Abstract
Background
Homeless and vulnerably housed individuals are at increased risk for multimorbidity compared with the general population. We assessed prevalence of brain infarcts on neuroimaging and associations with vascular risk factors and cognitive performance in a prospective study of residents living in marginal housing.
Methods and Results
Two hundred twenty‐eight participants underwent structured clinical interviews, targeted clinical, laboratory, and neuropsychological assessments, and magnetic resonance imaging with T1, T2‐fluid‐attenuated inversion recovery and susceptibility‐weighted images. Subjects underwent cognitive testing to assess premorbid IQ, verbal learning and memory, inhibition, sustained attention, mental flexibility, and decision making. In this sample (mean age 44.0 years [SD 9.4], 77% male), prevalence of conventional vascular risk factors was lower than in the general population apart from tobacco use (94%). Ten‐year Framingham risk for any cardiovascular event was 11.4%±9.2%. Brain infarcts were present in 25/228 (11%). All were ischemic (40% cortical, 56% lacunar, 4% both). Participants with infarcts were older than those without (48.9±9.4 versus 43.4±9.2, P=0.006). In a multivariable regression analysis, only age remained a significant predictor of brain infarcts (odds ratio 1.08, 95% CI1.02–1.14, P=0.004). After controlling for age and education, the presence of infarct was a significant predictor of impaired decision making on the Iowa Gambling Task of decision making (β −28.2, 95% CI −42.7 to −14.1, P<0.001).
Conclusions
Prevalence of infarcts on neuroimaging in this disadvantaged, community‐dwelling cohort was much higher than expected for age and was associated with impaired decision making. Further research is needed to identify individuals at highest risk who may benefit from targeted preventative strategies.
Keywords: cognition, drug abuse, health disparities, homeless people, infarct or infarction
Subject Categories: Risk Factors, Cognitive Impairment, Magnetic Resonance Imaging (MRI), Epidemiology, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
In a younger (mean age 44 years) community‐dwelling cohort of participants living in marginal housing and without a major burden of conventional vascular risk factors, brain infarcts on baseline neuroimaging were highly prevalent (11%) and mostly (92%) in the absence of a self‐reported history of stroke.
After adjustment for age and education, infarcts on neuroimaging were associated with worse performance on the Iowa Gambling Task, a task of complex decision making and risk‐taking.
What Are the Clinical Implications?
Clinicians caring for individuals living in marginal housing or without a fixed address should be aware of the high prevalence of “silent” brain infarcts and their association with impaired decision making, and should advocate for housing and community services to help optimize access to preventative care.
Introduction
Socioeconomic disparities are associated with burden of vascular risk factors as well as stroke incidence, outcomes, and recurrence.1, 2, 3, 4 In high‐income nations, individuals who are homeless or living in marginal housing represent the most disadvantaged end of the socioeconomic spectrum. Homeless and vulnerably housed individuals may have reduced access to health care, and therefore may have suboptimal management of modifiable vascular risk factors.2 Furthermore, these individuals may have additional health issues, including substance use and dependence, mental illness, antipsychotic use, and chronic viral infections such as HIV and hepatitis. Previous studies examining the relationship between homelessness and vascular risk have yielded inconsistent results. Some reported an increased risk of future cardiovascular events,5 while others found that risk was comparable to that of the general population.6, 7
Single room occupancy (SRO) hotels are found in many North American urban centers. They are a form of marginal housing that is frequently subsidized in Canada, with low barriers to entry, and are often the only alternative to homelessness for individuals with very low incomes. Longitudinal health studies are more feasible with SRO residents than with homeless individuals. SRO residents have been shown to share a similar likelihood to homeless individuals8 of having unmet healthcare needs and high mortality rates.9 The Hotel study is a longitudinal investigation of multimorbidity in a cohort of SRO residents in the Downtown Eastside of Vancouver, Canada.10 This neighborhood has one of the lowest median incomes in Canada and is home to a vulnerable urban population.11 We explored the prevalence of brain infarcts on baseline neuroimaging and their association with measured vascular risk factors and cognitive performance. Based on any positive findings concerning infarcts, our goal was to develop hypotheses for future analyses or prospective studies, including the possibility of a comprehensive evaluation of small vessel disease. To our knowledge, there have been no other comparable cohorts of participants who are homeless or living in tentative housing that have included neuroimaging.
Methods
Study Population
All participants were adults over the age of 18 years and living in SRO hotels in Vancouver's Downtown Eastside. Staggered recruitment took place at 4 sites from November 2008 to July 2011. The study was approved by the Clinical Research Ethics Board at the University of British Columbia and Simon Fraser University in accordance with Canadian Tri‐Council policy. All participants provided written consent at enrollment. Baseline assessment methods were described previously.10 Briefly, upon recruitment, participants underwent a structured interview including demographic characteristics, general medical, psychiatric, social, and occupational history, including cardiovascular risk factors, prior stroke and lifetime substance exposure (including tobacco, alcohol, and prescribed as well as nonprescribed drugs and substance use). The Global Assessment of Function score was rated by a research assistant based on this interview. Participants were tested for HIV, hepatitis B virus and hepatitis C virus serology, hemoglobin A1C percentage, and LDL concentrations. Qualitative polymerase chain reaction was collected for those positive for the hepatitis C virus antibody. Height and weight were measured, and blood pressure was recorded 3 times via an automatic blood pressure measurement device with subjects in seated position, and the mean of these 3 measurements was used for further analysis.
Neuroimaging
The neuroimaging protocol is detailed in Table S1. T1, T2‐fluid‐attenuated inversion recovery, and susceptibility‐weighted images sequences were obtained on a Philips Achieva 3T MRI scanner (software Version 2.6.3.5). Software version and coil selection were maintained throughout the entire course of the study to ensure consistency of data quality.
Neurocognitive Assessments
Detailed neurocognitive assessment methodology has been previously published.12 Briefly, testing was carried out by trained research assistants supervised by a psychologist (A.E.T.). Tests included measures of estimated premorbid intelligence (Using Wechsler Test of Adult Reading), verbal learning and memory (Hopkins Verbal Learning Test Revised), inhibition (Stroop Color‐Word Test), sustained attention (Rapid Visual Information Processing subtest), mental flexibility (Intra‐Dimensional Extra‐Dimensional subtest, and decision making (Iowa Gambling Task [IGT]). Results from sessions closest in time to the date of baseline imaging were used for analysis. All subjects had neurocognitive testing within 1 year of their baseline scan. Cognitive testing occurred on the same day as neuroimaging in 89% of participants, and cognitive testing within 1 month of neuroimaging occurred in 98% of participants.
Analysis
The Framingham multiple risk factor equation was used to calculate 10‐year risk for vascular events (including coronary death, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral artery disease, and heart failure). Morbidity from self‐reported medical history questionnaires was used to generate a modified Charlson Comorbidity Index score (details in Data S1). Since brain infarction was the outcome of interest in our study, points on the Charlson Comorbidity Index given for previous stroke were removed to adjust for multimorbidity for subsequent analysis.
Scans were reviewed separately by a neuroradiologist (A.T.V.) and a team of 2 neurologists (T.S.F., W.J.P.). Infarct type (ischemic versus intracerebral hemorrhage) and location and morphology (lacunar, cortical, both, other, uncertain) were documented. Disagreements were identified in 7/228 subjects and settled by adjudication by an additional senior neuroradiologist (M.K.S.H.). For those study participants with infarcts or other findings of clinical significance on their neuroimaging, their family physicians received a letter from the study team recommending further clinical assessment as required.
Those with and without neuroradiological evidence of previous infarction were compared on vascular risk factor variables using t tests, Mann–Whitney U test, or χ2 tests as appropriate. A logistic regression model was constructed to examine the effects of variables identified as significant on univariable analyses (P<0.05) on the odds of having an infarct on neuroimaging. Multiple imputation using chained equations accounting for possible monotonicity was completed for missing values. Application of multiple imputation using chained equations mitigates statistical uncertainty within the imputations and allows for inclusion of continuous or binary data.12
A multivariable linear regression was used to explore the association between infarction on neuroimaging and cognitive performance, controlling for effects of age and education. Multimorbidity using the modified Charlson Comorbidity Index was also considered as a covariable for the model but was excluded because it was not significantly correlated with performance of any of the 5 tasks.
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 21 (Armonk, NY). Statistical tests were 2‐sided with significance level set at P<0.05 with no adjustments for multiple comparisons. Study data are available from the corresponding author upon reasonable request.
Results
Of 406 residents who were approached, 308 agreed to participate, and 228 completed baseline neuroimaging and were included in the present analysis (Table 1). Participants were predominantly male (77%) and mainly in young adulthood and middle age (Figure S1). Three‐quarters had a prior history of homelessness. Compared with the general Canadian population of working age, a much lower proportion had completed at least a high school education (25% versus 80%).13 Diagnosis of lifetime history of substance dependence was common, especially for injected drugs (82% with history of regular use) but also for alcohol (48%). A substantial proportion of the cohort had positive serology for HIV (17%), hepatitis B virus (38% core antibody positive), and hepatitis C virus (68%) infections.
Table 1.
Participant Demographics
| No Infarct on Imaging (N=203) | Infarct on Imaging (N=25) | Canadian Population 2011 | |
|---|---|---|---|
| Age (y) (missing=0) | |||
| Mean | 43.4 (SD 9.2) | 48.9 (SD 9.4) | |
| Range | 23.3–63.2 | 33–62.4 | |
| Sex (missing=0) | |||
| Male | 156/203 (77%) | 20/25 (80%) | 51% |
| Race‐ethnicitya (missing=0) | |||
| White | 118/203 (58%) | 15/25 (60%) | 67.3% |
| First Nations | 63/203 (31%) | 6/25 (24%) | 2.1% |
| First Nation Mixed | 12/203 (6%) | 1/25 (4%) | 0.9% |
| Other | 9/203 (4%) | 3/25 (12%) | 30.1% |
| Monthly income, Canadian $ (missing=4) | |||
| Median | $871 (SD 538) | $919 (SD 476) | $2150b |
| Range | $200–$5600 | $235–$2700 | |
| History of homelessness (missing=3) | |||
| Yes | 154/201 (76.6%) | 11/24 (46%) | |
| Total y of homeless (missing=4) | |||
| Mean | 3.4 (SD 5.4) | 1.8 (SD 3.16) | |
| Range | 0–39 y | 0–11.4 y | |
| Highest educational attainment (missing=0) | |||
| 0–8 y | 39/203 (19%) | 6/25 (24%) | 6.4% |
| 8–11 y | 112/203 (55%) | 14/25 (56%) | 13.4% |
| 12 y | 28/203 (14%) | 4/25 (16%) | 19.8% |
| >12 y | 24/203 (12%) | 1/25 (4%) | 60.4% |
| Body mass index (missing=4) | |||
| Mean | 22.8 (SD 4.2) | 23.6 (SD 3.6) | |
| ≥25 | 45/203 (22%) | 7/25 (28%) | 34.1% |
| ≥30 | 0 | 0 | 18.4% |
| HgA1c (missing=8) | |||
| ≥6.5% | 3/195 (1.5%) | 1/25 (4%) | |
| ≥7.0% | 2/195 (1%) | 1/25 (4%) | |
| Self‐reported diabetes mellitus (missing=8) | |||
| Yes | 7/196 (3.6%) | 3/24 (13%) | 6.1% |
| LDL cholesterol in mmol/L (missing=11) | |||
| Mean | 2.2 (SD 0.83) | 2.5 (SD 0.96) | |
| ≥2 mmol/L | 99/192 (52%) | 16/25 (64%) | |
| ≥3.5 mmol/L | 14/192 (7.3%) | 3/25 (12%) | |
| Self‐reported dyslipidemia (missing=9) | |||
| Yes | 3/195 (1.5%) | 1/24 (4.2%) | |
| Systolic blood pressure (missing=45) | |||
| Mean (mm Hg) | 115 (SD 13.8) | 117 (SD 13.1) | |
| ≥130 mm Hg | 22/164 (13.4%) | 3/19 (16%) | |
| ≥140 mm Hg | 8/164 (4.8%) | 1/19 (5%) | |
| Diastolic blood pressure (missing=45) | |||
| Mean (mm Hg) | 75 (SD 11.3) | 78 (SD 8.5) | |
| ≥80 mm Hg | 47/164 (25.7%) | 9/19 (47%) | |
| ≥90 mm Hg | 16/164 (8.7%) | 2/19 (10.5%) | |
| Self‐reported hypertension (missing=9) | |||
| Yes | 12/199 (6%) | 4/25 (16%) | 17.6% |
| Pack y (missing=1) | |||
| Mean | 19.6 (SD 15.9) | 27.8 (SD 26.6) | |
| Range | 0–111 | 0–109 | |
| >10 pack‐y | 138/202 (68%) | 19/25 (76%) | |
| Active smoker (missing=1) | |||
| Yes | 191/202 (94.6%) | 22/25 (88%) | 19.9% |
| History of regular use of injected drugs (missing=1) | |||
| Yes | 165/202 (81.7%) | 22/25 (88%) | |
| History of alcohol dependence (missing=1) | |||
| Yes | 95/202 (47%) | 15/25 (60%) | |
| History of marijuana dependence (missing=1) | |||
| Yes | 92/202 (46%) | 11/25 (44%) | |
| HIV status (missing=0) | |||
| HIV not on ARV | 13/203 (6.4%) | 1/25 (4%) | |
| HIV on ARV | 22/203 (11.3%) | 3/25 (8%) | |
| Hepatitis C virus status (missing =9) | |||
| Ab pos, PCR neg | 35/195 (18%) | 3/24 (13%) | |
| PCR positive | 99/195 (51%) | 13/24 (54%) | |
| Hepatitis B virus status (missing=3) | |||
| Core ab positive | 74/200 (38%) | 12/25 (48%) | |
| Surface ag positive | 3/200 (1.5%) | 0/25 | |
| Modified Charlson score (missing=0) | |||
| Mean | 3.47 (SD 2.99) | 3.44 (SD 2.79) | |
| 0 | 35/203 (17%) | 3/25 (12%) | |
| ≥5 | 60/203 (30%) | 7/25 (28%) | |
| Global assessment of function (missing=0) | |||
| Mean | 38 (SD 35) | 35 (10.2) | |
| Range | 15–70 | 19–58 | |
| Estimate of IQ (WTARb) (missing=5) | |||
| Mean | 96 (SD 8.6) | 97 (SD 9.9) | |
| Range | 75–122 | 78–113 | |
| 10‐y Framingham CVD (excluded 23 for age ≤30 y, missing component 38) | |||
| Mean | 10.9 (SD 8.5%) | 15.4 (SD 13.0%) | |
| <10% | 92/148 (62%) | 8/19 (42%) | |
| 10%–20% | 56/148 (38%) | 11/19 (58%) | |
| >20% | 22/148 (15%) | 5/19 (26%) | |
| Range | 1.2%–44.4% | 1.7%–48.9% | |
HgA1c indicates hemoglobin A1c; ARV, antiretroviral drugs; CVD, cardiovascular disease; PCR, polymerase chain reaction; WTAR, Wechsler Test of Adult Reading.
Reported for Vancouver, BC; proportions of individuals by ethnicity are highly variable between different Canadian cities.
Based on annual income of unattached individuals.
Conventional vascular risk factors including hypertension, diabetes mellitus, and obesity were lower than in the general Canadian population. Rates of tobacco smoking, however, were very high at 94%. Detailed age‐specific rates of morbidity for these conditions for the general population were not available from Statistics Canada to calculate age‐ and sex‐standardized expected prevalence and a meaningful standardized morbidity ratio. In those 167 participants with complete data for a Framingham risk calculation (also excluding participants under the age of 30 years, for whom the equation was not validated), the mean 10‐year risk of a cardiovascular event was estimated to be 11.4%±9.2% (range 1.2–48.9%). The median modified Charlson Comorbidity Index score was 3 (mean 3.43, SD 2.95, range 0–11) with 39% of participants having a score ≥5. The median Global Assessment of Function score for this cohort was 35, commensurate with major impairment in several spheres, such as judgment, thinking, mood, or interpersonal relationships (mean 38, SD 11, range 15–70).
Prevalence of infarction on brain magnetic resonance imaging (MRI) was 11% (Table 2). This was more than double the rate of self‐reported history of prior stroke (4%). All infarcts were ischemic (Figure). Cortical morphology was present in 36% and 56% met Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria14 for lacunar infarction. Details on infarct location are available in Table S2. Comparing participants with lacunar to cortical infarcts, there tended to be a longer history of intravenous drug use in those with lacunar infarcts (Table 3) with no significant differences in other classes of substance use.
Table 2.
Prevalence of Infarcts on MRI
| Infarct on Baseline Imaging | No Infarct | |
|---|---|---|
| Self‐reported history of stroke | 2 | 7 |
| No reported history of stroke | 23 | 193 |
| Missing | 0 | 3 |
MRI indicates magnetic resonance imaging.
Figure 1.
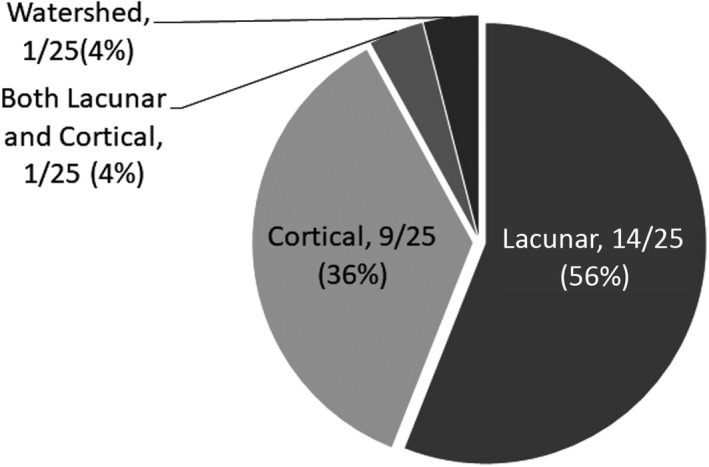
Infarct morphology.
Table 3.
Substance Use and Infarct Morphology
| Years of Substance Use | |||||||
|---|---|---|---|---|---|---|---|
| Alcohol | Cannabis | Cocaine | Amphetamine | Hallucinogen | Opiate | IVDUa | |
| Cortical (n=9) | |||||||
| Median | 5 | 10 | 7.5 | 0.5 | 0 | 0 | 3.5 |
| Mean | 9.50 | 13.78 | 9.94 | 4.50 | 1.86 | 3.94 | 5.38 |
| Lacunar (n=14) | |||||||
| Median | 20 | 4.5 | 10 | 3.5 | 0 | 6 | 20 |
| Mean | 18.09 | 8.79 | 14.45 | 4.65 | 0.50 | 12.75 | 20.36 |
| P value of Mann–Whitney U test | 0.364 | 0.734 | 0.904 | 0.962 | 0.837 | 0.11 | 0.041 |
IVDU indicates intravenous drug use.
Participants with infarcts were older compared with those without (48.9±9.4 versus 43.4±9.2 years, P=0.006) and had a shorter duration of total homelessness (1.84±3.16 versus 3.39±5.44 years, P=0.019). No other variables differed significantly between the 2 groups (Table 4). Multiple imputations for missing values did not change our results. Age and duration of homelessness were not significantly correlated. With multivariable logistic regression, only age remained a significant predictor of infarcts seen on baseline imaging (odds ratio 1.08, 95% CI 1.02–1.14, P=0.004) (Table S3).
Table 4.
Univariate Risk Factor Comparisons
| Variable | No Infarct on Imaging | Infarct on Imaging | Univariate Comparisons | |
|---|---|---|---|---|
| (N=203) | (N=25) | |||
| Age (missing =0)a | Mean (y) | 43.4 (SD 9.2) | 48.9 (SD 9.4) | T Score=−2.818, P=0.005 |
| Sex (missing=0)b | ||||
| Male | 156/203 (77%) | 20/25 (80%) | χ2=0.126, P=0.719 | |
| Race‐ethnicity (missing=0)b | ||||
| White | 118/203 (58%) | 15/25 (60%) | χ2=2.929, P=0.491 | |
| First Nations | 63/203 (31%) | 6/25 (24%) | ||
| First Nation mixed | 12/203 (6%) | 1/25 (4%) | ||
| Other | 9/203 (4%) | 3/25 (12%) | ||
| Total y of homeless (missing=4)c | Median | 1.3 (IQR 5) | 0 (IQR 2) | Z score=−2.341, P=0.019 |
| Highest educational attainment (missing=0)c | Median | 10 (IQR 3) | 10 (IQR 3) | Z score=−0.981, P=0.326 |
| Body mass index (missing=4)c | Median | 22.2 (IQR 4.1) | 22.9 (4.7) | Z score=−1.288, P=0.198 |
| HgA1c (missing=8)c | Median | 5.5 (IQR 0.5) | 5.4 (IQR 0.6) | Z score=−0.117, P=0.907 |
| LDL cholesterol (missing=11)c | Median (mmol/L) | 2.1 (SD 1.1) | 2.5 (IQR 1.3) | Z score=−1.612, P=0.107 |
| Systolic blood pressure (missing=45)c | Median (mm Hg) | 114 (IQR 18) | 121 (IQR 16) | Z score=−1.103, P=0.270 |
| Diastolic blood pressure (missing=45)c | Median (mm Hg) | 75 (IQR 12) | 81 (IQR 13) | Z score=−1.265, P=0.206 |
| Pack y (missing=1)c | Median | 17.0 (IQR 22.5) | 16.8 (IQR 17.9) | Z score=−1.198, P=0.231 |
| History of regular use of injected drugs (missing=1)b | Yes | 165/202 (81.7%) | 22/25 (88%) | χ2=0.737, P=0.638 |
| History of alcohol dependence (missing=1)b | Yes | 95/202 (47%) | 15/25 (60%) | χ2=1.628, P=0.420 |
| History of marijuana dependence (missing=1)b | Yes | 92/202 (46%) | 11/25 (44%) | χ2=0.145, P=0.881 |
| HIV status (missing=0)b | ||||
| HIV not on ARV | 13/203 (6.4%) | 1/25 (4%) | χ2=0.241, P=0.875 | |
| HIV on ARV | 22/203 (11.3%) | 3/25 (8%) | ||
| HCV status (missing =9)b | ||||
| Ab pos, PCR neg | 35/195 (18%) | 3/24 (13%) | χ2=0.442, P=0.924 | |
| PCR pos | 99/195 (51%) | 13/24 (54%) | ||
| HBV status (missing=3)b | ||||
| Core ab positive | 74/200 (38%) | 12/25 (48%) | χ2=1.801, P=0.491 | |
| Surface ag positive | 3/200 (1.5%) | 0/25 | ||
| Modified Charlson Score (missing=0)c | Median | 3 (IQR 4) | 4 (IQR 5) | Z score=−0.264, P=0.792 |
HgA1c indicates hemoglobin A1c; ARV, antiretroviral drugs; IQR, interquartile range; HBV, hepatitis B virus; HCV, hepatitis C virus; PCR, polymerase chain reaction.
T scores; independent samples t test.
Z scores; Mann–Whitney U test for continuous variable felt to have significant deviations from normal distribution.
χ2 for comparison of frequencies.
Performance on a task of decision making (IGT) was significantly poorer in those with infarcts after controlling for effects of age and education (β −28.2, 95% CI −42.7 to −14.1, P<0.001, ΔR2 −0.072). IGT was also the only task for which the combined model of age and education alone was not significantly predictive of performance (Table 5).
Table 5.
Neurocognitive Testing
| Stroop | Delayed Hopkins Verbal Learning Test | Rapid Visual Information Processing | Iowa Gambling Task | Log Transformed Intra‐Dimensional Extra‐Dimensional Set Shift | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infarct − | Infarct + | − | + | − | + | − | + | − | + | |
| N | 193 | 24 | 198 | 24 | 186 | 24 | 180 | 23 | 193 | 24 |
| Mean | 35.99 | 31.83 | 6.09 | 5.96 | 0.87 | 0.84 | −1.50 | −30.70 | −1.58 | −1.73 |
| SD | 10.01 | 10.99 | 2.85 | 3.11 | 0.06 | 0.06 | 32.36 | 24.92 | 0.38 | 0.28 |
| Model 1 R 2 with age and education | 0.099 | 0.054 (0.0537) | 0.056 | 0.012 | 0.116 | |||||
| Model 2 R 2 with age, education, and infarct | 0.104 | 0.054 (0.0544) | 0.064 | 0.084 | 0.121 | |||||
| ∆R 2 With stroke effect | 0.005 | 0.0007 | 0.009 | 0.072 | 0.004 | |||||
| ∆R 2 P valuea | 0.291 | 0.690 | 0.167 | <0.001 | 0.300 | |||||
| Age | B=−0.226 (95% CI −0.371 to −0.081), P=0.002 | B=−0.054 (95% CI −0.095 to −0.014), P=0.009 | B=−0.001 (95% CI −0.002 to 0.000), P=0.020 | B=−0.122 (95% CI −0.600 to 0.356), P=0.616 | B=−0.011 (95% CI −0.016 to −0.006), P<0.001 | |||||
| Education | B=1.094 (95% CI 0.493 to 1.694), P<0.001 | B=−0.204 (95% CI −0.039 to 0.369), P=0.016 | B=0.004 (95% CI 0.001 to 0.007), P=0.024 | B=0.900 (95% CI −1.051 to 2.851), P=0.364 | B=0.032 (95% CI 0.011 to 0.054), P=0.003 | |||||
| Presence of infarct | B=−2.278 (95% CI −6.518 to 1.963), P=0.291 | B=0.247 (95% CI −0.974 to 1.469), P=0.690 | B=−0.018 (95% CI −0.043 to 0.008), P=0.167 | B=−28.204 (95% CI −42.270 to −14.138), P<0.001 | B=−0.081 (95% CI −0.235 to 0.073), P=0.300 | |||||
Adjusted for age and education.
Discussion
Within this socioeconomically disadvantaged, community‐dwelling cohort of relatively young age (mean age 44.0 ±9.4 years), there was a very high prevalence of chronic ischemic infarcts (11%) found on baseline MRI. Of the 25 patients with infarcts on neuroimaging, 23 did not report a history of stroke. Silent brain infarcts (SBI) are associated with cognitive decline15, 16 and increased risk of recurrent stroke.17, 18 The prevalence of SBI on MRI in community‐dwelling cohorts varies widely based on age and geography, in addition to imaging modality.19 In a Canadian population‐based neuroimaging cohort, 2.8% of 40‐ to 49‐year‐olds and 5.9% of 50‐ to 59‐year‐olds had silent brain infarcts on MRI.20 Within a Korean cohort of healthy volunteers of an age similar to our cohort (mean age of 49.0±7.7 years), prevalence of SBI on MRI was 5.8%.21 A recent meta‐analysis found an SBI prevalence in community‐dwelling cohorts (mean age 50–55 years) of 5%.19 In contrast to community‐based cohorts with symptomatic stroke, where cardioembolic and large artery cortical infarcts are each as common as lacunar infarcts,22, 23 asymptomatic lacunar infarcts are the most frequent subtype of SBI.
The majority of infarcts in this cohort were also lacunar. Lacunar infarcts are generally caused either by intracranial large artery disease occluding a penetrating artery, or occlusion of a single penetrating artery by microatheroma or lipohyalinosis.24 Conventional vascular risk factors such as hypertension, diabetes mellitus, dyslipidemia, and smoking are associated with increased risk of both lacunar and atheroembolic stroke.25 However, similar to a study of homeless participants from Toronto,6 the only traditional vascular risk factor more common in this cohort compared with the Canadian general population was tobacco use. The mean calculated 10‐year Framingham risk for cardiovascular disease in this study population was 11.4%, compared with the general Canadian population at 8.9%.26 However, this metric likely underestimates vascular risk for this cohort because it does not account for risks associated with other factors, such as chronic infection, injection drug use, and social determinants of cardiovascular health.
Given the high prevalence of injection drug use in this cohort and secondary risk of endocarditis, one explanation for the high prevalence of lacunar over cortical infarcts may be the “healthy survivor” effect. Cortical strokes may be more debilitating than lacunar events. Individuals with significant poststroke disability would be unable to live independently in a SRO. This explanation may also apply to the increased prevalence of infarcts in those with shorter duration of homelessness (a major risk factor for early mortality),27 and may also explain why no intracerebral hemorrhages, which are more disabling than ischemic strokes,28 were observed in this cohort. In contrast, intracerebral hemorrhages and cerebral microbleeds, a marker of intracerebral hemorrhage risk, were found to be highly prevalent in a Boston‐based inner‐city cohort of young stroke patients with a median age of 44 years, similar to our population.29
Cognitive impairment is common both in homeless populations, and after stroke. One recent meta‐analysis estimated the prevalence of cognitive impairment among homeless cohorts to be 25%,30 with some studies reporting much higher prevalences, up to 72%.31 This study used a battery of neurocognitive tasks that offer a more comprehensive assessment than the Montreal Cognitive Assessment or Mini‐Mental State Examination, which are screening measures limited by ceiling effects. Interestingly, presence of an infarct on MRI was not associated with poorer performance on verbal learning and memory, inhibition, sustained attention, or mental flexibility but did significantly impact performance on a relatively complex decision making task involving risk assessment. In our cohort, IGT performance overall was poor in participants both with and without infarcts, but those with infarcts demonstrated an increased preference for “risky” decks compared with safe ones. Impaired performance on the IGT has been associated both with poverty32, 33 as well as history of substance use disorders.34 There are little data on IGT testing after stroke, but 1 small study found that subjects with frontal infarcts showed a persistent preference for riskier decks compared with healthy controls, where as those with cerebellar infarcts did not.35 The relatively few observations within this cohort did not allow us to examine for the effect of location, laterality, or stroke mechanism. Further research with robust poststroke data is needed to interpret the clinical significance of our findings on cognitive tasks. Multiple additional structural brain factors beyond infarcts may modify cognitive performance in this cohort. The relationship between burden of white matter hyperintensities and other markers of cerebral small vessel disease are associated with cognitive performance36 and are being characterized in our study cohort. We have previously shown that other neuroimaging findings in this study population, such as white matter diffusion alterations37 as well as congenital anomalies,38 are also associated with cognitive performance.
Our study has limitations. First, participation in the Hotel study is voluntary, and the demographics of our cohort may not fully reflect that of all SRO residents in the DTES (downtown Eastside of Vancouver). Like other voluntary community‐dwelling cohorts, there is potentially “healthy participant” bias and the burden of cerebrovascular disease measured may be an underestimate of the true prevalence. The initial response rate of all residents approached was 76%, but 80 of those 308 participants did not complete baseline imaging for a variety of reasons including contraindications for MRI (extracranial metal detected by x‐ray, intracranial metal because of previous neurosurgery), movement disorder too severe to allow imaging, death, and dropout before baseline imaging. Despite the extensive involvement required of participants and the additional complexities of arranging neuroimaging in a cohort with tentative housing, a final participation rate of 56% is comparable to that of other contemporary voluntary community‐based cohorts.39, 40, 41, 42 Of interest, many long‐standing studies such as the Behavioral Risk Factor Surveillance Survey have reported a declining trend of participation since the time of their establishment, with 2017 response rates close to 45%43 compared with 71% in 1993.44 It is particularly important to be mindful of this declining participant rate and possible variable impact of the “healthy participant” bias when interpreting epidemiological trends across time. Reassuringly, the baseline demographics of our study is similar to those reported in other studies of SRO residents on Vancouver's Downtown Eastside (Table S4)45, 46, 47, 48 and other vulnerably housed and homeless participants in other Canadian cities (Table S5) conducted within the same time frame.47, 48
Second, our cohort may be underpowered to show significant differences in other risk factors in participants with and without brain infarcts. Furthermore, the number of infarcts observed in the cohort did not allow for sufficient power to properly explore interactions that may have impacted the relationship between cognitive performance and presence of infarction, such as age and educational attainment. Next, because this was a retrospective analysis, some relevant information related to risk factor assessment for stroke and SBI, including vascular imaging and information on high‐risk cardioembolic sources, was not captured. Last, the demographic characteristics of Vancouver's SRO population may differ from that of other vulnerably housed North American cohorts, potentially limiting generalizability.
Conclusion
The prevalence of brain infarcts on baseline neuroimaging was 11% in a cohort of residents of marginal housing within Vancouver, with 92% of the lesions representing SBIs. The prevalence of brain infarction in our cohort is far in excess of those in healthy community‐dwelling cohorts of older individuals. Those with infarcts showed worse performance on a task of complex decision making. Our findings suggest that vulnerably housed individuals, despite having low rates of conventional modifiable vascular risk factors, are a high‐risk group for stroke in need of targeted preventative health strategies.
Sources of Funding
This work was supported by Canadian Institutes for Health Research (CBG‐101827, MOP‐137103), and the British Columbia Mental Health and Substance Use Services (an Agency of the Provincial Health Services Authority). TSF is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada, a Clinician‐Scientist Award from the Vancouver Coastal Health Research Institute, and a Michael Smith Health Professional Scholar Award. WGH is supported by the Jack Bell Chair in Schizophrenia.
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. Neuroimaging Protocol
Table S2. Infarct Location
Table S3. Logistic Regression Model
Table S4. Demographics in Vancouver Studies of Vulnerably Housed and Homeless Participants
Table S5. Demographics in Cross‐Canada Studies of Vulnerably Housed and Homeless Participants
Figure S1. Age distribution of participants.
Acknowledgments
The authors are grateful for the outstanding support of the Hotel study team, and thank the participants for their invaluable contributions. Additionally, we would like to thank Zoe O'Neill and Thomas Soroski for their help in organizing the supplemental materials.
(J Am Heart Assoc. 2019;8:e011412 DOI: 10.1161/JAHA.118.011412.)
References
- 1. Choiniere R, Lafontaine P, Edwards AC. Distribution of cardiovascular disease risk factors by socioeconomic status among Canadian adults. CMAJ. 2000;162:S13–S24. [PMC free article] [PubMed] [Google Scholar]
- 2. Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, Wolfe CDA, McKevitt C. Socioeconomic status and stroke: an updated review. Stroke. 2012;43:1186–1191. [DOI] [PubMed] [Google Scholar]
- 3. Pennlert J, Asplund K, Glader EL, Norrving B, Eriksson M. Socioeconomic status and the risk of stroke recurrence: persisting gaps observed in a nationwide Swedish study 2001 to 2012. Stroke. 2017;48:1518–1523. [DOI] [PubMed] [Google Scholar]
- 4. Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015;14:1206–1218. [DOI] [PubMed] [Google Scholar]
- 5. Gozdzik A, Salehi R, O'Campo P, Stergiopoulos V, Hwang SW. Cardiovascular risk factors and 30‐year cardiovascular risk in homeless adults with mental illness. BMC Public Health. 2015;15:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee TC, Hanlon JG, Ben‐David J, Booth GL, Cantor WJ, Connelly PW, Hwang SW. Risk factors for cardiovascular disease in homeless adults. Circulation. 2005;111:2629–2635. [DOI] [PubMed] [Google Scholar]
- 7. Kim DH, Daskalakis C, Plumb JD, Adams S, Brawer R, Orr N, Hawthorne K, Toto EC, Whellan DJ. Modifiable cardiovascular risk factors among individuals in low socioeconomic communities and homeless shelters. Fam Community Health. 2008;31:269–280. [DOI] [PubMed] [Google Scholar]
- 8. Argintaru N, Chambers C, Gogosis E, Farrell S, Palepu A, Klodawsky F, Hwang SW. A cross‐sectional observational study of unmet health needs among homeless and vulnerably housed adults in three Canadian cities. BMC Public Health. 2013;13:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Honer WG, Cervantes‐Larios A, Jones AA, Vila‐Rodriguez F, Montaner JS, Tran H, Nham J, Panenka WJ, Lang DJ, Thornton AE, Vertinsky T, Barr AM, Procyshyn RM, Smith GN, Buchanan T, Krajden M, Krausz M, MacEwan GW, Gicas KM, Leonova O, Langheimer V, Rauscher A, Schultz K. The hotel study‐clinical and health service effectiveness in a cohort of homeless or marginally housed persons. Can J Psychiatry. 2017;62:482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vila‐Rodriguez F, Panenka WJ, Lang DJ, Thornton AE, Vertinsky T, Wong H, Barr AM, Procyshyn RM, Sidhu JJ, Smith GN, Buchanan T, Krajden M, Krausz M, Montaner JS, William MacEwan G, Honer WG. The hotel study: multimorbidity in a community sample living in marginal housing. Am J Psychiatry. 2013;170:1413–1422. [DOI] [PubMed] [Google Scholar]
- 11. Krausz M, Jang K. Lessons from the creation of Canada's poorest postal code. Lancet Psychiatry. 2015;2:e5. [DOI] [PubMed] [Google Scholar]
- 12. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Statistics Canada . Table 8: Educational attainment of working‐age population, 2006 to 2016. 2017; Catalogue No. 12‐581‐X.
- 14. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw F‐E, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, van Oostenbrugge R, Pantoni L, Speck O, Stephan BCM, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 16. Longstreth WT Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ Jr, O'Leary D, Carr J, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the cardiovascular health study. Stroke. 2002;33:2376–2382. [DOI] [PubMed] [Google Scholar]
- 17. Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, Wright C, Beiser AS, Seshadri S, Pandya A, Kamel H. Silent brain infarction and risk of future stroke: a systematic review and meta‐analysis. Stroke. 2016;47:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coutts SB, Eliasziw M, Hill MD, Scott JN, Subramaniam S, Buchan AM, Demchuk AM. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke. 2008;3:3–10. [DOI] [PubMed] [Google Scholar]
- 19. Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population‐based cohorts. BMC Med. 2014;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith EE, O'Donnell M, Dagenais G, Lear SA, Wielgosz A, Sharma M, Poirier P, Stotts G, Black SE, Strother S, Noseworthy MD, Benavente O, Modi J, Goyal M, Batool S, Sanchez K, Hill V, McCreary CR, Frayne R, Islam S, DeJesus J, Rangarajan S, Teo K, Yusuf S; PURE Investigators . Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol. 2015;77:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee SC, Park SJ, Ki HK, Gwon HC, Chung CS, Byun HS, Shin KJ, Shin MH, Lee WR. Prevalence and risk factors of silent cerebral infarction in apparently normal adults. Hypertension. 2000;36:73–77. [DOI] [PubMed] [Google Scholar]
- 22. Rincon F, Sacco RL, Kranwinkel G, Xu Q, Paik MC, Boden‐Albala B, Elkind MSV. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan stroke study. Cerebrovasc Dis. 2009;28:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Androulakis XM, Kodumuri N, Giamberardino LD, Rosamond WD, Gottesman RF, Yim E, Sen S. Ischemic stroke subtypes and migraine with visual aura in the ARIC study. Neurology. 2016;87:2527–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arboix A, Blanco‐Rojas L, Marti‐Vilalta JL. Advancements in understanding the mechanisms of symptomatic lacunar ischemic stroke: translation of knowledge to prevention strategies. Expert Rev Neurother. 2014;14:261–276. [DOI] [PubMed] [Google Scholar]
- 25. Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, Anderson CS, Hankey GJ, Jamrozik K, Appelros P, Sudlow CLM. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–629. [DOI] [PubMed] [Google Scholar]
- 26. Statistics Canada . Prevalence of cardiovascular disease (CVD) risk, by sex, age and cardiovascular risk factors, household population aged 20 to 79, Canada excluding territories, 2007 to 2011Population health impact of statin treatment in Canada. Health reports. 2016; Catalogue no. 82‐003‐X Statistics Canada ISSN 1209‐1367.
- 27. Hwang SW, Wilkins R, Tjepkema M, O'Campo PJ, Dunn JR. Mortality among residents of shelters, rooming houses, and hotels in Canada: 11 year follow‐up study. BMJ. 2009;339:b4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lekander I, Willers C, von Euler M, Lilja M, Sunnerhagen KS, Pessah‐Rasmussen H, Borgström F. Relationship between functional disability and costs one and two years post stroke. PLoS One. 2017;12:e0174861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shoamanesh A, Catanese L, Romero JR, Lau H, Babikian VL, Benavente OR, Kase CS, Pikula A. High prevalence of cerebral microbleeds in inner city young stroke patients. J Stroke Cerebrovasc Dis. 2016;25:733–738. [DOI] [PubMed] [Google Scholar]
- 30. Depp CA, Vella L, Orff HJ, Twamley EW. A quantitative review of cognitive functioning in homeless adults. J Nerv Ment Dis. 2015;203:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stergiopoulos V, Cusi A, Bekele T, Skosireva A, Latimer E, Schutz C, Fernando I, Rourke SB. Neurocognitive impairment in a large sample of homeless adults with mental illness. Acta Psychiatr Scand. 2015;131:256–268. [DOI] [PubMed] [Google Scholar]
- 32. Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. [DOI] [PubMed] [Google Scholar]
- 33. Ursache A, Raver CC. Iowa gambling task performance and executive function predict low‐income urban preadolescents’ risky behaviors. Pers Individ Dif. 2015;79:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barry D, Petry NM. Predictors of decision‐making on the Iowa gambling task: independent effects of lifetime history of substance use disorders and performance on the trail making test. Brain Cogn. 2008;66:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cardoso Cde O, Branco LD, Cotrena C, Kristensen CH, Schneider Bakos DD, Fonseca RP. The impact of frontal and cerebellar lesions on decision making: evidence from the Iowa gambling task. Front Neurosci. 2014;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, Redmond P, Starr JM, Deary IJ, Wardlaw JM. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gicas KM, Cheng A, Rawtaer I, Willi TS, Panenka WJ, Lang DJ, Smith GN, Vila‐Rodriguez F, Leonova O, Giesbrecht CJ, Jones AA, Barr AM, Procyshyn RM, Buchanan T, William MacEwan G, Su W, Vertinsky AT, Rauscher A, O'Rourke N, Thornton WL, Thornton AE, Honer WG. Diffusion tensor imaging of neurocognitive profiles in a community cohort living in marginal housing. Brain Behav. 2019:;e01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gicas KM, Giesbrecht CJ, Panenka WJ, Lang DJ, Smith GN, Vila‐Rodriguez F, Leonova O, Jones AA, Barr AM, Procyshyn RM, Buchanan T, MacEwan GW, Su W, Vertinsky AT, Rauscher A, Honer WG, Thornton AE. Structural brain markers are differentially associated with neurocognitive profiles in socially marginalized people with multimorbid illness. Neuropsychology. 2017;31:28–43. [DOI] [PubMed] [Google Scholar]
- 39. Nurses Health Study History. Available at: http://www.nurseshealthstudy.org/about-nhs/history. Accessed October 28, 2018.
- 40. MESA Coordinating Center . MESA Exam 1 Participation Rate. Available at: http://www.mesa-nhlbi.org/participation.aspx. Accessed October 28, 2018.
- 41. Thorpe LE, Gwynn RC, Mandel‐Ricci J, Roberts S, Tsoi B, Berman L, Porter K, Ostchega Y, Curtain LR, Montaquila J, Mohadjer L, Frieden TR. Study design and participation rates of the New York City Health and Nutrition Examination Survey, 2004. Prev Chronic Dis. 2006;3:A94. [PMC free article] [PubMed] [Google Scholar]
- 42. Ganna A, Ingelsson E. 5 year mortality predictors in 498,103 UK Biobank participants: a prospective population‐based study. Lancet. 2015;386:533–540. [DOI] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System (BRFSS) 2017 Summary Data Quality Report. Available at: https://www.cdc.gov/brfss/annual_data/2017/pdf/overview-2017-508.pdf. Accessed October 28, 2018.
- 44. Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–653. [DOI] [PubMed] [Google Scholar]
- 45. Lewis M, Boyes K, McClanaghan D, Copas J. Downtown eastside demographic study of sro and social housing tenants. April 2008. Available at: http://www.vancouveragreement.ca/wp-content/uploads/080600_DTES-Demographic-Study-Final.pdf. Accessed November 6, 2018.
- 46. Shannon K, Ishida T, Lai C, Tyndall MW. The impact of unregulated single room occupancy hotels on the health status of illicit drug users in Vancouver. Int J Drug Policy. 2006;17:107–114. [Google Scholar]
- 47. Palepu A, Gadermann A, Hubley AM, Farrell S, Gogosis E, Aubry T, Hwang SW. Substance use and access to health care and addiction treatment among homeless and vulnerably housed persons in three Canadian cities. PLoS One. 2013;8:e75133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hwang SW, Aubry T, Palepu A, Farrell S, Nisenbaum R, Hubley AM, Klodawsky F, Gogosis E, Hay E, Pidlubny S, Dowbor T, Chambers C. The health and housing in transition study: a longitudinal study of the health of homeless and vulnerably housed adults in three Canadian cities. Int J Public Health. 2011;56:609–623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Neuroimaging Protocol
Table S2. Infarct Location
Table S3. Logistic Regression Model
Table S4. Demographics in Vancouver Studies of Vulnerably Housed and Homeless Participants
Table S5. Demographics in Cross‐Canada Studies of Vulnerably Housed and Homeless Participants
Figure S1. Age distribution of participants.


