Abstract
This trial (20010168) studied how body weight (BW) and body mass index (BMI) influenced the pharmacokinetics (PK) of anakinra. Subjects (n = 32) were assigned to four groups (n = 8) according to BW and BMI. Randomization was according to a four‐treatment, four‐period, four‐sequence crossover design. The four anakinra injections were 100, 150, and 300 mg s.c. and 100 mg i.v. Plasma samples were measured by enzyme‐linked immunosorbent assay and noncompartmental PK parameters estimated. BW demonstrated the following effects: after i.v. administration, significant effects (P < 0.05) were observed for exposure (area under the concentration–time curve from zero to infinity (AUC0–∞)), peak plasma concentration (Cmax), volume of distribution at steady state, and clearance; whereas after s.c. administration, significant effects (P < 0.05) were observed for Cmax, AUC0–∞, terminal half‐life, and estimated apparent clearance. Mean AUC was reduced 24% and 33% for heavier (BW ≥ 100 kg) vs. lighter subjects (BW ≤ 90 kg) after i.v. and s.c. administration, respectively. BMI increased clearance for heavier subjects. For example, mean (SD) plasma clearance of i.v. anakinra increased from 1.17 ± 0.29 to 1.62 ± 0.24 mL/minute/kg (P < 0.05) for larger (> 100 kg) obese (BMI > 36) vs. larger (> 100 kg) less obese (BMI < 35) subjects, respectively. Similarly, results following s.c. supported those after i.v. administration. Derived half‐lives increased with higher BW and higher BMI ranging from 3.63 hour for less obese, lighter‐weight subjects to 7.62 hour for obese, heavier‐weight subjects. Absolute bioavailability ranged from 80–92% and was unrelated to BW or BMI. Anakinra exposure is statistically significantly related to BW and to a lesser extent BMI.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ There is no direct information on how body weight (BW) or degree of adiposity alter the pharmacokinetics (PK) of i.v. or s.c. anakinra. There is a substantial body of literature on how body mass index (BMI) and BW influence disposition of small molecules. However, data for biologics are limited. Results from this study are likely to be extrapolated to other therapeutic proteins.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ The study question was “how do BW and BMI influence the PK of anakinra?”
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The study demonstrated that absolute bioavailability was not altered in obese subjects. The PK of anakinra showed that, as anticipated, exposure was higher (and clearance lower) in lighter‐weight subjects. BMI altered clearance and exposure in heavier subjects.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The results provide insight into how differences in BW and BMI alter the PK profile of a given therapeutic protein and are likely to be extrapolated to other similar compounds. The modest PK changes in the face of a reasonable safety margin provide supportive evidence for fixed (as opposed to weight based) dosing of proteins that do not have a narrow therapeutic index. In other words, biologics should, as a base case, be administered as a fixed dose when first introduced into humans.
Anakinra is the methionylated, nonglycosylated recombinant form of the naturally occurring interleukin‐1 (IL‐1) receptor antagonist (IL‐1ra). IL‐1 serves a key role in the pathophysiology and progression of rheumatoid arthritis (RA).1 Anakinra blocks the biologic activity of IL‐1 by competitively inhibiting IL‐1 binding to the IL‐1 type I receptor, which is expressed in a wide variety of tissues and organs.2, 3 In patients with active RA, anakinra is efficacious and well tolerated when used as monotherapy or in combination therapy with methotrexate.1
IL‐1 production is induced in response to inflammatory stimuli and mediates various physiologic responses, including inflammatory and immunological responses. IL‐1 contributes to cartilage degradation by its induction of the rapid loss of proteoglycans, bone, and periarticular tissues, as well as stimulation of bone resorption.4 The levels of the naturally occurring IL‐1ra in synovium and synovial fluid from patients with RA are not sufficient to compete with the elevated amount of locally produced IL‐1.5, 6, 7
The pharmacokinetics (PK) of anakinra have been well described. The absolute bioavailability (F) after a 70‐mg s.c. bolus injection into healthy subjects is 95%.8 In subjects with RA, maximum observed plasma concentrations (Cmax) of anakinra occurred within 3–7 hours after s.c. administration, with a terminal half‐life (t1/2) ranging within 4–6 hours. Population PK analyses at doses of 30 mg, 70 mg, and 150 mg of s.c. injected anakinra up to 24 weeks indicated that the estimated apparent clearance (CL/F) of anakinra after s.c. administration increased with increasing creatinine clearance and body weight (BW).9 Gender differences also occur, most likely related to differences in BW.10 Plasma clearance of anakinra occurs predominantly via glomerular filtration and tubular reabsorption.11
Anakinra is administered daily in standard, prefilled syringes containing 100 mg anakinra for s.c. injection. Accordingly, it is important to understand whether dose adjustment should be considered for those with extremes of BW or composition, in particular those with obesity. One question posed was whether there could be sequestration in fat tissue relating to the observation from population PK analyses that heavier patients with RA had an increase in clearance.9 It is unclear whether or how an increase in adiposity would alter the kinetics of s.c. absorption. A thorough understanding of the PK of anakinra according to BW and composition (as assessed by body mass index (BMI)) is likely to help determine whether a strong case can be made for dose adjustment. In this study, we selected four groups of subjects according to their BW (≤ 90 kg vs. ≥ 100 kg; lighter vs. heavier) and BMI (< 35 kg/m2 vs. ≥ 36 kg/m2; less obese vs. obese). The rationale for the four groups was to select samples of subjects typifying body types (large frame, obese with large frame, small frame, and obese with small frame). This study compared the PK parameters and linearity of anakinra in heavier‐weight and lighter‐weight healthy subjects after three single s.c. doses and one i.v. dose of anakinra.
Methods
Study design
This was a single‐center, open‐label, crossover study of healthy subjects grouped into one of four categories based on BW and BMI (Figure 1). Eight subjects were assigned to each group, and within each group subjects were randomized according to a four‐period, four‐sequence, crossover design to receive each of four treatments: 100 mg anakinra administered by a 1‐minute i.v. infusion; 100, 150, and 300 mg anakinra administered by s.c. injections. The s.c. doses were administered into the right upper quadrant of the abdomen; the 300 mg s.c. dose required two separate 150‐mg injections into the same area. Each dose of anakinra was separated by a 7‐day washout period, not to have exceeded 14 days. The t1/2 of anakinra is 4–6 hours; therefore, 7 days was considered an adequate washout period. During each treatment period, subjects were admitted to the research facility on the day before dosing, at least 12 hours before anakinra administration. Doses were administered in the morning of day 1 of each treatment period. Subjects remained in the facility until day 3 and were released after completion of the last blood sample collection and all study‐related procedures for each treatment period. Length of study participation per subject was ~ 30 days. The protocol for this study was reviewed and approved by the Research Ethics Committee of Royal Adelaide Hospital, Australia.
Figure 1.
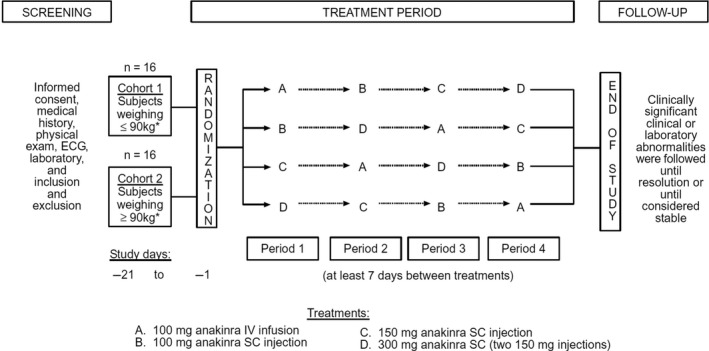
Study design and treatment schema. BMI, body mass index; BW, body weight; ECG, echocardiogram.
Subjects
All subjects provided written informed consent before any study‐related procedures were performed. Men and nonpregnant, nonbreastfeeding women were between the ages of 35 and 75 years and had to have BW < 90 kg or > 100 kg and BMI outside the range of 35.0–35.9 kg/m2 at the time of screening.
Subjects were healthy and tested negative for hepatitis B surface antigen and hepatitis C antibody. Subjects were excluded from the study for any of the following reasons: unstable medical condition, clinically significant skin allergies, use of certain prescription or over‐the‐counter medication within 2 weeks before randomization, use of an investigational agent within 30 days before randomization, or history of drug or alcohol abuse within the past year. Subjects known to have tested positive for HIV antibody or who donated blood products within 90 days before randomization, and subjects who smoked > 10 cigarettes per day at screening, were also excluded.
Investigational product
Anakinra was supplied as a clear colorless‐to‐white liquid in single‐use, 3‐mL glass vials containing 1.00 mL (withdrawal volume) of 100 mg/mL anakinra. Preparation of the 150‐mg dose required two vials to achieve a total injection volume of 1.5 mL, and the 300‐mg dose required four vials to achieve two injections of 1.5 mL each.
PK sample analysis
Plasma samples to determine predose anakinra concentrations were obtained on day 1 of each treatment period, within 30 minutes before anakinra administration. Postdose samples were obtained at the following time points: 1, 5, 10, 15, and 30 minutes, and 1, 2, 4, 5, 6, 7, 8, 10, 12, 16, 24, 30, 36, and 48 hours after completion of the i.v. infusion, or 0.5, 1, 2, 4, 6, 8, 10, 12, 16, 24, 30, 36, and 48 hours after s.c. administration. Plasma samples were stored at ~ −70°C until analysis by a validated antibody‐capture enzyme‐linked immunoassay; the lower limit of quantitation was 20 ng/mL.
PK data analysis
The primary end points of this study were F after s.c. administration of anakinra, area under the concentration–time curve (AUC), and Cmax. Secondary PK end points were time of maximum plasma concentration (Tmax), t1/2, CL/F, volume of distribution at steady state (Vss), and clearance (CL). High random variability was observed in the individual anakinra concentration–time profile for many subjects. This high variability was unexpected based on PK data of anakinra from previous clinical studies. To smooth out the concentration–time profiles, the raw data were subjected to compartmental analysis. A two‐compartmental disposition model with a linear clearance process was found to best describe the i.v. data. This is the reason noncompartmental data were not used in these instances.
Anakinra concentration data from individuals after each s.c. dose were modeled simultaneously with the concentration data after i.v. administration. The absorption of anakinra after s.c. administration was described by two first‐order absorption processes, one of which involved a delayed compartment.
The compartmental analysis was performed using SAAM II (version 1.1.2; SAAM Institute, University of Washington, Seattle, WA). Compartmental models were optimized to plasma profiles with a fractional SD of 10% assigned to each datum, using relative weighting based on observed concentrations. There were no adjustments for multiplicity. Computational settings included a minimum of 50 calculations, the Rosenbrock integrator with relative error of 0.001, and a convergence criterion of 0.0001. Goodness‐of‐fit was evaluated by weighted residual plots. The Akaike information criterion and the Schwarz‐Bayesian information criterion were used to evaluate which model was best able to fit the data with the minimum number of parameters. The PK parameters of anakinra based on the fitted profile for each subject were extracted by using the PK/pharmacodynamic/noncompartmental analysis Analysis Wizard in WinNonlin Professional (Version 3.3, Pharsight, Mountain View, CA).
Statistical methods
Prespecified hypotheses tested in this study included whether obesity altered absolute bioavailability or clearance.
Power analyses for numbers of subjects were based on data from a previous study of anakinra (study 20000196), in which the between‐subject variability for AUC, Cmax, and the ratio of dose A to dose B for AUC and Cmax does not exceed 14% for AUC and 27% for Cmax. Using these estimates, it was determined that a sample size of 32 would provide an 80% chance (power) of detecting at the 5% level of significance a 14% difference in AUC parameters and a 32% difference in Cmax parameters between the two BMI classifications (≥ 36 or < 35; n = 16) or the two BW classifications (≤ 90 or ≥ 100; n = 16). Using the same estimates for the differences between any two groups (n = 8), this study had an 80% chance of detecting at the 5% level of significance, a 22% difference in AUC, and a 50% difference in Cmax.
The derived PK parameters after i.v. treatment administration were compared using a factorial analysis of variance; the BMI classification, BW classification, and the interaction of BMI and BW were evaluated. Each s.c. dose was evaluated using the same model. The primary contrasts of interest were the estimates of F, AUC, and Cmax for the two BMI and two BW classifications.
The between‐subject effects of sequence, BMI classification, BW classification, and the interaction of BMI and BW were extracted. The within‐subject effects of period, treatment, and the interaction of treatment with BMI classification, BW classification, and the interaction of BMI and BW were also extracted. Secondary contrasts of interest were analysis of variance (ANOVA) comparisons among the three s.c. doses (for each of the four BW/BMI classifications); a crossover ANOVA was used to compare dose‐adjusted Cmax and AUC after each s.c. dose. The i.v. data were compared using a factorial ANOVA with the between‐subject effects noted above. No adjustments for multiple comparisons were planned.
Safety
Safety parameters were monitored throughout the study and included incidences of adverse events (AEs), electrocardiograms, vital signs, physical examinations, and clinical laboratory values. AEs were coded by preferred term and body system using a modified World Health Organization Adverse Reaction Terms dictionary. All subjects receiving anakinra were included in the safety analyses.
Results
Baseline characteristics
The baseline characteristics of the 33 subjects who participated in this study are summarized in Table 1. Although 33 subjects were enrolled, 32 subjects (11 men and 21 women) completed the study. One subject withdrew after the initial blood collection; therefore, data for this subject were excluded from PK analyses. This subject was replaced with another assigned to the same treatment sequence. All subjects were white, and the mean age was 47.8 with a range of 35–74 years. Per protocol, subjects were categorized by BW and BMI (Figure 1). The lowest mean BW was 66.3 kg in cohort 3, and the highest mean BW was 125.2 kg in cohort 2. BMI mean values ranged from 25.3 kg/m2 in cohort 3 to 44.1 kg/m2 in cohort 2. Estimated glomerular filtration rate (eGFR) was calculated for each cohort using Chronic Kidney Disease Epidemiology Collaboration12 and is included in Table 1.
Table 1.
Subject characteristics
| Characteristic | BMI < 35 BW ≥ 100 kg | BMI ≥ 36 BW ≥ 100 kg | BMI < 35 BW ≤ 90 kg | BMI ≥ 36 BW ≤ 90 kg |
|---|---|---|---|---|
| N | 8 | 9 | 8 | 8 |
| Female | 1 | 7 | 6 | 8 |
| White | 8 | 9 | 8 | 8 |
| Age (years)a | 41.9 (5.6) | 44.9 (8.0) | 48.4 (12.3) | 56.3 (10.1) |
| Height (cm)a | 183.3 (7.1) | 168.6 (6.8) | 161.4 (8.9) | 153.6 (3.3) |
| BW (kg)a | 109.6 (9.3) | 125.2 (14.5) | 66.3 (13.2) | 86.9 (3.0) |
| eGFR | 61.0 (45.0–73.1) | 69.7 (53.0–86.2) | 53.8 (35.7–81.1) | 50.9 (32.9–63.6) |
BMI, body mass index; BW, body weight; eGFR, estimated glomerular filtration rate.
The eGFR rate (mL/minute/1.73 m2) as median (range) calculated by Chronic Kidney Disease Epidemiology Collaboration.
Data are presented as mean (SD).
Overall PK results
BW demonstrated the following effects: after i.v. administration, significant effects (P < 0.05) were observed for exposure (AUC0–∞), Cmax, Vss, and CL, whereas after s.c. administration, significant effects (P < 0.05) were observed for Cmax, AUC0–∞, t1/2, and CL/F.
PK after i.v. administration
Median plasma anakinra concentration–time profiles after i.v. administration of 100 mg anakinra declined with similar slopes and t1/2 values for the different BW and BMI groups, varying from 1.67−1.86 hours (Figure 2 and Table 2). Irrespective of BMI category, AUC was 24% lower in subjects with BW ≥ 100 kg than in subjects with BW ≤ 90 kg. The CL value, which is inversely proportional to AUC, was higher for the heavier subjects than for the lighter subjects; however, after being normalized by BW (CL/BW), it was 16% higher for the lighter subjects than for the heavier subjects. In the same BW category (> 100 kg), CL/BW was higher for BMI < 35 than for the more obese BMI ≥ 36 (Table 2). These results indicate that exposure to anakinra is related to both BW and BMI. The t1/2 was independent of BW, as the Vss value also increased with increasing BW in a similar manner to CL.
Figure 2.
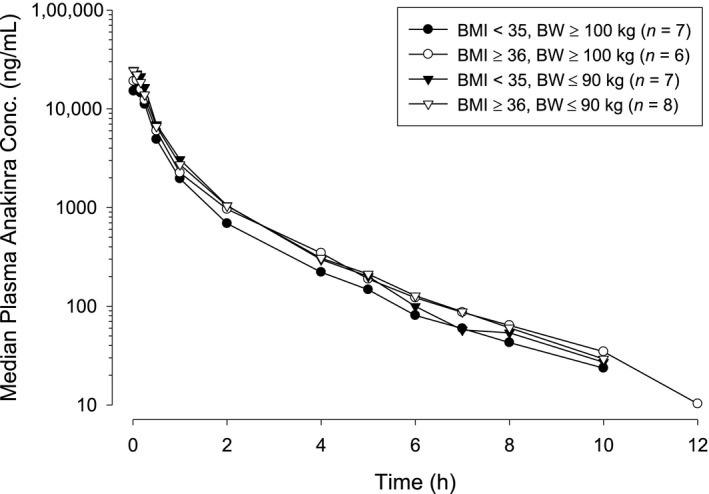
Median plasma anakinra concentration–time profiles after 100‐mg i.v. administration. BMI, body mass index; BW, body weight.
Table 2.
Mean (SD) pharmacokinetic parameters in heavier‐weight and lighter‐weight subjects after i.v. administration of 100 mg anakinra
| Parameter | BMI < 35 BW ≥ 100 kg | BMI ≥ 36 BW ≥ 100 kg | BMI < 35 BW ≤ 90 kg | BMI ≥ 36 BW ≤ 90 kg |
|---|---|---|---|---|
| N | 7 | 6 | 7 | 8 |
| Cmax (ng/mL) | 21,532 (6,332) | 25,336 (4,984) | 32,193 (6,426) | 29,259 (7,913) |
| AUC(0–∞) (ng·hour/mL) | 9,533 (1,702) | 12,487 (2,822) | 14,658 (3,461) | 14,263 (4,398) |
| CL (mL/minute) | 180 (38) | 138 (26) | 122 (42) | 124 (28) |
| CL/BW (mL/minute/kg) | 1.62 (0.24) | 1.17 (0.29) | 1.82 (0.44) | 1.43 (0.32) |
| Vss (L) | 10.38 (2.35) | 8.47 (1.36) | 6.29 (1.50) | 7.70 (1.58) |
| t1/2 (hour) | 1.67 (0.12) | 1.86 (0.39) | 1.69 (0.42) | 1.74 (0.38) |
AUC0–∞, area under the concentration–time curve from time 0 to infinity; BMI, body mass index; BW, body weight; CL, plasma clearance after i.v. administration; CL/BW, body weight–normalized CL; Cmax, maximum plasma concentration; t1/2, terminal half‐life; Vss, volume of distribution at steady state after i.v. administration.
As seen in Table 2, the total number analyzed was 28 of the potential 32 subjects. However, for evaluation of PK parameters after i.v. administration, four subjects were omitted from the analyses. Unusually low plasma anakinra concentrations after i.v. administration were observed for three subjects. Specifically, all three of these anakinra concentrations were either below the lower limit of quantitation or were much lower than the median concentrations for other subjects who were within the same BW and BMI categories and had received the same dose. Therefore, concentration data after i.v. administration for these subjects were excluded from PK and statistical analyses, and the absolute bioavailability for these subjects was not estimated. Additionally, following the 100‐mg i.v. dose, the concentration–time profile for one subject was substantially lower than those for the rest of the subjects. The clearance value of this subject was 2.9‐fold higher than the mean value for the rest of the subjects. The estimated F after s.c. administration for this subject was unexpectedly out of range: 218% at 100 mg, 225% at 150 mg, and at 212% at 300 mg. Based on the CL and F values, this subject's PK profile after i.v. administration was classified as an outlier. We are unable to explain these results and cannot exclude dosing error for the i.v. portion. Therefore, the PK parameters after i.v. administration and the F after s.c. administration for this subject were also excluded from statistical analyses.
PK after s.c. administration
The median plasma concentration–time profiles after s.c. administration of 100 mg, 150 mg, and 300 mg of anakinra are presented in Figure 3. Similar to the observations after i.v. administration, the maximal concentrations of anakinra were lower in subjects with BW ≥ 100 kg than in subjects with BW ≤ 90 kg. After s.c. administration of 100 mg anakinra, the mean Cmax value was 46% lower and the mean AUC value was 33% lower for subjects with BW ≥ 100 kg than for subjects with BW ≤ 90 kg. Mean CL/F values were higher for subjects with BW ≥ 100 kg than for subjects with BW ≤ 90 kg, and BMI seemed to have an impact only for heavier subjects. As seen in Table 3, t1/2 increased with both higher BW and higher BMI ranging from 3.63 hours for less obese, lighter‐weight subjects to 7.62 hours for obese, heavier‐weight subjects. F was independent of both BW and BMI and ranged from 80−92% (Table 3). Overall, results after s.c. administration supported the results seen with i.v. administration and indicated that exposure to anakinra after s.c. administration is related to both BW and BMI.
Figure 3.
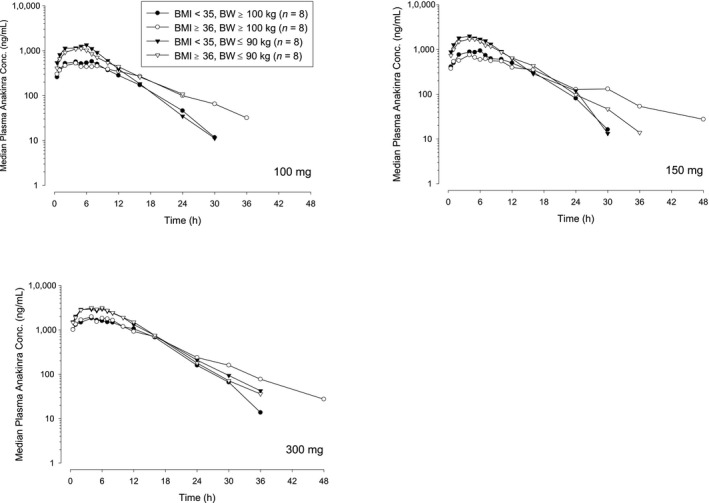
Median plasma anakinra concentration–time profiles in heavier‐weight and lighter‐weight subjects after increasing doses of s.c. administration. BMI, body mass index; BW, body weight.
Table 3.
Mean (SD) (and median (range) for Tmax) pharmacokinetic parameters in heavier‐weight and lighter‐weight subjects after s.c. administration of 100 mg anakinra
| Parameter | BMI < 35 BW ≥ 100 kg | BMI ≥ 36 BW ≥ 100 kg | BMI < 35 BW ≤ 90 kg | BMI ≥ 36 BW ≤ 90 kg |
|---|---|---|---|---|
| N | 8 | 8 | 8 | 8 |
| Tmax (hour)a | 3.7 (3.0–7.5) | 4.0 (2.0–6.5) | 4.3 (2.0–5.0) | 3.7 (3.0–4.5) |
| Cmax (ng/mL) | 612 (177) | 696 (397) | 1,326 (477) | 1,086 (307) |
| AUC0–∞ (ng·hour/mL) | 7,569 (836) | 9,905 (3,151) | 13,266 (3,510) | 12,625 (2,316) |
| CL/F (mL/minute) | 223 (27) | 182 (52) | 134 (36) | 136 (28) |
| t1/2 (hour) | 5.47 (2.28) | 7.62 (3.66) | 3.63 (0.89) | 5.76 (1.86) |
| F (%) | 80.2 (7.5)b | 87.2 (11.4)c | 86.9 (13.9)b | 91.9 (18.7) |
AUC0–∞, area under the concentration–time curve from time 0 to infinity; BMI, body mass index; BW, body weight; CL/F, plasma clearance after s.c. administration; Cmax, maximum observed plasma concentration; F, absolute bioavailability; t1/2, half‐life associated with the terminal phase; Tmax, time at which Cmax occurred.
aData are presented as median (range). bOne subject was excluded (n = 7). cTwo subjects were excluded (n = 6).
Regardless of BMI and BW, the exposure to anakinra after s.c. administration increased proportionally relative to dose. When the dose was normalized vs. the 100‐mg dose, Cmax and AUC0‐∞ were similar in a dose range for 100 mg, 150 mg, and 300 mg (Figure 4).
Figure 4.
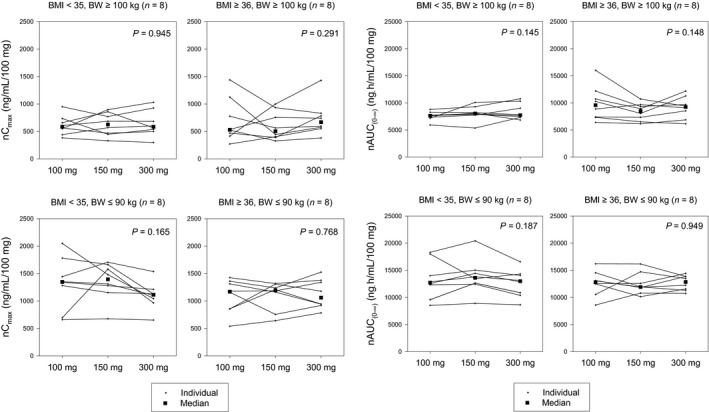
Dose‐normalized peak plasma concentration (Cmax) and area under the concentration–time curve from zero to infinity (AUC0–∞) in heavier‐weight and lighter‐weight subjects after increasing doses of s.c. administration. BMI, body mass index; BW, body weight.
Safety
Anakinra was well tolerated by the healthy subjects who participated in this study. No new safety concerns were observed. A total of 21 subjects (64%) experienced the most common AE, a reaction at the site of s.c. injection. However, because injection site reactions are the most common category of events seen in large clinical studies of anakinra, this does not represent a new safety finding. No deaths, serious AEs, or withdrawals because of AEs were reported in this study.
The administered dose affected the frequency of AEs. There was a noticeable dose–response relationship with injection site reactions within the s.c. doses. The 100‐mg s.c. dose showed the lowest occurrence rate of injection site reactions at 22%, and the highest rate was observed with the 300‐mg s.c. dose at 33%.
Discussion
Anakinra is an IL‐1ra demonstrating well‐defined PK parameters in clinical trials with healthy subjects.11, 13 In the current study, the PK profile of anakinra was investigated in select groups of healthy subjects with varying levels of BW and BMI. After s.c. administration of 100 mg of anakinra, bioavailability ranged from 80−92% and was independent of BW and BMI. No differences were found in F indicating lack of support for the hypothesis that obese subjects have reduced bioavailability of anakinra.
The median plasma anakinra concentration–time profiles after i.v. administration of 100 mg of anakinra declined with similar slopes and t1/2 values for the different BW and BMI groups. As would be anticipated for a fixed dose therapeutic, AUC was reduced more in heavier than in lighter‐weight subjects. Accordingly, CL increased with BW. However, when the clearance was normalized (CL/BW), the obese subjects had lower clearance. These results indicated that i.v. exposure to anakinra was related to both BW and BMI. The terminal t1/2 was independent of BW, as the Vss value also increased with increasing BW in a similar manner to CL.
Anakinra exposure after s.c. administration was 33% lower for subjects with higher BW than for subjects with lower BW. This type of difference would have been expected for fixed dosing of a protein therapeutic in which the initial volume of distribution approximates plasma volume. Generally, subjects with higher BW have a larger plasma volume and will have lower concentrations than subjects with lower BW.
Anakinra is known to exhibit flip‐flop kinetics after s.c. administration. Specifically, the terminal phase of the concentration–time profile is represented by the absorption process because s.c. absorption is rate limiting in the disposition of anakinra. After s.c. administration, the t1/2 is longer relative to i.v. administration (5.24 hours vs. 2.64 hours).11 In this study, the t1/2 was also shorter after i.v. administration, ranging from 1.67−1.74 hours. After s.c. administration, the t1/2 increased significantly (P < 0.05) with both BW and BMI with values ranging from 3.63−7.62 hours. Because of the inverse relationship, these results suggested that the absorption rate constant decreases with increasing BW and BMI. It is notable that the more obese subjects had longer half‐lives, suggesting that subjects with more adipose tissue have slower anakinra transport and consequently less rapid systemic s.c. absorption.
Plasma clearance normalized to bioavailability (CL/F) was significantly different between heavier‐weight and lighter‐weight subjects. After s.c. administration, lighter subjects had comparable mean values of 134 and 136 mL/minute for less obese and obese subjects, respectively. However, heavier subjects had mean clearance values of 223 and 182 mL/minute for less obese and obese subjects, respectively. Because there is a relationship between glomerular filtration rate and body size, these results could have been anticipated as anakinra is predominantly renally cleared. These values were in the range previously observed (170 mL/minute) for healthy subjects.11 In a more recent study, CL/F values ranged from 60−226 mL/minute in subjects with RA.14 In the current study, there were differences in baseline eGFR more notably for the heavier subjects (median 61.0 and 69.7 mL/minute/1.73 m2 for nonobese and obese subjects, respectively). BW and eGFR are clearly interrelated, and no prespecified analyses were planned to account for differences. However, differences would likely have been even greater had adjustments for baseline differences been made (body surface area was slightly greater for the obese vs. nonobese subjects for the heavier cohorts). For the lighter subjects, baseline eGFR values were similar but body surface area was substantially greater for the obese vs. nonobese subjects.
The PK differences demonstrated in this study are modest for a drug with a wide therapeutic index. The benefits of fixed dosing (reduced medication error) seem to outweigh the potential reduction in PK variance, which could be anticipated if anakinra were administered according to BW. The results are likely to be able to be extrapolated to thinner subjects, given that the nonobese lighter subjects had a mean weight of 66 kg and BMI of 25 kg/m2. In summary, the PK of fixed‐dose anakinra is related to BW with lighter‐weight subjects demonstrating modestly higher exposure relative to heavier‐weight subjects. BMI also affected the PK of anakinra with heavier obese subjects having higher exposure. Absolute bioavailability of s.c. anakinra was independent of BW and BMI.
Funding
This study was funded by Amgen Inc.
Conflict of Interest
B.‐B.Y. was previously an employee and is currently a stockholder, and J.T.S. is currently an employee and stockholder, of Amgen Inc. Thousand Oaks, CA. P.G. is an employee and stockholder of Swedish Orphan Biovitrum AB (Sobi) Stockholm.
Author Contributions
B.‐B.Y., J.T.S., and P.G. wrote the manuscript. B.‐B.Y. and J.T.S. designed the research. B.‐B.Y. and J.T.S. performed the research. B.‐B.Y. and J.T.S. analyzed the data.
Acknowledgments
The authors thank Alan Moskwa, MB, BS, Kensington Park SA, Australia, Maggi Salfi, MS, Leslie LeScale‐Matys PhD, and Alan Forsythe, PhD, for their contributions to this investigation, and Julie Wang, DPM, Anna Kido, BS, and Keith Del Villar, PhD, for their assistance with writing this manuscript.
References
- 1. Furst, D.E. Anakinra: review of recombinant human interleukin‐I receptor antagonist in the treatment of rheumatoid arthritis. Clin. Ther. 26, 1960–1975 (2004). [DOI] [PubMed] [Google Scholar]
- 2. Hannum, C.H. et al Interleukin‐1 receptor antagonist activity of a human interleukin‐1 inhibitor. Nature 343, 336–340 (1990). [DOI] [PubMed] [Google Scholar]
- 3. Arend, W.P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J. Clin. Invest. 88, 1445–1451 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Lent, P.L. , Van De Loo, F.A. , Holthuysen, A.E. , Van Den Bersselaar, L.A. , Vermeer, H. & Van Den Berg, W.B. Major role for interleukin 1 but not for tumor necrosis factor in early cartilage damage in immune complex arthritis in mice. J. Rheumatol. 22, 2250–2258 (1995). [PubMed] [Google Scholar]
- 5. Chomarat, P. et al Balance of IL‐1 receptor antagonist/IL‐1 beta in rheumatoid synovium and its regulation by IL‐4 and IL‐10. J. Immunol. 154, 1432–1439 (1995). [PubMed] [Google Scholar]
- 6. Deleuran, B.W. et al Localization of tumor necrosis factor receptors in the synovial tissue and cartilage‐pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 35, 1170–1178 (1992). [DOI] [PubMed] [Google Scholar]
- 7. Firestein, G.S. et al Synovial interleukin‐1 receptor antagonist and interleukin‐1 balance in rheumatoid arthritis. Arthritis Rheum. 37, 644–652 (1994). [DOI] [PubMed] [Google Scholar]
- 8. Kineret [package insert]. (Amgen Inc., Thousand Oaks, CA, 2005). [Google Scholar]
- 9. Yang, B.B. , Frazier, J. , McCabe, D. & Young, J.D. Population pharmacokinetics of recombinant interleukin‐1 receptor antagonist (anakinra) in subjects with rheumatoid arthritis (RA). Arthritis Rheum. 43, S153 (2000). [Google Scholar]
- 10. Gandhi, M. , Aweeka, F. , Greenblatt, R.M. & Blaschke, T.F. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 44, 499–523 (2004). [DOI] [PubMed] [Google Scholar]
- 11. Yang, B.B. , Baughman, S. & Sullivan, J.T. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin. Pharmacol. Ther. 74, 85–94 (2003). [DOI] [PubMed] [Google Scholar]
- 12. Levey, A.S. et al A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Granowitz, E.V. et al Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin‐1 receptor antagonist in healthy humans. Cytokine 4, 353–360 (1992). [DOI] [PubMed] [Google Scholar]
- 14. Chang, D.M. , Chang, S.Y. , Yeh, M.K. & Lai, J.H. The pharmacokinetics of interleukin‐1 receptor antagonist in Chinese subjects with rheumatoid arthritis. Pharmacol. Res. 50, 371–376 (2004). [DOI] [PubMed] [Google Scholar]


