We appreciate the opportunity to respond to the comments provided by Ferrara and colleagues.
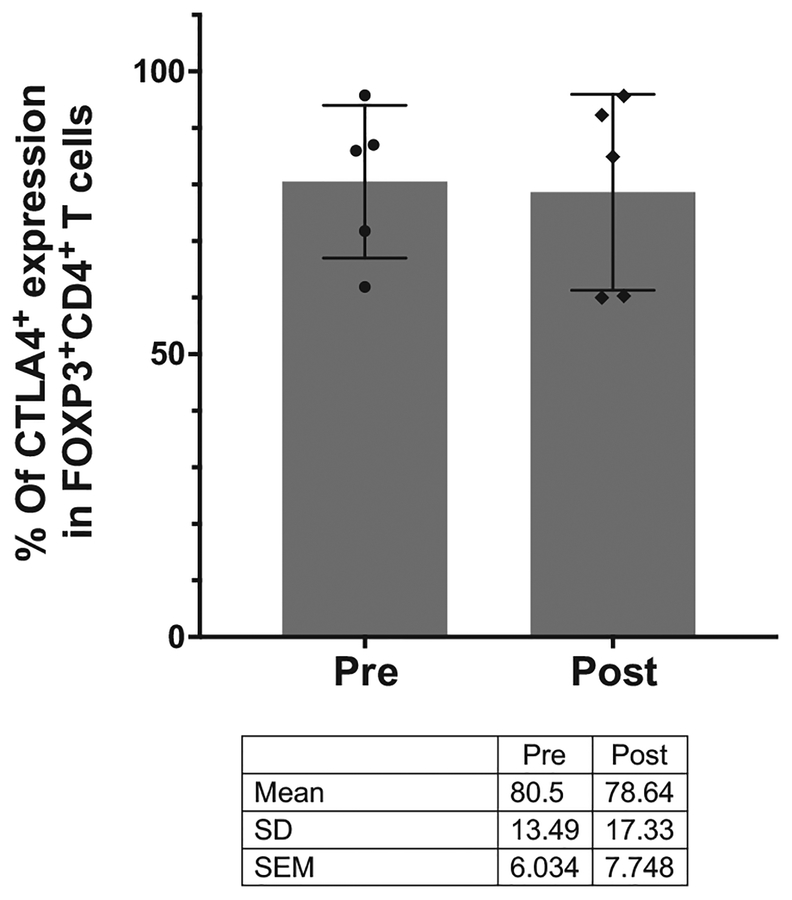
First, Ferrara and colleagues state that “a minority of intratumoral FOXP3+CD4+ T cells (regulatory T cells [T regs]) express membrane CTLA-4”. This statement is in contrast to our previous findings demonstrating that a majority (~60%–90%) of murine intratumoral FOXP3+CD4+ T cells express cell surface CTLA-4 (1). In addition, Ferrara and colleagues referenced a study by Marabelle and colleagues, who have shown in another murine model that approximately 50% intratumoral FOXP3+CD4+ T cells express cell surface CTLA-4 (2). Also, we reevaluated our CyTOF data from paired pre- and post-treatment human melanoma tissues as described in Supplementary Fig. S6 of our paper (3), and we generated additional data (Fig. 1), which did not show any evidence for depletion of FOXP3+CD4+ Tregs expressing CTLA-4 (intracellular and membrane-bound). Furthermore, since FOXP3 is used to identify Tregs, and the majority of FOXP3+CD4+ T cells express CTLA-4, we relied on FOXP3 staining in our IHC studies to determine whether Tregs were depleted by anti–CTLA-4 therapy.
Figure 1.
Effect of ipilimumab on CTLA-4–expressing FOXP3+CD4+ T cells in human melanoma. The proportion of CTLA-4–expressing FOXP3+CD4+ T cells was compared using multiparametric CyTOF analysis in pre- and post-ipilimumab-treated melanoma samples (N = 5). The dot plots represent mean with SD, and each dot represents an individual patient sample.
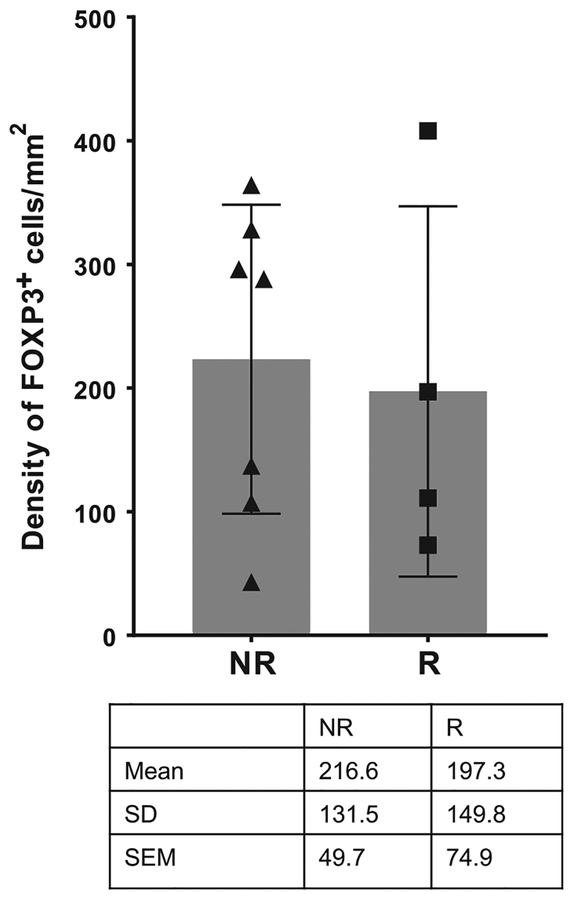
In addition, Ferrara and colleagues suggest that intratumoral Treg depletion may be more prevalent in responders versus nonresponders. However, our previously published data evaluating tremelimumab-treated melanoma samples did not show any significant difference in the density of intratumoral FOXP3+ cells between responders and nonresponders (4). Likewise, we evaluated 11 patients from our published manuscript (3) to correlate the density of intratumoral FOXP3+ cells with clinical response to ipilimumab in post-treatment melanoma samples. As shown in Fig. 2, we did not observe any difference in the density of intratumoral FOXP3+ cells between responders (N = 4) and nonresponders (N = 7).
Figure 2.
Effect of ipilimumab on density of intratumoral FOXP3+ cells in responders versus nonresponders. Ipilimumab-treated melanoma samples from nonresponders (NR; N = 7) and responders (R; N = 4) were analyzed by IHC for the density of intratumoral FOXP3+ cells. The dot plots represent mean with SD, and each dot represents an individual patient sample.
Ferrara and colleagues also referenced Romano and colleagues (5), who evaluated intratumoral FOXP3+ cells in pre- and post-ipilimumab melanoma samples (5 responders and 8 nonresponders). Their conclusion was that anti–CTLA-4 therapy depletes intratumoral Tregs in responders. However, it should be noted that Romano and colleagues evaluated FOXP3+ cells as a percentage of “total” tumor-infiltrating lymphocytes, which consists of subsets such as effector CD4+ and CD8+ T cells. Therefore, in the setting of anti–CTLA-4 therapy, which tends to increase the “total” number of tumor-infiltrating lymphocytes, especially in responders, the actual number of FOXP3+ cells may not change, but the percentage of FOXP3+ cells may appear to be less. We discussed this issue in our paper (3), and for this reason we chose to quantify intratumoral immune subpopulations using density rather than percentage.
Furthermore, Ferrara and colleagues emphasize that prior studies in in vivo mouse models have shown that Treg depletion is rapid (within days) following anti–CTLA-4 therapy (1, 5–7). This is true in mouse models where the murine antibodies have a short half-life, but the human anti–CTLA-4 antibodies have a much longer half-life and different levels of penetration into human tumors, which makes it unlikely to have similar kinetics in patients. Moreover, to address the issue of early depletion of Tregs, we would need to consider the ethical ramifications of multiple invasive procedures within short intervals to obtain longitudinal tumor samples from patients. We addressed this issue as best as we could as shown in Supplementary Fig. S4 of our published manuscript (3), and we did not observe a correlation between timing of post-treatment biopsy and the density of intratumoral FOXP3+ cells.
Finally, we do agree with Ferrara and colleagues that future clinical studies should include proportion and phenotype of all antibody-dependent cellular cytotoxicity performing cells and their FCγR repertoire within the tumor microenvironment. However, this will likely be difficult in human studies given the limited amounts of tissue available from tumor biopsies.
Footnotes
Disclosure of Potential Conflicts of Interest
S.K. Subudhi reports receiving speakers bureau honoraria from Valeant Pharmaceuticals International and the University of Texas Health Science Center at Taylor; holds ownership interest (including patents) in Apricity Health LLC; and is a consultant/advisory board member for Bayer Health-Care Pharmaceuticals, Dendreon, and Driver Inc. J.A. Wargo reports receiving speakers bureau honoraria from Dava Oncology, Illumina, Roche Genentech, Bristol-Myers Squibb, Merck, Gilead, MedImmune, and PHE; and is a consultant/advisory board member for Novartis, Bristol-Myers Squibb, AstraZeneca, Roche Genentech, and Merck. J.P. Allison holds ownership interest (including patents) in Jounce, Neon, Forty-Seven, ImaginAb, Tvardi Therapeutics, Constellation Pharmaceuticals, BioAtla, Polaris Pharmaceuticals, Hummingbird Bioscience, Merck, Amgen, Aparicity Therapeutics; has immediate family members who hold ownership interest in Jounce, Neon, Constellation, Oncolytics Biotech, Merck, BioMX, BioAtla, Forty-Seven, Aparicity, Polaris, Marker, ImaginAb, Hummingbird, Pieris Pharmaceuticals, Amgen, and Codiak; is a consultant/advisory board member for Jounce, Neon, BioAtla, Codiak, Tvardi, Amgen, Forty-Seven, ImaginAb, Merck, and Hummingbird; and has immediate family members who are consultant/advisory board members for Jounce, Constellation, Neon, BioAtla, Pieris, Oncolytics, Merck, BioMX, Forty-Seven, Aparicity, Polaris, Marker Therapeutics, Codiak, Hummingbird, AstraZeneca, Amgen, and GlaxoSmithKline. A. Ribas is a consultant/advisory board member for Merck, Bristol-Myers Squibb, Amgen, Chugai, Arcus, FLX-Bio, Five Prime, and Compugen. P. Sharma holds ownership interest in Jounce, Neon, Constellation, Oncolytics Biotech, BioAtla, Forty-Seven, Aparicity, Polaris, Marker, ImaginAb, Hummingbird, Optera, Dragonfly and Codiak and is a consultant/advisory board member for Jounce, Constellation, Neon, BioAtla, Pieris, Merck, Forty-Seven, Aparicity, Polaris, Marker Therapeutics, Codiak, Hummingbird, ImaginAb, Optera and Dragonfly; she also reports an immediate family member holding ownership interest in Jounce, Neon, BioAtla, Forty-Seven, Aparicity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Hummingbird, Tvardi Therapeutics, Optera, and Dragonfly and consultant/advisory board membership in Jounce, BioAtla, Neon, Amgen, Forty-Seven, Aparicity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Tvardi Therapeutics, Hummingbird, Merck, BMS, Optera, and Dragonfly. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells codefines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013;123:2447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Subudhi SK, Blando J, Scutti J, Vence L, Wargo J, et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin Cancer Res 2018;25: 1233–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang RR, Jalil J, Economou JS, Chmielowski B, Koya RC, Mok S, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res 2011;17: 4101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A 2015;112:6140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1:32–42. [DOI] [PubMed] [Google Scholar]
- 7.Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 2018;33:649–63. [DOI] [PMC free article] [PubMed] [Google Scholar]