Abstract
Objective
Numerous studies have explored the role of methylenetetrahydrofolate reductase gene (MTHFR) C677T polymorphism and homocysteine (Hcy) concentration in obesity, but the results are inconsistent. Hence, we performed a meta-analysis implementing Mendelian randomization approach to test the assumption that the increased Hcy concentration is plausibly related to the elevated risk of obesity.
Methods
Eligible studies were selected based on several inclusion and exclusion criteria. Correlations between MTHFR C677T and obesity risk, MTHFR C677T and Hcy concentration in obesity, Hcy concentration, and obesity were estimated by ORs, effect size and standard mean difference with their corresponding 95% CIs, respectively. Furthermore, Mendelian randomization analysis was performed to estimate the relationship between Hcy level and obesity.
Results
Consequently, this meta-analysis implemented with Mendelian randomization approach was conducted among 8,622 cases and 29,695 controls. The results indicated that MTHFR C677T is associated with an increased risk of obesity (for T vs C: OR=1.06, 95% CI=1.02–1.10; for TT vs CC: OR=1.13, 95% CI=1.03–1.24). Moreover, in obese subjects, the pooled Hcy concentration in individuals of TT genotype was 2.91 mmol/L (95% CI: 0.27–5.55) higher than that in individuals of CC genotype. Furthermore, the pooled Hcy concentration in subjects with obesity was 0.74 mmol/L (95% CI: 0.36–1.12) higher than that in controls. The evaluated plausible OR associated with obesity was 1.23 for 5 μmol/L Hcy level increase.
Conclusions
Through this meta-analysis, we emphasize a strong relationship between Hcy level and obesity by virtue of MTHFR C677T polymorphism.
Keywords: homocysteine, MTHFR, obesity, polymorphism
Introduction
Nowadays, many chronic diseases including cardiovascular diseases, hypertension and diabetes mellitus are closely related to obesity and being overweight/obese could strongly elevate the likelihood of these chronic diseases, which is becoming a serious public health issue globally.1 Twin, family, and adoption studies indicated that the rate of heritability of body mass index (BMI) is high, accounting for 40–70%,2 indicating that genetic factors have a pivotal role in the pathophysiology of obesity. Thus, detecting genetic factors which caused overweight/obesity could be of great significance not only in comprehending the developmental pathogenesis of this disease but also in providing more effective intervention programs to reduce the incidence of obesity.
Methylenetetrahydrofolate reductase (MTHFR) is a key rate-limiting enzyme accounting for the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which serves as a crucial enzymatic process in the remethylation of homocysteine (Hcy) to methionine.3 For the MTHFR C677T polymorphism, a single base pair C to T substitution causes an alanine into valine change. As a result, the homozygous MTHFR 677TT genotype expresses a heat-sensitive enzyme with reduced activity, which leads to reduced folate level and elevated plasma Hcy level.3,4 Previous epidemiological studies have indicated that Hcy concentration and folate level were associated with an enhanced risk of overweight/obesity.5–7 The mechanisms with respect to these observations remain unclear. Nevertheless, some researchers have speculated that enhanced Hcy concentrations might influence the development of obesity by means of controlling body fat storage in the epigenetic regulation of gene expression because the Hcy metabolism pathway is strongly related to methylation of DNA and amino acid residues on histones.8–11 Moreover, recent researches from genetic studies and animal experiments could stand by this hypothesis.12–14
In recent years, there exist numerous studies exploring the association between MTHFR C677T polymorphism and obesity. However, it is difficult to draw a definitive conclusion to date because of the controversial results. Additionally, the associations of MTHFR C677T polymorphism, Hcy level and obesity are still equivocal. For providing more evidence of the underlying association, we carried out a meta-analysis of the published articles with regard to the risk of obesity associated with an enhanced Hcy concentration and the MTHFR C677T polymorphism to obtain pooled estimates of these associations. Moreover, a Mendelian randomization approach, which is acknowledged as an epidemiological method based on the random assignment of an individual’s genotype from his/her parental genotypes, was performed to test the assumption that enhanced Hcy concentration is plausibly associated with the elevated risk of obesity.
Materials and methods
Selection of studies
Studies that evaluated the association between the MTHFR gene 677 C > T polymorphism and Hcy level with the development of obesity were included in this meta-analysis. A detailed literature retrieval was conducted independently by two investigators for publication from PubMed, Embase and Web of Science by using the following terms: “MTHFR”, “rs1801133”, “MTHFR C677T”, “homocysteine”, “Hcy”, “obesity” and “obese”, up to 21 September 2018.
The following criteria were used to select the eligible studies: 1) case-control, cross-sectional or case-cohort designed studies; 2) providing the distributions of the MTHFR C677T genotypes in obesity and in controls free of obesity, respectively. Reviews or letters, abstract and editorials were excluded. The language was restricted to English.
Data extraction
Data were carefully drawn by two independent investigators, and any disagreements were resolved after discussion with a third investigator. Following information was extracted from each study: 1) the surname of the first author, the publication year, the country and ethnicity of subjects; 2) number of cases, number of controls, the diagnostic standard of cases and controls, genotype distribution in all groups. For articles including different study populations, data were extracted, respectively. Extracted data were analyzed by using the Stata, version 12.0 (StataCorp LP, College Station, TX, USA).
Statistical analysis
For controls in each study, the Chi-squared test was employed to evaluate whether the Hardy–Weinberg equilibrium (HWE) was violated. Sensitivity analysis by removing one study with controls not in HWE was performed to assess the stability of the results.
Four genetic models including homozygous codominant model (TT vs CC), allelic model (T vs C), dominant model (TT+TC vs CC) and recessive model (TT vs TC+CC) are considered, and associations were represented as ORs with their matching 95% CIs for each study. Based on the individual ORs, a pooled OR was concluded. For each of those four models, Metan command in Stata was performed to evaluate the mean difference between MTHFR 677TT group and MTHFR 677CC group in obese subjects. Hcy level was pooled to compute the standardized mean difference with its corresponding 95% CI for comparing the obese subjects with the healthy ones. Cochrane’s Q test15,16 was carried out to test the between-study heterogeneity (significance at I2>50.0% and P<0.10). If there is no heterogeneity, we fitted the fixed-effects model to the data; otherwise, we employed the random-effects model.17 For the meta-analysis of the association between MTHFR C677T and obesity, subgroup analyses by ethnicity and age range (defined age ≥18 as adults, <18 as children) were also performed. We used Begg’s funnel plot and Egger’s regression test (P<0.05 was considered statistically significant) to estimate publication bias.18
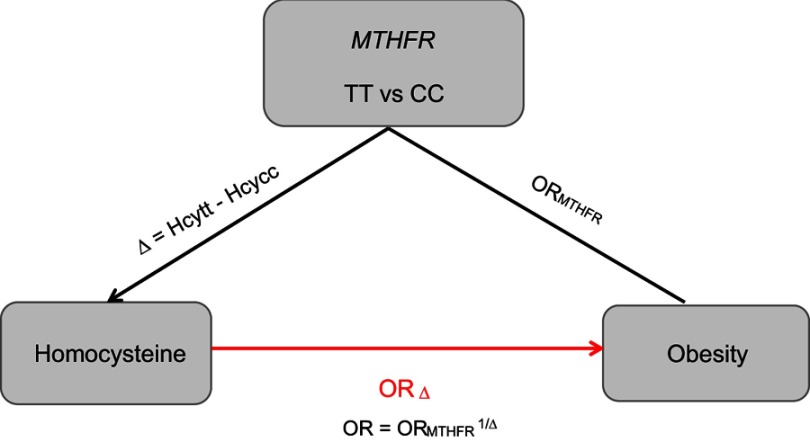
Mendelian randomization analysis integrates the information of genotype-intermediate phenotype and genotype-disease association into an analytical framework, which can provide an unbiased estimate of the intermediate phenotype-disease association. For the genetic variant MTHFR C677T to be a valid instrumental variable in Mendelian randomization, three conditions are to be satisfied: 1) the MTHFR C677T has to be associated with Hcy level robustly; 2) confounding factors, which could bias the association of Hcy level and obesity, should not be associated with the genotype in the MTHFR; 3) variant of MTHFR 677C > T has an influence on the obesity only through the specific intermediate Hcy level.19 MTHFR C677T may meet all these conditions well based on the available evidence.9,14,20,21 Therefore, Mendelian randomization coefficient evaluated by utilizing MTHFR C677T as an instrument should make a plausible reference to Hcy level and obesity. Compared to MTHFR 677CC, the genotype of MTHFR 677TT is associated with the increased risk of obesity, and its effect is gauged by the ORTT vs CC. Further, compared with CC, TT is associated with the mean difference (Δ) of Hcy level. OR1=(ORTT vs CC)1/Δ would be an unconfounded estimate of the effect of obesity due to one unit change on the Hcy level. In this analysis, we adopted ORk=(ORTT vs CC)k/Δ for an increase of k units,22 and we thus analyzed 5 μmol/L increment in Hcy level to assess the OR.23
Results
Characteristics of the studies
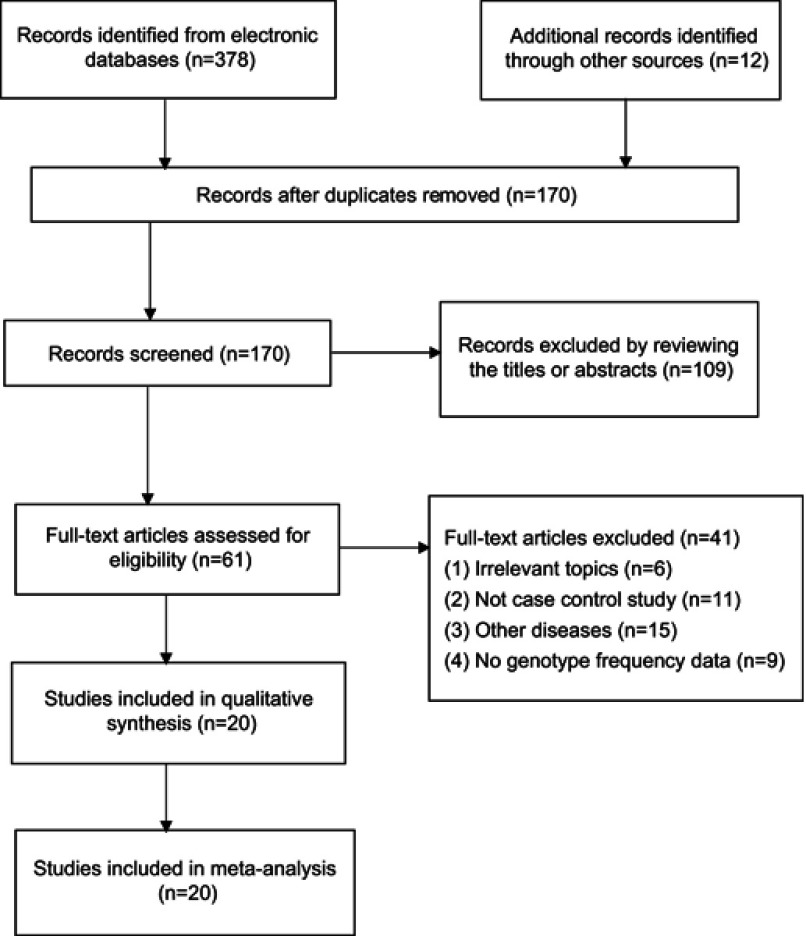
The detailed information of screening various studies in the meta-analysis is described in Figure 1. The 20 studies provided 8,622 cases and 29,695 controls,9,11,24–38 which supplied the genotypes to estimate the association of MTHFR C677T and obesity. In these studies, the frequencies of the TT genotype were the lowest in cases and in controls, while that for genotype CC was the highest. Five studies only described the association between MTHFR C677T and Hcy level in obese patients.39–42 In two studies,9,31 the genotype distribution in the control subjects was not in accordance with HWE (P<0.05) (Table 1).
Figure 1.
PRISMA flow diagram for selection of studies in the meta-analysis.
Table 1.
The genotypic and allelic distributions of MTHFR C677T for cases and controls
| First author | Year | Country | Ethnicity | Age range | Genotype distribution | Allele frequency | P-value HWE | Number of cases/controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||||||||||||
| CC | CT | TT | CC | CT | TT | C | T | C | T | |||||||
| Glueck24 | 2003 | America | White | Adults | 13 | 12 | 3 | 5 | 5 | 0 | 38 | 18 | 15 | 5 | 0.292 | 28/10 |
| Terruzzi9 | 2007 | Italy | White | Adults | 18 | 54 | 12 | 14 | 33 | 5 | 90 | 78 | 61 | 43 | 0.026 | 84/52 |
| Lewis(BWHHS)11 | 2008 | UK | White | Adults | 360 | 410 | 112 | 1165 | 1086 | 283 | 1130 | 634 | 3416 | 1652 | 0.214 | 882/2543 |
| Lewis(ALSPAC for women)11 | 2008 | UK | White | Adults | 163 | 155 | 38 | 2707 | 2713 | 715 | 481 | 231 | 8127 | 4143 | 0.375 | 356/6135 |
| Lewis(ALSPAC for children)11 | 2008 | UK | White | Children | 115 | 93 | 25 | 2155 | 2190 | 552 | 323 | 143 | 6500 | 3294 | 0.902 | 233/4897 |
| Lewis(CCHS)11 | 2008 | Denmark | White | Adults | 588 | 574 | 107 | 3812 | 3356 | 736 | 1750 | 788 | 10,980 | 4828 | 0.946 | 1269/7904 |
| Settin25 | 2009 | Saudi | Asian | Adults | 89 | 34 | 5 | 69 | 36 | 5 | 212 | 44 | 174 | 46 | 0.912 | 128/110 |
| Tavakkoly Bazzaz26 | 2010 | Iran | Asian | Adults | 44 | 21 | 9 | 113 | 80 | 14 | 109 | 39 | 306 | 108 | 0.975 | 74/207 |
| Gara28 | 2011 | Tunisian | African | Children | 15 | 14 | 2 | 9 | 12 | 1 | 44 | 18 | 30 | 14 | 0.228 | 31/22 |
| Bokor27 | 2011 | France | White | Children | 97 | 99 | 17 | 130 | 154 | 33 | 293 | 133 | 414 | 220 | 0.2 | 213/317 |
| Tabassum30 | 2012 | Indian | Asian | Children | 290 | 144 | 20 | 581 | 218 | 31 | 724 | 184 | 1380 | 280 | 0.068 | 454/830 |
| Yin31 | 2012 | China | Asian | Adults | 354 | 341 | 56 | 471 | 441 | 66 | 1049 | 453 | 1383 | 573 | 0.006 | 751/978 |
| Chauhan29 | 2012 | Indian | Asian | Adults | 348 | 185 | 29 | 272 | 148 | 16 | 881 | 243 | 692 | 180 | 0.451 | 562/436 |
| Hernandez-Guerrero32 | 2013 | Mexico | Mestizo | Adults | 18 | 38 | 19 | 15 | 28 | 10 | 74 | 76 | 58 | 48 | 0.63 | 75/53 |
| Fan33 | 2015 | China | Asian | Adults | 115 | 244 | 158 | 160 | 375 | 206 | 474 | 560 | 695 | 787 | 0.662 | 517/741 |
| Chedraui34 | 2016 | Ecuador | White | Adults | 51 | 43 | 17 | 35 | 39 | 7 | 145 | 77 | 109 | 53 | 0.399 | 111/81 |
| Kupcinskiene35 | 2016 | Lithuania | White | Adults | 156 | 135 | 28 | 159 | 129 | 15 | 447 | 191 | 447 | 159 | 0.082 | 319/303 |
| Shen36 | 2016 | Canada | White | Adults | 190 | 182 | 51 | 181 | 212 | 53 | 562 | 284 | 574 | 318 | 0.447 | 423/446 |
| Zhi37 | 2016 | China | Asian | Adults | 287 | 633 | 434 | 198 | 438 | 249 | 1207 | 1501 | 834 | 936 | 0.838 | 1354/885 |
| Fu38 | 2018 | China | Asian | Children | 121 | 364 | 273 | 531 | 1353 | 861 | 606 | 910 | 2415 | 3075 | 0.99 | 758/2745 |
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; HWE, Hardy-Weinberg equilibrium test; The P-value for HWE testing for controls is shown; BWHHS, British Women’s Heart and Health Study; ALSPAC, Avon Longitudinal Study of Parents and Children women cohort study; CCHS, Copenhagen City Heart Study.
The mean Hcy concentration difference between MTHFR genotypes in obese patients
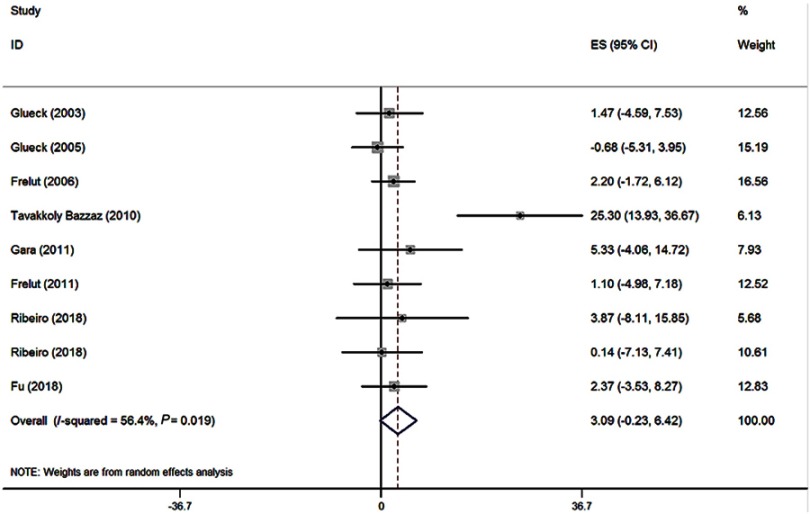
According to the inclusion criteria, nine studies (8 references, 420 obese patients)24,26,28,38–42 were selected, and they reported the Hcy concentration in different genotypic groups by means of the arithmetic mean and the corresponding SD in obese patients. In all these studies, none of them was not in HWE, and the mean Hcy level was higher in MTHFR 677TT subjects than that in the other genotypes. The pooled mean Hcy level in MTHFR 677TT subjects was 2.91 μmol/L (95% CI: 0.27–5.55) higher than that in MTHFR 677CC subjects (P=0.031) (Figure 2). Meanwhile, the MTHFR 677TT subjects had 3.09 μmol/L (95% CI: (−0.23)-6.42) greater Hcy level than MTHFR 677CT subjects (P=0.068) (Figure 3).
Figure 2.
Forest plot of the evaluation for the effect size (ES) in Hcy level between the MTHFR genotypes (TT vs CC) in obese patients.
Figure 3.
Forest plot of the evaluation for the effect size (ES) in Hcy level between the MTHFR genotypes (TT vs CT) in obese patients.
The association between MTHFR C677T and the risk of obesity
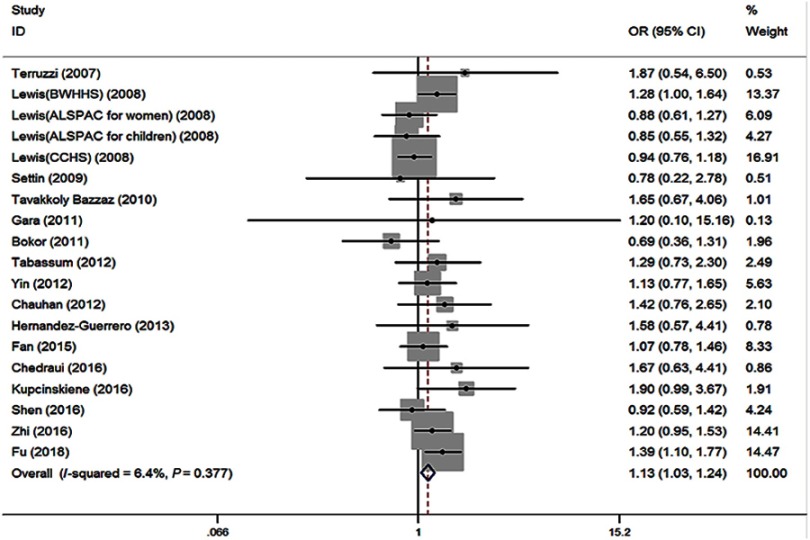
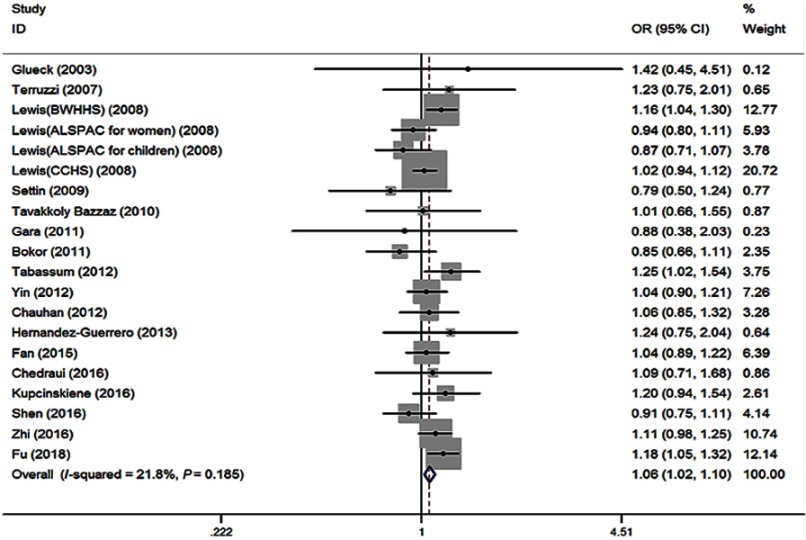
No significant heterogeneity (I2=6.4%, P=0.377) among 19 studies was shown in the primary outcome for revealing the association of MTHFR 677TT with the risk of obesity, compared to MTHFR 677CC. The fixed effect (FE) pooled OR was significant: FE OR=1.13 (95% CI: 1.03–1.24) (P=0.007) (Figure 4). Above all, the T allele in the MTHFR C677T conferred a higher risk of obesity (FE OR=1.06 [95% CI: 1.02–1.10] [P=0.003], I2=21.8%, P=0.185) (Figure 5). MTHFR 677TT revealed a significantly greater risk for obesity compared with CC+CT genotype (FE OR=1.12 [95% CI: 1.04–1.22] [P=0.003], I2=0, P=0.558). MTHFR 677 TT+CT also revealed a significantly greater risk for obesity compared with CC genotype (FE OR=1.06 [95% CI: 1.00–1.12] [P=0.045], I2=21.0%, P=0.194). Subgroup analysis by the ethnicity demonstrated its correlations under recessive, homozygous codominant, and allelic models in Asian (TT vs CC+CT: OR=1.20, 95% CI=1.09–1.33, P<0.001; TT vs CC: OR=1.24, 95% CI=1.09–1.41, P=0.001; T vs C: OR=1.11, 95% CI=1.04–1.17, P=0.001). Moreover, subgroup analysis by the age showed the associations under recessive, homozygous codominant, and allelic models in adults (TT vs CC+CT: OR=1.11, 95% CI=1.02–1.22, P=0.022; TT vs CC: OR=1.12, 95% CI=1.01–1.24, P=0.036; T vs C: OR=1.05, 95% CI=1.01–1.10, P=0.023) and under recessive and allelic genetic models in children (TT vs CC+CT: OR=1.16, 95% CI=1.00–1.34, P=0.05; T vs C: OR=1.09, 95% CI=1.00–1.19, P=0.047) with the risk of developing obesity (Table 2).
Figure 4.
Forest plot of the MTHFR C677T associated with obesity risk (under homozygous codominant model: TT vs CC).
Abbreviations: BWHHS, british women’s heart and health study; ALSPAC, avon longitudinal study of parents and children women cohort study; CCHS, copenhagen city heart study.
Figure 5.
Forest plot of the MTHFR C677T associated with obesity risk (under allelic model: T vs C).
Abbreviations: BWHHS, british women’s heart and health study; ALSPAC, avon longitudinal study of parents and children women cohort study; CCHS, copenhagen city heart study.
Table 2.
Stratified analysis of associations of MTHFR C677T polymorphisms with obesity
| Subgroup | Dominant | Recessive | Homozygous Codominant | Allelic Model | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Ph | OR (95% CI) | Ph | OR (95% CI) | Ph | OR (95% CI) | Ph | |
| Overall | 1.06 (1.00–1.12) | 0.194 | 1.13 (1.04–1.22) | 0.558 | 1.13 (1.03–1.24) | 0.377 | 1.06 (1.02–1.10) | 0.185 |
| Ethnicity | ||||||||
| Asian | 1.09 (0.99–1.19) | 0.282 | 1.20 (1.09–1.33) | 0.954 | 1.24 (1.09–1.41) | 0.872 | 1.11 (1.04–1.17) | 0.509 |
| White | 1.04 (0.97–1.12) | 0.111 | 1.02 (0.90–1.15) | 0.283 | 1.03 (0.91–1.18) | 0.152 | 1.03 (0.97–1.08) | 0.117 |
| Others | 1.04 (0.55–1.98) | 0.448 | 1.46 (0.65–3.28) | 0.996 | 1.52 (0.59–3.92) | 0.842 | 1.13 (0.74–1.74) | 0.491 |
| Age | ||||||||
| Adults | 1.05 (0.99–1.12) | 0.605 | 1.11 (1.02–1.22) | 0.45 | 1.12 (1.01–1.24) | 0.511 | 1.05 (1.01–1.10) | 0.616 |
| Children | 1.08 (0.95–1.23) | 0.018 | 1.16 (1.00–1.34) | 0.504 | 1.19 (0.98–1.43) | 0.151 | 1.09 (1.00–1.19) | 0.018 |
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; OR, odds ratio; CI, confidence interval; Ph, P-value for heterogeneity test.
The associations of plasma Hcy level with obesity
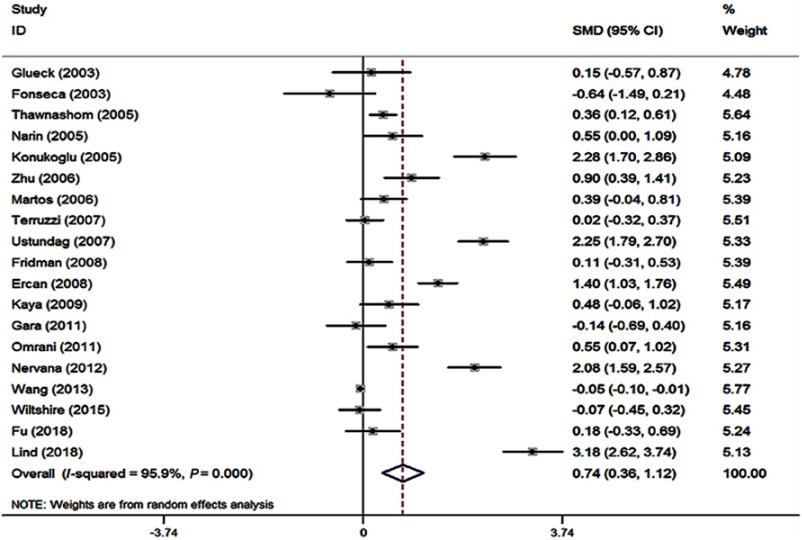
The standard mean difference (SMD) of Hcy level between the subjects with and without obesity indicated the effect on obesity. A forest plot is displayed in Figure 6. In this meta-analysis, there was significant heterogeneity (I2=95.9%, P<0.001) among the included studies. In 19 of these studies,7,9,24,28,38,43–56 the mean Hcy level was higher in the obese group than that in the control group (Figure 6). The pooled mean Hcy level in the obese group was 0.74 μmol/L (95% CI: 0.36–1.12) higher than that in the control group for the random-effects model (P<0.001). The subgroup analysis by ethnicity was carried out, and all the corresponding results from White, Asian, and others showed significant differences in Hcy concentration between obese subjects and control ones. Moreover, both Begg’s and Egger’s tests were performed to see whether there is potential publication bias. No evidence of substantial publication bias was found for the Hcy–obesity association (data not shown).
Figure 6.
Forest plot of standardized mean difference (SMD) in Hcy levels between obese patients and control subjects in included studies.
The plausible relationship between Hcy level and obesity via Mendelian randomization
By means of MTHFR C677T as an instrument variable for Hcy level, the Hcy level per unit increment associated with the predicted OR of obesity by indirect or direct measurement is shown in Figure 7. The Hcy concentrations were positively associated with the risk of developing obesity. The evaluated plausible OR was 1.23 (95% CI: 1.05–1.45) for 5 μmol/L Hcy concentration increase.
Figure 7.
Forest plot of standardized mean difference (SMD) in Hcy levels between obese patients and control subjects in included studies.
Discussion
This study indicated that MTHFR 677T allele was significantly associated with the increased plasma Hcy level. Moreover, the mean Hcy level in obese patients was higher than that in those without obesity. The findings by means of Mendelian randomization method reinforced the hypothesis that the increased Hcy concentration plausibly influenced the elevated risk of obesity.
MTHFR C677T is a point mutation that changes cysteine into thymine nucleotide, which results in the substitution of alanine to valine in the MTHFR enzyme.57 Because of the reduced activity of the enzyme, the variant in the MTHFR gene decreases the thermostability of the enzyme, especially at 37°C or greater. Compared to the normal nonmutated controls, the activity of MTHFR enzyme in homozygous subjects is lower close to 50–60% at 37°C and 65% at 46°C.58,59 The deactivation of this enzyme leads to the increased Hcy level in the homozygous subjects. Thus, the Hcy concentration of homozygous subjects is higher than those of heterozygous mutated subjects, and the heterozygous subjects have mildly elevated the Hcy concentration compared to the nonmutated controls.59 The findings of our meta-analysis supported the hypothesis that the MTHFR C677T was strongly linked with the Hcy level in obese subjects. The homozygous subjects have significantly greater Hcy concentration than that of the heterozygous subjects in obese patients, as previously reported in the literature.26
Previous studies have reported inconsistent results with regard to the altered Hcy concentrations in obese patients. Recently, a research on 3,833 obese patients and 3,367 normal controls found that the level of Hcy in obese patients was lower than that in normal controls.54 However, other studies indicated that the Hcy concentrations were significantly greater in obese patients than that in subjects without obesity.52,53,56 The present meta-analysis mainly analyzed the weighted mean difference of Hcy levels between obese cases and normal controls, which suggested that the absolute pooled mean Hcy level in obesity was significantly greater than that in controls. Due to the heterogeneity of subjects encompassed in these studies concerning the ethnicity of different regions and the coexistence of obesity-related disease,52–54,56 we applied a random-effects model to reduce the heterogeneity. Hcy level has a crucial influence on the process of regulating the correlation between methylation of DNA and amino acid residues on histones. This process has been recognized as one of the epigenetic mechanisms that regulate the gene expression.9–11,13,14 The improved Hcy concentrations might affect the progress of developing obesity by means of regulating gene expression in body fat accumulation. Recently, research on genetics and animal experiments seem to elucidate this hypothesis.12–14 Overall, the homocysteine metabolism pathway might have a substantial role in leading to obesity.
MTHFR C677T was first identified as a significant variant associated with obesity in a Thai population.46 Subsequently, another study suggested that MTHFR 677T allele had an elevated obesity risk with a 1.24-fold compared with MTHFR 677C allele in Indian children.30 Although a large number of studies have assessed the associations between MTHFR C677T and overweight/obesity, the results are controversial in different populations.9,11,25,26,28,30,33,46,60 Our previous study attempted to investigate the relationship between MTHFR C677T and obesity-related traits in a Chinese children population. As a result, we demonstrated that MTHFR 677T had an effect on elevating obese risk in Chinese children.38 The reasons why contradicted results exist in studies concerning MTHFR C677T and obesity are still unclear, but a vital reason might be the racial heterogeneity in the included studies. The distribution frequency of the 677TT genotype was greatest in the Italian and Hispanic.61 However, the homozygous frequency was very low for Blacks in Brazil and American.62–64 Furthermore, the study design flaws, small sample size or other biases seem to be more common factors for the discrepancies comprising in different studies concerning genetic factors.65,66 On the basis of case–control, cross-sectional or case–cohort designed studies, the overall results of the present meta-analysis suggested that MTHFR C677T is associated with obesity and MTHFR TT genotype has an influence on increasing the risk of obesity. In addition, sensitivity analysis suggested that an omission of studies that depart from HWE did not change the magnitude of the observed effect, indicating that the results were generally reliable and robust.
To the best of our knowledge, this is the first meta-analysis aggregating all the data available for evaluating the association between MTHFR-linked Hcy level and obesity. Thus, it has provided the most substantial data on this issue. However, several limitations should be addressed. First, findings from this meta-analysis pooled individual unadjusted results, while potential confounders including age and gender should be taken into account for a more accurate estimate. Second, Hcy measurement via different HPLC and immunoassay approaches showed discrepancies among different laboratories,67 and they may differ in their sensitivity and cutoff values. Therefore, different methods of Hcy measurement seem to result in variation among studies, and this cannot be ignored as an obvious flaw. Third, the genotyping of this SNP was performed by different analytic methods, which could influence the results. Note that both studies,9,31 where the genotype distribution in the control groups was not in accordance with HWE employed the same genotyping method. However, by removing the two studies that depart from HWE, we confirmed the result did not change a lot. Finally, only English articles were included, which might ignore the publication bias, although both Begg’s and Egger’s tests showed no evidence for the existence of substantial publication bias concerning the small effect size.
In conclusion, our results provided sufficient evidence that the TT genotype in MTHFR C677T plausibly leads to the susceptibility of obesity. Through Mendelian randomization, the findings supported the assumption that increased Hcy concentration is strongly linked to elevated risk of obesity. To some degree, the presence of gene–environment interactions may contribute to the discordance of results encompassed in the genetic association studies. Therefore, future prospective studies that explore the gene–environment interaction with larger sample size are expected to help further illuminate the genetics of obesity.
Acknowledgments
This study was supported by grants to YQH from the National Natural Science Foundation of China (grant nos. 11571082 and 11171075), Key Research Project of the Ministry of Science and Technology of China (grant no. 2016YFC0904400), and Scientific Research Foundation of Fudan University.
Author contributions
Study concept and design: LF and YQH; acquisition of data: LF, YQH, and YL; analysis and interpretation of data: LF and YL; drafting of the manuscript: LF and YL; critical revision of the manuscript for important intellectual content: LF, YL, DL, SD and YQH. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.Van Gaal LF, Maggioni AP. Overweight, obesity, and outcomes: fat mass and beyond. Lancet. 2014;383(9921):935–936. doi: 10.1016/S0140-6736(13)62076-0 [DOI] [PubMed] [Google Scholar]
- 2.Bray MS, Loos RJ, McCaffery JM, et al. NIH working group report-using genomic information to guide weight management: from universal to precision treatment. Obesity. 2016;24(1):14–22. doi: 10.1002/oby.21381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111 [DOI] [PubMed] [Google Scholar]
- 4.Pereira AC, Schettert IT, Morandini Filho AA, Guerra-Shinohara EM, Krieger JE. Methylenetetrahydrofolate reductase (MTHFR) c677t gene variant modulates the homocysteine folate correlation in a mild folate-deficient population. Clin Chim Acta. 2004;340(1–2):99–105. [DOI] [PubMed] [Google Scholar]
- 5.Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001;73(3):613–621. doi: 10.1093/ajcn/73.3.613 [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Manini R, Bianchi G, et al. Homocysteine and psychological traits: a study in obesity. Nutrition. 2002;18(5):403–407. [DOI] [PubMed] [Google Scholar]
- 7.Martos R, Valle M, Morales R, Canete R, Gavilan MI, Sanchez-Margalet V. Hyperhomocysteinemia correlates with insulin resistance and low-grade systemic inflammation in obese prepubertal children. Metabolism. 2006;55(1):72–77. doi: 10.1016/j.metabol.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 8.Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15(5):490–495. doi: 10.1016/j.gde.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Terruzzi I, Senesi P, Fermo I, Lattuada G, Luzi L. Are genetic variants of the methyl group metabolism enzymes risk factors predisposing to obesity? J Endocrinol Invest. 2007;30(9):747–753. doi: 10.1007/BF03350812 [DOI] [PubMed] [Google Scholar]
- 10.Williams KT, Schalinske KL. New insights into the regulation of methyl group and homocysteine metabolism. J Nutr. 2007;137(2):311–314. doi: 10.1093/jn/137.2.311 [DOI] [PubMed] [Google Scholar]
- 11.Lewis SJ, Lawlor DA, Nordestgaard BG, et al. The methylenetetrahydrofolate reductase C677T genotype and the risk of obesity in three large population-based cohorts. Eur J Endocrinol. 2008;159(1):35–40. doi: 10.1530/EJE-08-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132(8 Suppl):2393s–2400s. doi: 10.1093/jn/132.8.2393S [DOI] [PubMed] [Google Scholar]
- 13.Melzner I, Scott V, Dorsch K, et al. Leptin gene expression in human preadipocytes is switched on by maturation-induced demethylation of distinct CpGs in its proximal promoter. J Biol Chem. 2002;277(47):45420–45427. doi: 10.1074/jbc.M208511200 [DOI] [PubMed] [Google Scholar]
- 14.Newell-Price J. Proopiomelanocortin gene expression and DNA methylation: implications for Cushing’s syndrome and beyond. J Endocrinol. 2003;177(3):365–372. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 16.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi: 10.1002/gepi.20048 [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glymour MM, Tchetgen Tchetgen EJ, Robins JM. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol. 2012;175(4):332–339. doi: 10.1093/aje/kwr323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T, Scheet P, Giusti B, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. 2009;84(4):477–482. doi: 10.1016/j.ajhg.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang T, Tucker KL, Lee YC, et al. Methylenetetrahydrofolate reductase variants associated with hypertension and cardiovascular disease interact with dietary polyunsaturated fatty acids to modulate plasma homocysteine in puerto rican adults. J Nutr. 2011;141(4):654–659. doi: 10.3945/jn.110.134353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson JR, Minelli C, Abrams KR, Tobin MD, Riley RD. Meta-analysis of genetic studies using Mendelian randomization–a multivariate approach. Stat Med. 2005;24(14):2241–2254. doi: 10.1002/sim.2100 [DOI] [PubMed] [Google Scholar]
- 23.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glueck CJ, Iyengar S, Goldenberg N, Smith LS, Wang P. Idiopathic intracranial hypertension: associations with coagulation disorders and polycystic-ovary syndrome. J Lab Clin Med. 2003;142(1):35–45. doi: 10.1016/S0022-2143(03)00069-6 [DOI] [PubMed] [Google Scholar]
- 25.Settin AA, Algasham A, Dowaidar M, Ismail H. Methylene tetrahydrofolate reductase and angiotensin converting enzyme gene polymorphisms related to overweight/obesity among Saudi subjects from Qassim Region. Dis Markers. 2009;27(2):97–102. doi: 10.3233/DMA-2009-0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavakkoly Bazzaz J, Shojapoor M, Nazem H, et al. Methylenetetrahydrofolate reductase gene polymorphism in diabetes and obesity. Mol Biol Rep. 2010;37(1):105–109. doi: 10.1007/s11033-009-9545-z [DOI] [PubMed] [Google Scholar]
- 27.Bokor S, Meirhaeghe A, Ruiz JR, et al. Common polymorphisms in six genes of the methyl group metabolism pathway and obesity in European adolescents. Int J Pediatr Obes. 2011;6(2–2):e336–344. doi: 10.3109/17477166.2010.500386 [DOI] [PubMed] [Google Scholar]
- 28.Gara S, Ochi H, Chango A, et al. C677t polymorphism of MTHFR and G80A polymorphism of RFC genes and their relation with homocysteine levels in obese Tunisian children. Tunis Med. 2011;89(6):565–568. [PubMed] [Google Scholar]
- 29.Chauhan G, Kaur I, Tabassum R, et al. Common variants of homocysteine metabolism pathway genes and risk of type 2 diabetes and related traits in Indians. Exp Diabetes Res. 2012;2012:960318. doi: 10.1155/2012/960318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabassum R, Jaiswal A, Chauhan G, et al. Genetic variant of AMD1 is associated with obesity in urban Indian children. PLoS One. 2012;7(4):e33162. doi: 10.1371/journal.pone.0033162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin RX, Wu DF, Miao L, et al. Several genetic polymorphisms interact with overweight/obesity to influence serum lipid levels. Cardiovasc Diabetol. 2012;11:123. doi: 10.1186/1475-2840-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez-Guerrero C, Romo-Palafox I, Diaz-Gutierrez MC, Iturbe-Garcia M, Texcahua-Salazar A, Perez-Lizaur AB. Prevalence of metilentetrahidrofolate reductase C677T polymorphism, consumption of vitamins B6, B9, B12 and determination of lipidic hydroperoxides in obese and normal weight Mexican population. Nutr Hosp. 2013;28(6):2142–2150. doi: 10.3305/nutrhosp.v28in06.6902 [DOI] [PubMed] [Google Scholar]
- 33.Fan SJ, Yang BY, Zhi XY, et al. Are MTHFR C677T and MTRR A66G polymorphisms associated with overweight/obesity risk? From a case-control to a meta-analysis of 30,327 subjects. Int J Mol Sci. 2015;16(6):11849–11863. doi: 10.3390/ijms160611849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chedraui P, Perez-Lopez FR, Escobar GS, et al. Polymorphisms of the FTO and MTHFR genes and vascular, inflammatory and metabolic marker levels in postmenopausal women. J Endocrinol Invest. 2016;39(8):885–890. doi: 10.1007/s40618-016-0443-7 [DOI] [PubMed] [Google Scholar]
- 35.Kupcinskiene K, Murnikovaite M, Varkalaite G, et al. Thrombosis related ABO, F5, MTHFR, and FGG gene polymorphisms in morbidly obese patients. Dis Markers. 2016;2016:7853424. doi: 10.1155/2016/7853424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen M, Chaudhry SH, MacFarlane AJ, et al. Serum and red-blood-cell folate demonstrate differential associations with BMI in pregnant women. Public Health Nutr. 2016;19(14):2572–2579. doi: 10.1017/S1368980016000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhi X, Yang B, Fan S, et al. Gender-specific interactions of MTHFR C677T and MTRR A66G polymorphisms with overweight/obesity on serum lipid levels in a Chinese Han population. Lipids Health Dis. 2016;15(1):185. doi: 10.1186/s12944-016-0354-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu L, Zhang M, Hu YQ, et al. Gene-gene interactions and associations of six hypertension related single nucleotide polymorphisms with obesity risk in a Chinese children population. Gene. 2018;679:320–327. doi: 10.1016/j.gene.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro MR, Lima RPA, Lisboa JVC, Chaves TR, Luna RCP. Influence of the C677T polymorphism of the MTHFR gene on oxidative stress in women with overweight or obesity: response to a dietary folate intervention. J Am Coll Nutr. 2018;27:1–8. [DOI] [PubMed] [Google Scholar]
- 40.Frelut ML, Nicolas JP, Guilland JC, de Courcy GP. Methylenetetrahydrofolate reductase 677 C->T polymorphism: a link between birth weight and insulin resistance in obese adolescents. Int J Pediatr Obes. 2011;6(2–2):e312–e317. doi: 10.3109/17477166.2010.486835 [DOI] [PubMed] [Google Scholar]
- 41.Frelut ML, Emery-Fillon N, Guilland JC, Dao HH, de Courcy GP. Alanine amino transferase concentrations are linked to folate intakes and methylenetetrahydrofolate reductase polymorphism in obese adolescent girls. J Pediatr Gastroenterol Nutr. 2006;43(2):234–239. doi: 10.1097/01.mpg.0000228110.83616.92 [DOI] [PubMed] [Google Scholar]
- 42.Glueck CJ, Goldenberg N, Golnik K, Sieve L, Wang P. Idiopathic intracranial hypertension: associations with thrombophilia and hypofibrinolysis in men. Clin Appl Thromb Hemost. 2005;11(4):441–448. [DOI] [PubMed] [Google Scholar]
- 43.Fonseca VA, Fink LM, Kern PA. Insulin sensitivity and plasma homocysteine concentrations in non-diabetic obese and normal weight subjects. Atherosclerosis. 2003;167(1):105–109. [DOI] [PubMed] [Google Scholar]
- 44.Konukoglu D, Serin O, Turhan MS. Plasma total homocysteine concentrations in obese and non-obese female patients with type 2 diabetes mellitus; its relations with plasma oxidative stress and nitric oxide levels. Clin Hemorheol Microcirc. 2005;33(1):41–46. [PubMed] [Google Scholar]
- 45.Narin F, Atabek ME, Karakukcu M, et al. The association of plasma homocysteine levels with serum leptin and apolipoprotein B levels in childhood obesity. Ann Saudi Med. 2005;25(3):209–214. doi: 10.5144/0256-4947.2005.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thawnashom K, Tungtrongchitr R, Petmitr S, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism (C677T) in relation to homocysteine concentration in overweight and obese Thais. Southeast Asian J Trop Med Public Health. 2005;36(2):459–466. [PubMed] [Google Scholar]
- 47.Zhu W, Huang X, Li M, Neubauer H. Elevated plasma homocysteine in obese schoolchildren with early atherosclerosis. Eur J Pediatr. 2006;165(5):326–331. doi: 10.1007/s00431-005-0033-8 [DOI] [PubMed] [Google Scholar]
- 48.Ustundag B, Gungor S, Aygun AD, Turgut M, Yilmaz E. Oxidative status and serum leptin levels in obese prepubertal children. Cell Biochem Funct. 2007;25(5):479–483. doi: 10.1002/cbf.1334 [DOI] [PubMed] [Google Scholar]
- 49.Ercan M, Konukoglu D. Role of plasma viscosity and plasma homocysteine level on hyperinsulinemic obese female subjects. Clin Hemorheol Microcirc. 2008;38(4):227–234. [PubMed] [Google Scholar]
- 50.Fridman O, Porcile R, Vanasco V, et al. Study on homocysteine levels and methylenetetrahydrofolate reductase gene variant (C677T) in a population of Buenos Aires City. Clin Exp Hypertens. 2008;30(7):574–584. doi: 10.1080/10641960802251958 [DOI] [PubMed] [Google Scholar]
- 51.Kaya C, Cengiz SD, Satiroglu H. Obesity and insulin resistance associated with lower plasma vitamin B12 in PCOS. Reprod Biomed Online. 2009;19(5):721–726. [DOI] [PubMed] [Google Scholar]
- 52.Omrani HQ, Shandiz EE, Qabai M, Chaman R, Fard HA, Qaffarpoor M. Hyperhomocysteinemia, folateo and B12 vitamin in Iranian patients with acute ischemic stroke. ARYA Atheroscler. 2011;7(3):97–101. [PMC free article] [PubMed] [Google Scholar]
- 53.Nervana MK, Bayoumy MM, Khaled A. Assessment of homocysteine plasma levels and insulin resistance among obese women with anovulatory infertility. Life Sci J. 2012;9(4):1599–1604. [Google Scholar]
- 54.Wang Y, Li X, Qin X, et al. Prevalence of hyperhomocysteinaemia and its major determinants in rural Chinese hypertensive patients aged 45-75 years. Br J Nutr. 2013;109(7):1284–1293. doi: 10.1017/S0007114512003157 [DOI] [PubMed] [Google Scholar]
- 55.Wiltshire EJ, Pena AS, MacKenzie K, Bose-Sundernathan T, Gent R, Couper JJ. A NOS3 polymorphism determines endothelial response to folate in children with type 1 diabetes or obesity. J Pediatr. 2015;166(2):319–325.e311. doi: 10.1016/j.jpeds.2014.10.050 [DOI] [PubMed] [Google Scholar]
- 56.Lind MV, Lauritzen L, Vestergaard H, et al. One-carbon metabolism markers are associated with cardiometabolic risk factors. Nutr Metab Cardiovasc Dis. 2018;28(4):402–410. doi: 10.1016/j.numecd.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg N, Murata M, Ikeda Y, et al. The frequent 5,10-methylenetetrahydrofolate reductase C677T polymorphism is associated with a common haplotype in whites, Japanese, and Africans. Am J Hum Genet. 2002;70(3):758–762. doi: 10.1086/338932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988;43(4):414–421. [PMC free article] [PubMed] [Google Scholar]
- 59.Rozen R. Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb Haemost. 1997;78(1):523–526. [PubMed] [Google Scholar]
- 60.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115. doi: 10.1371/journal.pgen.0030115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151(9):862–877. doi: 10.1093/oxfordjournals.aje.a010290 [DOI] [PubMed] [Google Scholar]
- 62.Stevenson RE, Schwartz CE, Du YZ, Adams MJ Jr. Differences in methylenetetrahydrofolate reductase genotype frequencies, between whites and blacks. Am J Hum Genet. 1997;60(1):229–230. [PMC free article] [PubMed] [Google Scholar]
- 63.Arruda VR, Siqueira LH, Goncalves MS, et al. Prevalence of the mutation C677 –> T in the methylene tetrahydrofolate reductase gene among distinct ethnic groups in Brazil. Am J Med Genet. 1998;78(4):332–335. [DOI] [PubMed] [Google Scholar]
- 64.Dilley A, Austin H, Hooper WC, et al. Relation of three genetic traits to venous thrombosis in an African-American population. Am J Epidemiol. 1998;147(1):30–35. doi: 10.1093/oxfordjournals.aje.a009363 [DOI] [PubMed] [Google Scholar]
- 65.Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG. Genetic associations in large versus small studies: an empirical assessment. Lancet. 2003;361(9357):567–571. doi: 10.1016/S0140-6736(03)12516-0 [DOI] [PubMed] [Google Scholar]
- 66.Ioannidis JP, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nat Genet. 2004;36(12):1312–1318. doi: 10.1038/ng1474 [DOI] [PubMed] [Google Scholar]
- 67.Pfeiffer CM, Huff DL, Smith SJ, Miller DT, Gunter EW. Comparison of plasma total homocysteine measurements in 14 laboratories: an international study. Clin Chem. 1999;45(8 Pt 1):1261–1268. [PubMed] [Google Scholar]