Abstract
Background
Familial hypercholesterolemia (FH), is a historically underdiagnosed, undertreated, high‐risk condition that is associated with a high burden of cardiovascular morbidity and mortality. In this study, we use a population‐based approach using electronic health record (EHR)‐based algorithms to identify FH. We report the major adverse cardiovascular events, mortality, and cost of medical care associated with this diagnosis.
Methods and Results
In our 1.18 million EHR‐eligible cohort, International Classification of Diseases, Ninth Revision (ICD‐9) code‐defined hyperlipidemia was categorized into FH and non‐FH groups using an EHR algorithm designed using the modified Dutch Lipid Clinic Network criteria. Major adverse cardiovascular events, mortality, and cost of medical care were analyzed. A priori associated variables/confounders were used for multivariate analyses using binary logistic regression and linear regression with propensity score–based weighted methods as appropriate. EHR FH was identified in 32 613 individuals, which was 2.7% of the 1.18 million EHR cohort and 13.7% of 237 903 patients with hyperlipidemia. FH had higher rates of myocardial infarction (14.77% versus 8.33%; P<0.0001), heart failure (11.82% versus 10.50%; P<0.0001), and, after adjusting for traditional risk factors, significantly correlated to a composite major adverse cardiovascular events variable (odds ratio, 4.02; 95% CI, 3.88–4.16; P<0.0001), mortality (odds ratio, 1.20; CI, 1.15–1.26; P<0.0001), and higher total revenue per‐year (incidence rate ratio, 1.30; 95% CI, 1.28–1.33; P<0.0001).
Conclusions
EHR‐based algorithms discovered a disproportionately high prevalence of FH in our medical cohort, which was associated with worse outcomes and higher costs of medical care. This data‐driven approach allows for a more precise method to identify traditionally high‐risk groups within large populations allowing for targeted prevention and therapeutic strategies.
Keywords: familial hypercholesterolemia, major adverse cardiovascular events, mortality, subclinical atherosclerosis risk factor, subclinical familial hypercholesterolemia
Subject Categories: Clinical Studies, Lipids and Cholesterol, Quality and Outcomes
Clinical Perspective
What Is New?
Familial hypercholesterolemia is characterized by lifelong elevation of circulating low‐density lipoproteins and is associated with high cardiovascular mortality and morbidity; however, because of the variability in its presentation and lack of awareness, the condition is often underdiagnosed.
Within our integrated health system, we evaluated novel ways of identifying and examining the sequelae of familial hypercholesterolemia in a population‐based manner.
This was done by using an electronic health record–based algorithm for detection and risk stratification of familial hypercholesterolemia and its associated comorbidities and cardiovascular outcomes and by using the total cost of care to examine the higher cost per year associated with the various categories of the familial hypercholesterolemia phenotype.
What Are the Clinical Implications?
This methodology can allow health systems to study the drivers of worse clinical outcomes and higher costs of care to identify areas of opportunity to target limited resources.
Introduction
The precision medicine model proposes customization of health care to individual patients, the success of which is largely dependent on early and accurate diagnosis. Familial hypercholesterolemia (FH)—a genetic disorder characterized by elevated low‐density lipoprotein cholesterol (LDL‐C) levels and early cardiovascular disease, is 1 of the 3 Centers for Disease Control and Prevention (CDC) designated Tier 1 public health genomic conditions, based on available evidence‐based guidelines.1 The major clinical manifestation of FH, premature atherosclerosis, is thought to result from the prolonged exposure of the vasculature to high levels of LDL‐C. Clinical cardiovascular heart disease occurs at a higher frequency and at an earlier age in patients with FH than in patients without FH or patients with polygenetic causes of elevated LDL‐C.2 Traditional estimates of FH in the general population vary significantly, from 1:500 to 1:137,3 with the added challenge of lack of specific International Classification of Diseases (ICD) coding until recently. Despite advances in our understanding of the pathophysiology of FH, significant numbers remain undiagnosed and undertreated in relation to LDL‐C targets.4 While the cost effectiveness of universal screening, early identification, and treatment is still evolving,5 availability of electronic health records (EHRs) and validated clinical criteria may offer a rapid and efficient approach to population‐based screening to identify high‐risk individuals for targeted interventions. Additionally, evaluation of interventions in FH is complicated by the paucity of relevant economic data.6 The goal of this study is to use EHR‐based algorithms to implement a population‐based screening approach to identify the hidden burden of FH and study the trends of major adverse cardiovascular events (MACE), mortality and cost of care associated with this diagnosis.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Extraction
We queried our EHR database of over 1.5 million Geisinger Health System (GHS) patients (January 2000 to August 2016) with ICD, Ninth Revision (ICD‐9) codes for hyperlipidemia (272.0, 272.1, 272.2, 272.3, 272.4). This project was approved as an expedited study on the basis of use of deidentified data by the Institutional Review Board at Geisinger Medical Center. We excluded patients aged <18 years or ≥85 years, leaving an eligible study population of 1.18 million patients. An internally validated algorithm (see below) developed at Geisinger using modified Dutch Lipid Clinic Network Criteria (DLCN) (Table S1) was applied to these patients to categorize them into definite, probable, possible, and unlikely FH categories.7 The DLCN criteria were modified (Tables S2 and S3) to suit the EHR‐derived data set. The first 3 of these discrete groups was defined to represent the hidden burden of FH in our population and were analyzed together as the “FH cohort.”
The EHR‐based algorithm based on the modified DLCN criteria for FH using a structured data set was validated internally by chart reviewing 250 randomly chosen case and control patients (definite, 2; probable, 13; possible, 111; unlikely, 124). A total of 125 of 126 patients were found to be accurately assigned to the positive group by the algorithm, for a positive predictive value of 99.21%. A total of 118 of 124 were found to be true‐negative patients (unlikely FH), for a negative predictive value of 95.16% (for details of diagnostic accuracy see Tables S4 and S5). Six patients moved from the unlikely category to possible, for a sensitivity score of 95.42%. One patient went from possible to unlikely, for a specificity score of 99.16% (Table S6). Ten patients had errors found but stayed unlikely, and 1 patient remained in the definite category. Six of the 10 algorithm definitions were found to have 100% accuracy after chart reviews. Our EHR‐based algorithm for identifying relevant family history in first‐degree relatives performed poorly. Ten additional patients were found to have a family history of heart disease during the chart review of clinic notes, and 2 patients were deemed to have a family history of hyperlipidemia.
Outcomes
Primary outcomes of interest were MACE, mortality, and cost of care.
Mortality
Mortality was defined as all‐cause mortality identified within the EHR. Our database interfaces with the Social Security Death Index on a biweekly basis to reflect updated information on vital status.
Major Adverse Cardiovascular Events
MACE was defined as a categorical variable denoting occurrence of the first instance of any of the following: all‐cause death, myocardial infarction, percutaneous coronary intervention, or coronary artery bypass. Each of these clinical events were identified by using ICD‐9 codes (Table S7) in patients’ EHR to obtain information relevant to MACE. Validation of the EHR‐derived MACE and clinical variables was done by manual chart review in 100 randomly chosen subjects and showed good diagnostic accuracy (95% myocardial infarction, 95% heart failure, 100% ischemic stroke, 100% percutaneous coronary intervention, 100% bypass grafting, and 100% ICD).
Cost of Care
To identify the hidden economic burden of FH on healthcare system, we sought to determine differences in medical care expenditures between FH and non‐FH hyperlipidemia cohorts on the basis of revenue data from medical care service use within our health system. Because of stepwise integration of several satellite sites within the system, financial data were not available for early years and some patients. We dealt with this by analyzing the clinical characteristics of patients with missing data to those with available data and using complete case analysis only. Inflation adjusted financial data for each year were calculated in terms of the 2015 data, according to the latest consumer price index data.8 We then compared adjusted median revenue between the 2 groups for the years 2005 through 2015. Because the revenue received by the healthcare system may vary depending on the payer mix, to calculate the absolute magnitude of difference in expenditure between the 2 groups we used a variable “Med Net Revenue,” which adjusts all payer revenues to Medicare rates for GHS. These data were available only for the years 2014 and 2015.
Data Analyses
Statistical Analysis System (SAS) Version 9.4 (SAS Institute Inc., Cary, NC) was used for statistical analyses. Descriptive variables were expressed as mean with standard deviation in cases of normal distribution, and median with interquartile range (IQR) in case of nonnormal distribution. Normality was assessed by the Kolmogorov–Smirnoff test. Categorical variables are expressed as counts with percentages. Comparisons between continuous variables were conducted with nonparametric Mann–Whitney test for mean ranks for those variables with nonnormal distribution, whereas the chi‐square test was performed to test the independence of categorical variables. To account for biases attributable to the observed confounders from baseline characteristics, the stabilized inverse probability of treatment weights was implemented by weighting the responses on the basis of their propensity scores in the regression model. The propensity scores were estimated on the basis of observed confounders that were significantly different between FH and non‐FH cohorts including age, sex, smoking history, comorbid conditions, and the lost to follow‐up indicator. Lost to follow‐up was defined as no visit in EHR 2 years before the end of the study. A priori associated variables/confounders were included in the logistic regression, after adjusting for age, sex, smoking status, diabetes mellitus, hypertension, and LDL‐C (maximum) values to evaluate if EHR FH significantly predicted outcomes.9 Odds ratios with corresponding 95% CIs were presented. By including the total follow‐up time (defined as the years between first and last encounter over 2005–2015) as the offset variable to normalize the total adjusted revenue to a per‐year basis, a negative binomial regression model using inverse probability of treatment weights was performed to evaluate the impact of FH on the total revenue after adjusting for a priori associated variables/confounders. Incidence rate ratios with corresponding 95% CIs were presented. A P value of <0.05 was considered significant.
Results
In our 1.18 million EHR‐eligible cohort, 237 903 patients had hyperlipidemia (49.5% female, 96.6% white). Median age of the entire cohort was 63 years (IQR, 20 years), with an overall available median EHR follow‐up of 10 years (IQR, 11 years). FH phenotype was identified using the EHR‐based algorithm in 32 613 individuals, which was 2.76% of the entire EHR cohort (definite, 0.03%; probable, 0.16%; possible, 2.55%) and constituted 13.7% of patients with hyperlipidemia (Figure 1).
Figure 1.
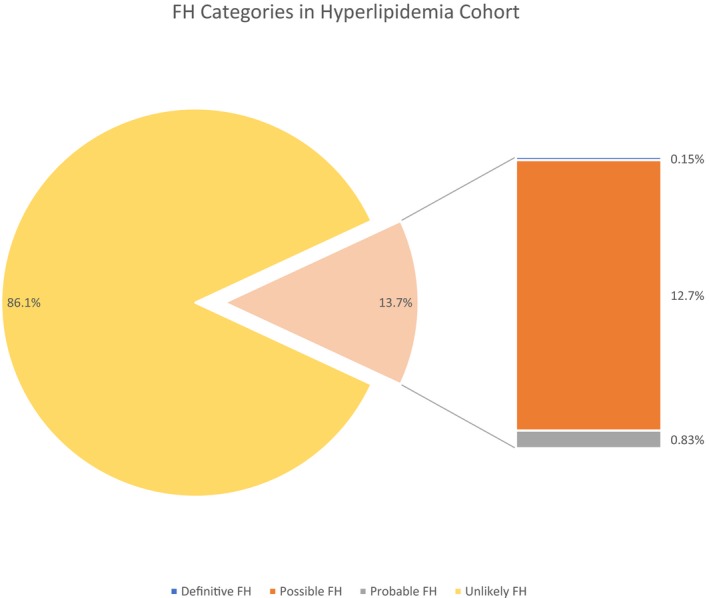
FH categories within hyperlipidemia cohort of 237 903 individuals. FH indicates familial hypercholesterolemia.
In univariate analysis, the FH cohort was noted to have a higher percentage of females (56.52% versus 48.51%; P<0.0001) and history of smoking (54.11% versus 50.73%; P<0.0001). Baseline demographic characteristics as well as use of medications and diagnostics tests in 2 groups are shown in Table 1. Deceased patients in the FH cohort were younger at the time of death compared with those in the non‐FH cohort (median, 70 years versus 75 years; P<0.0001). Cumulatively, a MACE event was observed at a younger age in the FH cohort (54 years versus 67 years; P<0.0001). Patients in the FH cohort experienced higher rate of specific MACE events, including myocardial infarction (14.77% versus 8.33%; P<0.0001) and heart failure (11.82% versus 10.50%; P<0.0001) (Table 2). Furthermore, total cholesterol, triglycerides, LDL‐C, and high‐density lipoprotein cholesterol levels were all higher in the FH cohort (Table 1). The FH cohort had a significantly higher median value of maximum LDL‐C levels (highest LDL‐C documented in EHR per patient) compared with that in non‐FH cohort (202 mg/dL versus 137 mg/dL; P<0.0001). Analysis of medication data revealed higher use of statins (79.10% versus 57.82%; P<0.0001), high‐potency statins (42.15% versus 19.49%; P<0.0001), and proprotein convertase subtilisin/kexin type 9 inhibitors (0.18% versus 0.02%; P<0.0001) in FH cohort compared with their non‐FH counterparts (Table 1). Overall, there were a total of 11.3% lost to follow‐up for MACE, and 12.5% lost to follow‐up for mortality. In the logistic regression model, by weighting the responses on the basis of their propensity scores (inverse probability of treatment weights), EHR FH correlated with MACE (incidence rate ratio, 4.02; 95% CI, 3.88–4.16; P<0.0001) and mortality (incidence rate ratio, 1.20; 95% CI, 1.15–1.26; P<0.0001) after adjusting for descriptive characteristics that differed between cohorts such as age, sex, smoking status, diabetes mellitus, hypertension, and maximum LDL (Figure 2).
Table 1.
Univariate Comparison of Baseline Characteristics Between FH and Non‐FH Hyperlipidemia Cohort
| FH Cohort | Non‐FH Cohort | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 32 613 | 13.71 | 205 290 | 86.29 | |
| Sex (male) | 14 179 | 43.48 | 105 696 | 51.49 | <0.0001 |
| Race (white, inclusive of Hispanic ethnicity) | 31 860 | 97.69 | 198 135 | 96.51 | <0.0001 |
| Smoking history (yes) | 17 647 | 54.11 | 104 132 | 50.73 | <0.0001 |
| Medications | |||||
| Statins | 25 796 | 79.10 | 118 688 | 57.82 | <0.0001 |
| High‐potency statinsa | 13 747 | 42.15 | 40 008 | 19.49 | <0.0001 |
| PCSK‐9 inhibitors | 59 | 0.18 | 51 | 0.02 | <0.0001 |
| β‐blockers | 13 288 | 40.74 | 68 062 | 33.15 | <0.0001 |
| Calcium channel blockers | 7490 | 22.97 | 44 186 | 21.52 | <0.0001 |
| ACE inhibitors | 14 034 | 43.03 | 79 250 | 38.60 | <0.0001 |
| Loop diuretics | 6349 | 19.47 | 35 288 | 17.19 | <0.0001 |
| Antiplatelets | 5837 | 17.90 | 22 431 | 10.93 | <0.0001 |
| Anticoagulants | 4712 | 14.45 | 28 585 | 13.92 | 0.01 |
| Diagnostic tests | |||||
| ECG | 23 740 | 72.79 | 132 775 | 64.69 | <0.0001 |
| Echocardiogram | 15 439 | 47.34 | 77 316 | 37.66 | <0.0001 |
| Median | IQR | Median | IQR | P Value | |
| Age | 61 | 17 | 63 | 19 | <0.0001 |
| Lipid profiles | |||||
| Total cholesterol | |||||
| Maximumb | 264 | 40 | 194 | 57 | <0.0001 |
| Averagec | 200 | 42 | 161 | 45 | <0.0001 |
| Deltad | 131 | 68 | 58 | 70 | <0.0001 |
| LDL cholesterol | |||||
| Maximumb | 202 | 27 | 137 | 49 | <0.0001 |
| Averagec | 148 | 35 | 110 | 38 | <0.0001 |
| Deltad | 111 | 61 | 42 | 59 | <0.0001 |
| HDL cholesterol | |||||
| Maximumb | 57 | 21 | 54 | 22 | <0.0001 |
| Averagec | 49 | 17 | 48 | 19 | <0.0001 |
| Deltad | 18 | 15 | 12 | 16 | <0.0001 |
| Triglycerides | |||||
| Maximumb | 237 | 163 | 181 | 144 | <0.0001 |
| Averagec | 171 | 105 | 143 | 100 | <0.0001 |
| Deltad | 133 | 142 | 75 | 122 | <0.0001 |
ACE indicates angiotensin‐converting enzyme; FH, familial hypercholesterolemia; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; PCSK‐9, proprotein convertase subtilisin/kexin type 9.
High potency is rosuvastatin ≥20 mg, atorvastatin ≥40 mg.
Maximum indicates highest value for a patient for the cholesterol type.
Average indicates (max+min)/2 for a patient for the cholesterol type.
Delta indicates difference between maximum and minimum values for a patient for the cholesterol type.
Table 2.
Univariate Comparison of Comorbidities Between FH and Non‐FH Cohort
| FH Cohort | Non‐FH Cohort | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 32 613 | 13.71 | 205 290 | 86.29 | |
| Hypertension | 21 318 | 65.37 | 133 137 | 64.85 | 0.07 |
| Renal disease | 5659 | 17.35 | 30 958 | 15.08 | <0.0001 |
| Cancer | 5938 | 18.21 | 37 488 | 18.26 | 0.82 |
| Angina | 2446 | 7.50 | 7354 | 3.58 | <0.0001 |
| Myocardial infarction | 4817 | 14.77 | 17 092 | 8.33 | <0.0001 |
| Heart failure | 3854 | 11.82 | 21 557 | 10.50 | <0.0001 |
| MACEa | 9202 | 28.22 | 40 832 | 19.89 | <0.0001 |
| Ischemic stroke | 2118 | 6.49 | 13 044 | 6.35 | 0.33 |
| Ventricular arrhythmias | 1701 | 5.22 | 9710 | 4.73 | 0.0001 |
| Percutaneous coronary intervention | 3086 | 9.46 | 8150 | 3.97 | <0.0001 |
| Coronary artery bypass grafting | 2488 | 7.63 | 10 427 | 5.08 | <0.0001 |
| Implantable cardioverter defibrillator | 577 | 1.77 | 2857 | 1.39 | <0.0001 |
FH indicates familial hypercholesterolemia; MACE, major adverse cardiovascular events.
MACE is a composite of death, myocardial infarction, percutaneous coronary interventions, and coronary artery bypass grafting.
Figure 2.
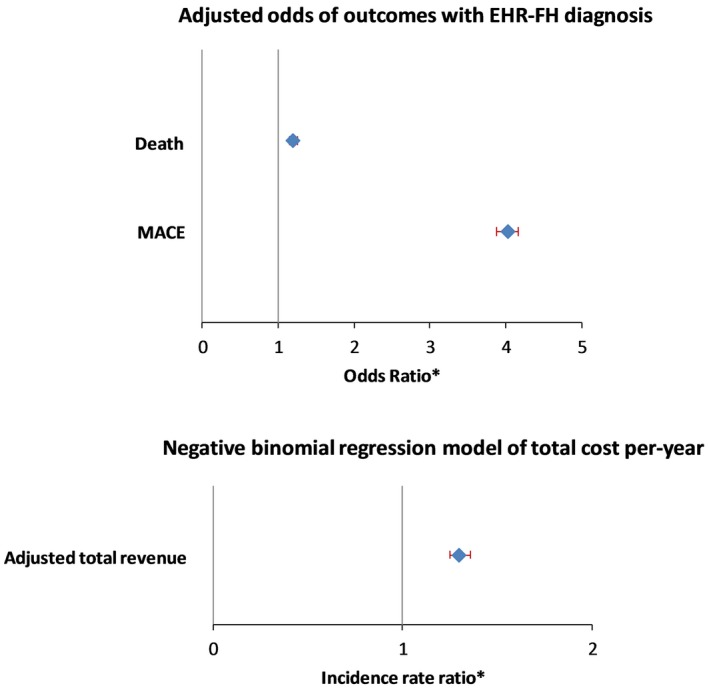
Multivariate models showing outcomes of MACE, mortality, and cost of care for FH diagnosis in the hyperlipidemia cohort. *Adjusted for age, sex, smoking, diabetes mellitus, hypertension and LDL (max). EHR indicates electronic health record; FH, familial hypercholesterolemia; IRR, incidence rate ratio; LDL, low‐density lipoprotein; MACE, major adverse cardiovascular events.
Cost Analysis
Using a complete case analysis methodology, inflation‐adjusted median annual revenue for the FH cohort was higher for each year from 2005 through 2015 (Table 3). The total follow‐up time for each patient during the study period for analysis (2005–2015) was used as an offset variable to normalize the total amount to a per‐year basis. The median follow‐up time was 11 years (IQR, 3 years) for the FH cohort and 10 years (IQR, 6 years) for the non‐FH cohort.
Table 3.
Cost of Care: Comparison of Yearly Revenue for the Years 2005–2015 (US$)
| Adjusted Revenue 2005–2015 | P Value | ||||
|---|---|---|---|---|---|
| FH | Non‐FH | ||||
| Median | IQR | Median | IQR | ||
| 2005 | 810 | 1914 | 687 | 1586 | <0.0001 |
| 2006 | 852 | 2048 | 724 | 1697 | <0.0001 |
| 2007 | 902 | 2186 | 752 | 1818 | <0.0001 |
| 2008 | 920 | 2210 | 790 | 1868 | <0.0001 |
| 2009 | 983 | 2461 | 847 | 2070 | <0.0001 |
| 2010 | 1043 | 2680 | 874 | 2184 | <0.0001 |
| 2011 | 1044 | 2627 | 867 | 2153 | <0.0001 |
| 2012 | 1063 | 2989 | 907 | 2439 | <0.0001 |
| 2013 | 1166 | 3370 | 974 | 2737 | <0.0001 |
| 2014 | 1294 | 4028 | 1093 | 3116 | <0.0001 |
| 2015 | 1307 | 3818 | 1005 | 2846 | <0.0001 |
| Total adjusted revenue (2005–2015) | 17 071 | 43 024 | 11 178 | 30 876 | <0.0001 |
| Med net revenue | |||||
| 2014 | 1026 | 2687 | 860 | 2152 | <0.0001 |
| 2015 | 1089 | 2788 | 850 | 2123 | <0.0001 |
FH indicates familial hypercholesterolemia; IQR, interquartile range.
In the negative binomial regression model, by including the total follow‐up time (defined as the years between first and last encounter over 2005–2015) as the offset variable to normalize the total adjusted revenue to a per‐year basis, the incidence rate ratio for the total revenue per year in the FH cohort was 1.30 (95% CI, 1.28–1.33; P<0.0001), after adjusting for age, sex, smoking, diabetes mellitus, hypertension, and maximum LDL‐C. There were no significant differences in baseline characteristics of patients with missing financial data compared with those for whom data were available.
Medicare rate‐adjusted median revenue for the FH cohort was 19% higher than the non‐FH cohort for the year 2014 ($1026 versus $860; P<0.0001) and 28% higher in 2015 ($1089 versus $850; P<0.0001). These trends were maintained when the analysis was repeated with exclusion of possible FH (Tables S8 and S9).
Discussion
Our study is one of few based on integrated health system data with population coverage of >3 million residents, including several areas designated as rural, with longitudinal follow‐up of >10 years. Using the modified DLCN criteria, an EHR‐based algorithm in our population was able to identify an FH cohort within an already high‐risk diagnosis of hyperlipidemia. Use of the modified DLCN criteria in this population‐based manner is methodology not unique to our study.10, 11 The designation of FH in the overall hyperlipidemia group had a prevalence of 13.7% and 2.7% of our entire EHR cohort. The prevalence of FH is variable4, 10, 12, 13 depending on the population studied and case definitions used, ranging from 1:137 (0.7%)10 to 1:250 (0.4%).3 The SEARCH (Screening Employees and Residents in the Community for Hypercholesterolemia) study by Safarova et al11 was based on 131 000 individuals seen in primary care practice and used a unique phenotyping algorithm using structured data and natural language processing for family history and presence of FH stigmata on physical examination for identification of FH in EHR and identified a prevalence of FH of 0.32%. This study comes the closest in methodology to our examination, but there are important differences. First, we used data from the entire spectrum of healthcare delivery systems, including outpatient as well as inpatient data, irrespective of the type of practice. Another notable distinction is our inclusion of “possible FH” in the FH cohort definition. Although this may lead to a degree of misclassification bias for FH (as evidenced by a total prevalence of 2.7% in the entire cohort), our approach was to be inclusive, as this strategy helped identify higher‐risk individuals within the larger EHR cohort. We felt that this was prudent, as the ability of EHR data alone to accurately differentiate between possible and probable FH may be limited. Furthermore, we believe that being inclusive better suits the objective to preemptively screen patients to identify those likely to be at higher risk who would be candidates for more intensive clinical phenotyping to confirm FH status. A combination of only definite and probable FH groups in our study would yield a prevalence of 0.19% in our entire EHR population and 0.98% in the hyperlipidemia cohort. We report a higher proportion of female subjects in our FH cohort as has been reported previously in a meta‐analysis of 6 large population‐based studies that included 37 889 patients.14
The EHR‐based FH designation in our study was associated with higher cholesterol levels and traditional comorbidities and correlated with MACE and all‐cause mortality. Benn et al10 examined the prevalence of FH and the risk of cardiovascular disease in a population of 69 016 individuals from the Danish general population and reported an odds ratio of 13.2 (95% CI, 10.0–17.4) for coronary artery disease for patients with FH not receiving lipid‐modifying therapies and 10.3 (95% CI, 7.8–13.8) for patients with FH receiving lipid‐modifying therapies, compared with patients without FH and not receiving lipid‐modifying therapies. Perak et al15 reported that the FH phenotype was associated with substantially elevated 30‐year coronary heart disease risk, with hazard ratios up to 5.0 (95% CI, 1.1–21.7). Similar patterns of results were found for total atherosclerotic cardiovascular disease risk, with hazard ratios up to 4.1 (95% CI, 1.2–13.4). The variable phenotypic expression of heterozygous FH is modulated in part by the underlying traditional cardiovascular risk factors,16 including obesity, diabetes mellitus, smoking, hypertension, male sex, and age, in addition to the risk associated with increased LDL‐C. Using an ICD‐based approach, we identified clinical outcomes that would represent the typical burden of atherosclerotic vascular disorder and combined them to define MACE for our study. To assess the utility of our EHR‐based FH assignment in correlating to outcomes, we decided a priori to adjust for traditional risk factors, namely, age, sex, smoking status, diabetes mellitus, hypertension, and maximum LDL‐C in our multivariate model. We observed odds ratios of 4.02 and 1.20 for MACE and mortality, respectively. These slightly lower odds ratios with regard to the studies cited above are expected, as our cohort is not derived from an FH disease‐specific database. More importantly, our comparison population also has hyperlipidemia, which constitutes a difference in methodology from the quoted studies and would lower the reported odds ratios.
Our purpose with this investigation was to show the viability of a large population‐based methodology to identify diverse FH phenotypes within an already high‐risk group of hyperlipidemias and show that such a methodology has consistency in identifying phenotypes associated with adverse outcomes and increased costs of care. We report on differences in death, ischemic heart disease, congestive heart failure, and implantable cardiac defibrillator use. While worse cardiac outcomes such as myocardial infarction have been reported in FH patients previously,17 the data on ischemic stroke have been variable.18 This more expansive assessment of comorbidities allowed us to attempt to explain the higher costs of care.
Only 79% of our EHR FH cohort was on statin treatment and only 42% on high‐potency statins. Undertreatment of FH as seen in our analysis has been widely reported previously.4, 19, 20, 21 These data again underline the role preemptive identification can play in early and adequate treatment of these individuals.
In our study, using revenue data, we determined that the FH designation was associated with consistently higher median costs of care across 12 years of longitudinal data. FH individuals can incur significant costs to the healthcare system over their lifetime attributable to premature atherosclerosis, and more so if not identified and treated.4 Prior studies exploring the economics of FH have been evaluations of screening strategies22 or therapeutic interventions,23 but less is known about the overall economic burden associated with FH. This is a needed area of research to support FH‐related economic evaluations. Our study provides evidence of higher total median costs (reflected in revenue) for FH patients almost twice that of their non‐FH hyperlipidemia counterparts. This differential is maintained across time and appears to be expanding. Using an inverse probability of treatment weights multivariable model, the EHR FH flag was associated with higher median total revenue per year (incidence rate ratio, 1.30; P<0.0001). These results add to current knowledge about higher morbidity and associated cost of care for this high‐risk population and can be helpful in studying the cost effectiveness of novel therapies. We believe that our approach is a novel way of assessing the hidden economic impact of FH in a hospital cohort and can be extrapolated to other health systems.
Cost‐effectiveness analyses of a targeted genetic (cascade) screening program for relatives of patients with FH have been extensively published previously.24, 25 However, the yield of an EHR‐based phenotyping algorithm to identify FH within the larger hyperlipidemia cohort as performed in our study is less common and could represent an important missed opportunity to use already available clinical data, which could then be advanced to the next level of targeted screening in the most cost‐effective manner. This potentially promising approach of using EHR‐based phenotyping to identify FH patients and then screen family members to efficiently achieve targeted population‐based FH screening is in need of additional evidence of effectiveness and costs for economic evaluation. Our approach is similar to that of the FH Foundation that recently launched the Find FH program, a machine‐learning algorithm used to identify individuals with probable FH using EHR data, laboratory results, and claims databases.26
Limitations
This is a retrospective observational study based on a population‐based cohort in a single healthcare system that is geographically limited and ethnically homogenous. Therefore, generalizability of our results to other, more diverse populations may be limited. However, generalizability of our methodology and approach to identify these high‐risk subjects is possible and desirable. Secondary to the retrospective nature of our study that used a preexisting general EHR, our FH phenotyping was likely affected by incomplete information regarding FH‐specific physical examination findings (in particular tendon xanthomas and arcus) and family history. These missing data may lead to an underestimate of the true prevalence of probable and definite FH in our population. Similarly, the use of EHR data to determine outcomes has its limitations regarding the potential for missing data, but manual review of the records validated that this approach was effective in our system. GHS may represent an ideal case in this regard given the high number of patients receiving most or all of their care at GHS. Finally, estimating the absolute increase in cardiovascular risk resulting from FH is complicated, as case ascertainment using healthcare system–based EHR data is likely to be biased toward patients experiencing symptoms and cardiovascular events.27 Nonetheless, this is the nature of the big‐data approach and is likely balanced by the real‐life pragmatic information being gathered by this methodology. Cost data were available for medical care utilization only within the GHS, and omitted medical care from other providers; however, it is unknown whether and the extent to which these additional services would change the relative cost outcomes.
Conclusion
Our study demonstrates a novel and pragmatic approach relying on standardized clinical criteria for identification of FH in a previously available EHR database. We were able to demonstrate the clinical significance of this approach by showing a statistically significant association between patients identified as having FH and adverse cardiovascular outcomes and higher costs of care, compared with those with hyperlipidemia not meeting the criteria for FH. The ever‐increasing use of EHR systems may broaden the appeal for such a population‐based approach to identify high‐risk patient groups, initiate guideline‐based interventions, and improve clinical outcomes.
Sources of Funding
This work was funded by internal faculty development grants from Geisinger Clinic, Danville, Pennsylvania.
Disclosures
None.
Supporting information
Table S1. Dutch Lipid Clinic Network Diagnostic Criteria for Familial Hypercholesterolemia*
Table S2. EHR‐Based Algorithm Based on Dutch Lipid Clinic Network Criteria to Identify Familial Hypercholesterolemia
Table S3. Count of Patients Meeting Each Definition
Table S4. PPV, NPV, Sensitivity, and Specificity to Definitions
Table S5. PPV, NPV, Sensitivity, and Specificity by FH Category
Table S6. Validation of DLCN‐Derived EHR FH Algorithm by Manual Chart Review
Table S7. International Classification of Diseases, Revision 9 Codes Used to Identify the Outcomes
Table S8. Cost Analysis—Definitive and Probable Categories Combined as FH and Compared With Unlikely (Possible Excluded)
Table S9. Cost Analysis—Definitive and Probable Combined as FH and Compared With Unlikely and Possible (Combined as Non‐FH)
Acknowledgments
Ms Kolinovsky and Drs Patel and Hu had full access to all the data in the study and take responsibility for its integrity and the data analysis.
(J Am Heart Assoc. 2019;8:e011822 DOI: 10.1161/JAHA.118.011822.)
References
- 1. Centers for Disease Control and Prevention . Available at: https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm. Accessed January 21, 2018.
- 2. Robinson JG, Goldberg AC. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S18–S29. [DOI] [PubMed] [Google Scholar]
- 3. de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016;133:1067–1072. [DOI] [PubMed] [Google Scholar]
- 4. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Borén J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg‐Hansen A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus Statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCrindle BW, Gidding SS. What should be the screening strategy for familial hypercholesterolemia? N Engl J Med. 2016;375:1685–1686. [DOI] [PubMed] [Google Scholar]
- 6. Norman R, Watts GF, Weintraub W, Gidding SS. Challenges in the health economics of familial hypercholesterolemia. Curr Opin Lipidol. 2016;27:563–569. [DOI] [PubMed] [Google Scholar]
- 7. Abul‐Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga‐Jauregui C, O'Dushlaine C, Leader JB, Lester Kirchner H, Lindbuchler DM, Barr ML, Giovanni MA, Ritchie MD, Overton JD, Reid JG, Metpally RP, Wardeh AH, Borecki IB, Yancopoulos GD, Baras A, Shuldiner AR, Gottesman O, Ledbetter DH, Carey DJ, Dewey FE, Murray MF. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016;354:aaf7000. [DOI] [PubMed] [Google Scholar]
- 8. Bureau of Labor Statistics Inflation Calculator. Available at: https://www.bls.gov/data/inflation_calculator.htm. Accessed December 15, 2017.
- 9. Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48:S114–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benn M, Watts GF, Tybjaerg‐Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol‐lowering medication. J Clin Endocrinol Metab. 2012;97:3956–3964. [DOI] [PubMed] [Google Scholar]
- 11. Safarova MS, Liu H, Kullo IJ. Rapid identification of familial hypercholesterolemia from electronic health records: the SEARCH study. J Clin Lipidol. 2016;10:1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lahtinen AM, Havulinna AS, Jula A, Salomaa V, Kontula K. Prevalence and clinical correlates of familial hypercholesterolemia founder mutations in the general population. Atherosclerosis. 2015;238:64–69. [DOI] [PubMed] [Google Scholar]
- 13. Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262–268. [DOI] [PubMed] [Google Scholar]
- 14. Pajak A, Szafraniec K, Polak M, Drygas W, Piotrowski W, Zdrojewski T, Jankowski P. Prevalence of familial hypercholesterolemia: a meta‐analysis of six large, observational, population‐based studies in Poland. Arch Med Sci. 2016;12:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd‐Jones DM. Long‐term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin MA, Hutter CM, Zimmern RL, Humphries SE. Familial hypercholesterolemia and coronary heart disease: a HuGE association review. Am J Epidemiol. 2004;160:421–429. [DOI] [PubMed] [Google Scholar]
- 17. Li S, Zhang Y, Zhu CG, Guo YL, Wu NQ, Gao Y, Qing P, Li XL, Sun J, Liu G, Dong Q, Xu RX, Cui CJ, Li JJ. Identification of familial hypercholesterolemia in patients with myocardial infarction: a Chinese cohort study. J Clin Lipidol. 2016;10:1344–1352. [DOI] [PubMed] [Google Scholar]
- 18. Huxley RR, Hawkins MH, Humphries SE, Karpe F, Neil HAW. Risk of fatal stroke in patients with treated familial hypercholesterolemia: a prospective registry study. Stroke. 2003;34:22–25. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins PN. Familial hypercholesterolemia—improving treatment and meeting guidelines. Int J Cardiol. 2003;89:13–23. [DOI] [PubMed] [Google Scholar]
- 20. Feldman DI, Blaha MJ, Santos RD, Jones SR, Blumenthal RS, Toth PP, Sperling LS, Martin SS. Recommendations for the management of patients with familial hypercholesterolemia. Curr Atheroscler Rep. 2015;17:473. [DOI] [PubMed] [Google Scholar]
- 21. Zafrir B, Jubran A, Lavie G, Halon DA, Flugelman MY, Shapira C. Clinical determinants and treatment gaps in familial hypercholesterolemia: data from a multi‐ethnic regional health service. Eur J Prev Cardiol. 2017;24:867–875. [DOI] [PubMed] [Google Scholar]
- 22. Nherera L, Marks D, Minhas R, Thorogood M, Humphries SE. Probabilistic cost‐effectiveness analysis of cascade screening for familial hypercholesterolaemia using alternative diagnostic and identification strategies. Heart. 2011;97:1175–1181. [DOI] [PubMed] [Google Scholar]
- 23. Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, van Hout B. Cost‐effectiveness of LDL‐C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliva J, López‐Bastida J, Moreno SG, Mata P, Alonso R. Cost‐effectiveness analysis of a genetic screening program in the close relatives of Spanish patients with familial hypercholesterolemia. Rev Esp Cardiol. 2009;62:57–65. [PubMed] [Google Scholar]
- 25. Marang‐van de Mheen PJ, ten Asbroek AH, Bonneux L, Bonsel GJ, Klazinga NS. Cost‐effectiveness of a family and DNA based screening programme on familial hypercholesterolaemia in the Netherlands. Eur Heart J. 2002;23:1922–1930. [DOI] [PubMed] [Google Scholar]
- 26. FIND FH Initiataive—The FH Foundation . Available at: https://thefhfoundation.org/find-fh-initiative. Accessed January 15, 2018.
- 27. Wong B, Kruse G, Kutikova L, Ray KK, Mata P, Bruckert E. Cardiovascular disease risk associated with familial hypercholesteolemia: a systematic review of the literature. Clin Ther. 2016;38:1696–1709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dutch Lipid Clinic Network Diagnostic Criteria for Familial Hypercholesterolemia*
Table S2. EHR‐Based Algorithm Based on Dutch Lipid Clinic Network Criteria to Identify Familial Hypercholesterolemia
Table S3. Count of Patients Meeting Each Definition
Table S4. PPV, NPV, Sensitivity, and Specificity to Definitions
Table S5. PPV, NPV, Sensitivity, and Specificity by FH Category
Table S6. Validation of DLCN‐Derived EHR FH Algorithm by Manual Chart Review
Table S7. International Classification of Diseases, Revision 9 Codes Used to Identify the Outcomes
Table S8. Cost Analysis—Definitive and Probable Categories Combined as FH and Compared With Unlikely (Possible Excluded)
Table S9. Cost Analysis—Definitive and Probable Combined as FH and Compared With Unlikely and Possible (Combined as Non‐FH)


