Abstract
Background
The C2HEST score (coronary artery disease or chronic obstructive pulmonary disease [1 point each]; hypertension [1 point]; elderly [age ≥75 years, 2 points]; systolic heart failure [2 points]; thyroid disease [hyperthyroidism, 1 point]) was initially proposed for predicting incident atrial fibrillation (AF) in the general population. Its performance in poststroke patients remains to be established, especially because patients at high risk for incident AF should be targeted for more comprehensive screening. This study aimed to evaluate this newly established incident AF prediction risk score in a post–ischemic stroke population.
Methods and Results
Validation was based on a hospital‐based nationwide cohort with 240 459 French post–ischemic stroke patients. Kaplan–Meier curves for incident rate of AF depict differences between varying risk categories. Discrimination of the C2HEST score was evaluated using the C index, the net reclassification index, integrated discriminatory improvement, and decision curve analysis. During 7.9±11.5 months of follow‐up, 14 095 patients developed incident AF. The incidence of AF increased from 23.5 per 1000 patient‐years in patients with a C2HEST score of 0 to 196.8 per 1000 patient‐years in patients with a C2HEST score ≥6. Kaplan–Meier curves showed a clear difference among different risk strata (log‐rank P<0.0001). The C2HEST score had good discrimination with a C index of 0.734 (95% CI, 0.732–0.736), which was better than the Framingham risk score and the CHA2DS2‐VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled], vascular disease, age 65 to 74 years, and female sex) (P<0.0001, respectively). The C2HEST score was also superior to the Framingham risk score and the CHA2DS2‐VASc score as shown by the net reclassification index, integrated discriminatory improvement (P<0.0001, respectively) and decision curve analysis.
Conclusions
The C2HEST score performed well in discriminating the individual risk of developing incident AF in a white European population hospitalized with previous ischemic stroke. This simple score may potentially be used as a risk stratification tool for decision making in relation to a screening strategy for AF in post–ischemic stroke patients.
Keywords: atrial fibrillation, cohort study, ischemic stroke, risk score
Subject Categories: Atrial Fibrillation, Ischemic Stroke
Clinical Perspective
What Is New?
The C2HEST score, a simple clinical risk stratification model, has been proposed to predict incident atrial fibrillation among Asian patients.
In the nationwide analysis of 240 459 patients with previous ischemic stroke in France, we found that the C2HEST score performed well in discriminating the individual risk of developing incident atrial fibrillation.
What Are the Clinical Implications?
The simple C2HEST score has potential to be used as a risk stratification tool for decision making in relation to a screening strategy for atrial fibrillation in poststroke non‐Asian patients.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, with increasing prevalence and incidence worldwide.1, 2, 3, 4 Many AF patients are asymptomatic or have nonspecific symptoms, and a large proportion remain undiagnosed.5 These asymptomatic patients may at higher risks of thromboembolic events and mortality compared with patient who have symptoms.6 Some would be identified with AF only after their presentation with a major complication, such as stroke or heart failure (HF).7
Individual risk evaluation for developing incident AF is important for the decision‐making process of early primary prevention and detection of AF, which may associate with better outcomes.8 A simple clinical risk‐evaluation tool may facilitate effective and cost‐effective prevention and screening strategies for incident AF. Such a tool may help identify patients at high risk for incident AF who can be targeted for more intensive screening programs and primary prevention strategies.
A simple clinical risk‐stratification model, the C2HEST score (coronary artery disease or chronic obstructive pulmonary disease [1 point each]; hypertension [1 point]; elderly [age ≥75 years, 2 points]; systolic HF [2 points]; thyroid disease [hyperthyroidism, 1 point]) was recently proposed to predict incident AF among Asian patients.9 This score was derived from a large cohort of 471 446 Chinese patients10 and was validated in the Korean National Health Insurance Service Health Screening cohort with 514 764 Korean patients.11 The risk of incident AF increased significantly with higher C2HEST score.9
Current guidelines recommend that poststroke patients need AF screening.12 However, diverse screening approaches may have different capabilities in detecting unrevealed AF; perhaps more aggressive screening methods should be used for patients who are more likely to develop incident AF,13 enabling an effective and cost‐effective screening strategy. The present study aimed to assess whether the newly established risk model, the C2HEST score, could predict AF in patients with previous ischemic stroke and without known prior AF and whether it could stratify poststroke patients into different risk groups for incident AF.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. This longitudinal cohort study was based on a national hospitalization database in France covering hospital care across the entire population. In France, each hospital discharge, whether from a public or a private hospital, must be registered in the National Hospital Discharge Database (PMSI [Programme de Médicalisation des Systèmes d'Information]).14 A standardized discharge summary is collected for every hospital stay in France and categorized into a single medical or surgical diagnosis‐related group based on the diagnosis and procedures coded, inspired by the US Medicare system.15 Since 2001, a unique patient identification number has made it possible to link multiple hospital stays corresponding to a single patient without revealing his or her identity. Since 2004, each hospital's budget has been linked to the medical activity described in this specific program, which compiles discharge abstracts related to all admissions for inpatients in the 1546 French healthcare facilities. The International Classification of Diseases, Tenth Revision (ICD‐10) has been used to code discharge diagnoses since 1996. The main outcome measure was the rate of incident AF.
Data for all patients admitted with ischemic stroke in France from January 2008 to December 2012 were collected from the PMSI using the annually updated versions of the ICD‐10 for the years 2008–2012. The reliability of PMSI data has already been assessed16 and used previously to study patients with stroke and AF.17, 18
The medical information contained in the database is anonymous and protected by professional confidentiality. Consequently, ethics review was not required. Patient consent was not sought. The study was conducted retrospectively, patients were not involved in its conduct, and there was no impact on their care. This type of study was approved by the institutional review board of the Pole Coeur Thorax Vaisseaux from the Trousseau University Hospital (Tours, France) on December 1, 2015, and registered as a clinical audit. Procedures for data collection and management were approved by the Conseil National de l'Informatique et des Libertés, the independent national ethics committee protecting human rights in France, which ensures that all information is kept confidential and anonymous (authorization no. 1749007). The study included adults (aged ≥18 years) with a diagnosis of acute ischemic stroke (code I63 and its subsections using ICD‐10) coded as the primary diagnosis (ie, the health problem that justified admission to hospital), the related diagnosis (ie, potential chronic disease or health state during hospital stay), or the significantly associated diagnosis (ie, comorbidity or associated complication) who were hospitalized between January 1, 2008, and December 31, 2012. We performed an analysis restricted to the patients seen after 2009, meaning that all patients had at least 1 year in which previous events were recorded to establish history of previous AF and comorbidities. Patients with no diagnosis of AF were considered to have sinus rhythm. Of note, asymptomatic cerebrovascular diseases and sequelae of stroke have different codes (I65, I66, and I69 with subdivisions) to be distinguished from acute strokes and were not included in our analysis. We calculated the CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, stroke [doubled], vascular disease, age 65 to 74 years, and female sex) and C2HEST scores, as described previously.9, 19 Because both hypo‐ and hyperthyroidism have been associated with AF,20, 21 we used a more general item of thyroid disease instead of hyperthyroidism when calculating the C2HEST score. We also performed a sensitivity analysis with the C2HEST score using hyperthyroidism only. We also calculated a modified Framingham risk score based on its initial description.22
Statistical Analysis
Qualitative variables were described using counts and percentages, and continuous quantitative variables were described as mean±SD or median (interquartile range). Comparisons were made using parametric or nonparametric tests, as appropriate: The Wilcoxon signed rank and Kruskal–Wallis tests were used for comparing values between 2 independent groups, and the χ2 test was used to compare categorical data. The population of individuals seen with ischemic stroke without prior AF was analyzed by calculating incidence rates of new‐onset AF and by multivariable Cox regression models. A proportional hazards model was used to identify independent characteristics associated with the occurrence of AF during follow‐up. Receiver operating characteristic curves were constructed, and Harrell C indexes (ie, area under the curve) were calculated as a measure of model performance and compared using the DeLong test. Integrated discriminatory improvement and net reclassification improvement were calculated according to the methods described by Pencina et al23 to assess the discrimination and reclassification performance of the scores. Clinical usefulness and net benefit of the C2HEST score in comparison to the CHA2DS2‐VASc score and the Framingham risk score were estimated using decision curve analysis.24, 25 In all analyses, P<0.05 was considered statistically significant. All analyses were performed using JMP 9.0.1 (SAS Institute) and STATA v12.0 (StataCorp).
Results
A total of 240 459 patients were included in the analysis. During follow‐up, 14 095 patients developed incident AF, which give us 158 302 person‐years of experience (mean follow‐up of 7.9±11.5 months). Baseline characteristics are presented in Table 1. Patients with AF were older than those without AF (P<0.0001), and more were female (P<0.0001). The prevalence of each comorbidity was higher in AF patients, including hypertension, diabetes mellitus, coronary arterial disease, valve disease, hyperlipidemia, vascular disease, chronic obstructive pulmonary disease, renal dysfunction, thyroid disease, and HF (P<0.0001, respectively). Patients who developed AF had higher CHA2DS2‐VASc scores at baseline than those who did not develop AF (P<0.0001).
Table 1.
Baseline Characteristics of 240 459 Patients Included in the Study
| Characteristics | Patients Without AF (n=226 364) | Patients With Incident AF (n=14 095) | P Value |
|---|---|---|---|
| Age, y, mean±SD | 70.8±15.7 | 77.6±10.6 | <0.0001 |
| Male sex, n (%) | 119 098 (53.0) | 7013 (50.0) | <0.0001 |
| Medical history, n (%) | |||
| Hypertension | 141 045 (62.3) | 11 745 (83.3) | <0.0001 |
| Diabetes mellitus | 50 977 (22.5) | 4083 (29.0) | <0.0001 |
| Coronary arterial disease | 39 652 (17.5) | 4969 (35.3) | <0.0001 |
| Valve disease | 15 121 (6.7) | 2780 (19.7) | <0.0001 |
| Hyperlipidemia | 69 428 (30.7) | 5793 (41.1) | <0.0001 |
| Vascular disease | 70 636 (31.2) | 6907 (49.0) | <0.0001 |
| COPD | 35 320 (15.6) | 3661 (26.0) | <0.0001 |
| Renal dysfunction | 38 618 (17.1) | 5393 (38.3) | <0.0001 |
| Hyperthyroidism | 3355 (1.5) | 646 (4.6) | <0.0001 |
| Thyroid disease | 19 720 (8.7) | 2525 (17.9) | <0.0001 |
| HF | 33 162 (14.7) | 6261 (44.4) | <0.0001 |
| CHA2DS2‐VASc score, median (IQR) | 5 (2) | 6 (2) | <0.0001 |
Thyroid disease comprises hypo‐ and hyperthyroidism. CHA2DS2‐VASc score is composed of congestive heart failure, hypertension, age ≥75 (doubled), diabetes mellitus, stroke (doubled), vascular disease, age 65 to 74 years, and female sex. AF indicates atrial fibrillation; COPD, chronic obstructive pulmonary disease; HF, heart failure; IQR, interquartile range.
Results of the Cox multivariable regression analysis for incident AF are shown in Table 2. On multivariable analysis, HF, age ≥75 years, coronary arterial disease, valve disease, chronic obstructive pulmonary disease, hypertension, and renal dysfunction were shown to be independently related to the development of incident AF. HF and age ≥75 years were the most potent risk factors for incident AF, with hazard ratios (HRs) >2.0.
Table 2.
HRs of Risk Factors for Incident AF
| Risk Factors | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| HF | 2.99 | 2.89–3.09 | <0.0001 | 2.21 | 2.13–2.30 | <0.0001 |
| Age ≥75 y | 2.54 | 2.45–2.63 | <0.0001 | 2.11 | 2.04–2.19 | <0.0001 |
| Coronary arterial disease | 1.70 | 1.64–1.76 | <0.0001 | 1.09 | 1.05–1.13 | <0.0001 |
| Valve disease | 2.26 | 2.18–2.36 | <0.0001 | 1.42 | 1.36–1.48 | <0.0001 |
| Thyroid disease | 1.71 | 1.64–1.79 | <0.0001 | 1.36 | 1.31–1.43 | <0.0001 |
| COPD | 1.42 | 1.36–1.47 | <0.0001 | 1.18 | 1.14–1.22 | <0.0001 |
| Hypertension | 1.90 | 1.81–1.98 | <0.0001 | 1.34 | 1.27–1.40 | <0.0001 |
| Renal dysfunction | 2.02 | 1.96–2.09 | <0.0001 | 1.21 | 1.17–1.26 | <0.0001 |
| Hyperlipidemia | 1.06 | 1.03–1.10 | 0.0005 | 0.87 | 0.84–0.90 | <0.0001 |
| Male sex | 0.83 | 0.80–0.86 | <0.0001 | 0.99 | 0.95–1.02 | 0.38 |
| Diabetes mellitus | 1.07 | 1.04–1.11 | <0.0001 | 0.95 | 0.91–0.98 | 0.002 |
| Vascular disease | 1.42 | 1.37–1.46 | <0.0001 | 0.93 | 0.90–0.97 | <0.0001 |
Thyroid disease comprises hypo‐ and hyperthyroidism. CHA2DS2‐VASc score is composed of congestive heart failure, hypertension, age ≥75 (doubled), diabetes mellitus, stroke (doubled), vascular disease, age 65 to 74 years, and female sex. AF indicates atrial fibrillation; COPD, chronic obstructive pulmonary disease; HF, heart failure; HR, hazard ratio.
The incident rate of AF increased significantly with higher C2HEST scores (Figure 1). The HRs for incident AF increased with higher score and risk group (Figure 2). When divided into 3 groups by baseline C2HEST score, annual incidence rates were 3.19% in the low‐risk group (0 or 1 point), 7.15% in the medium‐risk group (2 or 3 points), and 14.64% in the high‐risk group (≥4 points). The Kaplan–Meier curves for the 3 risk categories showed a graded increased risk for incident AF across risk groups (log‐rank P<0.0001; Figure 3).
Figure 1.
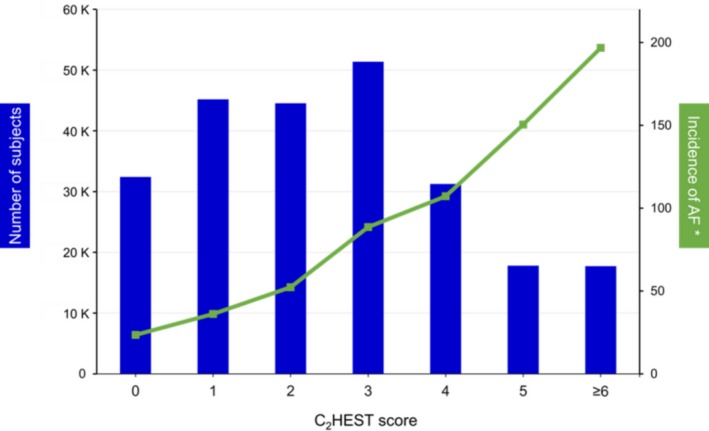
Prevalence of the C2 HEST scores and incident rate of atrial fibrillation (AF). *Per 1000 person‐years.
Figure 2.
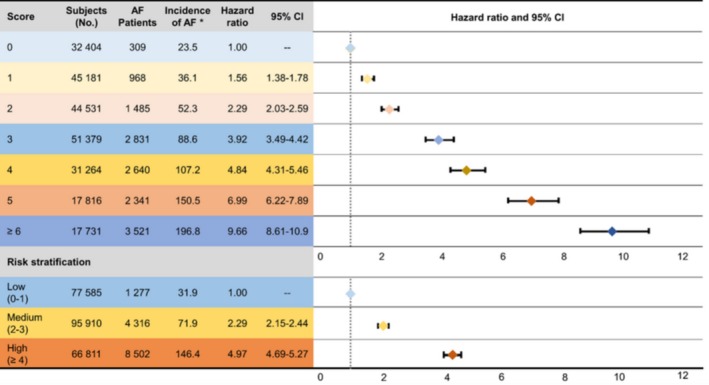
Annual incidence of atrial fibrillation (AF) by C2 HEST score. *Per 1000 person‐years.
Figure 3.
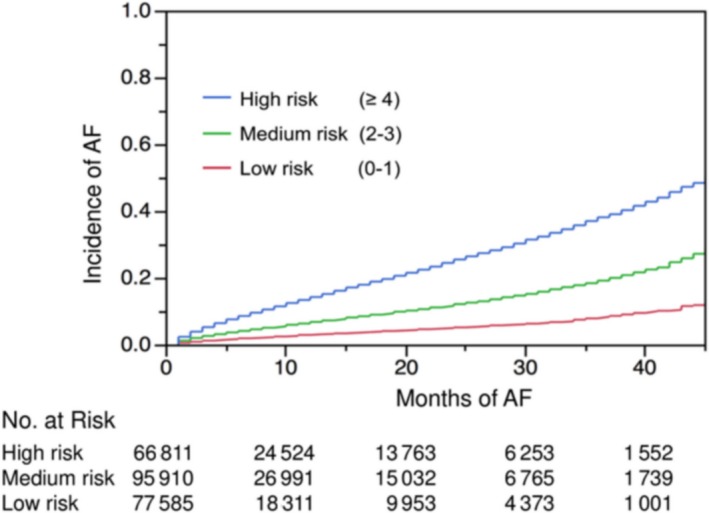
Kaplan–Meier curves of incidence of atrial fibrillation (AF) regarding different risk strata: low, 0 or 1 point; medium, 2 or 3 points; high, ≥4 points.
The C2HEST score showed good discriminative ability with a C index of 0.734 (95% CI, 0.732–0.736), which was significantly better than the CHA2DS2‐VASc score (0.703; 95% CI, 0.701–0.704; P<0.0001) and the Framingham risk score (0.720; 95% CI, 0.718–0.722; P<0.0001; Figure 4A). These results with the C2HEST score using an item of thyroid disease including hypo‐ or hyperthyroidism were marginally better than the sensitivity analysis with the score calculated using hyperthyroidism only (C index: 0.716; 95% CI, 0.714–0.718). The discriminative ability of the C2HEST score was also assessed with regard to sex, showing satisfactory results in both men (C index: 0.741; 95% CI, 0.735–0.747) and women (C index: 0.724; 95% CI, 0.718–0.729). Among elderly patients (aged ≥75 years), the C2HEST score could also discriminate for different risk strata in relation to incident AF (C index: 0.694; 95% CI, 0.689–0.700; for patients aged <75 years: C index: 0.735; 95% CI, 0.728–0.743).
Figure 4.
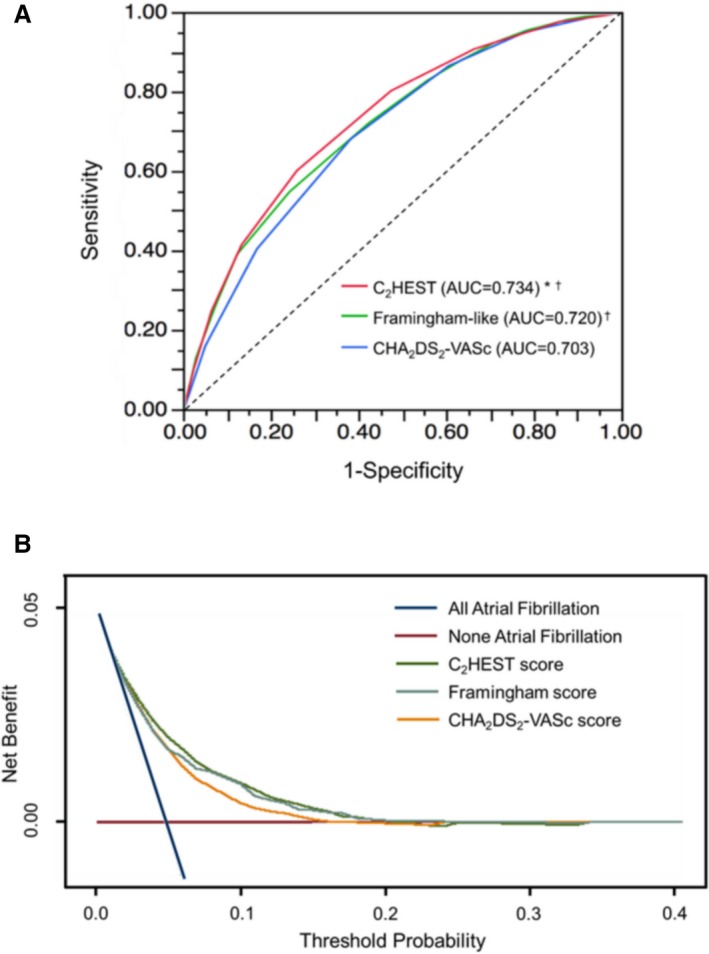
A, Receiver operating characteristic curves of incident atrial fibrillation developing during follow‐up. *P<0.0001 vs Framingham risk score; † P<0.0001 vs CHA 2 DS 2 VASc score. B, Decision curve analyses for the C2 HEST, CHA 2 DS 2‐VASc, and Framingham risk scores. AUC indicates area under the curve.
The C2HEST score had positive net reclassification improvement and integrated discriminatory improvement compared with the CHA2DS2‐VASc score (23.6% [P<0.0001] and 31.0% [P<0.0001], respectively) and the Framingham risk score (6.7% [P<0.0001] and 12.0% [P<0.0001], respectively). Using decision curve analysis, the C2HEST score showed better clinical usefulness compared with the CHA2DS2‐VASc and Framingham risk scores (Figure 4B).
Discussion
This study is the first to externally validate the newly established C2HEST score, a simple risk prediction model for incident AF, in a European cohort by using a nationwide (French) hospital‐based white European population admitted with ischemic stroke. We found that the C2HEST score performed well in discriminating the individual risk of developing incident AF in a white European population hospitalized with previous stroke. Given that poststroke patients at high risk incident AF should be targeted for more comprehensive screening, this simple score has the potential to be used as a risk‐stratification tool for decision making in relation to a screening strategy for AF in poststroke patients.
The predictive performance of this risk score was statistically better than that of the CHA2DS2‐VASc and Framingham risk scores, which have previously been shown to be useful for AF prediction.22, 26, 27 As demonstrated by integrated discriminatory improvement and net reclassification improvement analyses, compared with CHA2DS2‐VASc, 23.6% more of the studied population was correctly classified into the right risk group28 and 31.0% more model sensitivity (with no loss of specificity) was obtained by the C2HEST score.29 Compared with the Framingham score, 6.7% of population was correctly reclassified and model sensitivity was increased by 12.0% with the C2HEST score.
The independent risk factors in this newly established C2HEST score were most common comorbidities among community and hospital‐based populations. The definitions of these risk factors are relatively clear and support accessible and easy evaluation of patients’ risk of developing incident AF.
On multivariable analysis, we found multiple independent risk factors for incident AF in our cohort, including coronary arterial disease, chronic obstructive pulmonary disease, hypertension, age ≥75 years, HF, and thyroid disease, all of which are constituents of the C2HEST score. HF and age ≥75 years had higher HRs (>2), which were also considered major risk factors for incident AF in the C2HEST score.9 Nevertheless, some differences exist in our study compared with the original Asian cohort describing the C2HEST score.9 Because both hypo‐ and hyperthyroidism have been associated with AF,20, 21 we used a more general item of thyroid disease instead of hyperthyroidism when calculating the C2HEST score, and this change showed slightly better predictive ability. We found that renal dysfunction was a risk factor in the present study but not an independent risk factor in Asian cohorts.9 This result may be because of the different risk factor profiles among the different populations. In the European population, renal dysfunction may be a stronger risk factor for incident AF than in Asian patients.30 For example, 2 studies from European populations reported renal impairment as an independent risk factor for incident AF, with HRs between 2.5 to 2.6.31, 32 In contrast, a report on a large cohort of 500 000 Asian patients found that renal dysfunction was not an independent risk factor (HR: 1.58; 95% CI, 0.78–3.20).10 In another cohort from Taiwan (n=15 947), renal dysfunction showed an association with incident AF but with a relatively lower HR (1.46; 95% CI, 1.31–1.61)33 compared with that reported in European populations. In this Taiwanese cohort, hemodialysis was analyzed as “renal dysfunction” but is a substantially more severe stage of this disease.33
We found the C2HEST score performed well in discriminating individual risk of incident AF, and this ability was consistent in both sexes and in different age strata. When divided into different point ranges, incidence of AF increased with increasing C2HEST scores. In addition, incidence of AF increased significantly with higher risk categorization, with an incident rate of 146.4 per 1000 person‐years in the high‐risk group (score ≥4). The C index for this score was also good in our white European cohort, consistent with the original derivation study from Asia.9
Several previously proposed risk models for predicting incident AF were derived from Western populations, including the Framingham risk score (Framingham Heart Survey),22 the ARIC (Atherosclerosis Risk in Communities) score,34 the CHARGE‐AF (Cohorts for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation) score,35 and the STAF (Score for the Targeting of Atrial Fibrillation) score.36 These risk scores had good discrimination for incident AF in their original studies; however, they require many instrumental and laboratory variables that might not be easily accessed in everyday practice. Furthermore, such complexity limits their daily application for operationalizing risk assessment in the real world, although they had good C indexes in the original studies.37 Compared with the Framingham risk score (slightly modified), the C2HEST score showed superiority for AF prediction in this poststroke patient population.
The CHADS2, CHA2DS2‐VASc, and HATCH (hypertension, age ≥75 years, transient ischemic attack or stroke [2 points], chronic obstructive pulmonary disease and HF [2 points]) scores also showed predictive capacity for incident AF in previous studies.26, 38 However, these scores were not derived for this purpose. Some components of these scores may not be risk factors for incident AF, such as female sex in the CHA2DS2‐VASc score. In addition, stroke or transient ischemic attack does not increase an individual's risk of developing AF but may be an indicator of undiagnosed AF. Nevertheless, we previously compared the performance of these scores in predicting incident AF and demonstrated the better discriminative ability of the new C2HEST score.9
In the present study, all patients had a history of ischemic stroke and thus should be candidates for screening of silent AF.12, 39 In the 2016 AF guidelines from the European Society of Cardiology, screening for AF using short‐term ECG recording followed by continuous ECG monitoring for at least 72 hours is recommended in patients with ischemic stroke (class I recommendation with level B evidence), and additional ECG monitoring by long‐term noninvasive ECG monitors or implanted loop recorders may be considered to document silent AF (class IIa recommendation with level B evidence).12 However, only a limited number of patients receive AF screening because of the uncertainty about cost‐effectiveness and the lack of robust evidence justifying the utility of AF screening.40
In our study, we found that patients with a C2HEST score ≥4 had extremely high risk of developing incident AF (14.6% per person‐year), justifying the need for more intensive ECG monitoring for silent or asymptomatic AF.39 The availability of simple risk stratification may lead to a more effective and cost‐effective, selective, opportunistic screening approach,41, 42 targeting patients at high risk of incident AF and its sequelae and supporting better adherence to guideline recommendations for AF screening. For instance, patients with extremely high C2HEST scores should receive more intensive heart‐rate monitoring, such as 1 to 2 weeks of Holter monitoring or an implantable loop recorder.
In the STROKESTOP (Massive Screening for Untreated Atrial Fibrillation) study, screening for asymptomatic AF in 75‐ or 76‐year‐old individuals was found to be cost‐effective.43 With a simple risk‐assessment model, it would be possible to increase efficiency by targeting a more intensive screening approach among those at higher risk of developing incident AF.44 In this study, we demonstrated that the C2HEST score could further discriminate those at‐risk elderly patients (aged ≥75 years) for AF. By initially calculating an individual C2HEST score, conducting a more focused and cost‐effective screening strategy may become more feasible.
Strengths and Limitations
This study is the first external validation of the C2HEST score in a large, nationwide, hospital‐based, European population (French) with prior stroke history. We found that the C2HEST score performed satisfactorily in evaluating the individual risk of developing incident AF after ischemic stroke, which may allow a targeted and tailored screening strategy in this population. Nevertheless, this study has some limitations. First, this hospital‐based cohort study in France may not represent the general population. Incidental AF might be marginally underestimated if it were identified in only some outpatients during follow‐up. Considering the way in which a history of AF might have been determined, a washout period of 1 year might be too short, and there is also a risk of underdiagnosis for prior AF in our population. We did not compare the performance of the C2HEST score with other previously established scores, such as the ARIC, CHARGE‐AF, or STAF scores, because some variables were unavailable in our data set to calculate those scores. For comparison with the Framingham risk score, we used a slightly modified model based on the original model, and this change may have introduced some difference from the original. Finally, some variables are known to influence the odds of detecting AF, such as chronic kidney disease, but are not included in the C2HEST score. By including hyperthyroidism, the score may be biased for identification of circumstantial and transient causes of AF that may not be substantially relevant in terms of the decision to anticoagulate.
Conclusion
The C2HEST score performed well in discriminating the individual risk of developing incident AF in a white European population hospitalized with previous stroke. This simple score has the potential to be used as a risk‐stratification tool for decision making in relation to a screening strategy for AF in poststroke patients.
Disclosures
Lip has been a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi‐Sankyo and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi‐Sankyo; no fees are directly received personally. Fauchier has been a consultant or speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim, Medtronic, and Novartis. Li has been a sponsored PhD trainee by the China Scholarship Council (201708110232). The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e012546 DOI: 10.1161/JAHA.119.012546.)
Contributor Information
Gregory Y. H. Lip, Email: gregory.lip@liverpool.ac.uk.
Laurent Fauchier, Email: laurent.fauchier@univ-tours.fr.
References
- 1. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Proietti M, Laroche C, Nieuwlaat R, Crijns H, Maggioni AP, Lane DA, Boriani G, Lip GYH; EORP‐AF General Pilot Registry; Euro Heart Survey on AF Investigators . Increased burden of comorbidities and risk of cardiovascular death in atrial fibrillation patients in Europe over ten years: a comparison between EORP‐AF pilot and EHS‐AF registries. Eur J Intern Med. 2018;55:28–34. [DOI] [PubMed] [Google Scholar]
- 3. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B, Lip GY. 10‐year nationwide trends of the incidence, prevalence, and adverse outcomes of non‐valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. [DOI] [PubMed] [Google Scholar]
- 4. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. [DOI] [PubMed] [Google Scholar]
- 5. Turakhia MP, Shafrin J, Bognar K, Trocio J, Abdulsattar Y, Wiederkehr D, Goldman DP. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One. 2018;13:e0195088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Sinagra G, Petrescu L, Tavazzi L, Maggioni AP, Lip GY. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP‐AF Pilot General Registry. Am J Med. 2015;128:509–518.e502. [DOI] [PubMed] [Google Scholar]
- 7. Chou PS, Ho BL, Chan YH, Wu MH, Hu HH, Chao AC. Delayed diagnosis of atrial fibrillation after first‐ever stroke increases recurrent stroke risk: a 5‐year nationwide follow‐up study. Intern Med J. 2018;48:661–667. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, Goette A, Hindricks G, Hohnloser S, Kappenberger L, Kuck KH, Lip GY, Olsson B, Meinertz T, Priori S, Ravens U, Steinbeck G, Svernhage E, Tijssen J, Vincent A, Breithardt G. Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J. 2007;28:2803–2817. [DOI] [PubMed] [Google Scholar]
- 9. Li YG, Pastori D, Farcomeni A, Yang PS, Jang E, Joung B, Wang YT, Guo YT, Lip GYH. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. 2019;155:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GYH. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147:109–119. [DOI] [PubMed] [Google Scholar]
- 11. Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, Kang HJ, Do CH, Song JS, Lee EJ, Ha S, Shin SA, Jeong SL. Cohort profile: the National Health Insurance Service‐National Health Screening Cohort (NHIS‐HEALS) in Korea. BMJ Open. 2017;7:e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 13. Miyazawa K, Mairesse GH, Lip GYH. Screening for atrial fibrillation: look harder, look longer, and improve stroke outcomes with oral anticoagulation. Europace. 2018;20:f278–f279. [DOI] [PubMed] [Google Scholar]
- 14. Grammatico L, Baron S, Rusch E, Lepage B, Surer N, Desenclos JC, Besnier JM. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital‐discharge data 2002–2003. Epidemiol Infect. 2008;136:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fetter RB, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis‐related groups. Med Care. 1980;18:iii, 1–53. [PubMed] [Google Scholar]
- 16. Couris CM, Polazzi S, Olive F, Remontet L, Bossard N, Gomez F, Schott A‐M, Mitton N, Colonna M, Trombert B. Breast cancer incidence using administrative data: correction with sensitivity and specificity. J Clin Epidemiol. 2009;62:660–666. [DOI] [PubMed] [Google Scholar]
- 17. Fauchier L, Clementy N, Pelade C, Collignon C, Nicolle E, Lip GY. Patients with ischemic stroke and incident atrial fibrillation: a nationwide cohort study. Stroke. 2015;46:2432–2437. [DOI] [PubMed] [Google Scholar]
- 18. Fauchier L, Chaize G, Gaudin AF, Vainchtock A, Rushton‐Smith SK, Cotte FE. Predictive ability of HAS‐BLED, HEMORR2HAGES, and ATRIA bleeding risk scores in patients with atrial fibrillation. A French nationwide cross‐sectional study. Int J Cardiol. 2016;217:85–91. [DOI] [PubMed] [Google Scholar]
- 19. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 20. Bruere H, Fauchier L, Bernard Brunet A, Pierre B, Simeon E, Babuty D, Clementy N. History of thyroid disorders in relation to clinical outcomes in atrial fibrillation. Am J Med. 2015;128:30–37. [DOI] [PubMed] [Google Scholar]
- 21. Jolobe OM. Thyroid heart disease should include the coincidental association of hypothyroidism and atrial fibrillation. Am J Med. 2015;128:e9. [DOI] [PubMed] [Google Scholar]
- 22. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 24. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saliba W, Gronich N, Barnett‐Griness O, Rennert G. Usefulness of CHADS2 and CHA2DS2‐VASc scores in the prediction of new‐onset atrial fibrillation: a population‐based study. Am J Med. 2016;129:843–849. [DOI] [PubMed] [Google Scholar]
- 27. Bisson A, Bodin A, Clementy N, Babuty D, Lip GYH, Fauchier L. Prediction of incident atrial fibrillation according to gender in patients with ischemic stroke from a nationwide cohort. Am J Cardiol. 2018;121:437–444. [DOI] [PubMed] [Google Scholar]
- 28. Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160:122–131. [DOI] [PubMed] [Google Scholar]
- 29. van Smeden M, Moons KGM. Event rate net reclassification index and the integrated discrimination improvement for studying incremental value of risk markers. Stat Med. 2017;36:4495–4497. [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Pastori D, Guo Y, Wang Y, Lip GYH. Risk factors for new‐onset atrial fibrillation: a focus on Asian populations. Int J Cardiol. 2018;261:92–98. [DOI] [PubMed] [Google Scholar]
- 31. Sciacqua A, Perticone M, Tripepi G, Miceli S, Tassone EJ, Grillo N, Carullo G, Sesti G, Perticone F. Renal disease and left atrial remodeling predict atrial fibrillation in patients with cardiovascular risk factors. Int J Cardiol. 2014;175:90–95. [DOI] [PubMed] [Google Scholar]
- 32. Molnar AO, Eddeen AB, Ducharme R, Garg AX, Harel Z, McCallum MK, Perl J, Wald R, Zimmerman D, Sood MM. Association of proteinuria and incident atrial fibrillation in patients with intact and reduced kidney function. J Am Heart Assoc. 2017;6:e005685 DOI: 10.1161/JAHA.117.005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen CH, Zheng CM, Kiu KT, Chen HA, Wu CC, Lu KC, Hsu YH, Lin YF, Wang YH. Increased risk of atrial fibrillation in end‐stage renal disease patients on dialysis: a nationwide, population‐based study in Taiwan. Medicine (Baltimore). 2016;95:e3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102 DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suissa L, Bertora D, Lachaud S, Mahagne MH. Score for the targeting of atrial fibrillation (STAF): a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40:2866–2868. [DOI] [PubMed] [Google Scholar]
- 37. Bisson A, Bodin A, Fauchier L. Why and how to screen for atrial fibrillation. Heart. 2018;104:1474–1475. [DOI] [PubMed] [Google Scholar]
- 38. Hu WS, Lin CL. Comparison of CHA2DS2‐VASc, CHADS2 and HATCH scores for the prediction of new‐onset atrial fibrillation in cancer patients: a nationwide cohort study of 760,339 study participants with competing risk analysis. Atherosclerosis. 2017;266:205–211. [DOI] [PubMed] [Google Scholar]
- 39. Sposato LA, Cipriano LE, Saposnik G, Ruiz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta‐analysis. Lancet Neurol. 2015;14:377–387. [DOI] [PubMed] [Google Scholar]
- 40. Orchard J, Lowres N, Neubeck L, Freedman B. Atrial fibrillation: is there enough evidence to recommend opportunistic or systematic screening? Int J Epidemiol. 2018;47:1361. [DOI] [PubMed] [Google Scholar]
- 41. Linker DT, Murphy TB, Mokdad AH. Selective screening for atrial fibrillation using multivariable risk models. Heart. 2018;104:1492–1499. [DOI] [PubMed] [Google Scholar]
- 42. Welton NJ, McAleenan A, Thom HH, Davies P, Hollingworth W, Higgins JP, Okoli G, Sterne JA, Feder G, Eaton D, Hingorani A, Fawsitt C, Lobban T, Bryden P, Richards A, Sofat R. Screening strategies for atrial fibrillation: a systematic review and cost‐effectiveness analysis. Health Technol Assess. 2017;21:1–236. [DOI] [PubMed] [Google Scholar]
- 43. Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al‐Khalili F, Friberg L, Frykman‐Kull V, Levin LA. Cost‐effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17:1023–1029. [DOI] [PubMed] [Google Scholar]
- 44. Davis RC, Hobbs FD, Kenkre JE, Roalfe AK, Iles R, Lip GY, Davies MK. Prevalence of atrial fibrillation in the general population and in high‐risk groups: the ECHOES study. Europace. 2012;14:1553–1559. [DOI] [PubMed] [Google Scholar]


