Summary
Introduction
TRC102 potentiates the activity of cancer therapies that induce base excision repair (BER) including antimetabolite and alkylating agents. TRC102 rapidly and covalently binds to apurinic/apyrimidinic (AP) sites generated during BER, and TRC102-bound DNA causes topoisomerase II-dependent irreversible strand breaks and apoptosis. This study assessed the safety, maximum-tolerated dose (MTD), pharmacokinetics and pharmacodynamics of TRC102 alone and in combination with pemetrexed.
Purpose
Patients with advanced solid tumors received oral TRC102 daily for 4 days. Two weeks later, patients began standard-dose pemetrexed on day 1 in combination with oral TRC102 on days 1 to 4. The pemetrexed-TRC102 combination was repeated every 3 weeks until disease progression.
Methods
Twenty-eight patients were treated with TRC102 at 15, 30, 60 or 100 mg/m2/d. The MTD was exceeded at 100 mg/m2/d due to grade 3 anemia in 50 % of patients. TRC102 exposure increased in proportion to dose with a mean t1/2 of 28 h. A pharmacodynamic assay confirmed that TRC102 binds to pemetrexed-induced AP sites at all doses studied. Stable disease or better was achieved in 15 of 25 patients evaluable for response (60 %), including one patient with recurrent metastatic oropharyngeal carcinoma that expressed high levels of thymidylate synthase, who achieved a partial response and was progression free for 14 months.
Conclusions
When administered with pemetrexed, the maximum tolerated dose of oral TRC102 is 60 mg/m2/d for 4 days. Randomized controlled studies are planned to evaluate the clinical benefit of adding TRC102 to pemetrexed and other agents that induce BER.
Keywords: TRC102, Base excision repair, Methoxyamine, Pemetrexed, First-in-human, Phase 1
Introduction
Base-excision repair (BER) is a fundamental cellular process employed by malignant cells to reduce the cytotoxicity of alkylating and antimetabolite chemotherapies. BER repairs individual DNA bases that have been damaged directly through alkylation as well as abnormal bases incorporated into DNA by the action of antimetabolite agents. Inhibition of BER has been proposed as a way to improve the therapeutic index of these agents, although, heretofore, no inhibitors of BER have been tested in clinical trials.
TRC102 (methoxyamine hydrochloride, TRACON Pharmaceuticals Inc.) is a small molecule inhibitor of BER with a unique mechanism of action that potentiates the activity of a wide variety of antimetabolite and alkylating agents in preclinical studies. [1–9] BER is a multiprotein pathway initiated by base-specific DNA glycosylases that generate common intermediates, apurinic and apyrimidinic (AP) sites, containing reactive aldehydes [10]. TRC102 rapidly and covalently binds the reactive aldehyde within the AP sites, thereby interrupting the BER pathway. Furthermore, TRC102 bound DNA is a topoisomerase II poison, such that DNA cleavage occurs without religation. The resulting accumulation of DNA strand breaks then triggers cellular apoptosis [8]. The induction of apoptosis by TRC102 is relatively selective for cancer cells, as they typically overexpress topoisomerase II. In nonmalignant cells with low topoisomerase II expression, TRC102-bound DNA is excised and replaced by the long patch DNA repair system [11, 12]. The activity of TRC102 is independent of tumor O6-methylguanine-DNA methyltransferase (MGMT) expression, DNA mismatch repair status, or p53 status and TRC102 potentiates the activity of other DNA repair inhibitors, including PARP inhibitors [1, 8–10, 13–15].
Pemetrexed induces BER in response to uracil incorporation into DNA, which is removed by uracil glycosylase [4]. TRC102 binds resulting AP sites and acts as a topoisomerase II poison, causing DNA strand breaks and apoptosis as described above. In mice with human non-small cell lung cancer xenografts, TRC102 extended median tumor growth delay from 2 days with pemetrexed alone to 9 days with the combination without added toxicity [16]. In repeat-dose toxicity studies, TRC102 caused anemia by extravascular red cell lysis (i.e., through splenic sequestration of erythrocytes) at a dose 10-fold higher than that required for activity. The anemia was reversible, not cumulative or severe and unaffected by coadministration of pemetrexed. (TRACON Pharmaceuticals, unpublished data).
This phase I study evaluated the maximum tolerated dose (MTD), safety profile, pharmacokinetics, and pharmacodynamics of TRC102 alone and in combination with standard-dose pemetrexed in patients with advanced cancer.
Patients and methods
Patient eligibility
Eligible patients had histologically proven advanced or metastatic solid cancer for which curative therapy was unavailable, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function as demonstrated by an absolute neutrophil count ≥1,500 cells/μL, hemoglobin ≥10 g/dL, platelets ≥100,000/μL, prothrombin time or international normalized ratio ≤1.5 times the institutional upper limit of normal (ULN), creatinine ≤1.5 times the ULN, bilirubin ≤1.5 mg/dL, and aspartate and alanine transaminases ≤2.5 times the ULN (or ≤5 times the ULN in patients with liver metastases). Patients were excluded if they had a known history of central nervous system disease, thromboembolic disease, clinically significant malignant effusions, or had received cancer therapy within 4 weeks prior to study entry. Patients were also excluded if they had received prior high dose chemotherapy associated with stem cell rescue, had a known disorder associated with hemolysis, or were pregnant or lactating. All patients signed an institutional review board-approved informed consent form prior to undertaking study-related procedures. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice (GCP) guidelines and all applicable local regulatory requirements and laws.
Study design and treatments
This was a multicenter open-label phase I study to evaluate the safety, maximum-tolerated dose, pharmacokinetics and pharmacodynamics of TRC102 dosed alone and in combination with pemetrexed (). TRC102 doses studied were 15, 30, 60, and 100 mg/m2/d. The starting dose was selected to be more than 10-fold lower than the dose that caused reversible anemia from extravascular hemolysis in the most sensitive preclinical species. The TRC102 dose was escalated in serial cohorts of patients using a standard 3+3 design. Dose-limiting toxicities were defined as any grade 3 nonhematologic adverse event, grade 3 anemia, grade 4 thrombocytopenia, grade 4 febrile neutropenia or grade 4 neutropenia lasting five or more days. Adverse events occurring in cycles 1 and 2 were considered in determining the maximum tolerated dose (MTD). Patients who discontinued for reasons other than dose-limiting toxicity prior to completion of cycle 2 were replaced to ensure an adequate safety assessment of each cohort. The MTD was defined as the highest dose that had an observed incidence of dose-limiting toxicity of less than 33 % of patients per cohort. Intrapatient dose escalation was not permitted. Patients were allowed to dose reduce for adverse events that resolved to grade 1 or baseline and were allowed to interrupt TRC102 or pemetrexed dosing following the 5 week dose-limiting toxicity evaluation period.
TRC102 is a small organic amine that is highly water soluble and nearly completely bioavailable after oral administration, and was supplied as a powder reconstituted in Crystal Light® lemonade by the study center pharmacist in single use bottles. In cycle 1, patients received daily oral TRC102 on days 1 to 4 of a 2-week cycle to allow for single-agent safety and pharmacokinetic analyses. In cycle 2 and beyond, patients received the same TRC102 regimen on days 1 to 4 concurrently with the approved dose of intravenous pemetrexed (500 mg/m2) on day 1 of 3-weekcycles. Patients were premedicated with vitamin B12 (1,000 mcg), folic acid (400 ug) and dexamethasone (4 mg twice daily the day prior to and day of dosing) according to the Alimta® package insert (Eli Lilly and Company, Indianapolis, IN). On day 1 of cycle 3, the TRC102 dose was held to permit assessment of the pharmacodynamics of pemetrexed when administered in the absence of TRC102. The two drug combination was continued every 3 weeks until disease progression, unacceptable toxicity, or withdrawal of consent. Colony stimulating factors including darbepoietin and erythropoietin were permitted following cycle 2.
Study assessments
Safety was evaluated at regular intervals at baseline, during treatment, and for 28 days after completing study therapy. Vitals signs were recorded before and after every infusion and weekly thereafter. Physical examination was performed every 2 weeks. Hematology, serum chemistry and coagulation parameters were analyzed at baseline and weekly thereafter. Adverse events were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Pharmacokinetic evaluation
Plasma for PK parameters were collected prior to dosing and 5, 15 and 30 min and 1, 2, 3, 4, 6, 8 and 24 h following the first and fourth doses of TRC102 in cycle 1 and following concurrent dosing of TRC102 with pemetrexed on cycle 2 day 1. TRC102 and pemetrexed concentrations were determined using validated pharmacokinetic methods [13, 17]. Pharmacokinetic properties including the maximum plasma concentration (Cmax), area under the plasma concentration versus time curve to the time of the last measurable concentration (AUClast), and terminal elimination half-life (t1/2), were analyzed for TRC102 in each cohort using a non-compartmental model in Winnonlin v. 5.2.1. A separate population pharmacokinetic analysis was performed on pooled TRC102 from C1D1 and C1D4 using a nonlinear mixed-effect modeling approach to characterize both the variability in pharmacokinetic parameters, and potential covariates.
Population pharmacokinetic analysis
Parameters were estimated by maximum likelihood estimation (MLE) with linearization using the stochastic approximation expectation maximization (SAEM) algorithm combined with a MCMC (Markov Chain Monte Carlo) procedure, in the Monolix software [18, 19]. A correlation matrix of clearance (CL/F) against potential covariates was used to identify initial covariates (Cov) for model development. Final covariate selection was performed by a forward stepwise procedure, based on a significant change (p<0.05) in the log-likelihood. Other factors, such as parameter precision, general interpretability, and clinical relevance were also considered in selecting covariates. The Wald test and the Bayesian information criterion (BIC) were used to test different covariate hypotheses regarding the final model. For the goodness-of-fit, observed and predicted concentrations versus time, observed concentrations vs. population predictions, weighted residuals vs time and weighted residuals vs. predictions were evaluated. A proportional error model was used to describe the residual variability, and inter-individual variability (HV) was modeled with covariates as exponential terms according to Individual Parameter (Pi)=Typical Value Population Parameter (θ) × (Cov)β × eη,Pi, where β was the change in Ln(Pi) per unit change in Ln(Cov), and the IIV is reported as the standard deviation (ω) of η error.
Pharmacodynamic analysis
Peripheral blood mononuclear cells were collected before treatment with pemetrexed alone on cycle 3 day 1 and then at four and 24 h following treatment, and at the same time points before and after treatment with pemetrexed and TRC102 on cycle 2 day 1. The number of AP sites in peripheral blood mononuclear cells was measured using an aldehyde reactive probe from Dojindo Molecular Technologies, Inc. (Gaithersburg, MD) as previously described and normalized to the pre-dose value determined for each patient [8].
Thymidylate synthase immunohistochemistry
Archival tumor samples from selected patients were sent to Caris Life Sciences (Irving, TX) for thymidylate synthase (TS) immunohistochemistry. Formalin-fixed, paraffin embedded tumors were cut into 4 μm sections and mounted on poly-L-lysine-coated slides, processed for epitope retrieval, and stained with a mouse anti-thymidylate synthase cocktail from Invitrogen Corporation (Carlsbad, CA) and appropriate controls as previously described [20]. Staining intensity was calculated as 0 (none), +1 (weak), +2 (distinct), or +3 (very strong). A value designated as the HSCORE was obtained for each tumor by multiplying the staining intensity times the percentage of cells that stain at that intensity. An HSCORE ≥30 was considered TS-positive based on survival correlations reported previously [21].
Evaluation of tumor response
Tumor response and progression were assessed using the original Response Evaluation Criteria in Solid Tumors (RECIST) [22]. Evaluations were performed at baseline, at the end of cycle 3 and then every two to three cycles or earlier if disease progression was suspected.
Statistical analysis
The safety population included all patients who received at least one dose of TRC102. Descriptive statistics (such as means, medians, standard deviations and ranges for continuous data and percentages for categorical data) were used to summarize patient characteristics, treatment administration, safety, efficacy, pharmacokinetic, and pharmacodynamic parameters.
Results
Patient characteristics and disposition
Between June 2008 and December 2009, 28 patients with advanced or metastatic solid tumors were enrolled and treated with escalating doses of TRC102 at 15, 30, 60 or 100 mg/m2 daily for days 1 through 4 of a 2 week cycle and then days 1 through 4 of a 3 week cycle in combination with 500 mg/m2 of pemetrexed on day 1. Baseline patient characteristics are summarized in Table 1. All patients enrolled received at least one dose of TRC102, and 25 of 28 completed the 5-week evaluation period for dose-limiting toxicity (DLT). Reasons for early withdrawal were unrelated adverse events in two patients (ischemic stroke and COPD exacerbation) and rapidly progressive disease in a patient with metastatic ovarian cancer. Twenty-six of 28 patients enrolled received at least two cycles of TRC102 (8 doses), 20 received at least three cycles (11 doses), 12 received at least four cycles (15 doses), and one patient at 30 mg/m2/d received 21 cycles (83 doses) of TRC102 therapy.
Table 1.
Patient characteristics
| Characteristic | Number of patients | % |
|---|---|---|
| Total number of patients treated | 28 | |
| Sex | ||
| Male | 12 | 43 |
| Female | 16 | 57 |
| Age, years | ||
| Median | 60 | |
| Range | 19–86 | |
| ECOG PS | ||
| 0 | 9 | 32 |
| 1 | 19 | 68 |
| Tumor type | ||
| Epithelial ovarian cancer | 4 | 14 |
| Non-squamous NSCLC | 3 | 11 |
| Squamous NSCLC | 3 | 11 |
| Pancreatic cancer | 3 | 11 |
| Colorectal cancer | 3 | 11 |
| Sarcoma | 3 | 11 |
| Othera | 9 | 32 |
| No. of prior chemotherapy regimens | ||
| Median | 3 | |
| Range | 1–11 | |
ECOG Eastern Cooperative Oncology Group; PS performance status; NSCLC non-small cell lung cancer
Other tumor types were adenoid cystic, bladder, breast, cholangiocar-cinoma, esophageal, head and neck, prostate, peritoneal and uterine
Safety and tolerability
Dose escalation proceeded stepwise until the top dose of 100 mg/m2/d was reached. Four patients were enrolled in the 15 mg/m2/d cohort, as one patient developed a COPD exacerbation and received only three doses of TRC102, making him unevaluable for DLT. Expansion of the 30 mg/m2/d cohort was required per protocol due to the occurrence of grade 3 anemia during cycle 2 in a patient with metastatic prostate cancer whose hemoglobin decreased from 10.4 g/dL prior to dosing to 7.9 g/dL at cycle 2 day 8. This 81 year old man with castrate resistant and chemotherapy refractory prostate cancer and metastases to bone, liver and lymph nodes continued on a reduced dose of TRC102 of 15 mg/m2/d for five cycles prior to disease progression. One additional patient at this dose level was unevaulable due to the occurrence of an unrelated ischemic stroke prior to completion of the DLT evaluation period.
The MTD of TRC102 was exceeded at 100 mg/m2/d due to DLT of grade 3 anemia in three of six patients (Table 2). All patients at this dose level developed grade 2 or 3 anemia, five of six patients required transfusion, and the one patient who was not transfused was treated with darbepoietin. Anemia was generally manifest by cycle 2 in these patients and four patients required dose reductions. One 47 year old woman with chemotherapy-refractory metastatic ovarian cancer dosed at 100 mg/m2/d developed grade 3 anemia during treatment with TRC102 alone in cycle 1 (hemoglobin decrease from 10.9 g/dL at screening 5 days prior to treatment, to 9.4 g/dL immediately prior to the initial TRC102 dose, to 7.6 g/dL 1 week following dosing). The patient remained on study for 7 cycles following transfusion and dose reduction to 50 mg/m2/d prior to developing progressive disease.
Table 2.
Frequency of grade 3/4 anemia, associated chemistry values and treatment over the full duration of the study by cohort (N=28)
| TRC102 Dose in mg/m2/d (n) | Grade 3 anemia | Grade 4 anemia | Haptoglobin decrease | Bilirubin increase | Red cell transfusion | Erythropoietin or darbepoietin | TRC102 dose-reduction |
|---|---|---|---|---|---|---|---|
| 15 (4) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 1 (25 %) | 0 (0 %) | 0 (0 %) |
| 30 (7) | 1 (14 %) | 0 (0 %) | 0 (0 %) | 1 (14 %) | 3 (43 %) | 1 (14 %) | 1 (14 %) |
| 60 (11) | 3 (27 %) | 1 (9 %) | 1 (9 %) | 4 (36 %) | 6 (54 %) | 3 (27 %) | 1 (9 %) |
| 100 (6) | 3 (50 %) | 0 (0 %) | 3 (50 %) | 4 (67 %) | 5 (83 %) | 2 (33 %) | 4 (67 %) |
n number of patients evaluable for safety.
Following determination of the MTD at 60 mg/m2/d, eight additional patients were enrolled at this dose level to further assess safety. One 74 year old woman with chemotherapy-refractory metastatic uterine carcinoma developed grade 3 anemia in cycle 1 following dosing with TRC102 alone. This patient with chemotherapy-associated anemia was granted an exception to enter the study with a hemoglobin level slightly lower than 10.0 g/dL and developed a decrease in hemoglobin from 9.7 g/dL prior to dosing to 7.9 g/dL over 1 week, prompting transfusion. The patient remained anemic throughout treatment, developing grade 4 anemia in cycle 4 that required further transfusion and she elected to discontinue treatment.
Anemia was the only DLT reported and was not accompanied by significant thrombocytopenia or leukopenia. Anemia was characterized by an elevated reticulocyte production index and laboratory signs of extravascular hemolysis (decreased plasma haptoglobin and increased total bilirubin without hemoglobinuria) that were more frequent as the dose of TRC102 was escalated (Table 2). Additional laboratory and clinical evaluations excluded common causes of anemia including blood loss, plasma volume expansion, iron deficiency, and vitamin B-12 or folate deficiency. The severity of anemia generally did not increase as the number of treatment cycles accumulated and the anemia was reversible and manageable with standard supportive measures including growth factors and packed red cell transfusion. The frequency of red blood cell transfusions and growth factor support increased with TRC102 dose, as did the need for dose reduction (Table 2).
Anemia was the only grade 3 or higher treatment-related adverse event noted following dosing with TRC102 alone in cycle 1, and treatment with TRC102 and pemetrexed in cycle 2 and beyond resulted in infrequent episodes of mye-losuppression similar to that reported for pemetrexed alone (Table 3). Treatment-related nonhematologic adverse events were generally mild to moderate (Table 3). The only exceptions were one patient with grade 3 fatigue and another with grade 3 diarrhea who received 100 mg/m2/d of TRC102 with pemetrexed.
Table 3.
Frequency of adverse events over the full duration of the study related to TRC102, excluding anemia (N= 28)
| Preferred terma | Grade 1 | Grade 2 | Grade 3 Grade 4 |
|---|---|---|---|
| Neutropenia | 1 | 3 | |
| Fatigue | 6 | 3 | 1b |
| Diarrhea | 1 | 1b | |
| Leukopenia | 1 | ||
| Nausea | 3 | 3 | |
| Vomiting | 3 | ||
| Anorexia | 6 | ||
| Mucosal inflammation | 3 | ||
| Pruritus | 3 | ||
| Pyrexia | 3 | ||
| Rash | 2 |
Includes events occurring in more than 1 patient, and all grade 3 and higher events
Patient was enrolled and treated at 100 mg/m2/d TRC102
Pharmacokinetics
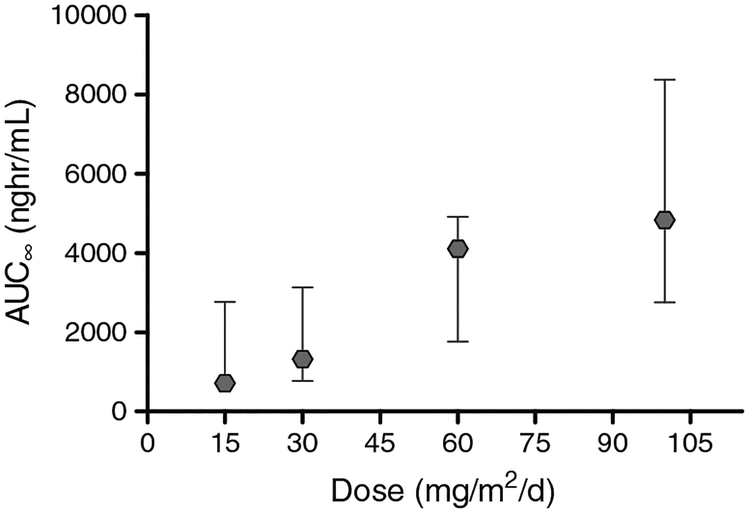
TRC102 pharmacokinetic properties are presented in Table 4. The overall mean half life (t1/2) of 28 h was unchanged with increasing doses (p=0.875) or cycle, and the exposure increased proportionally with dose (Fig. 1, AUC/Dose, ANOVA p=0.98). The target AUC was exceeded in all patients who received at least four doses of TRC102. Pharmacokinetic parameters were similar when TRC102 was administered without pemetrexed on cycle 1 day 1 or with pemetrexed on cycle 2 day 1. TRC102 accumulated with daily dosing; the target Cmax of 50 ng/mL for in vivo activity was achieved by the fourth daily dose in two of three patients treated at 15 mg/m2/d and all patients receiving 30 mg/m2/d and higher doses. In addition, pemetrexed exposure analyzed following dosing in cycle 2 day 1 (with coadministration of TRC102) was similar to published exposures (Alimta® package insert (Eli Lilly and Company, Indianapolis, IN); data not shown).
Table 4.
TRC102 pharmacokinetic properties by cohort
| No. | Cmax (ng/mL) | t1/2 (hr) | AUC0−∞ (hr-ng/mL) | |
|---|---|---|---|---|
| Cycle 1 Day 1 | ||||
| 15 mg/m2/d | 3 | 19.7 (12.7–57.4) | 37.3 (34.1–41.2) | 287 (219–1132) |
| 30 mg/m2/d | 6 | 64.3 (22.7–205.0) | 21.8 (15.9–25.5) | 1041 (263–3462) |
| 60 mg/m2/d | 3 | 119.0 (63.7–126.0) | 21.9 (17.6–29.9) | 1991 (1093–2161) |
| 100 mg/m2/d | 5 | 152.0 (83.5–417) | 26.8 (14.1–44.0) | 2312 (987–5331) |
| Cycle 1 Day 4 | ||||
| 15 mg/m2/d | 3 | 68.1 (27.0–129) | 41.5 (36.0–52.2) | 1136 (188–2580) |
| 30 mg/m2/d | 6 | 126.0 (73.2–282.0) | 30.9 (26.5–59.4) | 1960 (1158–5230) |
| 60 mg/m2/d | 3 | 327.0 (155.0–626.0) | 26.9 (25.8–44.8) | 5812 (2632–11075) |
| 100 mg/m2/d | 5 | 247.0 (148.0–435.0) | 25.0 (16.4–36.6) | 3105 (2066–7969) |
| Cycle 2 Day 1 | ||||
| 15 mg/m2/d | 3 | 24.6 (18.2–56.0) | 34.3 (5.6–55.4) | 297 (126–1186) |
| 30 mg/m2/d | 6 | 57.4 (25.6–137.0) | 21.9 (12.6–43.3) | 943 (381–2071) |
| 60 mg/m2/d | 3 | 103.0 (93.6–239.0) | 26.7 (25.4–28.0) | 1654 (1332–4556) |
| 100 mg/m2/d | 5 | 225.0 (74.9–385.0) | 37.0 (25.3–45.5) | 3697 (940–6176) |
All values expressed as medians with ranges in parentheses.
Cmax maximum concentration; t1/2 half-life; AUC0−∞ area under the serum concentration-time curve for zero to infinity.
Fig. 1.
Relationship between dose of TRC102 and median AUC∞ for nineteen patients treated on C1D1. The exposure of TRC102 was proportional to dose across the range of 15 to 100 mg/m2/d
The population pharmacokinetics of TRC102 were evaluable in 19 patients who received doses on C1D1 thru C1D4. The structural PK model was satisfied as one compartment with first-order absorption (Ka) and without lag-time. The fixed and random effects determined in the final model are listed in Table 5. Interindividual variability was estimated for the elimination rate constant (Kel) and the apparent volume of distribution (V/F). Significant covariates were identified to be related to the variability in V/F, namely, sex (M, F), baseline alanine transaminase (ALT), baseline serum creatinine (SrCr) and baseline serum albumin (Albu), whereas Kel was identified as significantly related to sex. The influence of SrCr on volume of distribution (and hence the Clearance) was greater than the other covariates. Table 6 summarizes the covariate model building steps for the significant covariates, which was effective at explaining variability in PK parameters for male patients, but not female patients in this study, as the inter-individual variability in Kel and V/F was reduced in males from by 25 and 56 %, respectively.
Table 5.
TRC102 population pharmacokinetic parameters on C1D1 and C1D4
| Population parameters | Base model estimate | ± s.e | Covariate model estimate | ± s.e. |
|---|---|---|---|---|
| Fixed effects θ | ||||
| Elimination Kel (hr−1) | 0.0359 | 0.0036 | 0.0399 | 0.0042 |
| Volume of Distribution V/F (L/m2) | 5.73 | 1.1 | 3.55 | 2.6 |
| Absorption Ka (hr−1) | 4.17 | 0.39 | 3.93 | 0.36 |
| Random effects η | ||||
| IIV ωKel (sex=F, n=11) | 0.288 | 0.09 | 0.278 | 0.093 |
| IIV ωKel (sex=M, n=8) | 0.685 | 0.19 | 0.431 | 0.013 |
| IIV ωV (sex=F, n=11) | 0.781 | 0.16 | 0.69 | 0.15 |
| IIV ωV (sex=M, n=8) | 0.716 | 0.18 | 0.161 | 0.084 |
| IIV Correlation (Kel,V/F) | 0.614 | 0.17 | 0.385 | 0.27 |
| Residual error | ||||
| σ | 0.378 | 0.014 | 0.38 | 0.014 |
| Covariates | ||||
| βV(SexM) | −0.866 | 0.24 | ||
| βV (ALT) | 0.0177 | 0.0033 | ||
| βV (SrCr) | −1.01 | 0.45 | ||
| βV (Albu) | 0.39 | 0.14 | ||
| βKel(SexM) | −0.445 | 0.033 | ||
| Parameters by group | ||||
| Kel (sex=F, n=11) | 0.0399 | 0.0042 | ||
| Kel (sex=M, n=8) | 0.0256 | 0.0046 | ||
| V/F (sex=F, n=11) | 3.55 | 1.6 | ||
| V/F (sex=M, n=8) | 1.49 | 0.74 |
SrCr serum creatinine, ALT alanine transaminase, Albu albumin
Table 6.
Covariate model build on V/F using log-likelihood (LL), and estimated fixed effects of the covariates (β) with the corresponding Wald test p-values (P)a
| Model | Cov 1 | Cov 2 | Cov 3 | Cov 4 | −2×LL | β1 | P1 | β2 | P2 | β3 | P3 | β4 | P4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Base Model | 3680.00 | |||||||||||
| 2 | Age | 3679.15 | 0.0043 | 0.67 | |||||||||
| 3 | Weight | 3678.90 | −0.0095 | 0.92 | |||||||||
| 4 | BSA | 3678.42 | −0.539 | 0.41 | |||||||||
| 5 | SrCr | 3678.36 | −0.691 | 0.36 | |||||||||
| 6 | CrCL | 3677.56 | −0.0067 | 0.27 | |||||||||
| 7 | Albumin | 3676.32 | 0.146 | 0.11 | |||||||||
| 8 | ALT | 3676.26 | 0.0122 | 0.015 | |||||||||
| 9 | Sex M | 3675.20 | −0.621 | 0.017 | |||||||||
| 10 | Albumin | ALT | 3673.43 | 0.382 | 0.018 | 0.0144 | 3.2e-005 | ||||||
| 11 | SrCr | ALT | 3672.68 | −1.37 | 0.016 | 0.0178 | 3e-005 | ||||||
| 12 | SrCr | Sex M | 3671.53 | −0.236 | 0.76 | −0.78 | 0.01 | ||||||
| 13 | Albumin | Sex M | 3669.04 | 0.42 | 0.068 | −0.802 | 0.0018 | ||||||
| 14 | ALT | Sex M | 3666.97 | 0.014 | 0.0036 | −1.02 | 0.00025 | ||||||
| 15 | Sex M | ALT | SrCr | 3665.03 | −0.88 | 0.0012 | 0.0177 | 3.8e-005 | −1.08 | 0.056 | |||
| 16 | Sex M | ALT | Albumin | 3662.91 | −1.00 | 4.5e-005 | 0.0147 | 8.5e-005 | 0.419 | 0.011 | |||
| Finalb | Sex M | ALT | SrCr | Albumin | 3661.50 | −0.866 | 0.00033 | −0.445 | 0.033 | 0.0177 | l.le-007 | 0.39 | 0.0056 |
Indicates significance of a linear model of log(parameter) on log (covariate) with β significantly different from zero
Model build for SexM on V/F included SexM on Kel, β =−0.445, P-value=0.033
Pharmacodynamics
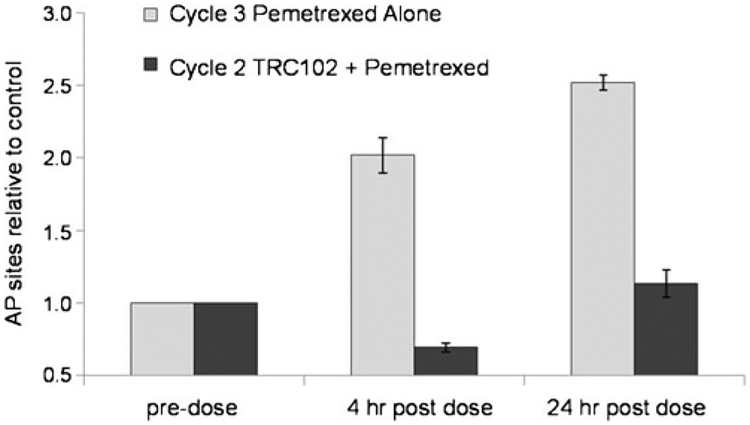
The binding of TRC102 to pemetrexed-induced AP sites was evaluated in peripheral blood mononuclear cells (PBMC) of a subset of patients. Comparison was made between AP site availability in PBMC on cycle 2 day 1 (when TRC102 and pemetrexed were administered concurrently) and cycle 3 day 1 (when TRC102 was held and pemetrexed was administered alone). As summarized in Table 7 and presented in Fig. 2, administration of pemetrexed in the absence of TRC102 on cycle 3 day 1 generally caused an increase in the number of AP sites (detected through binding of a reactive aldehyde group) relative to baseline, indicating that pemetrexed induced the BER pathway. These sites were masked during concurrent administration of pemetrexed and TRC102 on cycle 2 day 1, indicating TRC102 bound AP sites could no longer were detectable by the aldehyde reactive probe. Increased AP sites in PBMC relative to baseline were noted in 10 of 11 patient samples assayed following treatment with pemetrexed alone, and the increase was reduced in PBMC collected following treatment with both agents.
Table 7.
Percentage increase (or decrease) of apurinic sites in peripheral blood mononuclear cells in the absence of TRC102 on cycle 3 day 1 compared to in the presence of TRC102 on cycle 2 day 1, at 4 and 24 hours following dosing of pemetrexed
| TRC102 Dose Group = 15 mg/m2 | ||
| Patient | 4 hr | 24 hr |
| 10063002 | 29 % | 16 % |
| 10033001 | 26 % | 0% |
| 10033003 | −50 % | −51 % |
| Mean | 2% | −12 % |
| TRC102 Dose Group=30 mg/m2 | ||
| Patient | 4 hr | 24 hr |
| 10063003 | 55 % | 11 % |
| 10013003 | 18 % | 42 % |
| 10013005 | 58 % | 2% |
| Mean | 44 % | 18 % |
| TRC102 Dose Group=60 mg/m2 | ||
| Patient | 4 hr | 24 hr |
| 10033004 | 50 % | 62 % |
| 10013006 | 191 % | 122 % |
| Mean | 121 % | 92 % |
| TRC102 Dose Group = 100 mg/m2 | ||
| Patient | 4 hr | 24 hr |
| 10063005 | 6% | 134 % |
| 10033005 | 133 % | 93 % |
| 10013007 | 22 % | 8% |
| Mean | 54 % | 78 % |
Fig. 2.
Representative pharmacodynamic data from a patient treated with pemetrexed plus 60 mg/m2/d TRC102. AP sites in peripheral blood mononuclear cells were detected using a fluorescent aldehyde-reactive probe, and results were expressed relative to pre-dose. The number of AP sites increased after dosing with pemetrexed alone (cycle 3 day 1) but not after dosing with pemetrexed and TRC102 (cycle 2 day 1). These findings are consistent with binding of TRC102 to pemetrexed-induced AP sites
Clinical activity
All 28 patients had RECIST-defined measurable disease, and 25 underwent at least one response assessment. Fifteen patients (60 %) had a best response of stable disease or better lasting ≥3 cycles, including a 61 year old woman with metastatic oropharyngeal adenoid cystic cancer treated previously with cetuximab, docetaxel and carboplatin, who had a partial response (with maximal reduction in the diameter of the mediastinal lymph node metastasis of 33 %) and remained progression free for 21 cycles (14 months). In addition, 14 patients had stable disease for ≥3 cycles including patients with squamous cell lung cancer (n=3), epithelial ovarian cancer (n=3), colorectal cancer (n=2), nonsquamous non-small cell lung cancer (n= 1), pancreatic cancer (n= 1), prostate cancer (n= 1), endometrial cancer (n= 1), head and neck cancer (n= 1) and breast cancer (n= 1). Patients with prolonged stable disease were noted across all four dose cohorts without clear dose dependency.
Thymidylate synthase immunohistochemistry
Archival formalin-fixed paraffin-embedded tumor tissue was assessed for thymidylate synthase immunohistochemistry in three patients who developed stable disease beyond 3 cycles with tumor types that may express high levels of TS. The patient with metastatic oropharyngeal adenoid cystic carcinoma who achieved a partial response had 1+ staining intensity in 30 % of tumor cells giving her a positive HSCORE indicating high thymidylate synthase expression and low likelihood of response to pemetrexed [21]. Two patients with squamous cell lung cancer, who remained on study for 9 cycles, had 2+ intensity in 1–2 % of tumor cells giving them negative HSCORES of <5 %.
Discussion
This study is the first to assess the pharmacological effects of TRC102 in humans, establishing a maximum tolerated daily oral dose in combination with the approved dose of pemetrexed chemotherapy. The MTD was 60 mg/m2/d for 4 days in combination with standard-dose pemetrexed every 3 weeks. At 100 mg/m2/d, the MTD was exceeded due to extravascular hemolytic anemia requiring transfusion and dose-reduction and was consistent with preclinical testing.
The anemia of TRC102 is remarkably predictable, occurring over a similar time course in rats, dogs, and cancer patients, typically within the first 3 weeks of treatment and not increasing in severity of anemia as the number of treatment cycles accumulated. In mice, TRC102 caused anemia at a dose 10-fold higher than those required for efficacy. Because of the large therapeutic window, TRC102 could be administered at a initial dose of 15 mg/m2/d that was projected to be efficacious. The wide safety margin is supported by pharmacodynamic data from the current study showing TRC102 bound to AP sites and achieving desired pharmacokinetic exposures at all doses studied with extravascular hemolysis limited to the higher doses.
The mechanism of TRC102-induced extravascular hemolysis is clearly different from the mechanism for activity since erythrocytes lack DNA and AP sites for TRC102 to bind. Hemolysis is believed to result from TRC102 binding to reactive aldehydes that accumulate on the plasma membrane of aging erythrocytes, thereby targeting them for early removal from the circulation by reticuloendothelial macrophages, primarily in the spleen [23].
Importantly, the current trial confirmed preclinical findings indicating that TRC102 does not potentiate chemotherapy-induced myelosuppression. In addition, grade 3 or higher drug-related nonhematologic toxicity was rare and observed only above the MTD of 60 mg/m2/d.
TRC102 pharmacokinetic parameters were dose-proportional with an overall mean t1/2>28 h. By the fourth daily TRC102 dose, all patients achieved the target plasma AUC and all but one patient achieved the desired Cmax of 50 ng/mL, that was associated with preclinical activity. Importantly, drug interactions between TRC102 and pemetrexed were not observed, which was consistent with preclinical testing. The variability in pharmacokinetics between patients were significantly influenced by both gender and baseline serum creatinine (see online Figure S6 and S7). The alanine transaminase and serum albumin were also significant covariates, however the beta coefficients were comparatively small and so were not considered heavily influential on the PK. Overall, we observed the PK parameters (Kel and V/F) were significantly larger in female patients, and therefore Clearance (CL=Kel*V/F) was much slower in male patients. The model provides an indication that these covariates were important for explaining PK variability among males in this cohort of patients, and are generally not considered predictive in other or larger cohorts.
Durable stable disease was achieved in a variety tumor types including patients with squamous and nonsquamous non-small cell lung cancer. A partial response lasting 21 cycles (14 months) was seen in a patient with metastatic oropharyngeal cancer that expressed high levels of thymidylate synthase. These findings suggest that TRC102 plus pemetrexed should be further evaluated in patients with cancers that are typically resistant to pemetrexed alone [24, 25].
In summary, TRC102 is a novel targeted therapy that is well-tolerated at clinically relevant doses in combination with pemetrexed chemotherapy, achieved desired PK and PD endpoints and was associated with activity when dosed with pemetrexed in patients unlikely to respond to pemetrexed treatment alone. The results of the current trial support rationally designed phase II studies to determine the clinical benefit of TRC102 in combination with pemetrexed in patients with non-small cell lung cancer and other cancer types. TRC102 is also undergoing clinical testing combination with temozolomide () and preclinical data support additional combination studies with wide variety of cancer therapies including antimetabolites, oxidizing agents, alkylating agents, and PARP inhibitors [1–9].
Supplementary Material
Acknowledgments
The authors would like to express their appreciation to the patients who participated in this investigational study and study staff.
Conflict of Interest Statement Ms. Adams, Dr. Leigh and Dr. Theuer are shareholders and employees of TRACON Pharmaceuticals. Dr. Gerson is a shareholder of TRACON Pharmaceuticals and he and Dr. Liu are inventors of patents relating to TRC102 that are assigned to Case Western Reserve University and licensed to TRACON Pharmaceuticals. The remaining authors declare that they have no conflict of interest.
Research Funding This Research was supported by TRACON Pharmaceuticals Inc San Diego CA
Footnotes
Presented in part at the following conferences 2009 ASCO Annual Meeting, Orlando, FL; 2010 AACR-IASLC Joint Conference on the Molecular Origins of Lung Cancer, Coronado, CA; 2010 IASLC Targeted Therapies for Lung Cancer Meeting, Santa Monica, CA; 2010 ASCO Annual Meeting, Chicago, IL
Trial registry number
Contributor Information
Michael S. Gordon, Pinnacle Oncology Hematology, 9055 E. Del Camino, Suite 100, Scottsdale, AZ 85258, USA
Lee S. Rosen, Los Angeles Division of Hematology-Oncology, University of California, Santa Monica, CA, USA
David Mendelson, Pinnacle Oncology Hematology, 9055 E. Del Camino, Suite 100, Scottsdale, AZ 85258, USA.
Ramesh K. Ramanathan, Virginia G. Piper Cancer Center Clinical Trials at Scottsdale, Healthcare (VGPCC)/TGen, Scottsdale, AZ, USA
Jonathan Goldman, Los Angeles Division of Hematology-Oncology, University of California, Santa Monica, CA, USA.
Lili Liu, Seidman Cancer Center, University Hospitals Case Medical Center, Cleveland, OH, USA; Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, USA.
Yan Xu, Seidman Cancer Center, University Hospitals Case Medical Center, Cleveland, OH, USA; Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, USA.
Stanton L. Gerson, Seidman Cancer Center, University Hospitals Case Medical Center, Cleveland, OH, USA Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, USA.
Stephen P. Anthony, Evergreen Hematology & Oncology, Spokane, WA, USA
William D. Figg, SAIC-Frederick, Inc., Center for Cancer Research, Frederick National Laboratory for Cancer Research, and Medical Oncology Branch, National Cancer Institute, NIH, Frederick, MD, USA
Shawn Spencer, SAIC-Frederick, Inc., Center for Cancer Research, Frederick National Laboratory for Cancer Research, and Medical Oncology Branch, National Cancer Institute, NIH, Frederick, MD, USA.
Bonne J. Adams, TRACON Pharmaceuticals, San Diego, CA, USA
Charles P. Theuer, TRACON Pharmaceuticals, San Diego, CA, USA
Bryan R. Leigh, TRACON Pharmaceuticals, San Diego, CA, USA
Glen J. Weiss, Virginia G. Piper Cancer Center Clinical Trials at Scottsdale, Healthcare (VGPCC)/TGen, Scottsdale, AZ, USA
References
- 1.Liu L, Yan L, Donze JR et al. (2003) Blockage of abasic site repair enhances antitumor efficacy of 1,3-bis-(2-chloroethyl)-1-nitrosourea in colon tumor xenografts. Mol Cancer Ther 2:1061–1066 [PubMed] [Google Scholar]
- 2.Yan T, Seo Y, Schupp JE et al. (2006) Methoxyamine potentiates iododeoxyuridine-induced radiosensitization by altering cell cycle kinetics and enhancing senescence. Mol Cancer Ther 5:893–902 [DOI] [PubMed] [Google Scholar]
- 3.Bulgar A, Miao Y, Borden E, et al. (2009) Enhancement of decita-bine cytotoxicity by methoxyamine via inhibition of base excision repair. AACR Meeting Abstracts (suppl; abstr 5547) [Google Scholar]
- 4.Weeks L, Bulgar A, Donze J et al. (2009) Induction of uracil DNA glycosylase (UDG) in human cancer cells in response to antimetabolites combined with methoxyamine. AACR Meeting Abstracts (suppl; abstr 3765) [Google Scholar]
- 5.Bulgar AD, Snell M, Donze JR et al. (2010) Targeting base excision repair suggests a new therapeutic strategy of fludarabine for the treatment of chronic lymphocytic leukemia. Leukemia 24:1795–1799 [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Bulgar A, Adams BJ et al. (2010) Combining pemetrexed with temozolomide and TRC102 (methoxyamine) causes synergistic cytotoxicity in melanoma cells. Proceedings of the 22nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics (Sup; abstr 517) [Google Scholar]
- 7.Bulgar AD, Miao Y, Gerson SL et al. (2010) Dual inhibition of BER by TRC102 and PARP inhibitor (ABT 888) synergistically enhances cytotoxicity of TMZ in human melanoma. AACR Meeting Abstracts (suppl; abstr 682) [Google Scholar]
- 8.Yan L, Bulgar A, Miao Y et al. (2007) Combined treatment with temozolomide and methoxyamine: blocking apurininc/pyrimidinic site repair coupled with targeting topoisomerase Ilalpha. Clin Cancer Res 13:1532–1539 [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Gerson SL (2004) Therapeutic impact of methoxyamine: blocking repair of abasic sites in the base excision repair pathway. Curr Opin Investig Drugs 5:623–627 [PubMed] [Google Scholar]
- 10.Liu L, Nakatsuru Y, Gerson SL (2002) Base excision repair as a therapeutic target in colon cancer. Clin Cancer Res 8:2985–2991 [PubMed] [Google Scholar]
- 11.Frosina G, Fortini P, Rossi O et al. (1994) Repair of abasic sites by mammalian cell extracts. Biochem J 304(Pt 3):699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horton JK, Prasad R, Hou E et al. (2000) Protection against methylation-induced cytotoxicity by DNA polymerase beta-dependent long patch base excision repair. J Biol Chem 275:2211–2218 [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Liu L, Gerson SL et al. (2003) Measurement of anti-cancer agent methoxyamine in plasma by tandem mass spectrometry with on-line sample extraction. J Chromatogr B 795:295–307 [DOI] [PubMed] [Google Scholar]
- 14.Taverna P, Liu L, Hwang HS et al. (2001) Methoxyamine potentiates DNA single strand breaks and double strand breaks induced by temozolomide in colon cancer cells. Mutat Res 485:269–281 [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Taverna P, Whitacre CM et al. (1999) Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents. Clin Cancer Res 5:2908–2917 [PubMed] [Google Scholar]
- 16.Liu L, Bulgar A, Donze J et al. (2007) Prevention of base excision repair by TRC102 (methoxyamine) potentiates the anti-tumor activity of pemetrexed in vitro and in vivo. J Clin Oncol 25(suppl; abst 13005) [Google Scholar]
- 17.Bobin-Dubigeon CAM, Herrenknecht C, Bard JM (2009) Development and validation of an improved liquid chromatography-mass spectrometry method for the determination of pemetrexed in human plasma. J Chromatogr B Analyt Technol Biomed Life Sei 877:2451–2456 [DOI] [PubMed] [Google Scholar]
- 18.http://software.monolix.org/; 2008,
- 19.Bauer RJ, Guzy S, Ng C (2007) A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J 9:E60–E83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano J, Huang C, Liu D et al. (2006) Evaluations of biomarkers associated with 5-FU sensitivity for non-small-cell lung cancer patients postoperatively treated with UFT. Br J Cancer 95:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CL, Yokomise H, Kobayashi S et al. (2000) Intratumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. Int J Oncol 17:47–54 [PubMed] [Google Scholar]
- 22.Duffaud F, Therasse P (2000) New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer 87:881–886 [PubMed] [Google Scholar]
- 23.Antonelou MH, Kriebardis AG, Papassideri IS (2010) Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus 8(Suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bepler G, Sommers KE, Cantor A et al. (2008) Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer. J Thorac Oncol 3:1112–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scagliotti GV, Ceppi P, Capelletto E et al. (2009) Updated clinical information on multitargeted antifolates in lung cancer. Clin Lung Cancer 10(Suppl 1):S35–S40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.