVEF), (D and E) remodeling indices in patients showing heart failure with reduced ejection fraction (HFrEF), (F) remodeling indices in patients showing heart failure with preserved ejection fraction (HFpEF), (G and H) biomarkers including NT‐proBNP and sST2. NT‐proBNP indicates N‐terminal pro–brain‐type natriuretic peptide; sST2, soluble suppressor of tumorigenesis‐2.
Figure S3. Forest plots for effect of ARNI on remodeling biomarkers (A) in contrast with ACEIs/ARBs (B). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor.
Figure S4. Subgroup analysis of ARNI effects on NYHA functional class according to different proportions of patients reaching target dosage of ARNI. ARNI indicates angiotensin‐receptor neprilysin inhibitor; NYHA, New York Heart Association.
Figure S5. Subgroup analysis of ARNI effects on 6MWD according to different (A) proportions of patients with MRA use and (B) follow‐up periods. ARNI indicates angiotensin‐receptor neprilysin inhibitor; 6MWD, 6‐minute walking distance; MRA, mineralocorticoid receptor antagonist.
Figure S6. Correlation analyses of LVEF and CRR indices, except LVEF, (A) LVESV, (B) LVEDV, (C) LVESD, (D) LVEDD, (E) LAV, (F) LVMI, respectively in patients following ARNI. LAV indicates left atrial volume; LVEDD, left ventricular end‐diastolic dimension; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index.
Figure S7. Forest plots for effects of ARNI on main left ventricular diastolic function indices. ARNI indicates angiotensin‐receptor neprilysin inhibitor.


