Abstract
Aim
Antibiotic abuse contributes to the emergence of methicillin-resistant Staphylococcus aureus (MRSA). It is increasingly important to screen new antimicrobial agents for the management of MRSA infections. G. chinensis, a nontoxic Chinese herbal medicine, is considered a potential antibacterial agent. The aim of this study was to investigate the bactericidal effects of the aqueous extracts of G. chinensis on MRSA. The potential mechanisms of G. chinensis aqueous extract inhibition of the pathogenicity of MRSA in vivo are also discussed.
Methods
G. chinensis aqueous extract was prepared and its antimicrobial activities were examined by determining its minimum inhibitory concentration (MIC). Biofilm biomass was determined by scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM). RNA sequencing (RNA-seq) was used to evaluate differentially expressed functional pathways in MRSA treated with G. chinensis aqueous extract. We validated the role of G. chinensis aqueous extract in the invasive ability and pathogenicity of MRSA in vivo using a rat infectious model.
Results
The results indicated that MRSA was sensitive to the G. chinensis aqueous extracts at concentration of 31.25μg/mL. G. chinensis extract led to a reduction in dextran-dependent aggregation and biofilm formation in MRSA. Based on the transcriptome analysis, G. chinensis aqueous extracts significantly downregulated the gene expression related to biofilm formation and carbohydrate metabolism. G. chinensis aqueous extract inhibited the invasive ability and the pathogenicity of MRSA in vivo.
Conclusion
The antimicrobial properties of G. chinensis aqueous extract are likely related to its modulation of MRSA biofilm formation and carbohydrate metabolism. G. chinensis aqueous extract is a promising supplementary therapy to lessen or eliminate the use of antibiotics and is a potential tool for the management of MRSA infections.
1. Introduction
Staphylococcus aureus (S. aureus) is a common bacterial genus in human caused infectious diseases from innocuous commensal to fatal infections [1]. As a type of gram-positive cocci and coagulase-positive coccoid bacterium of the Firmicutes phylum, S. aureus is carried by approximately 20%–30% of healthy humans [2]. With the acquisition of the gene mecA, the methicillin-resistant Staphylococcus aureus (MRSA) is emerged constitutively. The mec operon is carried by the Staphylococcal cassette chromosome mec and mostly originated from horizontal transfer from coagulase-negative Staphylococcal species [3].
Microbial biofilm cells show significantly less susceptibility to antimicrobial agents than planktonic cells [4]. Correspondingly, more biofilm is identified in MRSA strains compared with methicillin-sensitive S. aureus strains [5]. Polysaccharide intercellular adhesion (PIA) is a vital component necessary for the biofilm organization of S. aureus [6]. The poly-N-acetyl glucosamine (PNAG) polysaccharide deposited on the surface of the cell wall, also referred to as PIA, is synthesized by glycosyl transferase enzymes that are encoded by the ica operon [7].
As the MRSA strains have been reported to be resistant to conventional antibiotics, new antimicrobial agents are urgently needed for the management of MRSA infections. Recently, it has become increasingly important to screen effective Chinese traditional medicines as potential sources of drugs for the management of Staphylococcus drug-resistance [8]. Galla chinensis (G. chinensis), a nontoxic Chinese herbal medicine, is naturally formed when Rhus chinensis Mill is parasitized by Melaphis chinensis Bell. G. chinensis is considered to be a potential antibacterial agent [9, 10]. Gallnuts are a group of very special natural plant-insect symbiont products measuring 2.5–9 cm in length and 1.5–4 cm in diameter as shown in Figure 1(a) [11, 12]. The compounds and extracts from G. chinensis are rich in gallic acid, gallotannin, and hydrolysable tannins and possess antimicrobial characteristics [10, 11, 13]. Furthermore, safety evaluation tests have shown that little acute or chronic toxicity is present when G. chinensis extracts are taken at lower doses [14].
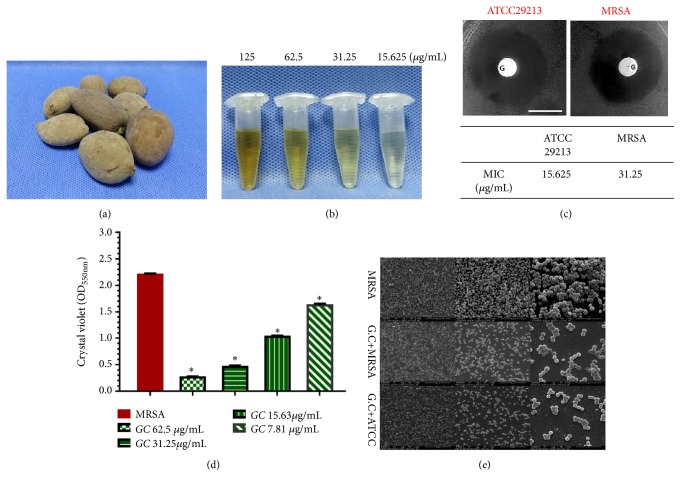
Figure 1.
Sensitivity of S. aureus to G. chinensis extract. (a) Morphology of dried Galla chinensis; (b) morphology of G. chinensis extract solutions; (c) the inhibition zone (the upper lane) and the minimum inhibitory concentration values (the lower lane) of G. chinensis extract solution for S. aureus; (d) crystal violet stain for methicillin-resistant S. aureus cells treated with different concentrations of G. chinensis extract for biomass comparison (∗p<0.05, n=10); (e) scanning electron microscopy (SEM) observation of the biofilm architecture of MRSA strain, MRSA treated with G. chinensis (1/2 MIC), and S. aureus ATCC with G. chinensis (1/2 MIC), respectively, at 24 h of growth. The MRSA biofilm treated with G. chinensis (1/2 MIC) presented a reduction in the extracellular matrices and only small microcolonies existing compared with untreated MRSA strains.
Previous investigations have demonstrated that components or extracts from G. chinensis have bactericidal activities against S. aureus, including growth-inhibitory and biofilm-reducing effects [9, 13]. However, our understanding of the antimicrobial characteristics of G. chinensis water extract to MRSA is still very limited. Taken together, the aims of this study were as follows: (1) to determine whether the aqueous extract of G. chinensis has an antibacterial effect on MRSA, (2) to perform transcriptome analysis of MRSA in response to treatment with G. chinensis aqueous extracts, and (3) to determine whether the aqueous extract of G. chinensis inhibited the pathogenicity of MRSA in vivo.
2. Materials and Methods
2.1. Preparation of an Aqueous Extract of G. chinensis
The fresh G. chinensis was harvested in autumn and placed in boiling water until the surface turned gray. Then, the gray Galla was dried in the air after removal of the larvae [11]. We purchased the G. chinensis from Zhewan Traditional Chinese Medicine of Limited by Share Ltd. (Galla chinensis, Certificate No. AH20150176). The obtained G. chinensis aqueous extract was processed as previously described [15, 16]. Briefly, the obtained G. chinensis was dried, powdered, and extracted with distilled water [16]. The solutions were concentrated using a vacuum falling filter evaporator (Iwai Co., Japan). The dried extract was dissolved in water to achieve a concentration of 10 g/L (W: V), then sterilized with a 0.2 μm syringe filter, and kept at 4°C for further experiments.
2.2. Bacterial Strains and Growth Conditions
S. aureus strains of ATCC 29213 (methicillin-sensitive S. aureus strain) and clinical isolated MRSA strains were obtained from the Department of Laboratory Medicine (West China Hospital, Sichuan University, Chengdu, China) and cultured on conventional Baird-Parker (BP) agar plate. The pure growth of single clones was achieved and Gram's staining was conducted for initial Staphylococcus strains identification [5]. For incubation, S. aureus strains were grown in Luria–Bertani (LB) liquid (Oxoid, Basingstoke, United Kingdom) at 37°C overnight. Then, the bacterial suspensions were cultured to mid-logarithmic growth phase (optical density at 600 nm of 0.5) in LB medium for further experiments.
2.3. Testing Planktonic Antimicrobial Susceptibility
The minimum inhibitory concentration (MIC) test was performed in LB medium via broth microdilution techniques in the presence of approximately 1 × 107 CFU/mL of S. aureus ATCC 29213 and MRSA strains. The LB medium contained serially diluted G. chinensis water extracts ranging from 3.9 μg/mL to 125 μg/mL. The MICs, the minimal inhibitory concentration, were defined as the concentration at which no visible turbid bacterial growth was observed. The determined MIC values were compiled for further investigations.
2.4. Biofilm Assays In Vitro
Biofilms were established after 24 h at 37°C in LB, GC (1/2 MIC) + LB media [17]. A crystal violet microtiter assay was used to quantitatively measure the biofilm biomass. Briefly, the biofilms were washed in phosphate buffered saline (PBS), dyed with crystal violet solutions, and then solubilized with the destaining solutions as previously described [5]. Then, the destaining solutions were transferred into a clean 96-well plate, and the optical density was measured at 600 nm [5].
The biofilm samples were washed twice in PBS and imaged with a scanning electron microscope (SEM, Inspect Hillsboro) following our previous procedures [5, 18]. The samples were serially dehydrated with ethanol solutions (30%, 50%, 70%, 95%, and 100%), dried in air, and coated with gold for imaging. Three randomly selected areas from each sample were imaged by SEM.
The anteromedial tibia cortex of the healthy rat was prepared and sliced into 4 × 4 mm specimens using a hard-tissue cutting machine (Buehler, Chicago, IL, USA). The bone samples were washed ultrasonically in distilled water for 10 min and stored in 10 mM PBS (pH 7.0) at 4°C. Then, the specimens were incubated with the MRSA strains, G. chinensis (1/2 MIC) + MRSA strains, and G. chinensis (1/2 MIC) + ATCC strains. After 24 h of coculture at 37°C, bone specimens were rinsed twice with PBS to remove the supernatants. The EPS matrix of S. aureus biofilms was stained with Alexa 647-labeled dextran conjugate (Invitrogen, Eugene, OR, USA) [5]. The bacterial cells in the biofilm were labeled with SYTO9 (Invitrogen, Carlsbad, CA, USA). Confocal laser scanning microscopy (CLSM, TSP SP2; Leica, Solms, Germany) was then performed [18]. For the fluorescence microscopy, the live and dead cells in the biofilms grown on the glass coverslips were distinguished with LIVE/DEAD BacLight™ Bacterial Viability Kit reagent (Invitrogen, Waltham, MA) and labeled in accordance with the manufacturer's instructions to assess the proportion of vital bacteria [18].
2.5. RNA Extraction and RNA Sequencing Performance
Bacterial total RNAs were extracted from mid-exponential phased planktonic methicillin-resistant S. aureus or the G. chinensis extract (1/2 MIC)-treated MRSA strain using the MasterPure™ RNA purification Kit (Epicenter Technologies, Epicenter, Madison, WI, USA) and purified with DNase I (Ambion) following the manufacturer's instructions. RNA quality and purity were analyzed by an Agilent 2100 bioanalyzer (Agilent Technologies). All RNA was determined to have an RNA integrity number (RIN) of 9.0 and above. Removal of rRNA was performed using a Ribo-Zero™ rRNA Removal Kit for gram-positive bacteria (Epicenter) in accordance with the supplier's specifications. The final quality and purity of the enriched bacterial mRNA were analyzed using an Agilent Bioanalyzer (Agilent Technologies) [19]. From enriched mRNAs, cDNA libraries were processed using a TruSeq™ RNA sample preparation kit (Illumina). Subsequently, RNA sequencing (RNA-Seq) was performed on a HiSeq 4000 (2x150 bp read length) at Majorbio Biotechnology Research (Shanghai, China). A Galaxy server was used to perform read mapping procedures with Bowtie 2 for Illumina [8].
2.6. Statistical Analysis of RNA Sequencing Data and Data Validation
Reads were mapped to the genome of methicillin-resistant S. aureus. Fold changes and significant differences in gene expression were calculated using edgeR (http://www.bioconductor.org/packages/2.12/bioc/html/edgeR.html) [20]. Significant differences in genes were defined as a fold-change > 2 and a threshold false discovery rate (FDR) of ≤0.05. The pathways were assigned by Gene ontology (GO) terms using Blast2GO [21]. Relative enrichment of GO terms compared with a background of GO terms was assessed using Fisher's exact tests. After combining all evidence from the gene expression level data, pathway enrichment analysis was performed, and the FDR procedure was used to correct for multiple hypothesis testing (FDR < 0.05). For the RNA-Seq data validation, quantitative real-time PCR assays were conducted to measure the expression levels of the genes (primers listed in Table A1). Briefly, total RNAs were isolated from cells harvested at mid-logarithmic growth phase and purified using the MasterPure™ RNA purification Kit (Epicenter Technologies, Epicenter, Madison, WI, USA) [5]. Contaminating genomic DNA was removed using Turbo RNase-free DNase I (Ambion). Any residual genomic DNA contamination was assessed and the quality of the RNA was determined. The reverse transcriptional reactions were processed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific) as previously described [5, 22].
2.7. Osteomyelitis Animal Model Construction and Micro-CT Imaging
Approved by the Institutional Animal Welfare Committee (West China Hospital, China, Approval No. 2018039A), 10 female Sprague-Dawley rats (260–280 g) were used in this study. Research was conducted in accordance with the nationally accepted principles for laboratory animal experiments. All animals were anesthetized by ketamine (60 μg/g) and xylazine (6 μg/g). Then, the right hind legs were shaved and disinfected with polyvinyl pyrrolidine-iodine. The anteromedial tibia cortex was exposed by incisions 1 cm in length, and a hole of 0.1 cm diameter was prepared on the medullary cavity as previously described [23]. The S. aureus clinical isolated MRSA strain was cultured on conventional Baird-Parker (BP) agar plate. The pure growth of single clones was achieved and Gram's staining was conducted for initial Staphylococcus strains identification. For incubation, MRSA strains were grown in Luria-Bertani (LB) liquid (Oxoid, Basingstoke, United Kingdom) at 37°C overnight. The untreated MRSA suspensions were cultured to mid-logarithmic growth phase (optical density at 600 nm of 0.5) in LB medium. The treated MRSA suspensions were grown to mid-logarithmic growth phase (optical density at 600 nm of 0.5) in LB medium and then cocultured with GC (1/2 MIC) for further experiments. For the bacterial injection, ten animals were divided into two groups, including the untreated group (n=5) injected with 100 μL of LB culture medium containing mid-exponential phase methicillin-resistant S. aureus only (1 × 107 CFU/mL) and the treated group (n=5) that was inoculated with 100 μL of a mixture of mid-exponential phase methicillin-resistant S. aureus suspension (1 × 107 CFU/mL) in LB culture medium cocultured with GC (1/2 MIC).
After suturing, all animals were observed for 4 weeks. To evaluate the infective tibias in rats, micro-CT analysis was performed using a Quantum GX Micro-CT System (PerkinElmer, Waltham, MA) as previously described [24]. The scanning conditions were as follows: kV = 90; CT μA = 72; 360° scan time = 8 sec [25]. The three-dimensional images were reconstructed using Analyze 12.0 (PerkinElmer). The ratios of BV/TV (trabecular and cortical bone volume (BV) per total volume (TV)) and cortical bone thickness (Ct. Th) were analyzed. Then, we split the rat tibia shaft longitudinally for the histological evaluations. Briefly, the tibias were fixed in 10% neutral buffered formalin, decalcified in 10% EDTA, and embedded in paraffin as previously described [26]. The 5 μm slices were Gram-stained to assess bacterial colonization.
2.8. Data Analysis
The homogeneity of data variances was assessed by Bartlett's test and the normal distribution of data was determined by the Shapiro-Wilk test. For parametric testing, the one-way analysis of variance model was used to compare the data followed by pairwise multiple comparisons.
3. Results
3.1. Sensitivity of Methicillin-Resistant S. aureus to G. chinensis Extracts
The MIC values of ATCC 29213 and methicillin-resistant S. aureus for G. chinensis aqueous extracts were 15.625 μg/mL and 31.25 μg/mL, respectively (Vancomycin as the positive control in Table A2). The methicillin-resistant S. aureus and ATCC 29213 diameters of the inhibition zones around G. chinensis (1 μg) disks were 20.2 ± 0.5 mm and 23.3 ± 0.3 mm (n=10, P<0.05, Figure 1(c)).
3.2. G. chinensis Suppressed Biofilm and Extracellular Matrix Formation of S. aureus
Crystal violet microtiter assay results revealed that biofilms of methicillin-resistant S. aureus treated with different concentrations of G. chinensis water solutions (62.5μg/mL 31.25 μg/mL, 15.625 μg/mL, and 7.81 μg/mL) were significantly impaired when compared with the control group (n=10, P<0.05, Figures 1(b) and 1(d)). SEM observation demonstrated that methicillin-resistant S. aureus cells were densely packed with extracellular matrix, whereas the methicillin-resistant S. aureus and ATCC strains treated with G. chinensis (1/2 MIC) showed reduced extracellular matrices in the biofilms interspersed among the “blank” areas, and only small microcolonies were randomly observed (Figure 1(e)). After incubation in LB medium with G. chinensis (1/2 MIC), both viable bacteria ratios of methicillin-resistant S. aureus and ATCC strains biofilms were observed by CLSM (Figure 2(a)). The proportion of viable methicillin-resistant S. aureus cells in the G. chinensis extract-treated methicillin-resistant S. aureus strains (33.6 ± 5.2%) was lower than the MRSA strains without intervention (60.89 ± 5.0%) (P<0.05, n=10, Figure 2(c)), which was similar to the ATCC 29213 strain treated by G. chinensis extract (28.8 ± 4.8%). By double-staining and CLSM observation, we found that EPS production in rat bone specimens clearly decreased in the G. chinensis-treated MRSA and ATCC groups (Figure 2(b)). These findings were further confirmed by quantitative data revealing that G. chinensis-treated MRSA cells exhibited a lower EPS/bacterial biomass (44 ± 5%) volume ratio than not-treated MRSA cells (76 ± 6%, P<0.05, n=10, Figure 2(d)).
Figure 2.
G. chinensis extract suppressed biofilm formation and extracellular matrix of S. aureus. (a) Double labeling of S. aureus biofilm. Green, viable S. aureus bacteria (SYTO 9); red, dead S. aureus bacteria (PI); scale bars, 100 μm; (b) double labeling of S. aureus biofilm formation on bone specimens. Green, total S. aureus bacteria (SYTO 9); red EPS (Alexa Fluor 647); scale bars, 100 μm; (c) percentage (%) of viable S. aureus cells in biofilm (n=10, ∗P<0.05); (d) volumetric ratio of the EPS matrix to the bacterial biomass in the biofilms of S. aureus strains (∗P<0.05, n=10).
3.3. Transcriptome Analysis Revealed that G. chinensis Modulates Carbohydrate Metabolism
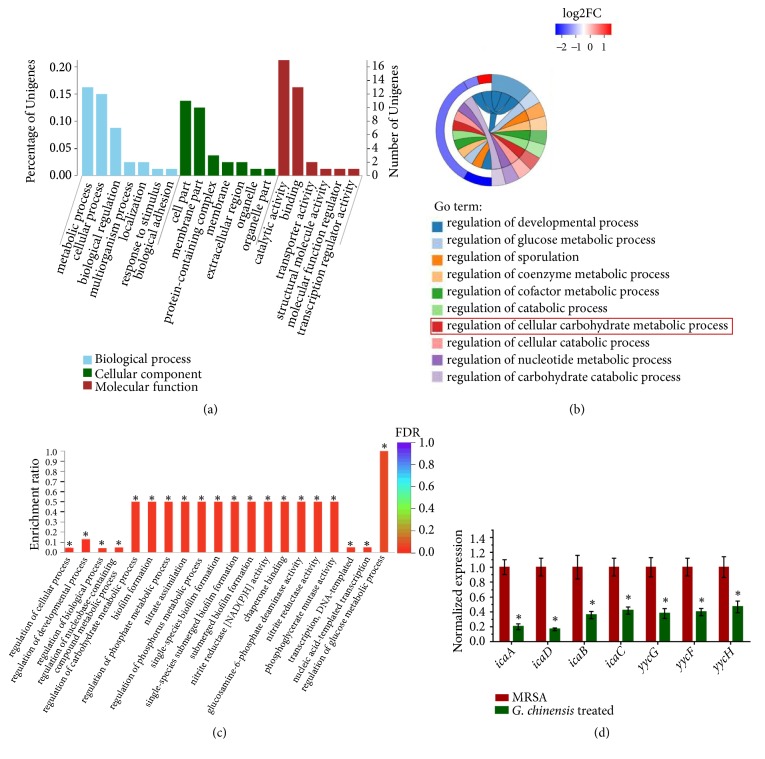
Using RNA-Seq, G. chinensis extract (1/2 MIC) treatment differentially regulated genes related to the regulation of carbohydrate metabolic processes, including glucose metabolic processes and biofilm formation processes (Figures 3(b) and 3(c)). GO enrichment showed altered carbohydrate metabolic processes and biofilm formation processes, suggesting that G. chinensis extract affected carbohydrate utilization by methicillin-resistant S. aureus. We next validated the gene expression of biofilm-associated genes by RT-qPCR (Figure 3(d)). In the G. chinensis-treated group, the mRNA expression levels of yycG, yycF, yycF, icaA, icaB, and icaD were significantly lower than those in the untreated group. In particular, the expression levels of icaA and icaD in the G. chinensis-treated group were five times lower than those in the untreated group. Consistently, these results demonstrated that G. chinensis suppressed the expression of S. aureus biofilm-associated genes and exopolysaccharide synthesis genes.
Figure 3.
Transcriptome analysis revealed that G. chinensis modulates carbohydrate metabolism. (a) Gene ontology terms annotation statistics; (b) Gene ontology enrichment analysis string diagrams; the majority of differentially regulated genes were related to carbohydrate metabolic processes, shown in red; (c) significant terms in Gene ontology enrichment analysis (∗FDR<0.05); (d) quantitative real-time PCR (qRT-PCR) validation for the expression changes of selected genes (∗P<0.05, n=10).
3.4. Inhibition Effect of G. chinensis on the Pathogenicity of S. aureus-Infected Osseous Tissue
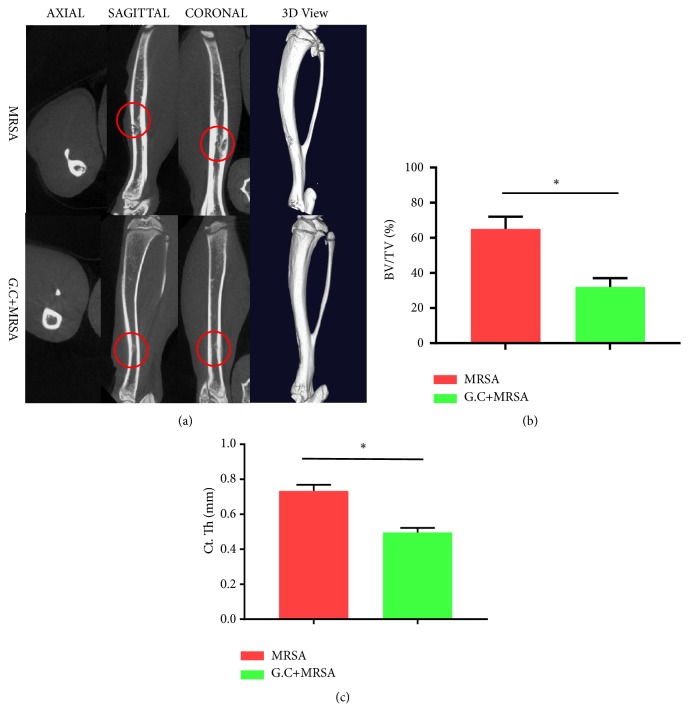
The micro-CT analysis showed significant osteolysis in the cortex and the thickness of the cortex was obviously increased in the methicillin-resistant S. aureus-infected group compared with the G. chinensis extract-treated group (Figure 4(a), upper lane). However, little reactive bone around the cortex was defined in the G. chinensis extract-treated group. This trend indicates that the G. chinensis-treated strains presented a limited capability to induce an infarct in infected bone tissues (Figure 4(a), lower lane). The quantitative data revealed that the average BV/TV value was 32 ± 5.2% in the G. chinensis extract-treated group, which was significantly lower than that in the methicillin-resistant S. aureus group, with a BV/TV value of 65 ± 7.1% (n=5, P<0.05, Figure 4(b)). Furthermore, the average value of cortical bone thickness (Ct. Th) was 0.73 ± 0.04 mm in the MRSA group, which was elevated compared with that in the G. chinensis group (0.49 ± 0.02 mm, n=5, P<0.05, Figure 4(c)). These data indicated that the G. chinensis extracts suppressed the pathogenesis of MRSA in a rat osteomyelitis model.
Figure 4.
Inhibition effect of G. chinensis on pathogenicity of methicillin-resistant S. aureus in vivo. (a) Micro-CT assessments and 3D images of rat tibias infected by methicillin-resistant S. aureus; the osteomyelitis caused by methicillin-resistant S. aureus and the infected regions are indicated (red circle); (b) the average BV/TV values in the methicillin-resistant S. aureus and G. chinensis extract-treated groups (∗P<0.05, n=10); (c) the average cortical bone thickness (Ct. Th) in the methicillin-resistant S. aureus and G. chinensis extract-treated groups (∗P<0.05, n=10).
4. Discussion
MRSA remains among the group of high-priority multidrug-resistant organisms that requires renewed efforts for the development of new antibiotics and innovative preventive approaches [27]. Conventional antibiotics may not be effective against the bacteria that develop resistance [28, 29]. Therefore, screening for Chinese herbal medicines that lessen the use of antibiotics may be a useful method for identifying compounds suitable for infection management. G. chinensis contains large amounts of hydrolysable tannins, which contribute to its effective and broad activities as a topical antibacterial agent [10]. According to Buziashvili et al., the main ingredients of G. chinensis are gallic acid (nearly 20%) and methyl gallate (7%) [30]. It was also reported that methyl gallate and gallic acid have significant growth-inhibitory activity towards the glucosyltransferase enzymes and biofilm formation [31]. On the other hand, G. chinensis contains large amounts of hydrolysable tannins with higher molecular weights, which contribute to its effective and broad activities as a topical antibacterial agent [10]. The components of gallotannins along with tannins make G. chinensis very useful in bacterial control as these effects of tannins may be precipitated by their binding to bacterial proteins [32]. The crude aqueous extracts of G. chinensis were characterized previously by high performance liquid chromatography (HPLC, Figure A1). Extracts from G. chinensis have antibacterial activities against S. aureus, Escherichia coli, and Streptococcus mutans, including growth-inhibitory and biofilm-reducing effects [9, 13].
According to previous reports, the exposure of specific pathogen free mice to G. chinensis extract at 40 mg/L is unlikely to result in significant toxicity [33]. For the antimicrobial susceptibility testing of S. aureus biofilm, our results showed that biofilms of S. aureus treated with different concentrations of G. chinensis extract solutions were significantly impaired when compared with the untreated group. The MIC value of MRSA for G. chinensis aqueous extract was 31.25 μg/mL, which is within the range of what is known to be safe. When treated with 1/2MIC of G. chinensis extract, the growth of the planktonic S. aureus strain was consistently inhibited and had a turbid bacterial suspension appearance (Figures A2 and A3).
The staphylococcal biofilm substance consists of polysaccharide intercellular adhesion (PIA), protein, and extracellular DNA (eDNA), which provides strength to the biofilm [34]. The present results indicated that the water extract of G. chinensis effectively inhibited the production of the extracellular substance matrix during biofilm formation. Additionally, our findings from CLSM showed that the water extracts of G. chinensis could inhibit the EPS/bacteria ratio in the biofilm aggregation on the bone specimens, which was probably related to the bactericidal effect against S. aureus. From this observation, we inferred that G. chinensis extract led to the downregulation of genes involved in biofilm formation and exopolysaccharide synthesis.
Interestingly, transcriptome analysis suggested that G. chinensis extract downregulated the expression of methicillin-resistant S. aureus biofilm-associated genes and exopolysaccharide synthesis genes. These findings are crucial since carbohydrate metabolism and exopolysaccharide synthesis are the key virulence factors for the biofilm formation of MRSA strains [5]. Transcriptome analysis confirmed the decrease in carbohydrate metabolism in the G. chinensis extract-treated methicillin-resistant S. aureus along with a reduced exopolysaccharide matrix in the biofilms. The YycFG TCS plays an essential role in cellular physiology, structure, and biofilm organization, particularly in cell wall metabolism [35]. The matrix of the three-dimensional staphylococcal biofilm is mainly composed of PIAs encoded by the ica operon (glycosyl transferase family protein) in S. aureus [36].
Osteomyelitis is a common disease of a major challenge for clinical treatment, particularly when infected by methicillin-resistant S. aureus, which often requires a combination of aggressive surgery and extended antibiotic therapy [37]. In this study, we validated the role of the G. chinensis extract in limiting the invasive ability and pathogenicity of methicillin-resistant S. aureus in vivo. We recorded reactive bone formation and bone infarction surrounding the bone tissues infected by MRSA strains in a rat model. The results indicated that G. chinensis extract inhibited the invasive ability and pathogenicity of MRSA in vivo. However, the limitation of the present study was a lack of the histology methods to observe, osteoblast, and osteoclast which would be considered in the future.
5. Conclusions
In summary, the results indicated the sensitivity of methicillin-resistant S. aureus to the G. chinensis water extracts. Furthermore, we showed that G. chinensis extract leads to a reduction in dextran-dependent aggregation and biofilm formation in S. aureus biofilms. Based on the transcriptome analysis, G. chinensis extract significantly affected the expression of several genes related to biofilm formation and influenced carbohydrate metabolism in methicillin-resistant S. aureus. Furthermore, we showed that G. chinensis extract inhibited the invasive ability and pathogenicity of methicillin-resistant S. aureus in vivo. Taken together, the antimicrobial properties of G. chinensis extract are probably linked to its modulation of methicillin-resistant S. aureus carbohydrate metabolism, which makes it a potential compound useful for the management of methicillin-resistant S. aureus infections.
Acknowledgments
We are most grateful to Professor Huiqi Xie for her excellent technical assistance. The study was supported by grants from the National Nature Science Foundation of China [No. 81800964], China Postdoctoral Science Foundation [2018M633380], and Sichuan Provincial Natural Science Foundation of China [Grant Nos. 2018SZ0125 and 2019YFS0270].
List of Abbreviations
- S. aureus:
Staphylococcus aureus
- G. chinensis:
Galla chinensis
- qRT-PCR:
Quantitative real-time PCR
- MIC:
Minimum inhibitory concentration
- SEM:
Scanning electron microscopy
- CLSM:
Confocal laser scanning microscopy
- RNA-seq:
RNA sequencing
- HPLC:
High performance liquid chromatography.
Data Availability
The data used to support the findings of this study are included within the article.
Ethical Approval
The West China Hospital of Sichuan University Biomedical Research Ethics Committee approved the animal experiments for this investigation. The Animal Experiments Committee at Sichuan University approved all experiments in this study (Approval No. 2018039A).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Supplementary Materials
Table A1: sequences of primers used for qRT-PCR analysis. Table A2: the minimum inhibitory concentration values for MRSA strain. Figure A1: culture on the solid medium and Gram's staining for S. aureus. Figure A2: chemical fingerprint of Galla chinensis by HPLC. Figure A3: planktonic S. aureus ATCC29213 treated with different concentrations of G. chinensis extract solutions. Figure A4: planktonic MRSA strains treated with different concentrations of G. chinensis extract solutions. Figure A5: the histology methods for the evaluation of the infective tibias in rats.
References
- 1.Boada A., Pons-Vigués M., Real J., Grezner E., Bolíbar B., Llor C. Previous antibiotic exposure and antibiotic resistance of commensal Staphylococcus aureus in Spanish primary care. European Journal of General Practice. 2018;24(1):125–130. doi: 10.1080/13814788.2018.1444748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Belkum A., Verkalk N. J., De Vogel C. P., et al. Reclassification of staphylococcus aureus nasal carriage types. The Journal of Infectious Diseases. 2009;199(12):1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 3.García-Álvarez L., Holden M. T. G., Lindsay H., et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. The Lancet Infectious Diseases. 2011;11(8):595–603. doi: 10.1016/s1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall C. W., Mah T. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiology Reviews. 2017;41(3):276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 5.Wu S., Huang F., Zhang H., Lei L. Staphylococcus aureus biofilm organization modulated by YycFG two-component regulatory pathway. Journal of Orthopaedic Surgery and Research. 2019;14(1):p. 10. doi: 10.1186/s13018-018-1055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer N. K., Mazaitis M. J., Costerton J. W., Leid J. G., Powers M. E., Shirtliff M. E. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence. 2011;2(5):445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rupp M. E., Fey P. D., Heilmann C., Götz F. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. The Journal of Infectious Diseases. 2001;183(7):1038–1042. doi: 10.1086/319279. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X., Liu Z., Liu Z., et al. Phenotype and RNA-seq-Based transcriptome profiling of Staphylococcus aureus biofilms in response to tea tree oil. Microbial Pathogenesis. 2018;123:304–313. doi: 10.1016/j.micpath.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Ahn Y.-J., Lee C.-O., Kweon J.-H., Ahn J.-W., Park J.-H. Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria. Journal of Applied Microbiology. 1998;84(3):439–443. doi: 10.1046/j.1365-2672.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen J.-C., Ho T.-Y., Chang Y.-S., Wu S.-L., Hsiang C.-Y. Anti-diarrheal effect of Galla Chinensis on the Escherichia coli heat-labile enterotoxin and ganglioside interaction. Journal of Ethnopharmacology. 2006;103(3):385–391. doi: 10.1016/j.jep.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 11.State Pharmacopoeia Committee of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China. Beijing, China: Chemical Industry Press China; 2015. [Google Scholar]
- 12.Giron D., Huguet E., Stone G. N., Body M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. Journal of Insect Physiology. 2016;84:70–89. doi: 10.1016/j.jinsphys.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Djakpo O., Yao W. Rhus chinensis and Galla Chinensis - Folklore to modern evidence: Review. Phytotherapy Research. 2010;24(12):1739–1747. doi: 10.1002/ptr.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang F., Peng L., Yin Z., et al. Acute and subchronic toxicity as well as evaluation of safety pharmacology of Galla chinensis solution. Journal of Ethnopharmacology. 2015;162:181–190. doi: 10.1016/j.jep.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Cheng L., Exterkate R., Zhou X., Li J., ten Cate J. Effect of galla chinensis on growth and metabolism of microcosm biofilms. Caries Research. 2011;45(2):87–92. doi: 10.1159/000324084. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L., Li J., Hao Y., Zhou X. Effect of compounds of Galla chinensis and their combined effects with fluoride on remineralization of initial enamel lesion in vitro. Journal of Dentistry. 2008;36(5):369–373. doi: 10.1016/j.jdent.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Nuño G., Alberto M., Arena M., Zampini I., Isla M. Effect of Zuccagnia punctata Cav. (Fabaceae) extract on pro-inflammatory enzymes and on planktonic cells and biofilm from Staphylococcus aureus. Toxicity studies. Saudi Journal of Biological Sciences. 2018;25(8):1713–1719. doi: 10.1016/j.sjbs.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei L., Shao M., Yang Y., Mao M., Yang Y., Hu T. Exopolysaccharide dispelled by calcium hydroxide with volatile vehicles related to bactericidal effect for root canal medication. Journal of Applied Oral Science. 2016;24(5):487–495. doi: 10.1590/1678-775720160014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng L., Burne R. A., Nojiri H. Sucrose- and Fructose-Specific Effects on the Transcriptome of Streptococcus mutans, as Determined by RNA Sequencing. Applied and Environmental Microbiology. 2015;82(1):146–156. doi: 10.1128/AEM.02681-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulos P., Hatzis P. Systematic integration of RNA-Seq statistical algorithms for accurate detection of differential gene expression patterns. Nucleic Acids Research. 2015;43(4):p. e25. doi: 10.1093/nar/gku1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Götz S., García-Gómez J. M., Terol J., et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Research. 2008;36(10):3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei L., Stipp R., Chen T., Wu S., Hu T., Duncan M. Activity of Streptococcus mutans VicR is modulated by antisense RNA. Journal of Dental Research. 2018;97(13):1477–1484. doi: 10.1177/0022034518781765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poeppl W., Tobudic S., Lingscheid T., et al. Daptomycin, fosfomycin, or both for treatment of methicillin-resistant staphylococcus aureus osteomyelitis in an experimental rat model. Antimicrobial Agents and Chemotherapy. 2011;55(11):4999–5003. doi: 10.1128/AAC.00584-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loc-Carrillo C., Wang C., Canden A., Burr M., Agarwal J., Chen C. Local intramedullary delivery of vancomycin can prevent the development of long bone Staphylococcus aureus infection. PLoS ONE. 2016;11(7):p. e0160187. doi: 10.1371/journal.pone.0160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovati A. B., Bottagisio M., Maraldi S., et al. Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clinical Orthopaedics and Related Research. 2018;476(6):1324–1338. doi: 10.1097/01.blo.0000534692.41467.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z., Wu S., Li Z., et al. Comparison of small intestinal submucosa and polypropylene mesh for abdominal wall defect repair. Journal of Biomaterials Science, Polymer Edition. 2018;29(6):663–682. doi: 10.1080/09205063.2018.1433419. [DOI] [PubMed] [Google Scholar]
- 27.Lee A. S., de Lencastre H., Garau J., et al. Methicillin-resistant Staphylococcus aureus. Nature Reviews Disease Primers. 2018;4(1):p. 18033. doi: 10.1038/nrdp.2018.34. [DOI] [PubMed] [Google Scholar]
- 28.Dale J. L., Cagnazzo J., Phan C. Q., Barnes A. M., Dunny G. M. Multiple roles for enterococcus faecalis glycosyltransferases in biofilm-associated antibiotic resistance, cell envelope integrity, and conjugative transfer. Antimicrobial Agents and Chemotherapy. 2015;59(7):4094–4105. doi: 10.1128/AAC.00344-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alekshun M. N., Levy S. B. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128(6):1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Buziashvili I. S., Komissarenko N. F., Kovalev I. P., Gordienko V. G., Kolesnikov D. G. The structure of gallotannins. Chemistry of Natural Compounds. 1973;9(6):752–755. doi: 10.1007/BF00565801. [DOI] [Google Scholar]
- 31.Shao D., Li J., Li J., et al. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. Journal of Food Science. 2015;80(6):M1299–M1305. doi: 10.1111/1750-3841.12902. [DOI] [PubMed] [Google Scholar]
- 32.Haslam E., Lilley T., Cai Y., Martin R., Mangnolato D. Traditional herbal medicines - the role of polyphenols. Planta Medica. 1989;55(01):1–8. doi: 10.1055/s-2006-961764. [DOI] [PubMed] [Google Scholar]
- 33.Iminjan M., Amat N., Li X.-H., Upur H., Ahmat D., He B. Investigation into the toxicity of traditional uyghur medicine quercus infectoria galls water extract. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0090756.e90756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadovskaya I., Vinogradov E., Flahaut S., Kogan G., Jabbouri S. Extracellular carbohydrate-containing polymers of a model biofilm-producing strain, Staphylococcus epidermidis RP62A. Infection and Immunity. 2005;73(5):3007–3017. doi: 10.1128/iai.73.5.3007-3017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubrac S., Bisicchia P., Devine K. M., Msadek T. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Molecular Microbiology. 2008;70(6):1307–1322. doi: 10.1111/j.1365-2958.2008.06483.x. [DOI] [PubMed] [Google Scholar]
- 36.O'Gara J. P. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiology Letters. 2007;270(2):179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt S. K. Osteomyelitis. Infectious Disease Clinics of North America. 2017;31:325–338. doi: 10.1016/j.idc.2017.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A1: sequences of primers used for qRT-PCR analysis. Table A2: the minimum inhibitory concentration values for MRSA strain. Figure A1: culture on the solid medium and Gram's staining for S. aureus. Figure A2: chemical fingerprint of Galla chinensis by HPLC. Figure A3: planktonic S. aureus ATCC29213 treated with different concentrations of G. chinensis extract solutions. Figure A4: planktonic MRSA strains treated with different concentrations of G. chinensis extract solutions. Figure A5: the histology methods for the evaluation of the infective tibias in rats.
Data Availability Statement
The data used to support the findings of this study are included within the article.