Abstract
Background & Aims:
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Progression from BE to cancer is associated with obesity, possibly due to increased abdominal pressure and gastroesophageal reflux disease, although this pathogenic mechanism has not been proven. We investigated whether environmental or dietary factors associated with obesity contribute to progression of BE to EAC in mice.
Methods:
Tg(ED-L2-IL1RN/IL1B)#Tcw mice (a model of BE, called L2-IL1B mice) were fed a chow (control) or high-fat diet (HFD), or crossbred with mice that express human IL8 (L2-IL1B/IL8 mice). Esophageal tissues were collected and analyzed for gene expression profiles and by quantitative PCR, immunohistochemistry, and flow cytometry. Organoids were established from BE tissue of mice and cultured with serum from lean or obese individuals, or neutrophils from L2-IL1B mice. Feces from mice were analyzed by 16s rRNA sequencing and compared to 16s sequencing data from patients with dysplasia or BE. L2-IL1B were mice raised in germ-free conditions.
Results:
L2-IL1B mice fed a HFD developed esophageal dysplasia and tumors more rapidly than mice fed the control diet; the speed of tumor development was independent of body weight. The acceleration of dysplasia by the HFD in the L2-IL1B mice was associated with a shift in the gut microbiota and an increased ratio of neutrophils to natural killer cells in esophageal tissues, compared to mice fed a control diet. We observed similar differences in the microbiomes from patients with BE that progressed to EAC vs patients with BE that did not develop cancer. Tissues from dysplasias of L2-IL1B mice fed the HFD contained increased levels of cytokines that are produced in response to CXCL1 (the functional mouse homolog of IL8, also called KC). Serum from obese patients caused organoids from L2-IL1B/IL8 mice to produce IL8. BE tissues from L2-IL1B mice fed the HFD and from L2-IL1B/IL8 mice contained increased numbers of myeloid cells and cells expressing Cxcr2 and Lgr5 mRNAs (epithelial progenitors) compared to mice fed control diets. BE tissues from L2-IL1B mice raised in germ-free housing had fewer progenitor cells and developed less dysplasia than in L2-IL1B mice raised under standard conditions; exposure of fecal microbiota from L2-IL1B mice on HFD to L2- IL1B mice on control diet accelerated tumor development.
Conclusions:
In a mouse model of BE, we found that a HFD promoted dysplasia by altering the esophageal microenvironment and gut microbiome, thereby inducing inflammation and stem cell expansion, independent of obesity.
Keywords: NK cells, neutrophils, cytokines, IL1B
Lay Summary:
In an inflammation induced mouse model of esophageal tumor development a diet rich in fat is leading to accelerated tumor growth in part caused by changes in the gut microbiome which induces an accelerated inflammatory microenvironment.
Introduction
BE is thought to arise as a consequence of gastro-esophageal reflux disease (GERD), which damages the esophageal mucosa, leading to a replacement of squamous epithelium by a metaplastic columnar epithelium above the gastroesophageal junction (GEJ)1. The increase in EAC incidence in the Western world has led to some uncertainty in the clinical management of BE and the estimation of its risk for EAC. While current strategies employ aggressive screening, surveillance and/or ablation procedures, the benefits of such procedures have been difficult to demonstrate2 and a better understanding of risk factors is needed.
In addition to male gender, age and tobacco use3, obesity is strongly associated with the development of EAC4 and is now endemic in Western countries and Asia. Obesity or a high waist circumference are thought to promote BE/EAC through increased abdominal pressure and GERD5, although this mechanism has not been proven and the degree of GERD correlates poorly with disease progression. Evidence linking obesity to cancer risk derives primarily from observational cohort studies, which may not establish directly causality, but identified associations of multiple obesity-related factors (hormones, cytokines, adipokines) with increased risk and poor outcome. In this context, obesity is mostly used as a proxy for an underlying but still undefined mechanism6. This leaves the possibility that environmental or dietary factors that are prevalent in obese patients influence the risk of BE progression, rather than increased body fat per se7, as recently demonstrated for colorectal cancer8. Changes in diet can lead to alterations in the gut microbiome9, with effects on stem cell biology10 and thus the susceptibility to gastrointestinal neoplasia. Furthermore, a Western-style diet itself can contribute to chronic inflammation8, 11, 12, which itself can favor the emergence of gut microbiota that favor carcinogenesis13.
Here, we utilize the L2-IL1B mouse model of BE14 to analyze dietary effects of a HFD on BE progression. The L2-IL1B mouse model expresses human interleukin 1β in the esophagus and forestomach squamous epithelium, resulting in inflammation with progression to metaplasia and dysplasia. We demonstrate that HFD leads to global changes in the gut microbiome that mirror closely those observed in BE patients, and facilitates the establishment of an inflammatory pro-carcinogenic microenvironment at the GEJ accelerating disease progression.
Important Methods
Animals
The mouse model (L2-IL1B, Tg(ED-L2-IL1RN/IL1B)#tcw)14, was backcrossed to C57BL/6J mice and fed a standard Chow diet from birth until weaning, and water ad libitum. Between 6 and 8 weeks of age, mice were started on HFD, control diet or remained on the Chow (ssniff). For bedding transfer experiments, cages were filled half with fresh bedding, while the other half was bedding from HFD cages starting at the age of 6 weeks until 9 months. IL8 mice15 and Mc4rX16 mice16 were bred with L2-IL1B, mice to obtain L2-IL1B/IL8Tg mice and L2-IL1B/Mc4R mice. Germfree mice were generated by embryo transfer rederivation of conventional L2-IL1B mice into germfree Swiss recipient mothers17 and maintained in sterile isolators on autoclaved food (RMH3000, Purina, USA) comparable to the standard chow and water (Suppl. Figure 8). Sterility was confirmed every other week by 16s PCR, aerobic, anaerobic and fungal culture as well as Gram stains of fecal smears. Animal experiments were approved by the District Government of Bavaria and performed in accordance with the German Animal Welfare and Ethical Guidelines and by the Committee on Animal Care at the MIT, USA.
Tissue preparation and disease evaluation
Macroscopic scoring of the squamocolumnar junction (SCJ) and the esophagus was performed and scores averaged as shown in Suppl. Fig. 1B, C. For further RNA or protein analysis the SCJ was macroscopically identified as the first 2 mm of columnar tissue and cut with a magnifying glass dissection-scope to eliminate squamous tissue contamination as good as possible. Mouse tissues were fixed in formalin and paraffin-embedded then cut and stained with H&E (Haematoxylins and eosin). Histopathological scores were performed by an experienced mouse pathologist by previously established criteria for the influx of immune cells per high-power field, metaplasia and dysplasia14 (Suppl. Fig 1D): Inflammation was scored by the percentage of different immune cells (mostly neutrophilic myeloid cells) in a defined tissue area of the SCJ in a high-power field evaluation. Metaplasia was evaluated by the abundance of mucus producing or cells per gland and the abundance of glands with mucus producing cells in the BE area at the SCJ. Dysplasia was evaluated by the amount of cellular atypia and the presence of low or high grade dysplasia in single or multiple glands as assessed by experienced mouse pathologists. Mucus production was assessed by Periodic Acid-Schiff- (PAS) staining and quantified as percentage of PAS positive area in BE regions. Crypt fission was quantified by counting fused crypts in the BE region.
Statistics
Statistical analysis was performed using Graph Prism 6 with Student’s t-tests or one-way or two-way ANOVA’s with p-values noted in each corresponding figure. For 2-way ANOVA, Sidak-Holm post-hoc was used, for 1 way ANOVA, Tukey post-hoc was used.
Results
HFD accelerates esophageal dysplasia in the L2-IL1B mouse model, independent of obesity
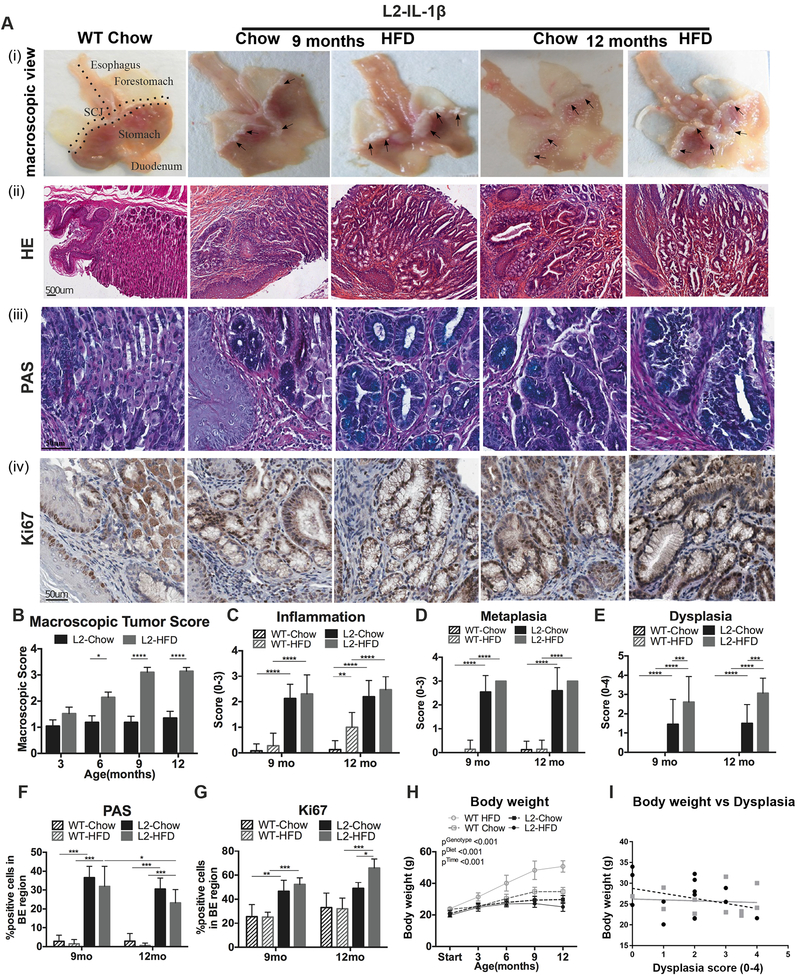
To study the effect of diet on BE and EAC, we fed wild type (WT) and L2-IL1B mice either Chow or high fat diet (HFD, 48% fat, Suppl. Fig. 1A). No pathological phenotype at the squamocolumnar junction (SCJ) in Chow and HFD fed WT mice (Fig. 1A–F, Suppl. Fig. 1B, C, D), in contrast to L2-IL1B mice on both diets. Compared to Chow fed L2-IL1B mice, a significant increase in macroscopic tumor burden at the SCJ and in the esophagus was observed in L2-IL1B mice on HFD at 9 and 12 months (Fig. 1A, B). Both L2-IL1B mice on HFD and Chow diet developed increasing but similar amounts of inflammation and columnar metaplasia over time (Fig. 1A, C, D, F). However, HFD-treated L2-IL1B mice exhibited a significant increase in proliferation only at the age of 12 months (Fig. 1A,G) compared to chow fed L2-IL1B mice. This correlated with markedly elevated dysplasia scores in HFD fed L2-IL1B mice as early as 9 months (Fig. 1A, E), demonstrating an accelerating effect of HFD on esophageal carcinogenesis in the mouse model.
Figure 1. HFD accelerates esophageal dysplasia in the L2-IL1B mouse model.
(A) (i) macroscopic image and epresentative pictures of (ii) Haematoxylin & Eosin (H&E), (iii) Periodic acid–Schiff (PAS) and (iv) Ki67 of the SCJ. A WT Chow mouse was used as a representative for all WT mice. Macroscopic tumor score at 3–12 months (mean ±SEM) (B), inflammation (C) metaplasia (D), and dysplasia scores (E) (n=8–12, mean ± SD). Quantification of (F) PAS and (G) Ki67 staining (n=8–12). (H) Body weight of L2-IL1B mice. (I) Correlations between body mass and dysplasia score. Quantification of 4 high-magnification-field (20X) of esophagus and SCJ tissue (****p<0.0001, ***p<0.001, **p<0.01, *p<0.05) For (B-E) 2-way ANOVA with Sidak-Holm post-hoc was used, for (F,G) a 1 way ANOVA with Tukey post-hoc and for (H,I) 2 way ANOVA with Tukey Post-hoc. BE region was defined as the region between squamous epithelium and oxyntic mucosa of the stomach. L2=L2-IL-1β, WT=wildtype, HFD=High fat diet
In contrast to HFD-induced obesity in WT mice (48% weight gain (p<0.001)), HFD-fed L2-IL1B mice did not gain body weight (p<0.001) (Fig. 1H). Both fat and lean mass were lower in L2-IL1B mice on HFD relative to WT mice on HFD (Suppl. Fig. 1E). Thus, acceleration of the BE phenotype occurred independent of body weight (Fig. 1I). To examine whether obesity alone without an additional inflammatory component was sufficient to influence BE progression, we interbred the L2-IL1B mice with mice lacking a functional melanocortin-receptor-4 (Mc4rW16X)16, which exhibit an obese phenotype comparable to HFD-induced obesity and are modeling the most common monogenic cause for obesity in humans18 (Suppl. Fig. 2A–C). L2-IL1B mice with a heterozygous (Mc4r+/− or Mc4rhet) or homozygous (Mc4r−/− or Mc4rki) loss of Mc4r showed a similar BE phenotype as L2-IL1B mice on a chow diet. In contrast to L2-IL1B mice on a HFD, Mc4rhet; L2-IL1B mice showed an absence of disease acceleration, with no increase in the level of inflammation, metaplasia or dysplasia (Suppl. Fig. 2A,D–G). There was no increase in the expression of inflammatory cytokine (data not shown), splenomegaly (Suppl. Fig. 2H) in L2-IL1B/Mc4rhet and L2-IL1B/Mc4rki mice, compared to L2-IL1B mice (Suppl. Fig. 2I)14. These data suggest that genetic obesity does not promote BE progression, and HFD accelerates the phenotype independent of body weight.
HFD induces an immune response leading to a distinct local inflammatory reaction
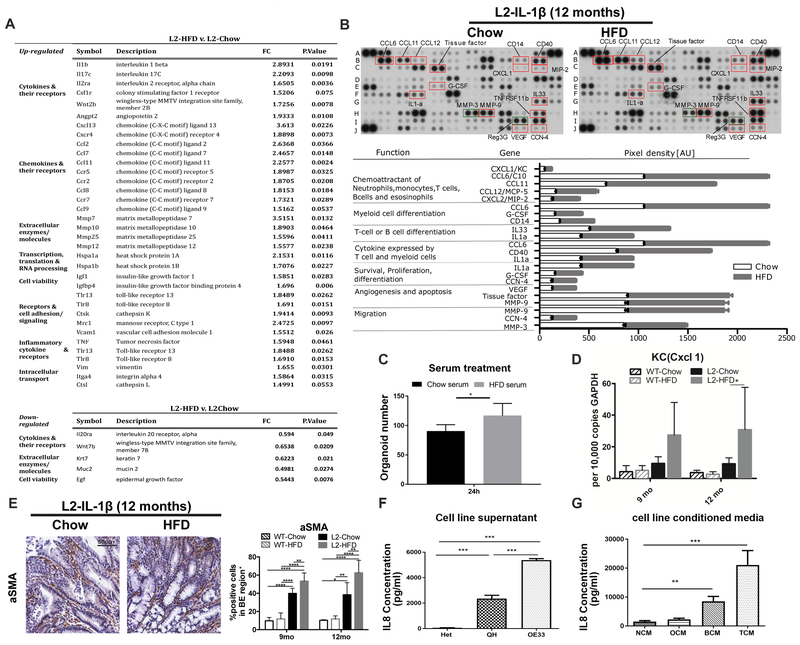
Previously, we showed that overexpression of IL1B in the esophagus leads to upregulation of pro- inflammatory cytokines14. HFD promotes chronic inflammatory responses, as has been demonstrated for colorectal carcinogenesis8. Thus, we postulated that the enhancement of dysplasia in L2-IL1B mice by HFD might be due primarily to a change of systemic and esophageal inflammation profiles. Although our tissue inflammation metric was not significantly altered in HFD L2 mice compared with chow fed L2 mice (Fig. 1C), Gene-expression (Fig. 2A) and Gene-Set-Enrichment-Analysis (GSEA) (Suppl. Fig. 3A) of SCJ tissue revealed changes in cytokine expression associated with HFD. Key mediators of this cytokine response were related to cell survival, proliferation, angiogenesis, apoptosis and importantly chemoattraction for the observed immune cells (Fig. 2A, B and Suppl. Fig. 3B). aSMA+ myofibroblasts within the SJC region were significantly increased in HFD fed L2-IL1B mice (Fig. 2E), indicating an activated stromal reaction associated with the inflammatory response. Serum from L2-IL1B mice fed with HFD significantly stimulated the growth of 3D mouse BE organoids (Fig. 2C), compared to serum from Chow mice, suggesting that systemic inflammatory components affect epithelial cell growth. Of note, serum analysis of 20 obese versus non-obese patients revealed that the pro-inflammatory cytokines IL-6 and leptin were elevated, and adiponectin decreased (Suppl. Fig. 4A). Additionally, we observed an upregulation of chemokines involved in recruitment and/or differentiation of myeloid cells and T cells, such as CCL6, CCL12 and G-CSF, (Fig. 2B, Suppl. Fig. 3B) in esophageal tissue in L2-IL1B mice on HFD. Importantly, the functional mouse homolog of IL8, KC or CXCL1, was among those cytokines in the chemokine array (Fig 2B), and was also upregulated 6-fold in esophagus tissue lysates from L2-IL1B mice on HFD for 12 months (Fig. 2D). Thus, HFD activates a systemic immune response that also affects the local microenvironment. Cytokine arrays of SCJ and esophageal tissue in Chow and HFD fed WT mice did not show any major changes; nevertheless, serum from WT-HFD showed a mainly obesity related phenotype, compared to chow fed WT mice, whereas HFD fed L2-IL1B mice showed an accelerated inflammatory phenotype compared to Chow fed L2-IL1B mice (Suppl. Fig.3C).
Figure 2. HFD induces a systemic immune response leading to a distinct local inflammatory microenvironment reaction.
(A) Gene expression analysis from SCJ tissue (n=3). (B) Cytokine array for chemoattractants of immune cells and cytokines/chemokines related to immune cell differentiation, cell survival, proliferation, angiogenesis and apoptosis in pooled esophageal tissue from12 month old L2-IL1B mice on Chow (n=4) or HFD (n=6). (C) Treatment with serum from L2-IL1B mice on HFD induces proliferation of 3D mouse BE organoids from L2-IL1B mice (Serum was collected and pooled from L2-IL1B mice maintained on Chow or HFD (n=3)). (D) qRT-PCR of CXCL1/KCin SCJ of L2-IL1B mice on HFD at 12 months (n=6). (E) a-SMA+ staining in L2-IL1B mice on HFD (n=6). (F) IL8 concentration in supernatant from esophageal squamous (Het), BE (QH) and EAC cell lines OE33 (n=3). (G) IL8 concentration in tissue conditioned medium from human normal esophagus, oesophagitis, BE and EAC. (n=10). NCM-normal conditioned medium, OCM-oesophagitis, BCM-BE, and TCM-EAC. Data are represented as mean ± SEM (****p<0.0001, ***p<0.001, **p<0.01, *p<0.05) For (C,D) 2 way ANOVA with Sidak-Holm post-hoc was used, for (E,F) 1 way Anova with Tukey post-hoc was used. (G) used a Kruskal-Wallis test against NCM and Dunns post-ho. L2=L2-IL-1β, WT=wildtype, HFD=High fat diet
HFD leads to levels of IL8/KC chemokines that promote esophageal dysplasia
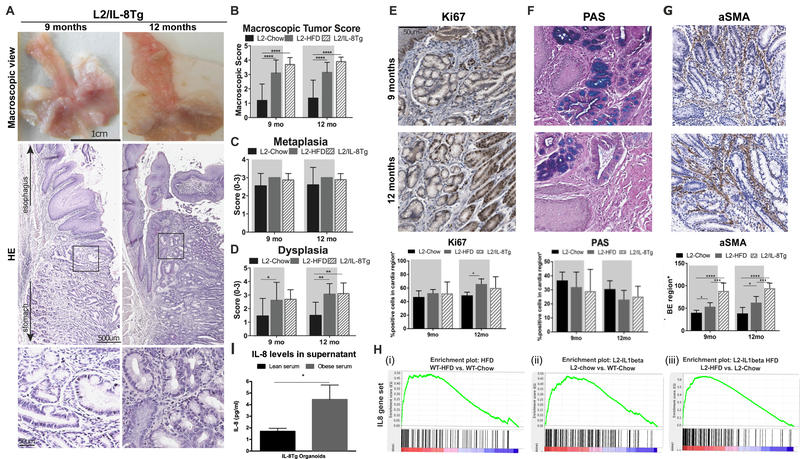
Esophageal tissue IL8 expression has been shown to be associated with histopathological inflammation in 50 BE patients and has been hypothesized to play a role in esophageal carcinogenesis19. Consistent with these earlier clinical observations, levels of the functional mouse IL8 homologue, KC or CXCL120, were significantly increased in the esophagus of HFD fed L2-IL1B mice (Fig. 2B, D). We also noted increased IL8 levels in conditioned media of a human EAC compared to BE or squamous cell lines (Fig. 2 F), and increased IL8 levels in conditioned media from BE and EAC esophageal biopsies compared to normal and esophagitis (Fig. 2G). Since rodents lack the IL8 gene, we crossed a human IL8-BAC-transgenic mouse model15, which exhibits tissue specific, physiologic IL8 gene regulation, with the L2-IL1B mice to generate L2-IL1B/IL8Tg mice. L2-IL1B/IL8Tg mice showed an accelerated phenotype, remarkably similar to that of L2-IL1B HFD mice (Fig. 3A–C, Suppl. Fig 4B), with a 2-fold increase in dysplasia scores (Fig. 3D), along with increased proliferation (Fig. 3E) and differentiation (Fig. 3F). Furthermore, we observed significant increases in aSMA+ stromal myofibroblasts (Fig. 3G).
Figure 3. IL8 is one of the key cytokines that accelerates esophageal dysplasia in HFD mice.
(A) Representative macroscopic pictures and Haematoxylin & Eosin (H&E) staining and (B) macroscopic tumor score in L2-IL1B/IL8Tg mice (n=10). (C) metaplasia and (D) dysplasia score in mice (n=8, mean ± SD). (E) Ki67 (F) PAS and (G) a-SMA+ staining in L2-IL1B/IL8Tg mice (n=6, mean ± SEM). (H) Gene Set Enrichment Analysis (GSEA): Differential expression between (i) WT HFD and Chow mice, (ii) L2-IL1B and WT Chow mice and (iii) L2-IL1B HFD and Chow mice (n=3) and analyzed with IL8 gene set, generated by utilizing gene expression data from L2-IL1B/IL8Tg mice and L2-IL-1β mice (n=3, mean ± SEM). (I) 3D mouse BE organoids from L2-IL1B/IL8Tg mice co-cultured with human lean or obese serum (pooled from 10 patients) for 48 hours. (****p<0.0001, ***p<0.001, **p<0.01, *p<0.05) For (B-D) a 2 way Anova with Sidak-Holm post-hoc was used. (E-G) use a 1 way Anova with Tukey post-hoc. (I) uses a t-test. L2=L2-IL-1β, L2/IL8Tg=L2-IL-1β/IL8Tg, HFD=High fat diet.
Utilizing gene expression data from L2-IL1B/IL8Tg compared to L2-IL1B mice, we generated an IL8 specific gene set including 150 upregulated and 110 downregulated genes. Genes stimulated by IL8 pathway activity were enriched in HFD fed WT and L2-IL1B mice compared to Chow fed mice (Fig. 3H), suggesting that the IL8/CXCL1 chemokine family contributes to the observed HFD L2-IL1B phenotype. However, we could not detect a difference in serum IL8 levels (Suppl.Fig. 4C) nor a correlation with obesity (Suppl. Fig. 4D) in patients with BE, LGD, HGD or EAC. Instead, elevated tissue concentrations of IL8 measured in conditioned medium from human patient biopsies (Fig. 2G), suggested that IL8 acts at a local level. When we treated BE organoids from L2-IL1B/IL8Tg mice with lean or obese human sera, obese sera stimulated growth of BE organoids (Suppl. Fig.4E). Relative to lean serum treatment, obese serum resulted in higher levels of L2-IL1B/IL8Tg mouse epithelial cell derived IL8 in the supernatant (Fig. 3I), indicating that serum components from obese subjects could induce local epithelial IL8 secretion. These data demonstrate that IL8 secretion in esophageal tissue is induced by HFD and plays an important role in stimulating BE cell proliferation in vitro and in vivo.
HFD increases neutrophils and decreases NK cells at the SCJ
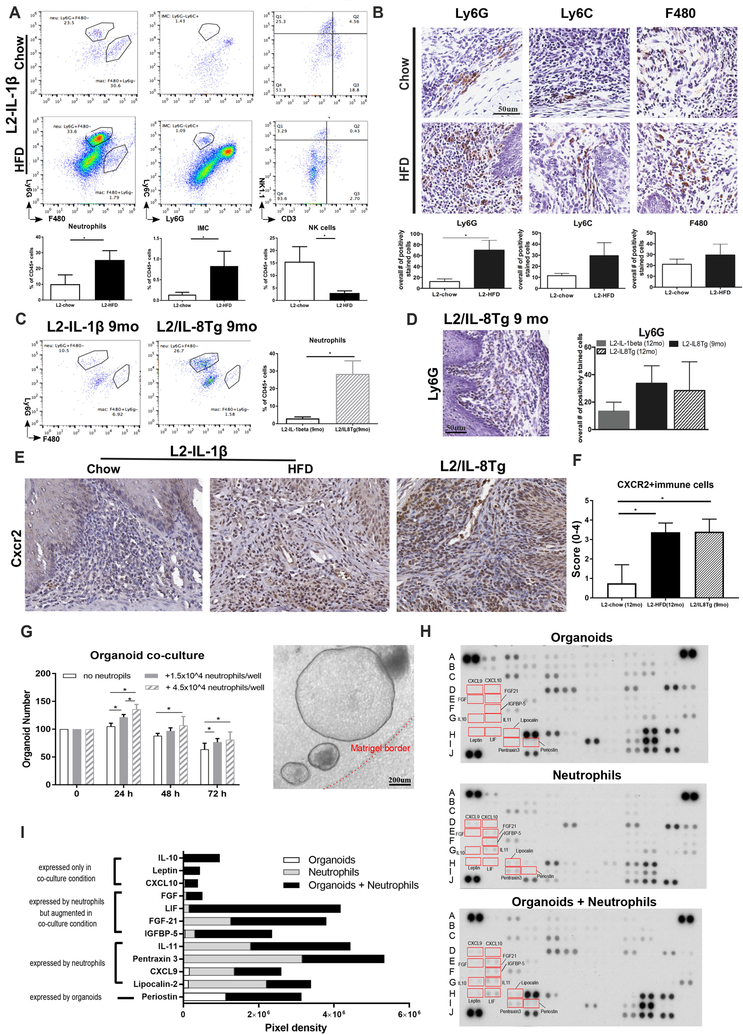
Assessment of the immune cell infiltrate in the lower esophagus using FACS (Suppl.Fig. 5A) in 12-month old L2-IL1B HFD mice with dysplasia showed a significant increase in neutrophils and immature myeloid cells (IMC) (Fig. 4A), previously linked to the IL8 pathway15. No differences in macrophages, CD4 helper T-cells, CD8 cytotoxic T-cells or gamma-delta T-cells were observed (Suppl. Fig. 5B). The influx of granulocytic myeloid cells was confirmed by IHC of the SCJ and lower esophagus (Fig. 4B) and was not observed in the glandular stomach (data not shown), highlighting the specificity of the response for the SCJ. Additionally, we observed a significant decrease in NK cells in HFD compared to chow fed mice (Fig. 4A), suggesting an inhibitory effect by neutrophils on NK cells, as previously reported21. We also found increased neutrophils in esophageal tissue of L2-IL1B/IL8Tg mice (Fig. 4C, D), consistent with the known role of IL8 as a recruiter of granulocytic immune cells15, while no difference could be detected for other immune cells (Suppl. Fig. 6A, B). Importantly, a significant increase of immune cells expressing IL8 receptor CXCR2 was observed in L2-IL1B HFD and L2-IL1B/IL8-Tg mice compared to L2-IL1B mice (Fig. 4E,F).
Figure 4. HFD induces a specific immune response with neutrophils infiltration and NK cells reduction.
(A) FACS of neutrophils, immature myeloid cells (IMC) and NK cells in the esophagus of HFD mice at 12 months (n=9–11). (B) IHC of neutrophils (Ly6G), immature myeloid cells (Ly6C) and macrophages (F4/80) in the squamous epithelium in 10 low power fields (n=3). (C) FACS of neutrophils in esophagus of L2-IL1B/IL8Tg mice (n=5–8). (D) IHC of neutrophils (Ly6G) in L2-IL1B/IL8Tgmice (n=3). (E) Cxcr2 expression and (F) score in the esophagus of L2-IL1B mice on HFD and in L2-IL1B/IL8Tg mice (n=8). (G) Proliferation of BE organoids of L2-IL1B mice co-cultured with neutrophils from the spleens of 9–12 months old L2-IL-1 β mice including a representative image of the organoids (n=3). (H) and (I) Cytokine arrays of BE organoid co-culture with neutrophils. Data are represented as mean ± SEM (*p<0.05) (A-C) use t-tests. (D,F) uses a 1 way Anova with Tukey post-hoc. (G) is a repeated measure 2 way Anova with Tukey post-hoc. L2=L2-IL-1β, L2/IL8Tg=L2-IL-1β/IL8Tg, HFD=High fat diet.
Co-cultured mouse BE organoids with neutrophils from the spleens of 9–12 months old L2-IL1B mice demonstrated a significant increase in proliferation and organoid growth in a dose-dependent fashion suggesting a direct stimulatory effect of neutrophils on BE epithelial cells (Fig. 4G). This response was mediated by inflammatory cytokines, such as leptin and IL-10, which were secreted solely under organoid-neutrophil co-culture conditions (Fig 4H,I).
The esophageal inflammatory niche recruits SCJ progenitor cells
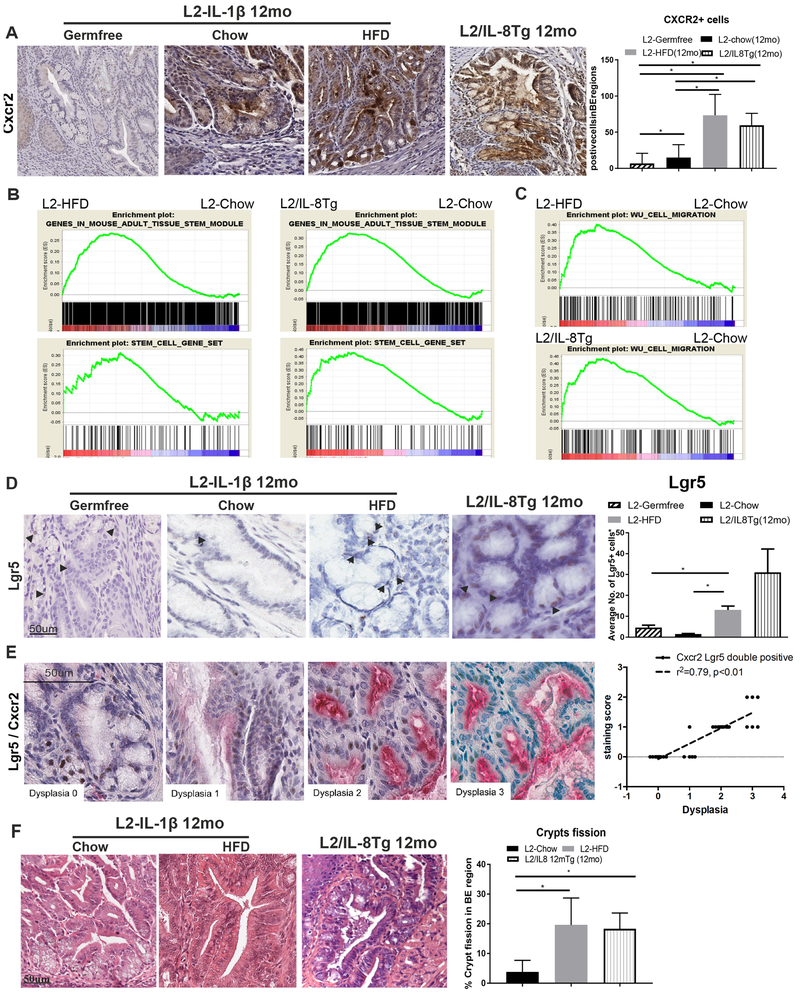
In addition to elevated CXCR2+ immune cells, we observed increased CXCR2 expressing epithelial cells in BE regions during disease progression (Fig. 5A), suggesting an effect of the inflammatory microenvironment on a distinct epithelial cell population. Previous studies by our group14 and others22 have pointed to the gastric SCJ23 as the origin of BE and EAC, with expansion of SCJ progenitor cells into the esophagus in response to chronic inflammation, thus giving rise to metaplastic and dysplastic lesions1. Indeed, when we analyzed gene expression changes related to HFD and IL8 expression, we observed a highly significant enrichment of stem cell signatures (Fig. 5B,C). In situ hybridization revealed a significant increase of the naturally present Lgr5+ progenitor cells24 in areas of BE at the SCJ in L2-IL1B mice on HFD, compared to Chow, and in L2-IL1B/IL8Tg mice (Fig. 5D). Lgr5+ cells could be found in the typical location at the bottom of the first SCJ crypt as well as in the BE region at the SCJ. These findings suggest that IL8/KC family members may directly stimulate through CXCR2 the expansion of Lgr5 progenitor cells at the SCJ. Indeed, Lgr5+ cells in HFD fed L2-IL1B mice highly expressed the IL8 receptor, CXCR2, and Lgr5+ cells increased with dysplastic progression (Fig. 5E). Additionally, analysis of BE specimens from 12-month old L2-IL1B HFD and L2-IL1B/IL8Tg mice showed significantly more crypt fission compared to age-matched L2-IL1B chow fed mice (Fig. 5F). These observations suggest a mechanism by which activation of SCJ progenitor cells by HFD/IL8 through CXCR2 could lead to crypt fission and expansion of SCJ glands into the esophagus, thus giving rise to metaplasia and dysplasia.
Figure 5. The esophageal inflammatory niche recruits SCJ progenitor cells.
(A) CXCR2 expression in BE in L2-IL1B mice on HFD and in L2-IL1B/IL8Tg mice and germfree L2-IL1B mice (n=4–8). GSEAs for (B) a mouse epithelial (cancer) stem cells gene signatures comparing L2-IL1B HFD vs. L2-IL1B Chow and L2-IL1B/IL8Tg vs. L2-IL1B Chow or a (C) human migratory cancer cell gene signature. (D) ISH for Lgr5+ progenitor cells at the SCJ in the BE region in L2-IL-1B mice on HFD and in L2-IL1B/IL8Tg mice compared to germfree L2-IL1B mice (n=5–7). (E) ISH show co-expression of Lgr5+ (brown) and CXCR2 (red) progenitor cells. (F) Crypt fission in L2-IL1B HFD mice and L2-IL1B/IL8Tg mice. Data are represented as mean ± SEM (*p<0.05). L2=L2-IL-1β, L2/IL8Tg=L2-IL-1β/IL8Tg, HFD=High fat diet.
HFD altered intestinal microbiota promote the accelerated immune response and dysplasia phenotype
Diet affects the composition of gut microbiota9 and dysbiosis is linked to cancer development13. Previously, a modest reduction in microbial diversity in BE tissue in the human esophagus was shown25, matching our results from 16S-rRNA gene sequencing of bacterial DNA isolated from the mouse SCJ. No clustering could be observed in the tissue from WT or L2-IL1B mice (Suppl. Fig. 7A) suggesting that esophageal microbes are difficult to categorize in their role during carcinogenesis.
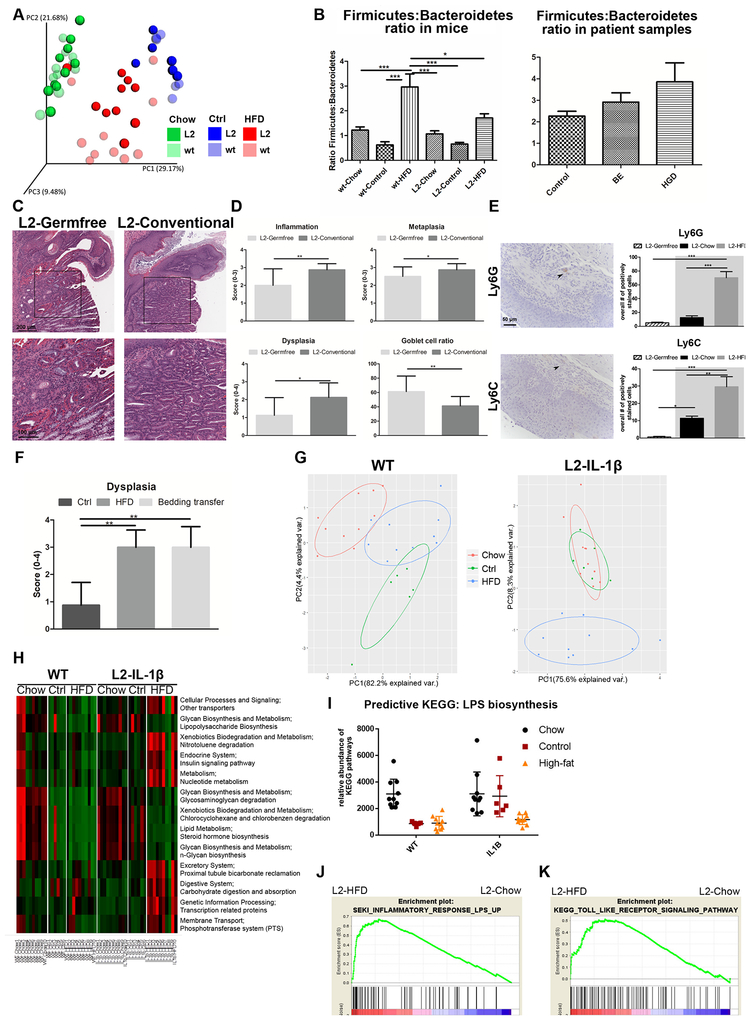
Since the lower GI tract harbors the majority of the intestinal microbiota, which mediate much of the dietary effects, we analyzed the intestinal microbiota by 16S-rRNA gene sequencing of fecal samples from 6-month-old WT and L2-IL1B mice on Chow, HFD, or nutrient-matched experimental diet26 (referred to as ‘Control’, Suppl. Fig. 8A). There was no difference in the SJC histopathology between Control and Chow, indicating that HFD, rather than the purified nutrient component, was responsible for the accelerated phenotype (Fig.6F, Suppl.Fig. 8B–E). Analysis of microbial β-diversity showed separate clustering of HFD fed L2-IL1B mice compared to all other groups, with a unique taxonomic profile, while WT and L2-IL1B harbored very distinct microbiota on control and on chow (Fig. 6A). Importantly, we observed an altered Firmicutes to Bacteroidetes ratio in HFD fed mice (Fig. 6B, Suppl. Fig. 7B,C), which correlated well with the increased Firmicutes: Bacteroidetes ratio in previously published patient (BE and HGD) data25 (Fig. 6B), although these data were from different tissue sites (feces versus esophagus) and comparisons of the microbiota between two species must be made cautiously.
Figure 6. The effect of HFD on the intestinal microbiome.
(A) Weighted Unifrac plot shows β-diversity after 16S-rRNA gene-sequencing of feces from L2-IL1B and WT mice on Chow (n=10), Control (n=6) and HFD (n=10) at the age of 6 months. (B) Ratio of the phylum Firmicutes to Bacteroidetes calculated after 16s-rRNA sequencing. Left: WT and L2-IL1B mice. Right: patient cytosponge samples (Control n=20, BE n=24, HGD n=23). (C) Representative H&E staining of SCJ and (D) Quantification of inflammation, metaplasia, dysplasia scores and goblet cell ratio in germfree (n=8, age 14 months, mean ± SD) and conventional L2-IL1B mice (n=16, age 11.5–16.5 months). (E) Representative pictures and quantification of IHC of neutrophils in the squamous epithelium in 10 low power fields (Ly6G) and IMCs (Ly6C) in germfree L2-IL1B mice (Germfree n=8, L2-IL1B-Chow n=3, L2-IL1B-HFD n=4). Groups with grey background are same mice as in Figure 4B. (F) Dysplasia score of L2-IL1B mice on control diet exposed to the old bedding of HFD L2-IL-1β mice (G) Principal component analysis of all significantly regulated KEGG pathways of the microbiome of WT (left) and L2-IL1B mice (right) at 6 months. (H) KEGG pathways of the microbiome in WT vs L2-IL1Bmice on Chow (n=10 each), Control (n=6 each) and HFD (n=10 each) predicting the microbiomes function with PICRUSt. (I) KEGG pathway prediction of LPS synthesis. (J and K) GSEAs show an enrichment of mouse derived LPS response and TLR signaling in L2-IL1B-HFD mice. Data are represented as mean ± SEM. *p<0.05, **p<0.01 ***p<0.001. (B,E,F) used a 1-way ANOVA with Tukey post-hoc testing. For (D) a t-test was used. L2=L2-IL-1β, wt=wildtype, HFD=High fat diet.
To investigate whether the gut microbiota in general influenced inflammation and tumor development in the L2-IL1B HFD mouse model, we re-derived L2-IL1B mice as germfree (GF) and found a marked reduction of inflammation, metaplasia and dysplasia, with an increase in goblet-like cells (Fig. 6C, D, Suppl.Fig 6C). This correlated with a significantly reduced influx of neutrophils and IMCs into the esophagus of germfree L2-IL1B mice (Fig. 6E), directly linking the elimination of enteric microbes to the inflammatory phenotype. Lgr5+ and Cxcr2+ cells at the SJC were significantly decreased under germfree conditions (Fig. 5A,D). When transferring bedding including feces from L2-IL1B mice on HFD to cages of L2-IL1B mice on Control diet over 9 months, the tumor phenotype could be transmitted, suggesting that the fecal microbial community is at least partly responsible for the SCJ phenotype (Fig. 6F and Suppl.Fig. 8F). Changes in the microbial community structure from HFD fed L2-IL1B mice pointed to unique functions, as the well-established PICRUSt pipeline27 in silico analysis of 16S-rRNA genes generated a predictive metagenome in KEGG pathway analysis. Clustering KEGG data showed that in L2-IL1B mice, HFD differentiated microbial function from Chow and Control diet (Fig. 6G). Functional pathway differences between HFD and Control in L2-IL1B mice27 (Fig. 6H, Suppl.Fig. 7D) suggest a microbial HFD associated component contributing to disease acceleration. One of the mostly regulated pathways in KEGG analysis in HFD fed L2-IL1B mice was bacterial Lipopolysaccharides (LPS) biosynthesis (Fig. 6I), known to have effects on inflammatory responses. GSEAs revealed an upregulation of the inflammatory response to LPS and Toll-Like Receptor (TLR) signaling in the SCJ of L2-IL1B HFD mice, suggesting that such gut microbiota changes are likely influenced through TLR signaling (Fig. 6J, K and Suppl. Fig 7E).
Discussion
The increase in BE and EAC in developed countries points to pathogenetic environmental influences. However, the increase in obesity32–35 did not precede this rise in EAC as one might suspect for a causative factor28. We provide evidence for an alternative hypothesis, suggesting that obesity and BE/EAC are secondary to the increasing consumption of a western-style or HFD7. HFD accelerated BE progression in the L2-IL1B mouse model through effects on gut microbiota, demonstrating the importance of the microbiome, and linking its influence in part to activity of the IL8/CXCL1 chemokine family within a distant altered inflammatory microenvironment.
HFD induced obesity in WT mice but not in L2-IL1B mice, where it accelerated the BE/EAC phenotype. The absence of weight gain in L2-IL1B mice on HFD was likely due to systemic effects of IL1B, but also raises the question as to whether increased body fat alone contributes to BE/EAC. In contrast to the HFD model, a genetic model of obesity (Mc4rx16) did not increase BE progression, arguing against body weight alone as a driver of esophageal carcinogenesis in mice. Instead, disease acceleration correlated with increased inflammation29, through pro-inflammatory cytokines and chemokines30 altering the local microenvironment with accumulation of immune cells and αSMA+ fibroblasts, which are known to recruit immune cells through specific factors (VEGF, HGF, MMP2, and IL8)31.
In our initial report of L2-IL1B mice, we noted upregulation of IL-6, a downstream target of IL1B, whose genetic ablation could attenuate the phenotype14. Here, we demonstrate a role for HFD in BE progression through inflammatory factors such as the IL8/CXCL1 family of chemokines14. The IL8/CXCL1 chemokine family is an additional well-defined downstream target of IL1B, and we observed elevated levels of the functional murine homolog CXCL1 at the SCJ of HFD treated L2-IL1B mice. Other studies have also suggested that chemokines such as IL-8 and IL1B are involved in esophageal carcinogenesis, and that CXCR1 and CXCR2 are expressed in esophageal mucosa32. During disease progression, human BE tissue produces abundant IL8, which is strongly overexpressed in EAC patients, pointing to an important role in human carcinogenesis19. Together with the observed elevation of CXCL1 gene and protein levels, and most importantly, the finding that immature myeloid cells and neutrophils were mainly attracted to the SCJ, this led us to further examine the effect of IL8 pathway in our mouse model. Indeed, reconstitution of the human IL8 gene in the BE mouse model resulted in acceleration of dysplasia, mimicking the HFD phenotype, confirming the importance of local IL-8 expression.33 In this study, we were not able to investigate comprehensively other inflammatory mediators, whether expressed locally or systemically. It has previously been demonstrated that circulating cytokines, including IL8, are associated with increased risk for BE34 and could be used as potential biomarker35. Nevertheless, IL8 likely plays a key local role during esophageal carcinogenesis19, with the IL8 specific gene signature being enriched in GSEA analysis of HFD fed L2-IL1B mice. IL8 is known to be upregulated by pro-inflammatory cytokines or pathogen-associated factors through toll-like receptors (TLRs)36, likely through an alteration of the gut microbiome. Although we cannot prove directly in our work that a specific microbial species is activating IL8 signaling in the esophagus, we provide evidence in our KEGG analysis: The bacterial community profile associated with LPS biosynthesis, known to activate TLR signaling, was upregulated in the SCJ of L2-IL1B HFD mice. We would therefore speculate, that gut microbiota changes on the immune system are influenced through TLR signaling, which in turn has an effect on IL8 activation, which is known to activate granulocytic myeloid cells that appear essential to the BE phenotype.
IL8 is a chemoattractant for myeloid cells through its cell surface receptors, CXCR1 and CXCR215. In HFD fed L2-IL1B and L2-IL1B/IL8Tg mice, we observed an accumulation of CXCR2+ IMCs and neutrophils. In combination with organoid culture data, these findings suggest that dietary fat components can result in increased local secretion of IL8/CXCL1 chemokines that then promote the accumulation of immature, granulocytic cells in the esophagus. These observations are consistent with previous studies that reported an increase in IMCs resulting from HFD feeding of WT mice37, and H. felis infection of WT mice11. Of note, bile acid treated L2-IL1B mice also exhibit a granulocytic shift, along with acceleration of dysplasia at the SCJ region14 pointing to the importance of neutrophils.
IMCs can carry out both, tumor promoting and immunosuppressive functions38. A subtype comprising CD11b+Ly6GhighLy6Clow neutrophils and CD11b+Ly6GlowLy6Chigh IMCs likely contributes to epithelial growth and progression, as demonstrated in 3D organoid cultures. However, myeloid cells may also possess immunosuppressive properties that could modulate tumor immunity. Tumor-derived G-CSF can promote the differentiation of IMCs into immunosuppressive neutrophils39 that inhibit cytotoxic T-cell responses, thereby facilitating tumor growth and metastasis40. Upregulation of G-CSF in HFD fed L2-IL1B mice underlines this possibility of increased myeloid suppressor cell recruitment and/or differentiation into an immunosuppressive local phenotype. While there was no significant change in cytotoxic T-cell numbers, increases in neutrophils were accompanied by reduced NK cells. Indeed, neutrophils protect against NK cell mediated clearance of tumor cells21 and have been proposed as a positive prognostic markers in esophageal cancer41. Although there are clearly limitations to studies of inflammation in a pro-inflammatory mouse model of BE, the findings suggest that an altered neutrophils to NK ratio may portend a poor prognosis, as shown in other cancers42.
Apart from IL8 effects on the esophageal inflammatory niche, we observed an epithelial effect of the IL8/CXCL1 chemokine family, with an expansion in the gastric SCJ of Cxcr2 expressing Lgr5+ progenitor cells with accelerated dysplasia. The increase in crypt fission at the SCJ is likely related to this expanded stem/progenitor cell pool. Thus, the transition of the normal SCJ niche to a distinct IL8 driven inflammatory niche may enhance the expansion of BE lesions into the esophagus, a scenario similar to that recently described in skin lesions43, providing a mechanism for HFD to influence tissue regeneration and cancer incidence44, 45.
The gut microbiome is modulated by diet46, and growing evidence indicates that both the microbiome9 and dietary components8 can potentiate cancer risk as shown for intestinal cancer13. However, such an interaction has not yet been reported for esophageal carcinogenesis. Esophageal carcinogenesis was attenuated under germfree conditions, with reduced influx of immune cells and decreased expansion of stem and epithelial cells. As suggested by previous studies47,48 and in line with reduced Cxcr2 expressing cells in germfree mice, epithelial IL8 secretion was likely induced by the gut microbiota. We propose that alterations in the esophageal immune niche might be related to body wide inflammatory effects of an altered gut microbiome as reported already for metabolic diseases49, and as such play a key role in esophageal carcinogenesis. The findings may be relevant to the care and treatment of patients with BE, as dietary modifications could change the gut microbial community and delay or prevent BE progression. Moreover, one could speculate that elimination of specific microbial species through antibiotics might provide a potential tool for cancer prevention. Nevertheless, such commensal bacteria have yet to be identified and further studies are needed to elucidate the role of the gut and/or esophageal microbiome in predicting the development of BE50. While GERD and bile acid reflux are known to induce inflammation in the esophagus and believed to contribute to the development of early BE, the gut microbiome may play an important role in malignant progression through acceleration of the inflammatory microenvironment.
While there are alterations in the composition of the microbial community in EAC, there were only modest reductions in microbial diversity in human BE samples25. In accordance, we found only modest changes in the microbiota at the SCJ in L2-IL1B mice, while microbial composition in the colon was changed upon feeding HFD, with an altered Firmicutes:Bacteroidetes ratio that correlated with BE progression. This ratio, together with a lower microbial α-diversity, have been associated with HFD46 and mirrored the changes seen in BE patients. Although the fecal material may not fully reflect the changes in the microbiota colonizing the gastroesophageal region, we would suggest that the fecal microbiota is highly relevant, and may have a systemic impact on BE progression. Changes in gut microbiota may account for the inflammatory phenotype observed in HFD L2-IL1B mice and importantly, can induce an increased cancer phenotype, if transferred to L2-IL1B mice on chow diet. 16S-rRNA gene profiling under diet-controlled conditions demonstrated that the HFD component was responsible for this effect. The esophageal microbiota in humans is thought to be transitory, and may change in association with esophagitis51, whereas the microbiota of the lower GI tract is quantitatively much greater, with potential systemically mediated inflammatory effects. Indeed, the “pathogenic microbial community” theory suggests that the entire microbial community contributes to pathogenicity, even though no individual community members can be clearly categorized as causative pathogens52. We noted a correlation between TLR signaling in GSEA and upregulation of TLR2 in the esophagus, linking the alteration of the gut microbiota to IL8 signaling. In terms of a preventive antibiotic treatment for patients with BE the knowledge of distinct components of the microbiome seems essential, as too long term antibiotic treatment seems to be a difficult option and broad short term antibiotic treatment will result in a fast reconstitution of commensal bacteria53.
In summary (Suppl. Fig. 9), we propose here that a diet enriched in fat, rather than increased body weight, is responsible for accelerated esophageal carcinogenesis. While IL-1B-dependent chronic inflammation on its own can cause dysplasia, HFD accelerates this disease phenotype by altering the intestinal microbiome and inducing a distinct inflammatory response in the esophagus marked by IL8/KC chemokines. Increased esophageal IL8 expression alters the neutrophils/NK cell ratio and promotes the expansion at Cxcr2+ progenitors that eventually progress to dysplasia. These findings offer some potential new strategies for prevention of BE and EAC and should be investigated further in patient cohorts.
Supplementary Material
Need to Know.
Background:
We investigated whether a high-fat diet and/or obesity contribute to progression of Barrett’s esophagus (BE) to esophageal cancer in a mouse model and by studying esophageal tissues from patients with BE.
Findings:
We found a high-fat diet to promote esophageal dysplasia by altering the intestinal microbiome and promoting inflammation and stem cell expansion, independent of obesity.
Implications for Patient Care:
Patients with BE should be encouraged to avoid a high-fat diet.
Funding:
The research leading to these results has received funding from the Deutsche Forschungsgemeinschaft (DFG) under Grant Agreement No. SFB 824 and has been funded by the Deutsche Krebshilfe with in the Max Eder Program to MQ. Additionally, TCW (PI) and MQ were funded by the NCI Barrett’s Esophageal Translational Research Network (2 U54 CA163004–06). JI, NSM, MQ, DH MK were funded by a graduate program by the German Research Fund (GRK 1482)
Abbreviations:
- BE
Barrett’s esophagus
- EAC
Esophageal adenocarcinoma
- GEJ
Gastroesophageal junction
- GERD
Gastro-esophageal reflux disease
- GF
germfree
- HFD
High fat diet
- NK cells
Natural killer cells
- SCJ
squamocolumnar junction
- WT
Wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests: none
Gene Expression Analysis:
Raw data have been deposited in National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) (GSE103616)
References
- 1.Quante M, Graham TA, Jansen M. Insights into the Pathophysiology of Esophageal Adenocarcinoma. Gastroenterology 2017. [DOI] [PubMed] [Google Scholar]
- 2.di Pietro M, Alzoubaidi D, Fitzgerald RC. Barrett’s esophagus and cancer risk: how research advances can impact clinical practice. Gut Liver 2014;8:356–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maret-Ouda J, El-Serag HB, Lagergren J. Opportunities for Preventing Esophageal Adenocarcinoma. CancerPrevRes(Phila) 2016;9:828–834. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: a case-control study. Gut 2014;63:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TH, Thrift AP, Ramsey D, et al. Risk factors for Barrett’s esophagus compared between African Americans and non-Hispanic Whites. Am J Gastroenterol 2014;109:1870–80. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin PJ, Chlebowski RT. Obesity and Cancer: Insights for Clinicians. J Clin Oncol 2016;34:4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thrift AP, Hilal J, El-Serag HB. Metabolic syndrome and the risk of Barrett’s oesophagus in white males. Aliment Pharmacol Ther 2015;41:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabung FK, Liu L, Wang W, et al. Association of Dietary Inflammatory Potential With Colorectal Cancer Risk in Men and Women. JAMAOncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyaz S, Mana MD, Roper J, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016;531:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ericksen RE, Rose S, Westphalen CB, et al. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1399–1412 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz MD, Atay C, Heringer J, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014;514:508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quante M, Bhagat G, Abrams JA, et al. Bile Acid and Inflammation Activate Gastric Cardia Stem Cells in a Mouse Model of Barrett-Like Metaplasia. Cancer Cell 2012;21:36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asfaha S, Dubeykovskiy AN, Tomita H, et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 2013;144:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolze F, Rink N, Brumm H, et al. Characterization of the melanocortin-4-receptor nonsense mutation W16X in vitro and in vivo. Pharmacogenomics J 2013;13:80–93. [DOI] [PubMed] [Google Scholar]
- 17.Lertpiriyapong K, Whary MT, Muthupalani S, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 2014;63:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farooqi IS, Yeo GS, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 2000;106:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald RC, Abdalla S, Onwuegbusi BA, et al. Inflammatory gradient in Barrett’s oesophagus: implications for disease complications. Gut 2002;51:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catusse J, Liotard A, Loillier B, et al. Characterization of the molecular interactions of interleukin-8 (CXCL8), growth related oncogen alpha (CXCL1) and a non-peptide antagonist (SB 225002) with the human CXCR2. Biochem Pharmacol 2003;65:813–21. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel A, Brooks MW, Houshyar S, et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov 2016;6:630–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 2011;145:1023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neil A, Petersen CP, Choi E, et al. Unique Cellular Lineage Composition of the First Gland of the Mouse Gastric Corpus. J Histochem Cytochem 2017;65:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott DR, Walker AW, O’Donovan M, et al. A non-endoscopic device to sample the oesophageal microbiota: a case-control study. Lancet Gastroenterol Hepatol 2017;2:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubeck R, Bonet-Ripoll C, Hoffmann C, et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol Metab 2016;5:1162–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013;31:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrams JA, Sharaiha RZ, Gonsalves L, et al. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev 2011;20:183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieder F, Bianchini P, Harnett K, et al. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. American Physiological Society 2010;298:G571–G581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardikar S, Onstad L, Song X, et al. Inflammation and oxidative stress markers and esophageal adenocarcinoma incidence in a Barrett’s esophagus cohort. Cancer Epidemiol Biomarkers Prev 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing F, Saidou J, Watade K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. 2011;15:166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrivastava MS, Hussain Z, Giricz O, et al. Targeting chemokine pathways in esophageal adenocarcinoma. Cell Cycle 2014;13:3320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook MB, Barnett MJ, Bock CH, et al. Prediagnostic circulating markers of inflammation and risk of oesophageal adenocarcinoma: a study within the National Cancer Institute Cohort Consortium. Gut 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett’s esophagus: a case-control study. Clin Gastroenterol Hepatol 2014;12:229–238 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharyya S, Borthakur A, Pant N, et al. Bcl10 mediates LPS-induced activation of NF-kappaB and IL-8 in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2007;293:G429–37. [DOI] [PubMed] [Google Scholar]
- 37.Adler BJ, Green DE, Pagnotti GM, et al. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLoS One 2014;9:e90639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016;16:431–46. [DOI] [PubMed] [Google Scholar]
- 39.Waight JD, Hu Q, Miller A, et al. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One 2011;6:e27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casbon A-J, Reynaud D, Park C, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA 2015;112:E566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu B, Chen L, Li J, et al. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget 2016;7:74904–74916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quail DF, Olson OC, Bhardwaj P, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol 2017;19:974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexeev V, Salas-Alanis JC, Palisson F, et al. Pro-inflammatory chemokines and cytokines dominate the blister fluid molecular signature in epidermolysis bullosa patients and affect leukocyte and stem cell migration. J Invest Dermatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray K High-fat diet influences intestinal stem cell biology. Nature Reviews Gastroenterology& Hepatology 2016;13:250–251. [DOI] [PubMed] [Google Scholar]
- 45.Duggan C, Onstad L, Hardikar S, et al. Association between markers of obesity and progression from Barrett’s esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2013;11:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HJ, Li H, Collins JJ, et al. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci USA 2016;113:E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schueller K, Riva A, Pfeiffer S, et al. Members of the Oral Microbiota Are Associated with IL-8 Release by Gingival Epithelial Cells in Healthy Individuals. Front Microbiol 2017;8:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med 2018;24:1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snider EJ, Compres G, Freedberg DE, et al. Barrett’s esophagus is associated with a distinct oral microbiome. Clin Transl Gastroenterol 2018;9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Lu X, Nossa CW, et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 2009;137:588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: Human gut microbes associated with obesity. Nature 2006;444:1022–1023. [DOI] [PubMed] [Google Scholar]
- 53.Weber D, Hiergeist A, Weber M, et al. Detrimental effect of broad-spectrum antibiotics on intestinal microbiome diversity in patients after allogeneic stem cell transplantation: Lack of commensal sparing antibiotics. Clin Infect Dis 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.