Abstract
Recent studies, in male rodents, have begun to elucidate a role for the GABAergic neurons in the tail of the ventral tegmental area (tVTA) in morphine withdrawal. To date, the mechanisms underlying morphine withdrawal have been studied almost exclusively in male animals. As a result, there is a considerable gap in our current understanding of the processes underlying sex differences in morphine withdrawal behaviors and its effects on cellular activity in the tVTA in females. The purpose of the present study was to investigate the influence of sex on the expression and duration of spontaneous somatic morphine withdrawal syndrome, and to characterize the relationship between spontaneous somatic withdrawal symptoms and cellular activation (measured as phosphorylated CREB; pCREB), in the GABAergic tVTA in male and female rats. Morphine-dependent adult male and female Long Evans rats underwent 72 hours of spontaneous withdrawal, and somatic withdrawal symptoms were assessed every 12 hours. Male morphine-dependent rats expressed more severe symptoms during the early phases of withdrawal compared to females. Although, females demonstrated lower overall symptom severity, their symptoms persisted for a longer period of time, thus demonstrating higher withdrawal-symptom severity than males during late withdrawal. pCREB activity in the tVTA was elevated in morphine-withdrawn rats and was positively correlated with the severity of withdrawal symptoms. These results demonstrate sex differences in the timing of the expression of somatic withdrawal. Our data add to the growing body of evidence demonstrating a role for the tVTA in morphine withdrawal and begin to establish a sex-dependent behavioral and molecular profile within this brain region.
Keywords: Opioid, dependence, somatic withdrawal, spontaneous withdrawal, time-course, CREB, RMTg
1. Introduction
Compared to men, women are more likely to more rapidly transition from casual opioid use to dependence, experience higher levels of craving and relapse during interludes of abstinence, consume larger amounts of drug during relapse, and are less likely to seek treatment for their addiction [1–3]. Moreover, during attempts to refrain from drug use, women report suffering significantly more distressing physical symptoms of withdrawal compared to men [4,5]. Overall, the current literature suggests that women may be more severely affected than men by the chronic use of opioid drugs.
Opioid drugs exhibit their initial rewarding effects through the inhibition of a subset of GABAergic interneurons, which leads to the excitation of ventral tegmental area (VTA) dopamine (DA) neurons [6,7]. It is now largely accepted that the source of this VTA-DA neuron excitation is the result of inhibition of GABAergic inputs from the “tail of the VTA” (tVTA;[8]), also called the “rostromedial tegmental nucleus” (RMTg;[9]). The tVTA/RMTg – a structure located just posterior to the VTA – is largely GABAergic and its neurons densely innervate DA cells of the VTA [10]. Mu-opioid receptors are densely packed on these GABAergic neurons of the tVTA [11–13] and, as a result, administration of morphine decreases their firing rate [11,13]. Consequently, DAergic neurons in the VTA become disinhibited to freely transmit DA to projection sites in the nucleus accumbens (NAc) and other limbic regions leading to an overstimulation of the reward pathway, which is associated with mediating addiction-related behaviors. Interestingly, withdrawal from chronic morphine has been shown to increase tVTA GABA release [13]. This increased GABA release is postulated to be one of the crucial steps that trigger the cascade of intracellular events that, ultimately, manifest as opiate withdrawal syndrome [14].
Withdrawal from chronic morphine upregulates the cyclic adenosine monophosphate (cAMP) pathway by increasing adenylyl cyclase and protein kinase A (PKA) activity and, thus, leading to an increase in the levels of phosphorylated CREB (pCREB) in reward-related brain regions [15–17]. In male rodents, cAMP-PKA-activated CREB is essential for the morphine-induced alterations in gene expression that precipitate withdrawal-associated behaviors [18,19]. Currently, there is a surprising gap in our understanding of the mechanisms underlying sex differences in opioid withdrawal behaviors. Additionally, the specific effects of opioid withdrawal on cAMP-PKA-CREB activity in the tVTA/RMTg are virtually unknown and no studies have been performed in females. Thus, the main objective of the current study is to examine potential differences in the behavioral and molecular signaling adaptations resulting from the interactions between morphine withdrawal and sex in a rodent model.
Here, we report that males exhibited more severe withdrawal symptoms than females immediately following cessation of morphine administration. However, in females, withdrawal symptoms persisted longer than in males. This sex difference in behavior was accompanied by increased pCREB expression in the tVTA of females and withdrawal symptom severity was positively correlated with increased pCREB expression in the tVTA of females, but not in males. Together, our data point to sex differences in the morphine withdrawal syndrome that may be mediated, at least in part, by activation of cAMP-PKA-CREB signaling cascade in the GABAergic neurons of the tVTA/RMTg.
2. Materials and Methods
2.1. Animals
Thirty-six experimentally naïve, adult (90-120 days old), male and female, Long Evans rats (source: the University of Texas at Arlington vivarium) were double housed with same-sex cage mates in a temperature/humidity-controlled environment under a 12h reversed light/dark cycle with lights on at 7p.m. and off at 7a.m. All rats had free access to food and water throughout the study and were maintained and cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All animal procedures were approved by the University’s IACUC.
2.2. Induction of Morphine Dependence
Morphine dependence was induced as previously described [20]. Briefly, rats were randomly assigned to either morphine or vehicle (0.01M phosphate buffered saline;PBS) treatment groups. Morphine sulfate (Spectrum Chemical, Los Angeles CA) was dissolved in PBS and administered at escalating doses by repeated twice-daily subcutaneous injections for 10 days: Days 1 and 2 = 2.5mg/kg;Days 3 and 4 = 5mg/kg;Days 5 and 6 = 10mg/kg;Days 7 and 8 = 20mg/kg;Days 9 and 10 = 40mg/kg. Control rats received twice daily injections of saline (1 ml/kg of body weight) for 10 days. All injections were spaced by 12h in order to prevent overdose and limit effects of inter-injection withdrawal.
2.3. Withdrawal Behavior Observations
Animals’ body weights were measured and signs of somatic withdrawal were observed over three days beginning 12h after the last morphine injection; measurements were taken 12, 24, 36, 48, 60, and 72h after the final morphine injection. The number of wet-dog shakes (shaking of the whole body: record total number of body shakes) and writhing movements (rat lies on floor with belly firmly pressed on surface, abdominal contractions present: record total number of contractions) were recorded for each animal [21] over a 10-minute observation period. A withdrawal score was calculated by adding scores for loss of body weight, wet-dog shakes, and writhing movements. These three measures were selected because they are all sensitive and reliable indicators of the degree of morphine dependence for rodents [22–28].
2.4. Immunohistochemistry
Immediately following the last somatic withdrawal assessment, rats were deeply anesthetized with chloral hydrate and transcardially perfused with 200ml of 0.01M PBS followed by 400ml of 4% paraformaldehyde in 0.01M PBS. Brains were post-fixed overnight, cryoprotected in 20% glycerol in PBS, and coronal sections (40μm) were collected on a freezing microtome. To identify co-localization of pCREB positive cells within GABA cells in the VTA/tVTA, brains were double-labeled for pCREB (Millipore #06-519 at 1:1500) and glutamic acid decarboxylase (GAD67; Millipore #MAB5406 at 1:500) as previously described [8]. Visualization was performed with fluorescent secondary antibodies using Cy2- and Cy3-conjugated secondary antibodies (Jackson Immuno Labs). Coronal slices −5.80 to −7.04 from Bregma were used to identify positive nuclei bilaterally in the tVTA. Analysis of GAD/pCREB nuclei was performed using a epifluorescent microscope; (colocalization was later confirmed via confocal analysis). Images (40x) from three equally spaced sections spanning the entire brain structure were captured and quantitative analysis of relative optical density was performed using Image-J software (NIH) by observers blind to each animal’s treatment condition. Because the GABAergic tVTA is a relatively small brain region expressing few pCREB positive cells under basal conditions, the quantification of easy-to-detect pCREB positive cells in throughout this entire region is simple and straightforward.
2.5. Statistical Analyses
Statistical analyses were performed using SPSS (version 23), with a significance level of p<0.05. All ANOVAs were followed with post-hoc tests or pre-planned comparisons between treatment groups and/or across days. Percentage loss in body weight for each rat across morphine exposure and withdrawal (Days 1-13) was calculated from their weight recorded on the first day of morphine treatment (i.e. animal weight on Day 1). Body-weight changes were compared across treatment conditions and both sexes using three-way mixed factor ANOVA. Statistical analyses of individual (i.e., writhing and wet-dog shakes) and collective (i.e. Withdrawal Score: %body weight lost + wet-dog shakes + writhing) signs of withdrawal were analyzed with three-way mixed factor ANOVAs (sex and treatment conditions as between-subjects factor and time after withdrawal as repeated-measures factor). To analyze pCREB immunoreactivity in GABAergic cells in the tVTA of morphine-withdrawn rats, a two-way factorial ANOVA was performed. Lastly, a simple linear regression analysis was used to test if levels of pCREB expression in the tVTA significantly predicted withdrawal scores in morphine-dependent rats.
3. Results
3.1. Sex differences in weight change
Weight gain patterns in male and female rats were differentially regulated by chronic morphine administration (Days 1-10) and spontaneous withdrawal from morphine (Days 11-13) thus demonstrating sex-specific physiological differences induced by morphine. A 2(sex) x 2 (treatment) x 13 (% weight change on different days as repeated measures variable) mixed-ANOVA was conducted to determine statistically significant morphine-induced sex-specific differences in weights of the rats. A significant three-way interaction between morphine treatment, sex, of the animal and weight change was observed (F(3.27, 104.51) = 16.52, p<0.001, partial η2=0.34). Since the assumption of sphericity was violated (χ277=289.40, p<0.001), Greenhouse-Geisser (ε = 0.272) correction was employed to the degrees of freedom while interpreting the ANOVA.
3.1.1. Chronic Escalating Morphine Exposure
Post-hoc tests employing the Bonferroni correction revealed that saline-treated control rats (both males and females) gained weight consistently throughout the course of the experiment. In contrast, morphine-treated male rats gained weight for the first 7 days of the drug treatment. However, the weight gain for morphine-treated male rats was significantly lower than that for the saline-treated controls (p<0.05). Thereafter, their weight began to decline such that on the last day of morphine treatment, their weight was equal to that observed on their first day of the drug treatment (Fig.1C and Table1). On the other hand, morphine-treated female rats continued to gain weight for all 10 days of morphine administration, and had significantly greater weight-gain than saline-treated counterparts (p<0.05; Fig.1B and Table1). Taken together, these results demonstrate that chronic morphine administration in male rats inhibits typical weight gain. In contrast, chronic morphine administration to female rats, potentiates the typical weight-gain pattern in female rats.
Figure 1. Effects of chronic escalating morphine treatment and morphine withdrawal on body mass of male and female rats.
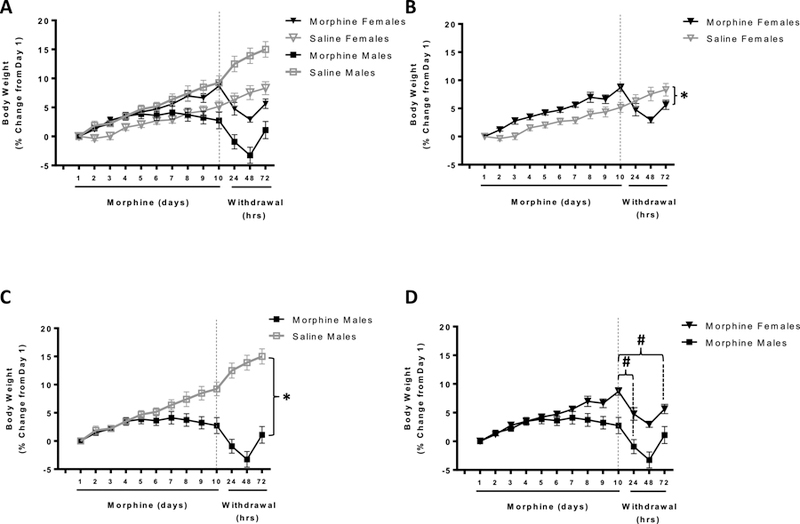
Mean body weight is expressed as percent (%) change in body weight throughout experimental timeline. The main figure (A) depicts all four experimental groups on the same graph (morphine-treated females, filled black triangles; saline-treated females, open grey triangles; morphine-treated males, filled black squares; saline-treated males, open grey squares). For purposes of clarity, these same data are replotted into mini-graphs B-D:B) females;C) males;D) morphine-treated male vs female rats. All saline-treated animals continued to gain weight throughout the course of the experiment (A). Morphine-treated male rats stopped gaining weight after 7 days of morphine treatment and the overall weight gain for morphine-treated male rats was significantly lower than that for saline-treated controls (*p<0.05; A,C). Female morphine-treated rats continued to gain weight for all 10 days of morphine administration and had significantly greater weight-gain than their saline-treated counterparts (*p<0.05; A,B). Withdrawal from chronic morphine immediately reduced body mass in both male and female animals (#p<0.001; A,B,C,D). While, both male and female rats showed a stark weight loss during the first 48h of withdrawal, female animals showed a greater percentage of weight loss than male animals during this time. Male animals seemed to recover from this much faster than female animals.
Table 1.
Mean ± SEM of % change in body weight from Day 1
| Treatment | Escalating morphine doses | Withdrawal | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Saline Males (n = 9) | Mean | 0.000 | 1.881 | 2.217 | 3.484 | 4.691 | 5.185 | 6.396 | 7.417 | 8.483 | 9.237 | 12.490 | 13.897 | 15.002 |
| SE | 0.000 | 0.498 | 0.508 | 0.558 | 0.613 | 0.643 | 0.770 | 1.049 | 0.982 | 1.046 | 1.187 | 1.115 | 1.180 | |
| Saline Females (n = 9) | Mean | 0.000 | −0.338 | 0.063 | 1.544 | 2.054 | 2.691 | 2.881 | 3.991 | 4.379 | 5.176 | 6.354 | 7.552 | 8.313 |
| SE | 0.000 | 0.498 | 0.508 | 0.558 | 0.613 | 0.643 | 0.770 | 1.049 | 0.982 | 1.046 | 1.187 | 1.115 | 1.180 | |
| Morphine Males (n = 8) | Mean | 0.000 | 1.465 | 2.179 | 3.483 | 3.859 | 3.617 | 4.113 | 3.730 | 3.236 | 2.744 | −0.925 | −3.252 | 1.100 |
| SE | 0.000 | 0.529 | 0.539 | 0.592 | 0.650 | 0.682 | 0.816 | 1.113 | 1.041 | 1.110 | 1.259 | 1.182 | 1.252 | |
| Morphine Females (n = 10) | Mean | 0.000 | 1.206 | 2.781 | 3.489 | 4.220 | 4.728 | 5.593 | 6.967 | 6.627 | 8.694 | 4.768 | 2.928 | 5.630 |
| SE | 0.000 | 0.473 | 0.482 | 0.530 | 0.582 | 0.610 | 0.730 | 0.995 | 0.932 | 0.993 | 1.126 | 1.058 | 1.120 | |
3.1.2. Withdrawal from Chronic Morphine Treatment
Spontaneous withdrawal from chronic morphine immediately induced significant weight loss in both morphine-treated male and female rats (p<0.05). Interestingly, sex-specific differences in the loss in body mass were observed in these rats over the period of withdrawal (Days 11-13) as well. Post-hoc tests employing the Bonferroni correction demonstrated that both morphine-treated male and female rats showed a stark weight loss during the first 48 hours of withdrawal. Specifically, female rats showed a significantly greater percentage of weight loss than male animals during the first 48 hours of withdrawal. However, male rats recovered much faster from this weight loss than female rats. This was evident because by 72h after withdrawal, male rats had regained all the weight they had lost during the first 48h of withdrawal. Female rats on the other hand were unable to gain weight as rapidly as their male counterparts by the end of the 72-hour withdrawal period. Taken together, this suggests that male rats experienced more severe short-term physiological imbalances whereas female rats experienced more long-lasting physiological imbalances, with respect to weight change during the withdrawal from morphine dependence (Fig.1D and Table1).
3.2. Wet-Dog Shakes
Spontaneous withdrawal from chronic morphine administration significantly induced wet-dog shakes in both male and female rats. A 2(sex: male vs female) x 2(treatment: saline vs morphine) x 6(time after withdrawal: 12, 24, 36, 48, 60, 72h) mixed-factor ANOVA revealed significant sex-dependent differences in wet-dog shakes during morphine withdrawal (F(5, 28) = 3.41; p<0.05, partial η2 = 0.379). Post-hoc tests employing the Bonferroni correction revealed that morphine-dependent male rats had more wet-dog shakes compared to morphine-dependent females at 12, 24, 36 and 60 hours after cessation of morphine treatment. Males and females did not differ in the number of wet-dog shakes 48 or 72 hours after the end of treatment (Fig. 2A).
Figure 2. Spontaneous withdrawal behaviors.
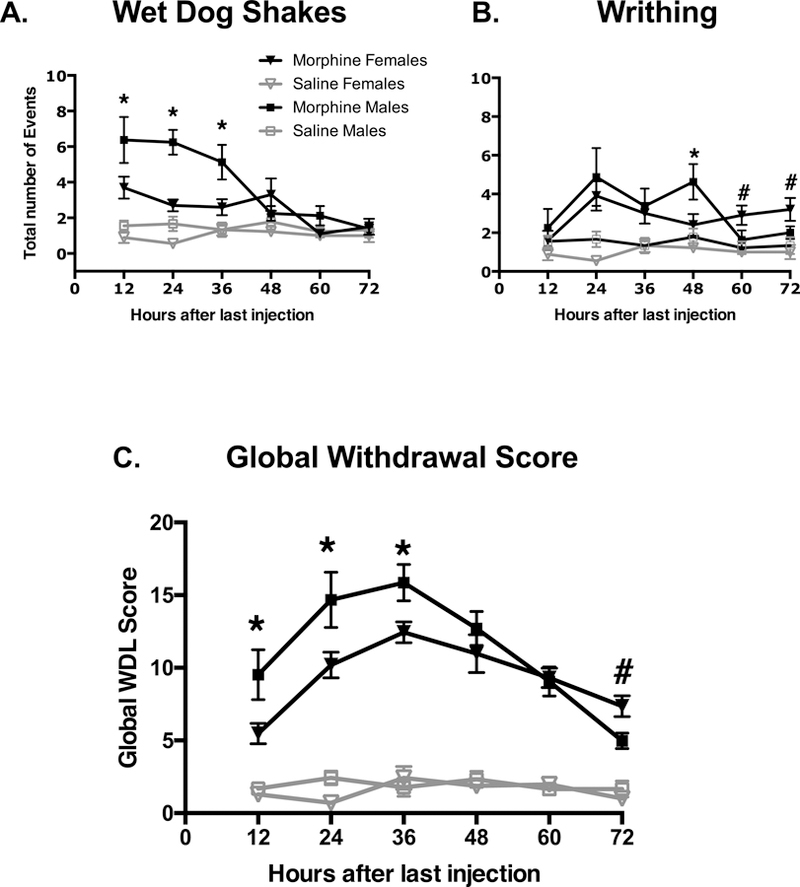
Behaviors were recorded 12, 24, 26, 48, 60, and 72 hours after the last injection. A) Spontaneous withdrawal from chronic morphine administration significantly induced wet-dog shake symptoms in all rats (p<0.001;B). Overall, morphine-dependent males had more wet-dog shakes compared to morphine-dependent females. Specifically, morphine-dependent male rats exhibited more wet-dog shakes compared to morphine-dependent females at 12, 24, 36 and 60 hours after cessation of morphine treatment (*male>female;p<0.05). B) Spontaneous withdrawal from chronic morphine administration significantly induced writhing symptoms in male and female rats compared to saline-paired controls (p<0.001). Morphine-dependent male rats had more writhing symptoms compared to females 48 hours after cessation of morphine treatment (*p<0.05); however, females had more writhing symptoms 60 and 72 hours after the end of treatment (#female>male;p<0.05). C) Difference in trends of collectively scored withdrawal behaviors at 12, 24, 36, 48, 60, and 72h after the last injection (n = 8-10/group for each group independently). Withdrawal scores for morphine-dependent male rats were significantly higher than those for morphine-dependent females at 12, 24, and 36 hours of withdrawal (*p<0.05) after which symptoms began to subside. Withdrawal scores for female rats remained elevated for up to 60 hours into morphine withdrawal. Global withdrawal scores for morphine-dependent female rats were remained elevated and exceeded those for males at the 72h time point (#p<0.05).
3.3. Writhing
Spontaneous withdrawal from chronic morphine administration significantly induced writhing symptoms in all rats. A similar 2X2X6 mixed-factor ANOVA revealed significant sex differences in writhing symptoms during morphine withdrawal (F(5,28) = 3.197; p<0.05, partial η2 = 0.363). Post-hoc tests employing the Bonferroni correction revealed that morphine-dependent male rats had more writhing symptoms compared to females 48 hours after cessation of morphine treatment; however, females had more writhing symptoms 60 and 72 hours after the end of treatment. Males and females did not differ in the amount of writhing 12, 24, or 36 hours after the end of treatment (Fig. 2B).
3.4. Withdrawal Score
In order to determine sex differences in withdrawal scores in morphine-treated animals a similar 2X2X6 mixed-factor ANOVA was conducted. Morphine withdrawal induced significant sex differences between saline and morphine-treated rats (F(5, 28) = 3.05, p = 0.025, partial η2 = 0.353). Post-hoc tests employing the Bonferroni correction demonstrated that male rats treated with morphine showed a swift and significant increase in withdrawal scores for the first 36 hours of withdrawal after which withdrawal scores began to decline. However, withdrawal scores for females remained significantly high for up to 60 hours after the last morphine dose. Morphine-treated male animals demonstrated higher withdrawal scores than females for 36h after the last morphine dose. However, withdrawal scores were significantly higher in morphine-treated female animals than males at 72h after the last morphine dose. These data demonstrate that males and females acquired morphine dependence similarly. Interestingly, male rats showed an extremely high intensity of withdrawal symptoms for the first 36 hours, whereas withdrawal symptoms for female animals were slightly less intense, but significantly more prolonged (Fig.2C).
3.5. pCREB expression in the tVTA
To begin identifying the underlying neurobiological mechanism for differential effects of chronic morphine in males versus females, pCREB expression within the tVTA was completed following 72-hours of morphine withdrawal. A 2(sex)X2(treatment) factorial ANOVA revealed a significant main effect of for treatment (F(1,23) = 12.578, p = 0.002, partial η2 = 0.398), but not sex. Additionally, no significant sex X treatment interaction was observed. Post-hoc analyses employing Bonferroni correction showed that morphine-dependent females had significantly higher pCREB counts compared to saline-treated females (p<0.01). Because no statistically significant differences were observed between any other groups (Fig.3), the main effect of treatment was likely driven by the differences in pCREB expression in females. However, we will need to conduct additional studies to substantiate this claim.
Figure 3. Sex differences in pCREB positive neurons of the VTA.
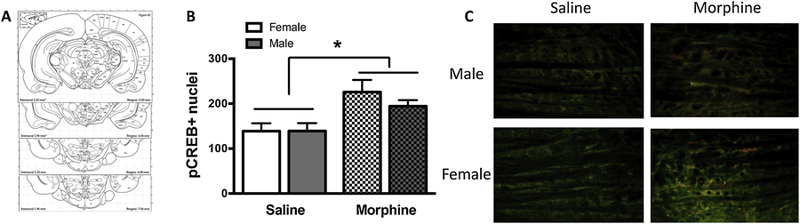
A) Schematics showing landmarks and position of tVTA from Begma. B) pCREB/GAD67 positive cell number quantification in saline-treated (clear bars) or morphine-dependent (checkered bars) animals. Note that pCREB levels after 72h of spontaneous withdrawal are significantly higher only in the tVTA of morphine-dependent females compared to saline-treated females (*p<0.05). C) 20x Photomicrographs depicting pCREB /GAD67 immunofluorescence.
3.6. VTA pCREB expression predicts withdrawal score
A simple/bivariate regression model was tested to determine whether tVTA-pCREB expression would predict withdrawal scores. The results indicated that tVTA pCREB expression accounted for a significant proportion of the variance in withdrawal scores (R2 = 0.251, F(1, 31) = 10.054, p = 0.003). tVTA-pCREB expression was a positive predictor of withdrawal scores (b = 0.02, SE = 0.006, t(31) = 3.171, p = 0.003). Additionally, a simple/bivariate regression model was tested to determine whether tVTA-pCREB expression would predict withdrawal scores differently in male and females. The results indicate that tVTA-pCREB expression accounted for a significant proportion of the variance in withdrawal scores of females (R2 = 0.489, F(1, 16) = 14.373, p = 0.002). tVTA-pCREB expression was a positive predictor of withdrawal scores (b = 0.03, SE = 0.007, t(16) = 3.791, p = 0.002). However in males, tVTA-pCREB expression could not significantly predict withdrawal scores (R2 = 0.002, F(1, 14) = 0.02, p = 0.89; Fig.4).
Figure 4. Correlation between pCREB positive cells and morphine withdrawal scores.
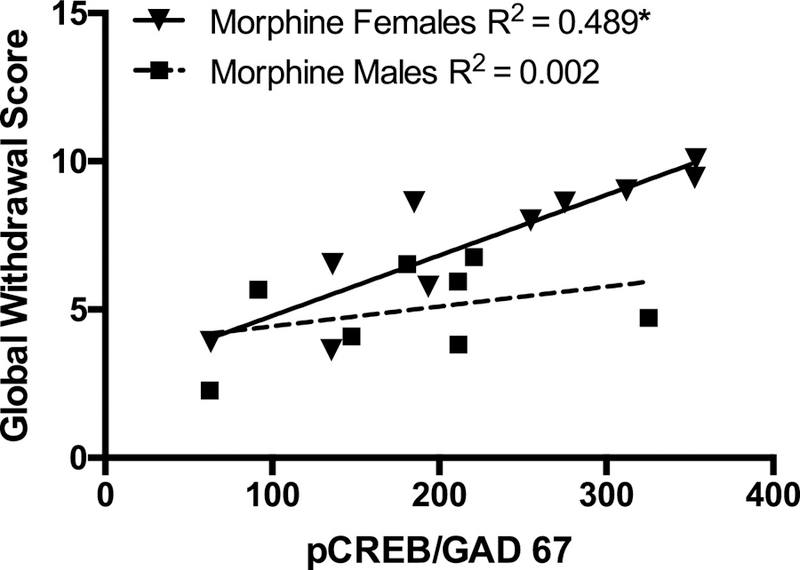
Solid inverted triangles represent global withdrawal scores correlated with pCREB/GAD67 positive cells of morphine-treated females; solid squares represent the same correlation scores for males. Regression analyses indicated that global withdrawal scores and number of pCREB/GAD67 positive cells were significantly positively correlated for morphine-treated females 72h after the last injection (*p<0.05).
4. Discussion
The main objective of the current study was to begin establishing potential sex differences in the behavioral, and molecular signaling adaptations within the tVTA, resulting from morphine withdrawal. The results of present study demonstrate sex differences in the expression and duration of spontaneous morphine withdrawal and provide some initial data characterizing the relationship between spontaneous somatic withdrawal symptoms and pCREB activation in the tVTA of morphine-dependent male and female rats. We report significant time- and sex-dependent differences in the severity of spontaneous morphine withdrawal symptoms, and that the severity of somatic withdrawal symptoms positively correlates with tVTA-pCREB expression in females.
Our data confirm and extend findings from earlier studies indicating that male rats express an overall more severe magnitude of somatic opioid withdrawal than females [29,30]. The effect we observed was largely driven by the males’ extreme plunge in body weight and higher number of wet-dog shakes. Weight loss after spontaneous morphine withdrawal in male rodents is well documented [31–34] and the loss in body weight loss has long been used as a highly predictive, single factor of withdrawal [35,36]. It has been postulated that the weight loss during withdrawal is associated with increased sympathetic tone, diarrhea, and salivation [37]. This is, in part, likely due to the relationship between stress-induced changes in metabolic states and HPA activity. A previous study demonstrated an early time course of weight loss in male rats accompanied by a rapid burst of corticotropin-releasing hormone (CRH) [34]. Suggesting that increases in CRH may be responsible for mediating some of the metabolic and behavioral effects of early withdrawal in male rats [38] 33]. Therefore, it is conceivable that males experience an enhanced HPA axis response in the earlier phases of morphine withdrawal compared to females, thus providing a potential mechanism for the higher expression of metabolic symptoms at earlier time points. However, to our knowledge, only one other study has incorporated body weight loss in female rats in measures of somatic morphine withdrawal [39]. Therefore, results from our study add empirical evidence towards the immediate physiological effects seen in morphine-dependent females during somatic withdrawal and also extend it by demonstrating that these physiological effects may be prolonged in females.
The second greatest sex difference in withdrawal symptoms, in the present study occurred as the expression of wet-dog shakes. Wet-dog shakes occur as a part of the withdrawal syndrome, a condition that develops as the result of hypothermia [40]. Sex differences in wet-dog shakes were previously reported [30] and attributed to established sex differences in morphine-induced hypothermia [41]. Based upon this literature, we also interpret our findings in wet-dog shakes to sex differences in thermoregulatory responses. This is an avenue of investigation we will incorporate into future studies.
In addition to male rats displaying an overall more severe withdrawal syndrome, the onset, severity, and duration of withdrawal for males occurred closer in proximity to the cessation of morphine treatment than in females. In other words, while both morphine-dependent male and female rats developed somatic symptoms within 12 hours of spontaneous withdrawal from morphine treatment, males consistently demonstrated more symptoms during the early stages of withdrawal compared to females. Remarkably, females exhibited a slower decline of symptoms compared to males over the 72-hour observation period. Although, the withdrawal syndrome for males was collectively more severe compared to females; the females seemed to be more symptomatic during the later stages of withdrawal (i.e., 72 hours after the last morphine treatment). We attribute much of this effect to (1) differences in body-weight loss over the withdrawal time course; although females lost a lower percentage of their overall bodyweight, males gained their pre-withdrawal weight back by the 72-hour time point; and (2) the persistence of stomach writhing symptoms in the females which continued well into late withdrawal. Consequently, global withdrawal scores for females exceeded those for males 72 hours after cessation of morphine treatment. Overall, the data here present an interesting finding which suggests that males and females have time-dependent differences in somatic withdrawal symptom onset and severity.
The neurobiological mechanism(s) underlying these sex differences in morphine-withdrawal profile are unknown. We report increases in pCREB within the tVTA in males and females, and that pCREB expression positively correlates and predicts severity of withdrawal in female, but not male rats. This is the first study to investigate sex differences in activation of CREB in the GABAergic tVTA after morphine dependence. As previously mentioned, withdrawal from chronic morphine has been shown to increase tVTA GABA release [13]. This increased GABA release is postulated to be one of the steps that triggers the cascade of intracellular events that ultimately manifest as morphine withdrawal syndrome [14]. Within the tVTA-VTA circuit, early withdrawal from morphine increases GABA release onto VTA-DA neurons and this effect has been shown to be dependent on cAMP pathway activity [14]. A recent study shows differential responding of the tVTA-VTA circuit during protracted versus early withdrawal. The alterations observed at the protracted time-point, indicate a long-term change in the functioning of the circuit, capacity of the tVTA to inhibit, but not disinhibit, DA cells which may contribute to long-term negative affective states during withdrawal [13]. It is possible that the mechanism by which we observed increased activation of CREB in the tVTA of morphine-withdrawn rats is indicative of the alterations which take place during “early” withdrawal periods. We are interested in identifying shorter-(hours) and longer-term (days) changes in cell signaling within this circuit. For example, a recent study in male rats demonstrated that acute naloxone-precipitated morphine withdrawal induced c-Fos expression in the tVTA [42]. In the context of our study, it is interesting to consider these results along with the electrophysiological findings demonstrating differential responding in the tVTA during initial/early phases of withdrawal (2-3 hours) versus longer-term protracted withdrawal states (14 days) [13,43]. We observed increases in pCREB positive cells in the tVTA of morphine-dependent animals at a relative “mid-range” withdrawal time-point (72h). Taken together, this indicates that rapid changes occur in the tVTA system within hours and days, and then persist for weeks after the cessation of opioid use. In addition, this also provides support for our hypothesis that CREB activity in the tVTA correlates with more severe symptoms of withdrawal. Of course, we will need to investigate these premises further, with more points on the withdrawal time course and additional molecular markers.
In conclusion, our data indicate that males suffer from more severe withdrawal symptoms sooner after the cessation of morphine treatment, while females experience a more protracted withdrawal syndrome lasting at least 72 hours after the last dose of morphine. In addition, our data demonstrate the link between activation of CREB in the tVTA and the severity of morphine withdrawal symptoms of females. Future studies will investigate sex-dependent differences in activation of the CREB pathways in GABAergic neurons in the tVTA and characterize the timescale and relationship between CREB activity and somatic withdrawal symptoms.
Highlights.
We observed sex differences in the onset and severity of withdrawal symptoms.
Males withdrawal symptoms were more severe earlier in withdrawal than females.
Withdrawal symptoms in females persisted for longer than in males.
tVTA pCREB activity is correlated with withdrawal symptom severity in females.
Acknowledgements
This research was supported by the National Institute on Drug abuse of the National Institutes of Health under Award Number R15DA040809 (LIP) and a graduate dissertation fellowship from the University of Texas at Arlington (SAMB). We graciously thank Michelle M. White and Ariel E. Elmore for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Bobzean SA, Dennis TS, Perrotti LI. Acute estradiol treatment affects the expression of cocaine-induced conditioned place preference in ovariectomized female rats. Brain Res Bull 2014;103:49–53. doi: 10.1016/j.brainresbull.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am 1999;22(2):241–52. [DOI] [PubMed] [Google Scholar]
- 3.Kosten TR, Rounsaville BJ, Kleber HD. Ethnic and gender differences among opiate addicts. Int J Addict 1985;20(8):1143–62. [DOI] [PubMed] [Google Scholar]
- 4.Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 2016;68(2):242–63. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology 2013;38(9):1532–44. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res 1983;277(1):119–27. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 1992;450:455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, et al. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci 2005;21(10):2817–24. doi: EJN4110 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 2009;61(5):786–800. doi: S0896-6273(09)00121-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourdy R, Sanchez-Catalan MJ, Kaufling J, Balcita-Pedicino JJ, Freund-Mercier MJ, Veinante P, et al. Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacology 2014;39(12):2788–98. doi: 10.1038/npp.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, et al. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A 2011;108(39):16446–50. doi: 10.1073/pnas.1105418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol 2009;513(6):566–96. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufling J, Aston-Jones G. Persistent Adaptations in Afferents to Ventral Tegmental Dopamine Neurons after Opiate Withdrawal. J Neurosci 2015;35(28):10290–303. doi: 10.1523/JNEUROSCI.0715-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhavan A, Bonci A, Whistler JL. Opioid-Induced GABA potentiation after chronic morphine attenuates the rewarding effects of opioids in the ventral tegmental area. J Neurosci 2010;30(42):14029–35. doi: 10.1523/JNEUROSCI.3366-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci 1997;17(2):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guitart X, Thompson MA, Mirante CK, Greenberg ME, Nestler EJ. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem 1992;58(3):1168–71. [DOI] [PubMed] [Google Scholar]
- 17.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 2001;81(1):299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 18.Walters CL, Kuo YC, Blendy JA. Differential distribution of CREB in the mesolimbic dopamine reward pathway. J Neurochem 2003;87(5):1237–44. doi: 2090 [pii]. [DOI] [PubMed] [Google Scholar]
- 19.Ren X, Lutfy K, Mangubat M, Ferrini MG, Lee ML, Liu Y, et al. Alterations in phosphorylated CREB expression in different brain regions following short- and long-term morphine exposure: relationship to food intake. J Obes 2013;2013:764742. doi: 10.1155/2013/764742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol 1996;49(4):636–45. [PubMed] [Google Scholar]
- 21.Seip KM, Reed B, Ho A, Kreek MJ. Measuring the incentive value of escalating doses of heroin in heroin-dependent Fischer rats during acute spontaneous withdrawal. Psychopharmacology (Berl) 2012;219(1):59–72. doi: 10.1007/s00213-011-2380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristow LJ, Hogg JE, Hutson PH. Competitive and glycine/NMDA receptor antagonists attenuate withdrawal-induced behaviours and increased hippocampal acetylcholine efflux in morphine-dependent rats. Neuropharmacology 1997;36(2):241–50. [DOI] [PubMed] [Google Scholar]
- 23.Busquets X, Ventayol P, Garcia-Sevilla JA. Naloxone-precipitated withdrawal in morphine-dependent rats increases the expression of alpha 2a-adrenoceptor mRNA in brain. Brain Res Mol Brain Res 1997;45(1):154–8. [DOI] [PubMed] [Google Scholar]
- 24.Caille S, Espejo EF, Reneric JP, Cador M, Koob GF, Stinus L. Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal. J Pharmacol Exp Ther 1999;290(2):881–92. [PubMed] [Google Scholar]
- 25.Jones KL, Zhu H, Jenab S, Du T, Inturrisi CE, Barr GA. Attenuation of acute morphine withdrawal in the neonatal rat by the competitive NMDA receptor antagonist LY235959. Neuropsychopharmacology 2002;26(3):301–10. doi: 10.1016/S0893-133X(01)00347-5. [DOI] [PubMed] [Google Scholar]
- 26.Tokuyama S, Ho IK. Inhibitory effects of diltiazem, an L-type Ca2+ channel blocker, on naloxone-increased glutamate levels in the locus coeruleus of opioid-dependent rats. Brain Res 1996;722(1-2):212–6. [DOI] [PubMed] [Google Scholar]
- 27.Tokuyama S, Wakabayashi H, Ho IK. Direct evidence for a role of glutamate in the expression of the opioid withdrawal syndrome. Eur J Pharmacol 1996;295(2-3):123–9. [DOI] [PubMed] [Google Scholar]
- 28.Tokuyama S, Zhu H, Oh S, Ho IK, Yamamoto T. Further evidence for a role of NMDA receptors in the locus coeruleus in the expression of withdrawal syndrome from opioids. Neurochem Int 2001;39(2):103–9. [DOI] [PubMed] [Google Scholar]
- 29.Cicero TJ, Nock B, Meyer ER. Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav 2002;72(3):691–7. doi: S0091305702007402 [pii]. [DOI] [PubMed] [Google Scholar]
- 30.Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology (Berl) 1999;143(1):1–7. [DOI] [PubMed] [Google Scholar]
- 31.Houshyar H, Manalo S, Dallman MF. Time-dependent alterations in mRNA expression of brain neuropeptides regulating energy balance and hypothalamo-pituitary-adrenal activity after withdrawal from intermittent morphine treatment. J Neurosci 2004;24(42):9414–24. doi: 10.1523/JNEUROSCI.1641-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maldonado R, Fournie-Zaluski MC, Roques BP. Attenuation of the morphine withdrawal syndrome by inhibition of catabolism of endogenous enkephalins in the periaqueductal gray matter. Naunyn Schmiedebergs Arch Pharmacol 1992;345(4):466–72. [DOI] [PubMed] [Google Scholar]
- 33.Navarro-Zaragoza J, Nunez C, Laorden ML, Milanes MV. Effects of corticotropin-releasing factor receptor-1 antagonists on the brain stress system responses to morphine withdrawal. Mol Pharmacol 2010;77(5):864–73. doi: 10.1124/mol.109.062463. [DOI] [PubMed] [Google Scholar]
- 34.Pinter-Kubler B, Ferenczi S, Nunez C, Zelei E, Polyak A, Milanes MV, et al. Differential Changes in Expression of Stress- and Metabolic-Related Neuropeptides in the Rat Hypothalamus during Morphine Dependence and Withdrawal. PLoS One 2013;8(6):e67027. doi: 10.1371/journal.pone.0067027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicero TJ, Meyer ER. Morphine pellet implantation in rats: quantitative assessment of tolerance and dependence. J Pharmacol Exp Ther 1973;184(2):404–8. [PubMed] [Google Scholar]
- 36.Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther 1978;205(3):536–46. [PubMed] [Google Scholar]
- 37.Kovacs GL, Telegdy G. Hypothalamo-neurohypophyseal neuropeptides and experimental drug addiction. Brain Res Bull 1988;20(6):893–5. [DOI] [PubMed] [Google Scholar]
- 38.Koob GF. A role for brain stress systems in addiction. Neuron 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav 2000;65(1):91–6. doi: S0091-3057(99)00174-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 40.Belknap JK. Components of the opioid withdrawal syndrome in mice are thermoregulatory responses. Pharmacol Biochem Behav 1989;34(2):241–5. [DOI] [PubMed] [Google Scholar]
- 41.Kasson BG, George R. Endocrine influences on the actions of morphine: IV. Effects of sex and strain. Life Sci 1984;34(17):1627–34. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Catalan MJ, Faivre F, Yalcin I, Muller MA, Massotte D, Majchrzak M, et al. Response of the Tail of the Ventral Tegmental Area to Aversive Stimuli. Neuropsychopharmacology 2017;42(3):638–48. doi: 10.1038/npp.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 2014;82(6):1346–56. doi: 10.1016/j.neuron.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


