Abstract
A major challenge in the repair and regeneration of soft tissue damage occurring as a result of aging, injury, or disease is recapitulating the biomechanical properties of the native tissue. Ideally, a candidate biomaterial for soft tissue engineering applications should be biocompatible, nonlinearly elastic to match soft tissue mechanical behavior, biodegradable to enable tissue remodeling, and tailorable to achieve a range of nonlinear elastic mechanical properties to match specific soft tissues. In addition, for cardiac and other applications, the biomaterial should have shape memory characteristics to facilitate minimally invasive and/or catheter-based delivery. Poly(glycerol dodecanoate) (PGD) is a shape memory material that has nonlinear elastic properties at body temperature and elastic-plastic behavior at room temperature. In this study, we investigated the effects of curing conditions on the nonlinear elastic, shape memory, and biocompatibility properties of PGD. Increased curing and crosslinking resulted in an increase in both the initial stiffness and the nonlinear strain stiffening behavior of PGD. After shape fixation at 60% strain, 100% shape recovery was achieved within 1 minute at body temperature for all conditions tested. Polymer curing had no adverse effects on the cellular biocompatibility or non-hemolytic characteristics of PGD, indicating the potential suitability of these formulations for blood-contacting device applications.
Keywords: PGD, shape memory, elastomer, bioresorbable, minimally-invasive
1. Introduction
Repair of soft tissue damage occurring as a result of aging, injury, or disease is challenging, due in part to difficulties matching the unique biomechanical properties of the native tissue matrix. Soft tissues throughout the body can be modeled as pseudoelastic, with a nonlinear stress-strain relationship and finite deformation under physiological loading conditions, but limited strain rate effects in many cases (1). Designing a biomaterial with similar nonlinear elastic properties could enable functional replacement of soft tissues, particularly those within mechanically dynamic environments, where appropriate mechanical properties are critical to tissue function. Appropriate bioresorption of an implanted material would enable natural remodeling processes to occur, allowing new tissue to replace the degrading polymer. Ideally, a candidate biomaterial would, in addition to matching the tissue mechanics and possessing an appropriate degradation rate, be tailorable to achieve a range of stiffnesses to suit various soft tissues. Finally, shape memory characteristics are increasingly desirable with the growing use of minimally invasive and catheter-based implant delivery.
The ability of nonlinear elastomeric materials to approximate the mechanical behavior of many tissues makes them uniquely suited for soft tissue engineering applications. Elastomers with biomimetic nonlinear elastic properties may transmit stimulatory mechanical signals to surrounding cells and extracellular matrix (ECM), promoting growth and functionality of the repaired tissue. In addition, many elastomers can withstand cyclic deformation at high strain levels, enabling them to replace the mechanical function of damaged or missing tissue within mechanically dynamic environments such as the lung, bladder, or cardiovascular system (2). Though many materials including protein-based hydrogels made from collagen and elastin have been considered as soft tissue replacements due to their similarity to the native ECM, it is difficult to finely control and alter the nonlinear elastic behavior of such natural materials (3). Furthermore, the tangent moduli of some crosslinked hydrogels are relatively low, potentially limiting their long-term mechanical stability. Synthetic polymers with readily tailorable nonlinear elastic properties would be beneficial for applications within a wide range of tissues with varying properties.
Besides mechanical properties that match those of the native ECM, biodegradability is another desirable characteristic of materials used for the repair and replacement of soft tissues. Bioresorbable polymers have been developed for multiple medical applications due to their ability to break down over time, allowing tissue to grow and replace the degrading polymer as healing progresses. There is no need for additional surgery to remove an implanted device made from a resorbable material, making it attractive for applications where only temporary support is required. The most prevalent biodegradable polymers, poly(lactide), poly(glycolide), and their copolymers, poly(lactide-co-glycolide) (PLGA), have been used for various applications including controlled drug delivery devices, bone plates and screws, vascular stents, and resorbable sutures. However, the bulk degradation process that these polyesters undergo results in an exponential loss of mechanical properties over time, and they cannot recover from large mechanical deformations, making them ill-suited for use within mechanically dynamic soft tissues when prolonged durability is required (4). Thus, considerable interest has been given to the development of biocompatible, biodegradable elastomeric polymers with tailorable physicochemical properties. The ability to adjust factors such as degradation time and mechanical properties could allow tailoring of elastomer characteristics to suit specific soft tissue sites.
Due to the desirability of biomaterials with tailorable physicochemical properties, multiple synthetic polymers with nonlinear elastic properties have been developed and characterized for tissue engineering (5). One such polymer, poly(glycerol sebacate) (PGS) (6), is a tough, biodegradable elastomer that has been investigated for multiple biomedical applications including nerve guidance (7), myocardial tissue (8), vascular grafts (9), cartilage (10), retinal tissue (11), and drug delivery (12). PGS is synthesized through polycondensation of glycerol and sebacic acid, forming a viscous prepolymer that is poured into a mold and cured at high temperatures. This process results in an elastomeric polymer that can recover from relatively large deformations, with ester bonds that enable slow hydrolytic degradation. Importantly, the mechanical properties of PGS can be tailored for specific applications by varying such processing parameters as curing time, curing temperature, or molar ratios of glycerol to sebacic acid (3, 13). However, PGS exhibits shape memory behavior near 10°C (14), which is much lower than body temperature and limits its applicability as part of catheter-based implant delivery systems.
Poly(glycerol dodecanoate) (PGD) is a thermosetting biodegradable polymer formed by polycondensation of non-toxic monomers (in this case, glycerol and dodecanedioic acid) (15). Unlike PGS, PGD has unique temperature-responsive characteristics that render it useful as part of minimally-invasive surgical applications. PGD is a shape memory material that has characteristics of a compliant nonlinear elastomer at body temperature (37°C), and those of a tough elastic-plastic material at room temperature (21°C), with a shape switching temperature at around 32°C. When used as part of implantable medical devices, PGD can be folded for delivery through narrow passages, and would retain a rigid shape, allowing the surgeon to easily manipulate and position the device. Once the PGD device is heated to body temperature, it would unfold to resume a functional shape, becoming soft and rubbery to better match the elasticity of the surrounding soft tissue. Such characteristics would enable PGD to be used as part of devices designed for insertion through a small incision, or for percutaneous delivery via catheter.
In addition to the original formulation of PGD previously reported (15), electrospun mats of PGD (16), PGD modified with fumaric acid (PGDF) (17), or gelatin-blended PGD (18) have been explored for neural tissue engineering. It is presently unclear, however, if the mechanical properties of PGD can be altered to produce shape memory materials with a range of tangent stiffness values to accommodate use within multiple soft tissues. In this work, we investigate the effects of specific pre-polymer curing conditions on the mechanical, thermal, and shape memory properties of PGD. As crosslinking density can affect polymer biocompatibility, the cured PGD was also assessed in terms of cellular proliferation and hemolytic activity. The development of multiple PGD formulations with shape memory properties and tailorable mechanical behavior could enable use as implants or as part of tissue engineering strategies for various soft tissues, including those within mechanically demanding environments such as the cardiovascular, gastrointestinal, or musculoskeletal systems.
2. Materials and Methods
2.1. PGD synthesis
PGD pre-polymer was synthesized by mixing glycerol and dodecanedioic acid in a 1:1 molar ratio, and stirring for 24 hr at 120°C under nitrogen as previously described (15). The viscous liquid pre-polymer was poured into silicone molds, and placed into a vacuum oven at 90°C for 4 hr under 90 mTorrs vacuum to remove any gas bubbles from the mold. The molds were then cured with vacuum maintained at 90 mTorrs to produce 3 cure levels as follows (Table 1): 48 hr at 120°C (“low” cured), 68 hr at 120°C (“medium” cured), or 48 hr at 130°C (“high” cured).
Table 1:
Curing conditions used to produce PGD with 3 different crosslinking levels.
| PGD Condition | Cure time (h) | Cure temperature (°C) |
|---|---|---|
| Low | 48 | 120 |
| Medium | 68 | 120 |
| High | 48 | 130 |
2.2. DSC for glass transition temperature
Differential Scanning Calorimetry (DSC) was performed using a Perkin Elmer DSC 7. PGD samples (N≥4) were heated to 90°C and held for 3 min, then cooled to −50°C and re-heated to 70°C. Temperature scans were performed at a rate of 10°C/min. All melting temperatures were determined from the second heating run.
2.3. Solvent swelling
Six-mm diameter (2 mm thickness) PGD disks were punched from PGD sheets and used for characterization testing. For solvent swelling measurements, PGD samples (N=4) were swollen in tetrahydrofuran (THF) at 37°C and weighed daily (8). Excess solvent was blotted from the swollen samples, and they were placed in sealed vials to minimize evaporation during weighing. After 3 days, no measurable weight increases were observed. Samples were weighed to determine their equilibrium swollen mass (ms), then dried for 5 days in a vacuum oven at 40°C and weighed again to determine the mass of the dried sample after extraction of solvent (md). The swelling ratio was determined as follows: (ms-md)/ms.
2.4. Mechanical testing and Ogden model fit
Compressive testing was conducted on 6-mm PGD disks within a custom-built temperature control chamber using an MTS system equipped with a 500 N load cell and a porous metal platen. Samples (N=7) were tested in water maintained at room temperature (21°C), or 5 degrees above the shape transition temperature of the PGD (37°C test temperature for medium and high cured PGD, and 44°C for low cured PGD). Compression was applied at a rate of 2 mm/min, and samples were compressed to 13.5% strain at 21°C and 60% strain (13) at 37 or 44°C. PGD crosslinking density was approximated from the tangent moduli at 37 or 44°C using the following equation for rubber elasticity (13): n=Ey/(3RT). The mechanical test data obtained at 37 or 44°C was fit to a one-term Ogden constitutive model for nonlinear hyperelastic materials using MATLAB as previously described (15).
2.5. Shape fixation and recovery
PGD disks were heated to 5°C above the shape transition temperature (to a test temperature of 37°C for high and medium cured PGD, and 44°C for low cured PGD) and compressed to 60% strain using an MTS system with a metal platen (N=3). Compression was maintained as the samples were cooled to room temperature, and then the applied force was removed. Cooled, compressed samples were removed from the test chamber and measured with calipers. To assess shape recovery, samples were re-heated to 37°C (high and medium) or 44°C (low), and allowed to recover for 1 minute. Final thickness was measured with calipers, and compared to original thickness measurements.
2.6. Hemocompatibility study
Hemolysis testing of PGD was conducted according to the ASTM F756–13 standard. The 3 cure levels of PGD were tested and compared to results with silicone (negative control) and water (positive control) (N=3). Briefly, blood from 3 rabbits was collected in citrate tubes and pooled for the hemolysis analysis. Total blood hemoglobin content was measured using Drabkin’s reagent according to manufacturer’s instructions and diluted with phosphate buffered saline (PBS) to a final hemoglobin concentration of 10 mg/ml. Disks of control or experimental samples were placed in tubes containing 7 ml PBS, and 1 ml of diluted blood was added to each tube. Samples were maintained at 37°C for 3 hr, with gentle inversion of the tubes every 30 min. At the end of the incubation, the tubes were centrifuged and the supernatant hemoglobin concentration was determined using Drabkin’s reagent. Percent hemolysis was corrected for blanks and calculated as [supernatant hemoglobin]/[total hemoglobin in tube] X 100.
2.7. Fibroblast proliferation assay
The metabolic activity of proliferating cells on PGD was monitored by a resazurin-based assay (Tox-8) following the manufacturer’s protocol. Culture plates (N=3) were prepared by coating 5 cm glass dishes with a 1% solution of PGD, or PLGA (5050 DLG 2A; Resomer) for controls, in THF. The THF was evaporated overnight, and then the PGD plates were cured as described above. The polymer-coated plates were UV sterilized for 15 min and rinsed with PBS prior to use. NIH 3T3 fibroblasts were seeded on the prepared plates at a density of 3500 cells/cm2 and cultured under standard culture conditions in growth medium consisting of Dulbecco’s Modified Eagle’s media (DMEM) containing 10% fetal bovine serum (FBS), 1% nonessential amino acids, and 1% penicillin/streptomycin. Absorbance was measured at 600 nm to determine the percentage of resazurin reduction on days 1, 4, and 7 after plating.
2.8. Statistical analysis
One-way ANOVA with Tukey’s post hoc tests was performed using GraphPad InStat 3.06 software, with values of p<0.05 considered statistically significant. All values are reported as mean ± standard deviation.
3. Results
3.1. DSC for glass transition temperature
DSC analysis indicated that PGD is a semi-crystalline material, with crystalline melting temperatures (Tm) that vary with the level of curing (Table 2). Low cured PGD exhibited 2 crystalline melting peaks (38.5 and 46.2°C), while the medium and high conditions each had a single melting peak (37.0 and 36.3°C, respectively). As observed empirically, the shape switching temperature for the medium and high cured PGD occurred at approximately 32°C, near the onset of the crystalline melt curves. The shape switching temperature for the low cured PGD occurred between the peak of the first melt curve and the onset of the second, at approximately 39°C.
Table 2:
Peak melting temperatures (Tm) of cured PGD obtained via DSC analysis, and approximate shape switching temperatures (Tsw). Tm1 represents the first melting peak on heating, and Tm2 represents the second melting peak (only applicable for low cured PGD).
| PGD Condition | Tm1 peak (°C) | Tm2 peak (°C) | Tsw (°C) |
|---|---|---|---|
| Low | 38.5 ± .7 | 46.2 ± .8 | 39 |
| Medium | 37.0 ± 1.1 | n/a | 32 |
| High | 36.3 ± .3 | n/a | 32 |
3.2. Solvent swelling
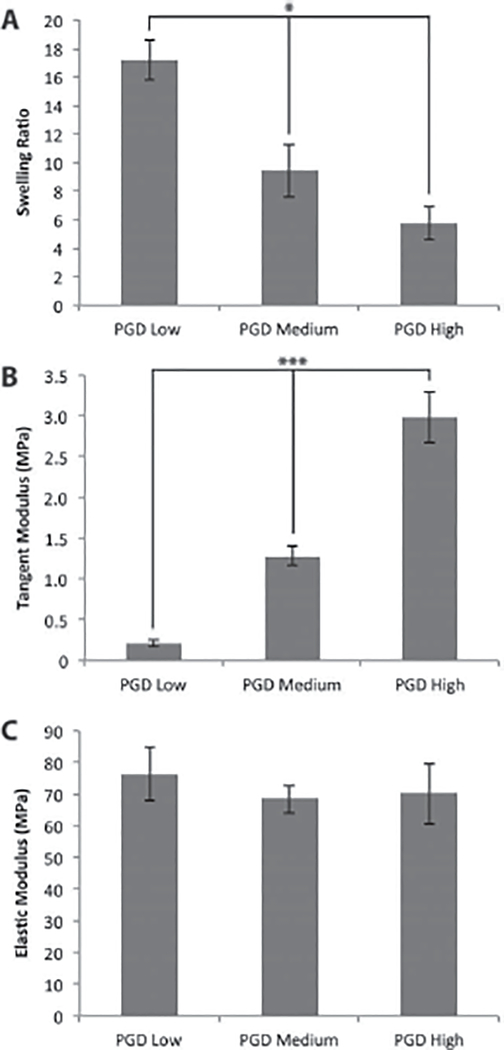
Solvent swelling measurements in THF indicated that the swelling ratios were significantly different for the 3 curing conditions tested, with the most swelling observed for the low cured PGD and the least swelling observed for the high cured PGD (Figure 1A).
Figure 1:
(A) Solvent swelling ratios of cured PGD, (B) tangent moduli measured at 12.5% strain at 37°C (medium and high cured PGD) or 44°C (low cured PGD), and (C) elastic moduli measured at 21°C. *p ≤ 0.05, ***p ≤ 0.001.
3.3. Mechanical testing and Ogden fit
Stress-strain curves generated from the mechanical testing data indicated a linear stress-strain relationship for PGD samples tested below the shape transition temperature, and a nonlinear relationship for samples tested above the shape transition temperature. Tangent modulus values were calculated at 12.5% strain for tests conducted at 37 or 44°C (Figure 1B). The tangent moduli were significantly different for the 3 curing conditions, with a trend of higher tangent modulus with increased curing. Approximate polymer crosslinking densities calculated from the tangent moduli are shown in Table 3. Young’s moduli were calculated from tests performed at 21°C, and were not significantly different for the 3 cure conditions (Figure 1C). The Young’s moduli at room temperature were at least 2 orders of magnitude higher than the moduli at 37 or 44°C for all PGD conditions. The one-term Ogden constants for high, medium, and low cured PGD tested above the shape transition temperature are reported in Table 4. The Baker-Eriksen inequality was satisfied for all model fits (15).
Table 3:
Estimated crosslinking densities of cured PGD based on tangent modulus measurements taken above the shape transition temperature.
| PGD Condition | Crosslinking density (mol/cm3) |
|---|---|
| Low | 3.852E-4 |
| Medium | 1.667E-4 |
| High | 0.278E-4 |
Table 4:
Constants (μ1, α1) and R2 error values for PGD mechanical test data at 37°C (medium and high cured PGD) or 44°C (low cured PGD) fit to a one-term Ogden model.
| PGD Condition | μ1 | α1 | R2 |
|---|---|---|---|
| Low | 0.36 ± 0.11 | 0.25 ± 0.07 | 0.999 ± 0.0005 |
| Medium | 0.64 ± 0.11 | 0.68 ± 0.24 | 0.995 ± 0.0021 |
| High | 1.03 ± 0.04 | 1.00 ± 0.04 | 0.996 ± 0.0021 |
3.4. Shape fixation and recovery
Following cooling of PGD disks compressed to 60% strain, all samples retained their compressed thickness after removal of the applied force, indicating a high degree of shape fixity in all conditions. When samples were heated and allowed to recover, samples from all 3 PGD curing conditions regained 100% of their original thickness within 1 minute, demonstrating a high degree of shape recovery.
3.5. Hemocompatibility study
Hemocompatibily analysis indicated that less than 1% hemolysis occurred in blood samples in contact with the PGD samples, comparable to the negative control material (Figure 2). This result indicates that PGD cured under the 3 conditions described here is non-hemolytic.
Figure 2:
Percent hemolysis of PGD samples compared to negative control material (silicone) and positive control (water). *p ≤ 0.05 vs. all other conditions.
3.6. Fibroblast proliferation assay
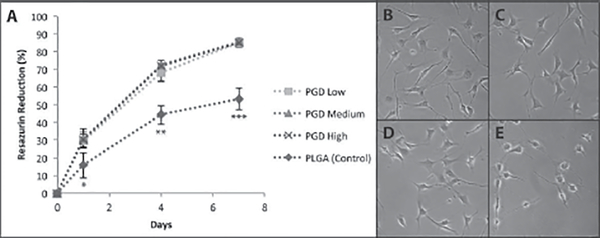
The metabolic activity of cells on PGD or PLGA (control) materials as measured by percentage of resazurin reduction is shown in Figure 3. PLGA was selected as a benchmark positive control for cell proliferation studies, as it is a widely used biodegradable polymer that is generally considered to be biocompatible (19). The cells in PGD-coated dishes had normal morphology (Figure 3B–D) and proliferated faster than those in control dishes (Figure 3E).
Figure 3:
(A) Percent resazurin reduction by NIH 3T3 cells proliferating on PGD or PLGA (control) at 1, 4, and 7 days. Photomicrographs of adherent cells on day 4 on (B) low cured PGD, (C) medium cured PGD, (D) high cured PGD, and (E) PLGA control. *p ≤ 0.05 vs. other conditions at day 1, **p ≤ 0.001 vs. other conditions at day 4, ***p ≤ 0.001 vs. other conditions at day 7.
4. Discussion
PGD is a biodegradable, shape memory polymer with applications for soft tissue engineering, particularly as part of minimally invasive medical devices. It is currently unknown, however, whether the physiochemical properties of PGD can be altered to produce materials with a range of properties suitable for use in multiple soft tissues. The focus of this study was to understand how curing conditions affected PGD properties, specifically the mechanical behavior, shape memory properties, and biocompatibility profile.
As demonstrated by DSC analysis, PGD is a semi-crystalline material with melting temperatures that vary with the specific curing conditions. Increased cure time and temperature appear to result in a more amorphous polymer, similarly to what has been reported with another biodegradable thermoset polymer, PGS(20). The positions of the crystalline melting peaks agree with physical observations of the shape switching temperature, and are accompanied by drastic changes in mechanical properties. Both the medium and high cured PGD are partially amorphous at 37°C, making these materials attractive for surgically implantable tissue engineered scaffolds. The low cured PGD formulation has a shape switching temperature of 39°C, and thus may not have direct utility for soft tissue applications. However, though this formulation of PGD is very soft and pliable at high temperatures, it is crosslinked enough to maintain its shape during further curing. Thus, the low cured PGD could be used as a “building-block” for PGD-based devices, making additional molds unnecessary.
It was anticipated that increasing the temperature or duration of curing would increase the polymer crosslinking level, as PGD is a thermally crosslinked material. The amount of swelling is indicative of the degree of crosslinking of a polymer network, and the observed swelling trends indicated that the low, medium, and high cured PGD had sequentially increasing crosslinking levels. This finding was in agreement with the crosslinking density values estimated from the mechanical testing data, which also indicated increased crosslinking with increased cure time or temperature. The range of tangent moduli for the PGD formulations tested was 0.22 to 2.98 MPa. This overlaps with the elastic moduli of multiple soft tissues within the body, including cardiac muscle (0.2 to 0.5 MPa at end of diastole) (8), cartilage (0.5 to 1 MPa) (21), and corneal tissue (0.3 to 7 MPa) (22).
The stress-strain data were a good fit to the Ogden one-term model for hyperelastic materials, with all model fits having an R2 error of more than .995, indicating that all 3 curing conditions result in PGD formulations that behave as nonlinear elastic materials above the shape switching temperature. Ogden fits also indicated that PGD had similar nonlinear behavior to PGS (3), poly(1,8 Octane-diol-citrate) (POC) (23), and our previous study of PGD (15). However, this was the first study to demonstrate that the nonlinear elasticity of PGD changed with curing conditions. Specifically, with increasing curing and crosslinking, both the initial stiffness and the nonlinear strain stiffening behavior increased, as reflected in the increasing Ogden coefficients. The linear elastic polymer stiffness dramatically increased at room temperature, and the linear stress-strain curves generated at 21°C indicated elastic-plastic behavior in agreement with what has previously been observed for PGD. Interestingly, the curing conditions tested had no effect on the mechanical properties of the material at room temperature. This is likely due to the semi-crystalline nature of the cured polymer at temperatures below the onset of the crystalline melting curves. This property could be beneficial for use as part of implantable shape memory PGD devices, as multiple formulations would have the same stiff plastic properties at room temperature, but would transition to a rubbery, compliant consistency with varied moduli at body temperature.
In this study, all 3 PGD formulations exhibited a high degree of shape fixity and shape recovery, indicating that they could have utility for procedures in which the implanted device must be reshaped into a lower profile to allow for delivery through small passages. The ability of PGD samples to recover from high compressive strains and regain 100% of their original dimensions suggests that a large degree of compaction for use in minimally invasive surgical applications (for example, loading within a catheter for percutaneous delivery) would permit complete shape recovery once within the body.
Varying the curing conditions did not appear to have any adverse effects on the biocompatibility profile of PGD. Proliferation of metabolically active fibroblasts was faster on PGD compared to PLGA controls, indicating that PGD is at least as cytocompatible as PLGA, one of the most commonly used bioresorbable polymers for medical device applications. Additionally, blood compatibility studies indicated that PGD is non-hemolytic, and that curing did not have any effect on the non-hemolytic properties of the material. PGD could therefore be used as part of devices with blood-contacting applications including intracardiac and intravascular devices.
5. Conclusions
These findings demonstrate that the physiochemical properties of PGD can be controlled by varying the curing time and temperature, resulting in materials with shape memory properties and a range of moduli comparable to soft tissues within the body. The shape switching temperatures of both the high and medium cured PGD formulations are maintained between room and body temperature, indicating that they could have application as part of minimally invasive medical devices designed for use within multiple soft tissues. The low cured PGD formulation could be used as a “building-block” for PGD-based devices, as it will not melt during further curing, eliminating the need for additional molds. Future studies will include in vitro and in vivo degradation analyses in order to assess effects of varied curing conditions on PGD degradation time and local tissue response.
Supplementary Material
Acknowledgements
The authors would like to thank Colleen Flanagan for technical assistance, and gratefully acknowledge funding from the University of Michigan McKay faculty development award of the Samuel and Jean Frankel Cardiovascular Center.
References
- 1.Fung YC. Structure and stress-strain relationship of soft tissues. Amer Zool 1984;24:13–22. [Google Scholar]
- 2.You Z, Wang Y. Bioelastomers in tissue engineering In: Burdick JA, Mauck RL, editors. Biomaterials for tissue engineering applications. Springer; Vienna; 2011. p 75–118. [Google Scholar]
- 3.Mitsak AG, Dunn AM, Hollister SJ. Mechanical characterization and non-linear elastic modeling of poly(glycerol sebacate) for soft tissue engineering. J Mech Behav Biomed Mater 2012;11:3–15. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate). J Biomed Mater Res A 2003;66:192–197. [DOI] [PubMed] [Google Scholar]
- 5.Shi R, Chen D, Liu Q, Wu Y, Xu X, Zhang L, Tian W. Recent advances in synthetic bioelastomers. Int J Mol Sci 2009;10:4223–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol 2002;20:602–606. [DOI] [PubMed] [Google Scholar]
- 7.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, Hadlock TA. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005;26:5454–5464. [DOI] [PubMed] [Google Scholar]
- 8.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008;29:47–57. [DOI] [PubMed] [Google Scholar]
- 9.Motlagh D, Yang J, Lui KY, Webb AR, Ameer GA. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials 2006;27:4315–4324. [DOI] [PubMed] [Google Scholar]
- 10.Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res A 2010;94:9–18. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard CD, Arner KM, Langer RS, Ghosh FK. Retinal transplantation using surface modified poly(glycerol-co-sebacic acid) membranes. Biomaterials 2010;31:7978–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun ZJ, Chen C, Sun MZ, Ai CH, Lu XL, Zheng YF, Yang BF, Dong DL. The application of poly (glycerol-sebacate) as biodegradable drug carrier. Biomaterials 2009;30:5209–5214. [DOI] [PubMed] [Google Scholar]
- 13.Pomerantseva I, Krebs N, Hart A, Neville CM, Huang AY, Sundback CA. Degradation behavior of poly(glycerol sebacate). J Biomed Mater Res A 2009;91:1038–1047. [DOI] [PubMed] [Google Scholar]
- 14.Cai W, Liu L. Shape-memory effect of poly (glycerol–sebacate) elastomer. Mater Lett 2008;62:2171–2173. [Google Scholar]
- 15.Migneco F, Huang YC, Birla RK, Hollister SJ. Poly(glycerol-dodecanoate), a biodegradable polyester for medical devices and tissue engineering scaffolds. Biomaterials 2009;30:6479–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai X, Huang YC. Electrospun fibrous scaffolds of Poly(glycerol-dodecanedioate) for engineering neural tissues from mouse embryonic stem cells. J Vis Exp 2014;(88). doi: 10.3791/51587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai X, Huang YC, Leichner J, Nair M, Lin WC, Li CZ. Peptide modified polymer poly (glycerol- dodecanedioate co-fumarate) for efficient control of motor neuron differentiation. Biomed Mater 2015;10:065013-6041/10/6/065013. [DOI] [PubMed] [Google Scholar]
- 18.Dai X, Kathiria K, Huang YC. Electrospun fiber scaffolds of poly (glycerol-dodecanedioate) and its gelatin blended polymers for soft tissue engineering. Biofabrication 2014;6:035005-5082/6/3/035005. Epub 2014 Apr 24. [DOI] [PubMed] [Google Scholar]
- 19.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev 1997;28:5–24. [DOI] [PubMed] [Google Scholar]
- 20.Jaafar IH, Ammar MM, Jedlicka SS, Pearson RA, Coulter JP. Spectroscopic evaluation, thermal, and thermomechanical characterization of poly(glycerol-sebacate) with variations in curing temperatures and durations. J Mater Sci 2010;45:2525–2529. [Google Scholar]
- 21.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res 1991;9:330–340. [DOI] [PubMed] [Google Scholar]
- 22.Duan X, Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials 2006;27:4608–4617. [DOI] [PubMed] [Google Scholar]
- 23.Jeong CG, Hollister SJ. Mechanical, permeability, and degradation properties of 3D designed poly(1,8 octanediol-co-citrate) scaffolds for soft tissue engineering. J Biomed Mater Res B Appl Biomater 2010;93:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.