Abstract
Background
Percutaneous mitral commissurotomy (PMC) was the first available transcatheter technique for treatment of mitral valve diseases. Experience has led to extending the indications to patients with less favorable characteristics. We aimed to analyze (1) the temporal trends in characteristic and outcomes of patients undergoing PMC in a single center over 30 years and (2) the predictive factors of poor immediate results of PMC.
Methods and Results
From 1987 to 2016, 1 full year for each decade was analyzed: 1987, 1996, 2006, and 2016. Poor immediate results of PMC were defined as a mitral valve area <1.5 cm2 or MR (mitral regurgitation) grade >2. Mitral anatomy was assessed using the Cormier classification and the fluoroscopic extent of calcification. Six hundred three patients were included: 111, 202, 205, and 85, respectively. Mean age increased >10 years over time (P<0.0001). Mitral anatomy was less favorable over the years: the presence of calcification increased from 25% of patients at the beginning of PMC to >40% during the past decade (P<0.0001) with a 3‐fold increase in severe mitral calcification. Consistently, the proportion of good immediate results decreased over time (P<0.05) but remained at 76% in 2016. Multivariate analysis showed 3 predictive factors of poor immediate results: smaller baseline mitral valve area (P<0.0001), pre‐PMC MR grade 2 (P<0.01), and the presence or amount of calcification (P<0.001).
Conclusions
This clinic's patients became significantly older with more frequent and severe calcification in the past decade. Predictive factors of poor immediate results were related to valve anatomy, including calcification. Despite challenges raised by severe calcification, PMC was still successful in >3 out of 4 patients in recent years.
Keywords: mitral stenosis, percutaneous procedure, temporal trends
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Rheumatic Heart Disease
Short abstract
See Editorial Palacios
Clinical Perspective
What Is New?
Over 30 years, rheumatic mitral stenosis is less frequently encountered in a large experienced Western country center with a >50% drop in the number of percutaneous mitral commissurotomy (PMC) performed, and the candidates for PMC are significantly older with more frequent valve calcification and, in particular, a 3‐fold higher rate of severe mitral calcification, which are usually considered a contraindication to PMC except as a palliative procedure.
What Are the Clinical Implications?
PMC may be considered in selected patients with severe calcification and high surgical risk, since as reported in our series, even in these patients with less favorable conditions, good immediate results of PMC were still achieved in more than 3 out of 4 patients, with very good safety of the procedure. These findings support the fact that good immediate results from PMC are really multifactorial, and that PMC should not be denied on the sole basis of anatomic criteria.
These good results may only be achieved after careful evaluation by the Heart Team in an expert center, to allow for multivalvular disease management and reduction of complication rates, and with the decrease in the number of candidates, PMC should be preferentially performed in high‐volume expert centers.
Mitral commissurotomy was one of the first available percutaneous techniques for the treatment of valvular diseases and has become the reference treatment for mitral stenosis, in particular in developing countries where most patients have favorable clinical and anatomical characteristics.1, 2 Percutaneous mitral commissurotomy (PMC) has thus opened the path for other percutaneous treatments such as transcatheter aortic valve implantation.3
Moreover, the technique initially described by Inoue in 1984 remains the reference.4 PMC thus represents a unique opportunity for the analysis of its temporal trends throughout 30 years of experience. The evolution over the years is largely because of the change in patients’ profile and also reflects changes in indications of PMC. This time period corresponds to a continuous decrease in the prevalence of rheumatic heart disease in industrialized countries.5 In parallel, there was an increase in degenerative mitral diseases with the aging of the population.6, 7
Growing experience of operators has led to extend PMC indications to older patients with rheumatic disease and less favorable anatomy, including patients with mitral valve calcification.
The aim of this study is to analyze the changes of patient presentation over the past 30 years in a single Western country center with extensive experience with PMC. We focused on the presence and the extent of mitral valve calcification. We also analyzed the predictive factors of poor immediate results (PIR) of PMC and evaluated the evolution of patients’ characteristics according to a previously described score.8
Methods
Population
The data that support the findings of this study are available from the corresponding author upon reasonable request.
From mid‐1986 to 2016, 1 full year for each decade was chosen to analyze the temporal trends over 30 years. Since the first PMC was performed in March 1986 in our institution, we instead chose 1987 from January 1 to December 31 to illustrate a full year of the beginnings of PMC. We then analyzed the years 1996, 2006, and 2016, from January 1 to December 31. All consecutive patients who underwent PMC during these 4 years were included in the study and their data were prospectively entered in a computerized database starting in 1986 as previously detailed.8, 9 All patients provided their written agreement before PMC, and independent review board approval was obtained by the local Ethics Committee.
PMC Procedure
All procedures were performed using an antegrade transvenous approach by the same team. The main difference in the material used over the years is the use of a single‐ or double‐balloon in 1987 and the adoption of the Inoue Balloon in 1990, as previously detailed.10
PMC was performed under echocardiographic guidance according to the stepwise technique when using the Inoue balloon. Contraindications to PMC have been previously described and correspond to the guidelines.1, 10
Measurements
Echocardiographic examinations were performed on the day before PMC and 24 to 48 hours after PMC by experienced echocardiographers over the 30 years. On the basis of transthoracic echocardiography, mitral valve anatomy was classified into 3 groups according to the Cormier classification as previously described.11 Cormier group 3 corresponds to the presence of mitral calcification of any extent confirmed by fluoroscopy. The extent of calcification was further graded into 4 groups, from small nodule (grade 1) to extensive calcification (grade 4) according to fluoroscopic examination, as previously described.12
The reference measurement for mitral valve area (MVA) was planimetry using 2D TTE (transthoracic echocardiography), as recommended,1, 2 and in case of missing data the use of Doppler half‐time measure. Mitral regurgitation was graded according to Sellers classification in 1987 and 1996 and to semiquantitative or quantitative echocardiographic analyses afterwards.
Definitions
Good immediate results of the PMC were defined by a composite criterion combining a final MVA ≥1.5 cm2 and MR (mitral regurgitation) ≤2/4, as previously described.8, 10
A previously described 13‐point score was calculated to evaluate the long‐term risk of poor functional results of PMC.8 Based on this score, 3 risk categories were defined: low‐risk group (score ≤2), intermediate‐risk group (score 3–5), and high‐risk group (score ≥6).
Statistical Analysis
Continuous variables were expressed as mean value±SD and qualitative variables as number and percentages.
Comparisons between more than 2 groups used an analysis of variance for quantitative variables and the χ2 test for qualitative variables. Post hoc analyses were performed between groups using a Bonferroni correction.
Comparisons before and after the PMC procedure in the same group of patients were performed using a paired t test.
Univariate analysis of the factors associated with PIR of the procedure was performed using a t test or a χ2 test as appropriate. Variables with P<0.10 were entered in a multivariable logistic model and selected by a backward procedure with a threshold of P=0.05. The results were considered significant when 2‐sided P values were <0.05. All analyzes were performed using the SPSS statistical software (SPSS V.23, Inc, Chicago, IL).
Results
Population
A total of 603 patients underwent PMC in our institution during the years 1987, 1996, 2006, and 2016, distributed as 111, 202, 205, and 85 patients, respectively.
The baseline clinical and echocardiographic characteristics of these patients are presented in Table 1, according to the year of their procedure. The chosen years were representative of their decades, as illustrated in Figure S1.
Table 1.
Baseline Characteristics of Patients
| Variables | 1987 (n=111) | 1996 (n=202) | 2006 (n=205) | 2016 (n=85) | P Value |
|---|---|---|---|---|---|
| Age, y | 42.4±15.1 | 49.1±15.3 | 53.7±15.6 | 55.4±16.8 | <0.0001 |
| Male sex | 27 (24) | 39 (19) | 39 (19) | 23 (27) | 0.33 |
| NYHA class 3 to 4 | 92 (83) | 128 (63) | 120 (59) | 44 (52) | <0.0001 |
| Atrial fibrillation | 36 (32) | 56 (28) | 71 (35) | 46 (54) | 0.0003 |
| History of commissurotomy | 18 (16) | 32 (16) | 52 (25) | 11 (13) | 0.03 |
| CHC | 15 (14) | 16 (8) | 13 (6) | 0 | |
| OHC | 3 (2) | 5 (2) | 7 (3) | 1 (1) | |
| PMC | 0 | 11 (5) | 32 (16) | 10 (12) | |
| Echocardiography before PMC | |||||
| MVA, cm2 | 1.04±0.23 | 1.03±0.22 | 1.03±0.24 | 1.07±0.26 | 0.42 |
| Mitral gradient, mm Hg | 10.8±5.4 | 10.2±4.1 | 9.5±4.5 | 10.1±4.7 | 0.13 |
| MR grade | |||||
| 0 | 63 (57) | 50 (25) | 49 (24) | 21 (24.5) | <0.0001 |
| 1 | 42 (38) | 134 (66) | 135 (66) | 54 (63.5) | |
| 2 | 6 (5) | 18 (9) | 21 (10) | 10 (12) | |
| Systolic PAP, mm Hg | 37.7±7.3 | 43.8±12.4 | 43.3±12.7 | 46.8±15.7 | <0.0001 |
| Valve anatomy (Cormier class) | |||||
| 1 | 33 (30) | 9 (5) | 9 (4) | 2 (2) | 0.003 |
| 2 | 49 (44) | 145 (72) | 100 (49) | 48 (56) | |
| 3 | 29 (26) | 48 (24) | 96 (47) | 36 (42) | |
| Calcification gradea | |||||
| 1 | 13 (11.5) | 21 (10) | 49 (24) | 5 (6) | <0.0001 |
| 2 | 9 (8) | 19 (9) | 33 (16) | 9 (11) | |
| 3 | 3 (3) | 8 (4) | 14 (7) | 10 (12) | |
| 4 | 4 (3.5) | 0 | 0 | 12 (14) | |
Results are presented as mean±SD or n (%). CHC indicates closed‐heart commissurotomy; MR, mitral regurgitation; MVA, mitral valve area; NYHA, New York Heart Association Class; OHC, open‐heart commissurotomy; PAP, pulmonary artery pressure; PMC, percutaneous mitral commissurotomy.
In patients with Cormier class 3.
Patients were significantly older over time, with a mean difference of more than 10 years between the initial experience (1987) and the latest procedures (2016) (P<0.0001). Moreover, the proportion of elderly patients ≥70 years old increased with time (from 7% in 1987 to 21% in 2016). There were significantly more patients with atrial fibrillation, in particular in 2016 (P=0.0003). On the other hand, patients were less frequently in NYHA class 3 to 4 over time (P<0.0001). The severity of mitral stenosis, as assessed by mean mitral gradient and MVA, did not significantly differ during the 30 years. Approximately 15% of patients had a history of commissurotomy and thus underwent PMC for mitral restenosis, with a transition from prior surgical (closed or open heart) commissurotomy to PMC over decades.
Mitral anatomy was less favorable over years, with only 2% of patients with a Cormier class 1 in 2016 versus 30% in 1987, while the presence of calcification (Cormier class 3) increased from 26% at the beginning of PMC to ≥40% during the past decades (P=0.003). The proportion of patients with severe calcification (grades 3–4) markedly increased in 2016 as compared with other years.
The evolution of these variables over time is illustrated in Figure 1.
Figure 1.
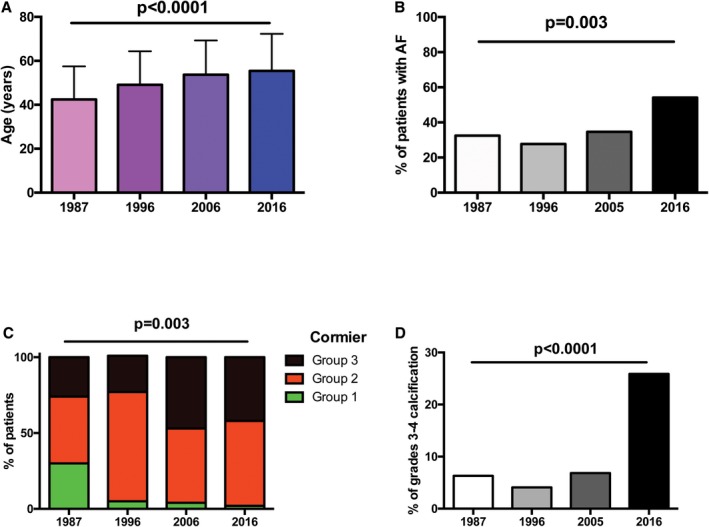
Evolution of age (A), presence of AF) (B), Cormier class (C), and severe calcification (D) over 30 years of percutaneous mitral calcification. AF indicates atrial fibrillation.
Results of PMC
Safety of the procedure
We report 10 complications over the 603 PMCs performed during the 4 years (1.7%), detailed as 1 cardiac tamponade (1987), 5 strokes (2 in 1996, 2 in 2006 and 1 in 2016), 2 severe MR requiring planned surgery (2016), and 2 deaths. One young 45‐year‐old woman without mitral calcification died because of septic shock (2006), while the other death occurred in a 86‐year‐old woman with severe mitral calcification and NYHA class 4 caused by esophageal perforation during a procedure needing transesophageal echocardiography guidance (2016). This patient was contraindicated for surgery and the PMC was attempted as a compassionate procedure after Heart Team evaluation. No other complication occurred in elderly patients with mitral calcification.
Immediate results of PMC
Good immediate results (GIR) were defined by a final MVA ≥1.5 cm2 and MR ≤grade 2. Among the 603 patients studied over the 3 decades, 519 (86%) experienced GIR of PMC. PIR of PMC were mainly because of a final MVA <1.5 cm2 accounting for 67%, 62%, 48%, and 80% of the cases for the 4 years, respectively.
The proportion of GIR slightly decreased in 2016 as compared with earlier years, as illustrated in Figure 2.
Figure 2.
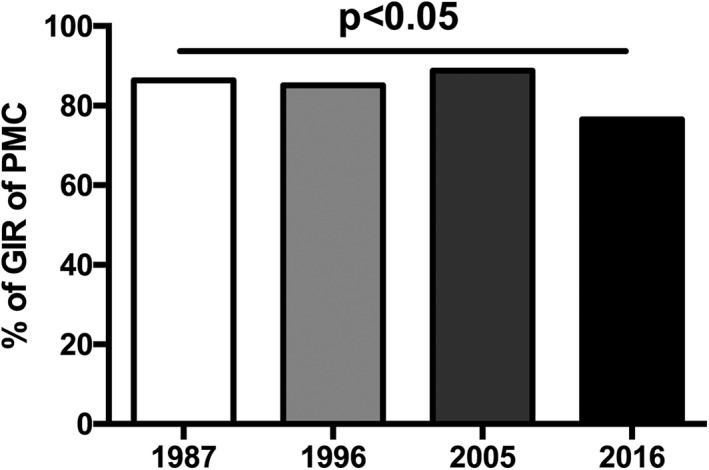
Proportion of GIR of PMC over years. GIR indicates good immediate results; PMC, percutaneous mitral commissurotomy.
Predictive Factors of Immediate Results of PMC
The baseline characteristics of patients according to immediate results of PMC are described in Table 2. Univariate analysis identified 9 variables related to PIR of PMC, including the presence of calcification assessed by echocardiography and the amount of calcification graded in fluoroscopy.
Table 2.
Univariate Analysis of the Factors Associated With PIR of PMC
| Variables | GIR (N=519) | PIR (N=84) | P Value |
|---|---|---|---|
| Age, y | 49.6±16.1 | 54.8±15.7 | 0.006 |
| Male sex | 114 (22) | 14 (17) | 0.27 |
| NYHA class 3 to 4 | 322 (62) | 62 (74) | 0.04 |
| Atrial fibrillation | 176 (34) | 33 (39) | 0.34 |
| History of PMC | 92 (18) | 21 (25) | 0.11 |
| Experience of PMC | 0.82 | ||
| <10 y | 96 (18) | 15 (18) | |
| ≥10 and <20 y | 176 (34) | 26 (31) | |
| ≥20 y | 247 (48) | 43 (51) | |
| Imaging data (pre‐PMC) | |||
| MVA, cm2 | 1.06±0.23 | 0.92±0.21 | <0.0001 |
| Mitral gradient, mm Hg | 9.9±4.6 | 11.2±4.7 | 0.02 |
| MR grade 2 vs 0 to 1 | 39 (8) | 16 (19) | 0.001 |
| sPAP, mm Hg | 42.2±11.6 | 47.4±16.7 | <0.0001 |
| Cormier group 3 vs 1 to 2 | 160 (31) | 49 (58) | <0.0001 |
| Calcification grade | |||
| 0 | 359 (69) | 35 (42) | |
| 1 | 72 (14) | 16 (19) | <0.0001 |
| 2 | 51 (10) | 18 (21) | |
| 3 | 26 (5) | 9 (11) | |
| 4 | 11 (2) | 6 (7) | |
Results are presented as mean±SD or n (%). MR indicates mitral regurgitation; MVA, mitral valve area; NYHA, New York Heart Association Class; PIR, poor immediate results; PMC, percutaneous mitral commissurotomy; sPAP, systolic pulmonary artery pressure.
Because of colinearity between these 2 variables, we performed 2 multivariate analyses: Model 1 included the variable “presence of calcification,” as assessed by a Cormier score 3, while Model 2 included the variable “calcification grade.” Multivariate Model 1 identified 3 factors independently associated with PIR of PMC: pre‐PMC MVA (adjusted odds ratio [OR]=0.09, 95% CI [0.06–0.13]; P<0.0001), preprocedural MR grade 2 (adjusted OR=3.19, 95% CI [1.6–6.3]; P=0.001), and the presence of mitral calcification (adjusted OR=2.8, 95% CI [1.8–4.5]; P<0.0001). When entering the variable “calcification grade” (Multivariate Model 2), the amount of calcification was an independent predictive factor of PIR of PMC (adjusted OR 1.6, 95% CI [1.3–1.9] per grade increase; P<0.0001), while pre‐PMC MVA and MR grade 2 remained statistically significant (OR 0.09, 95% CI [0.06–0.13]; P<0.0001 and OR 4.0, 95% CI [2.0–8.0]; P<0.0001, respectively).
Detailed Results of PMC Among Patients With GIR
The results of PMC are detailed in Table 3 for patients who had GIR of the procedure.
Table 3.
Detailed Results of PMC Among Patients With Successful Procedure
| Variables | 1987 (n=96) | 1996 (n=176) | 2006 (n=182) | 2016 (n=65) | P Value |
|---|---|---|---|---|---|
| Final MVA, cm2 | 2.01±0.35 | 1.87±0.21 | 1.87±0.24 | 1.86±0.29 | <0.0001 |
| Mean MVA increase, cm2 | 0.95±0.34 | 0.82±0.25 | 0.83±0.25 | 0.73±0.34 | <0.0001 |
| Final mitral gradient, mm Hg | 4.6±2.0 | 4.5±1.6 | 4.5±1.8 | 4.8±1.9 | 0.68 |
| Mean gradient decrease, mm Hg | 5.6±4.2 | 5.5±3.9 | 4.9±4.0 | 5.1±4.4 | 0.41 |
| Final MR grade | |||||
| 1 | 45 (47) | 113 (64) | 113 (62) | 45 (69) | <0.0001 |
| 2 | 12 (13) | 53 (30) | 63 (35) | 17 (26) | |
| MR increase of ≥1 grade | 30 (31) | 73 (42) | 78 (43) | 20 (31) | 0.12 |
| Mean PMC score | 3.4±2.7 | 3.9±2.4 | 4.6±2.6 | 5.4±3.3 | <0.0001 |
| PMC score: group | |||||
| 1 | 40 (44) | 53 (30) | 41 (23) | 15 (23) | <0.0001 |
| 2 | 42 (46) | 83 (47) | 77 (43) | 20 (31) | |
| 3 | 9 (10) | 39 (22) | 63 (35) | 30 (46) | |
Results are presented as mean±SD or n (%). MR indicates mitral regurgitation; MVA, mitral valve area; PMC, percutaneous mitral commissurotomy.
The mean MVA increase was less important over years with a significant difference between the beginning of PMC (1987) and the most recent year (2016): 0.95±0.34 versus 0.73±0.34 cm2, respectively, P<0.0001 (Figure 3A).
Figure 3.
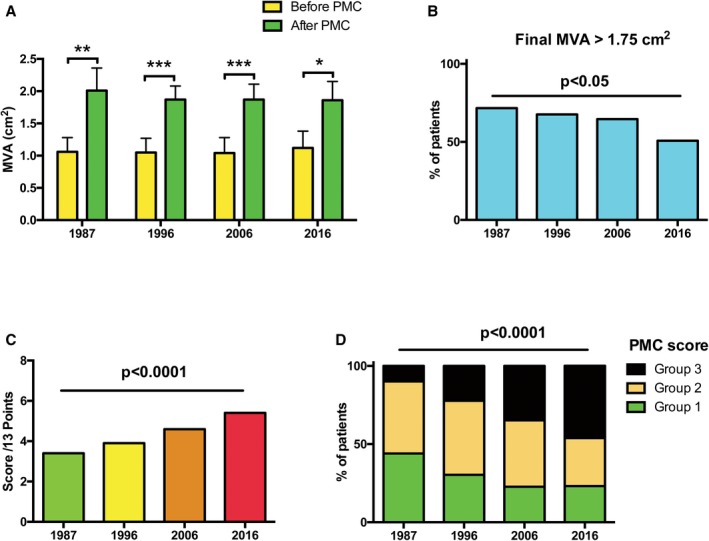
Mean MVA before and after PMC (A), proportion of patients with final MVA >1.75 cm2 (B), and post‐PMC scores (C and D), among patients with good immediate results. MVA indicates mitral valve area; PMC, percutaneous mitral commissurotomy.
In line with these results, the proportion of patients with a final MVA >1.75 cm2 significantly decreased over time, with only 50% of patients in 2016 reaching this threshold (Figure 3B). The final mean mitral gradient did not significantly differ between years, nor did the increase in final MR grade. Age, mitral calcification, and final MVA were components of the PMC score used to estimate the risk of poor long‐term results after the procedure; this later significantly increased from 1987 to 2016 (P<0.0001), with a higher score corresponding to a worse late prognosis (Figure 3C). The 3 groups of PMC score, from low‐ to high‐risk are represented in Figure 3D, with a significant increase of higher‐risk patients over time.
Discussion
Temporal Trends of PMC Over 30 Years
The number of PMC performed in our institution decreased over the past decades, as described in other series.13, 14 Indeed, only 85 patients underwent a PMC in our institution in 2016 as compared with >200 procedures in 1996 and 2006. This >50% drop in PMC numbers may reflect the decrease in the number of patients with rheumatic heart disease in industrialized countries.5 Over the years, patients who underwent PMC in France were significantly older. The proportion of elderly patients >70 years old increased with time. There were more patients with atrial fibrillation and less frequency of NYHA class 3 to 4 over time. The severity of mitral stenosis as assessed by gradient and MVA did not change significantly during the 30 years.
Mitral anatomy was less favorable over the years, with only 2% of patients with Cormier class 1 in 2016 versus 30% in 1987, in particular with more frequent valve calcification (from 26% at the beginning to 40% during the past decade) and larger extent of calcification. This evolution confirms and strengthens the previous trends observed in our experience.15 The recognition of PMC as the reference technique for symptomatic rheumatic mitral stenosis led to earlier referral of patients, as illustrated by the constant decrease in NYHA class 3 to 4 patients over time. Indeed, only 52% of patients were in NYHA class 3 to 4 in 2016 as compared with 83% in 1987.
Finally, surgical commissurotomy has almost disappeared, supplanted by PMC, given the comparable outcome between the 2 techniques added to a lower morbidity‐mortality with PMC.16 Indeed, whereas mitral restenosis occurred in 16% of patients with previous surgical commissurotomy in 1987 and 11% in 1996, only 1 patient had a history of surgical commissurotomy in 2016.
Changes in patient characteristics, in particular increasing age and more frequent valve calcification, is a consequence of the decrease of the prevalence of rheumatic heart disease in Western countries. However, these changes may also reflect the extension of indications of PMC from ideal to less favorable anatomical conditions, in particular valve calcification, as a consequence of the experience acquired with PMC.
Immediate Results of PMC
GIR of PMC were achieved in 86% of the whole population over the 30 years. PIR were mostly because of a final MVA <1.5 cm2. The rate of GIR was less important in 2016, consistent with less favorable clinical and anatomical characteristics of patients. However, >75% of patients still exhibited GIR in recent years.
The predictive factors of PIR were a smaller pre‐PMC MVA, a pre‐PMC MR grade 2, and the presence and larger extent of mitral calcification. Mitral calcification has previously been associated with PIR of PMC.10, 12 Although the choice of the first‐line treatment of patients with mitral calcification remains debated, the multifactorial prediction of PMC results lead to considering PMC as an initial treatment in patients with mild‐to‐moderate calcification who otherwise have favorable clinical characteristics.2, 17, 18, 19
Challenges Raised by Elderly Patients and Severe Mitral Calcification
Besides rheumatic mitral stenosis, degenerative calcific mitral disease is more frequently encountered in aging patients.6 Degenerative mitral stenosis is characterized by calcification of the mitral annulus without commissural fusion and is therefore not amenable to PMC. Given the technical challenges raised by extensive mitral calcification with less satisfying surgical results, less invasive approaches are particularly attractive and new percutaneous treatments for high‐risk patients may be considered.20, 21, 22 However, transcatheter mitral valve implantation is not applicable to rheumatic heart disease since calcification is not located on the mitral annulus but deeper in the left ventricle and cannot provide a safe prosthesis anchoring.
We found in this study that patients ≥70 years old were more frequent over time. Despite less favorable conditions, GIR of PMC were still achieved in 80% of patients ≥70 years old. The safety of the procedure remained very good in elderly patients, since only 1 experienced a death‐related procedure during a compassionate procedure and there was no severe complication of PMC such as cardiac tamponade or stroke.
The presence of severe calcification is considered a usual contraindication to PMC, and only patients at high risk or contraindicated for surgery may benefit from PMC, according to guidelines.2 In our study, 50 patients exhibited severe calcification but were contraindicated or considered at high risk for surgery, or refused to undergo surgical treatment. We report GIR of PMC in 76% of these patients. These findings support the fact that PMC should not be denied on the sole basis of anatomic criteria. These findings are in agreement with previous data from our group and with other investigators demonstrating that the GIR from PMC are multifactorial including anatomic, clinical, procedural, and demographic factors associated with GIR from PMC. In recent years, more than 1 out of 4 patients referred for PMC had severe calcification and with the aging population, this situation is expected to become more common.
The question about whether to perform first‐line PMC in these patients is therefore relevant, given the evolution of the presentation of patients with rheumatic mitral stenosis.
Long‐Term Perspectives
With time, there was a progressive increase in the proportion of patients classified at higher risk of poor late functional results according to a previously described score.8 This is consistent with the evolution of the components of the score (older age, more frequent valve calcification, higher final mean gradient, and lower final MVA). Indeed, MVA increase between pre‐ and post‐PMC was less prominent in recent years, and only 50% of patients reached a final MVA >1.75 cm2 in 2016. As previously shown, a lower final MVA was associated with poorer long‐term results.8 Patients classified in the higher‐risk group are expected to experience less good outcome in the long run. However, even in these patients, we can expect that half of them would still experience sustained good functional results at 10‐year follow‐up.8 These results in patients with nonfavorable characteristics emphasize the usefulness of PMC in postponing surgery.
In the context of extending indications of PMC, the individual identification of patients at higher risk of deterioration using this simple score is therefore of particular interest to allow for a closer follow‐up and timely surgery if needed.
With regard to healthcare organization, PMC patients should be referred to high‐volume expert centers, given the continuous decrease in the number of candidates and the changes toward less favorable conditions. Moreover, population aging has led to an increase in multivalvular disease, whose diagnosis and management are even more challenging. Besides expertise by the Heart Team, this is likely to reduce complication rates.23
Limitations of the Study
Given the single‐center nature of this study, we cannot exclude referral bias in a large experienced center. On the other hand, the homogeneity in the evaluation of patients and in the realization of PMC ensures accurate analysis of differences over time. Similarly, it is difficult to generalize some of the findings past the particular hospital involved, and this study mostly reports our center's experience. However, temporal trends appear to be in agreement with other studies and are likely to reflect general trends. We could not report a continuous analysis of the 30 years corresponding to the time period analyzed, since our local database was interrupted between 2007 and 2015. We therefore chose 1 year for each decade as detailed in the Methods section.
We used the validated Cormier's classification to evaluate mitral anatomy,24 which may limit comparison with other studies using the Wilkins score. However, the Cormier classification was prospectively used from the beginning of our experience and to ensure homogeneity in the evaluation, we continued with the same method over the years.
Another limitation is the absence of location of mitral valve calcification. Indeed, fluoroscopy is the reference method for the analysis of the presence and extent of calcification12, 25 but lacks accuracy for assessing their location.
Sources of Funding
This study was supported and funded by the French Society of Cardiology.
Disclosures
None.
Supporting information
Figure S1. Histograms representing temporal trends for age (A), echocardiographic Cormier classification (B), and the presence of atrial fibrillation (C) between 1987 and 2006.
(J Am Heart Assoc. 2019;8:e012031 DOI: 10.1161/JAHA.119.012031.)
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 3. de Freitas Campos Guimaraes L, Urena M, Wijeysundera HC, Munoz‐Garcia A, Serra V, Benitez LM, Auffret V, Cheema AN, Amat‐Santos IJ, Fisher Q, Himbert D, Garcia Del Blanco B, Dager A, Le Breton H, Paradis JM, Dumont E, Pibarot P, Rodes‐Cabau J. Long‐term outcomes after transcatheter aortic valve‐in‐valve replacement. Circ Cardiovasc Interv. 2018;11:e007038. [DOI] [PubMed] [Google Scholar]
- 4. Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. [PubMed] [Google Scholar]
- 5. Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30:962–970. [DOI] [PubMed] [Google Scholar]
- 6. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol. 2015;66:1934–1941. [DOI] [PubMed] [Google Scholar]
- 7. Sud K, Agarwal S, Parashar A, Raza MQ, Patel K, Min D, Rodriguez LL, Krishnaswamy A, Mick SL, Gillinov AM, Tuzcu EM, Kapadia SR. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation. 2016;133:1594–1604. [DOI] [PubMed] [Google Scholar]
- 8. Bouleti C, Iung B, Laouenan C, Himbert D, Brochet E, Messika‐Zeitoun D, Detaint D, Garbarz E, Cormier B, Michel PL, Mentre F, Vahanian A. Late results of percutaneous mitral commissurotomy up to 20 years: development and validation of a risk score predicting late functional results from a series of 912 patients. Circulation. 2012;125:2119–2127. [DOI] [PubMed] [Google Scholar]
- 9. Bouleti C, Iung B, Himbert D, Brochet E, Messika‐Zeitoun D, Detaint D, Garbarz E, Cormier B, Vahanian A. Long‐term efficacy of percutaneous mitral commissurotomy for restenosis after previous mitral commissurotomy. Heart. 2013;99:1336–1341. [DOI] [PubMed] [Google Scholar]
- 10. Iung B, Cormier B, Ducimetiere P, Porte JM, Nallet O, Michel PL, Acar J, Vahanian A. Immediate results of percutaneous mitral commissurotomy. A predictive model on a series of 1514 patients. Circulation. 1996;94:2124–2130. [DOI] [PubMed] [Google Scholar]
- 11. Cormier B, Vahanian A, Michel PL, Starkman C, Enriquez L, Kulas A, Vitoux B, Acar J. Evaluation by two‐dimensional and Doppler echocardiography of the results of percutaneous mitral valvuloplasty [Article in French]. Arch Mal Coeur Vaiss. 1989;82:185‐191. [PubMed] [Google Scholar]
- 12. Bouleti C, Iung B, Himbert D, Messika‐Zeitoun D, Brochet E, Garbarz E, Cormier B, Vahanian A. Relationship between valve calcification and long‐term results of percutaneous mitral commissurotomy for rheumatic mitral stenosis. Circ Cardiovasc Interv. 2014;7:381–389. [DOI] [PubMed] [Google Scholar]
- 13. Badheka AO, Shah N, Ghatak A, Patel NJ, Chothani A, Mehta K, Singh V, Patel N, Grover P, Deshmukh A, Panaich SS, Savani GT, Bhalara V, Arora S, Rathod A, Desai H, Kar S, Alfonso C, Palacios IF, Grines C, Schreiber T, Rihal CS, Makkar R, Cohen MG, O'Neill W, de Marchena E. Balloon mitral valvuloplasty in the United States: a 13‐year perspective. Am J Med. 2014;127:1126.e1–12. [DOI] [PubMed] [Google Scholar]
- 14. Meneguz‐Moreno RA, Costa JR Jr, Gomes NL, Braga SLN, Ramos AIO, Meneghelo Z, Maldonado M, Ferreira‐Neto AN, Franca JID, Siqueira D, Esteves C, Sousa A, Sousa JE, Abizaid A. Very long term follow‐up after percutaneous balloon mitral valvuloplasty. JACC Cardiovasc Interv. 2018;11:1945–1952. [DOI] [PubMed] [Google Scholar]
- 15. Iung B, Nicoud‐Houel A, Fondard O, Hafid A, Haghighat T, Brochet E, Garbarz E, Cormier B, Baron G, Luxereau P, Vahanian A. Temporal trends in percutaneous mitral commissurotomy over a 15‐year period. Eur Heart J. 2004;25:701–707. [DOI] [PubMed] [Google Scholar]
- 16. Ben Farhat M, Ayari M, Maatouk F, Betbout F, Gamra H, Jarra M, Tiss M, Hammami S, Thaalbi R, Addad F. Percutaneous balloon versus surgical closed and open mitral commissurotomy: seven‐year follow‐up results of a randomized trial. Circulation. 1998;97:245–250. [DOI] [PubMed] [Google Scholar]
- 17. Palacios IF, Sanchez PL, Harrell LC, Weyman AE, Block PC. Which patients benefit from percutaneous mitral balloon valvuloplasty? Prevalvuloplasty and postvalvuloplasty variables that predict long‐term outcome. Circulation. 2002;105:1465–1471. [DOI] [PubMed] [Google Scholar]
- 18. Nunes MC, Nascimento BR, Lodi‐Junqueira L, Tan TC, Athayde GR, Hung J. Update on percutaneous mitral commissurotomy. Heart. 2016;102:500–507. [DOI] [PubMed] [Google Scholar]
- 19. Cruz‐Gonzalez I, Sanchez‐Ledesma M, Sanchez PL, Martin‐Moreiras J, Jneid H, Rengifo‐Moreno P, Inglessis‐Azuaje I, Maree AO, Palacios IF. Predicting success and long‐term outcomes of percutaneous mitral valvuloplasty: a multifactorial score. Am J Med. 2009;122:581.e11–9. [DOI] [PubMed] [Google Scholar]
- 20. Himbert D, Bouleti C, Iung B, Nejjari M, Brochet E, Depoix JP, Ghodbane W, Fassa AA, Nataf P, Vahanian A. Transcatheter valve replacement in patients with severe mitral valve disease and annular calcification. J Am Coll Cardiol. 2014;64:2557–2558. [DOI] [PubMed] [Google Scholar]
- 21. Guerrero M, Rihal C. Taking transcatheter mitral valve replacement to the next level. Circ Cardiovasc Interv. 2018;11:e007369. [DOI] [PubMed] [Google Scholar]
- 22. Lim ZY, Boix R, Prendergast B, Rajani R, Redwood S, Hancock J, Young C, Bapat VV. First reported case of transcatheter mitral valve implantation in mitral annular calcification with a fully repositionable and self‐expanding valve. Circ Cardiovasc Interv. 2015;8:e003031. [DOI] [PubMed] [Google Scholar]
- 23. Nobuyoshi M, Arita T, Shirai S, Hamasaki N, Yokoi H, Iwabuchi M, Yasumoto H, Nosaka H. Percutaneous balloon mitral valvuloplasty: a review. Circulation. 2009;119:e211–e219. [DOI] [PubMed] [Google Scholar]
- 24. Rahimtoola SH, Durairaj A, Mehra A, Nuno I. Current evaluation and management of patients with mitral stenosis. Circulation. 2002;106:1183–1188. [DOI] [PubMed] [Google Scholar]
- 25. Tuzcu EM, Block PC, Griffin B, Dinsmore R, Newell JB, Palacios IF. Percutaneous mitral balloon valvotomy in patients with calcific mitral stenosis: immediate and long‐term outcome. J Am Coll Cardiol. 1994;23:1604–1609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Histograms representing temporal trends for age (A), echocardiographic Cormier classification (B), and the presence of atrial fibrillation (C) between 1987 and 2006.


