SYNOPSIS
Fetal development occurs in a relatively hypoxemic environment, and birth represents significant oxidative stress. Premature infants are disadvantaged due to a lack of maternal antioxidant transfer and impaired endogenous antioxidant responses. O2 metabolism is essential for life and its biochemical reactions are dynamic, compartmentalized, and difficult to characterize in vivo. There is a growing appreciation for the role of reactive oxygen species in non-pathologic processes including regulation of cell signaling and mitochondrial function. There are several gaps in our knowledge about the role of reactive oxygen species in normal development and how oxidative stress alters normal signaling and subsequent development.
Keywords: oxygen, prematurity, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, glutathione, antioxidants, mitochondria
Introduction
Fetal development occurs normally in a relatively hypoxic (~20–25 Torr) environment in utero meaning that the transition into room air at birth represents significant oxidative stress for the prematurely born neonate.1,2 Unfortunately, the transition from the hypoxic environment of the womb to the relatively hyperoxic extrauterine environment occurs during a period of marked susceptibility to oxidative stressors. Preterm neonates are more susceptible to the effects of O2 toxicity due to developmental deficits in antioxidant defenses and developmental impairments in the ability to mount rapid antioxidant responses to hyperoxia.3–7 In general, the toxicities of O2 during the neonatal period have been characterized by macromolecular indices of oxidative protein, lipid, and/or DNA damage. An expanding body of evidence has defined the molecular effects of hyperoxia on developmental pathways that guide organogenesis.8,9 The sudden and dramatic increase in lung and systemic O2 tension upon preterm delivery significantly influence transcription factor activation and related downstream pathways. Unfortunately, the global impact of O2 toxicity in preterm neonates is incompletely characterized due to the lack of sensitive and specific redox biological techniques that adequately capture these complex biochemical reactions that undoubtedly contribute to the observed morbidity and mortality in this highly vulnerable patient population.
Basic tenets of oxidative stress
Sources of Reactive O2 Species
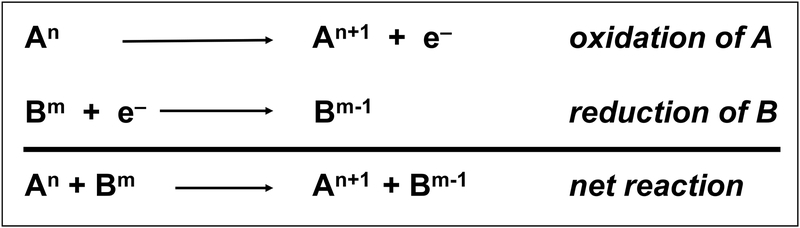
An oxidation-reduction or “redox” reaction refers to a transfer of electrons between molecules. It is essential to remember that matter is neither created nor destroyed in chemical transformations. In the simplified scheme (Figure 1), molecule A loses an electron and becomes oxidized while molecule B accepts an electron and becomes reduced. Thus, the net reaction is simply the transfer of the electron from molecule A to molecule B. In the illustration, “n” and “m” refer to the oxidation state of molecules A and B, respectively. When electrons are lost, the oxidation number increases (An+1). Conversely, when electrons are gained, the oxidation number decreases (Bm−1).
Figure 1.
Basic scheme of oxidation-reduction (redox) reactions. Molecule A loses an electron and becomes oxidized while molecule B accepts an electron and becomes reduced. Thus, the net reaction is simply the transfer of the electron from molecule A to molecule B. In the illustration, “n” and “m” refer to the oxidation state of molecules A and B, respectively. When electrons are lost, the oxidation number increases (An+1). Conversely, when electrons are gained, the oxidation number decreases (Bm−1).
In order to fully comprehend the effects of O2 tension on neonatal pathophysiology, the complexities of redox biology must be appreciated. Conceptually, this understanding must extend beyond the “oxidant/antioxidant balance” concept which is that “oxidative stress” represents a deficiency of antioxidants in a setting of enhanced oxidant generation. This overly simplistic model suggests that oxidative stress can be overcome by exogenously administered antioxidants to restore “balance”. In reality, the complex biochemical reactions responsible for the reduction of O2 are dynamic, highly compartmentalized, sensitive to clinically relevant factors such as pH and temperature, and extremely difficult to characterize in vivo with currently available techniques.10
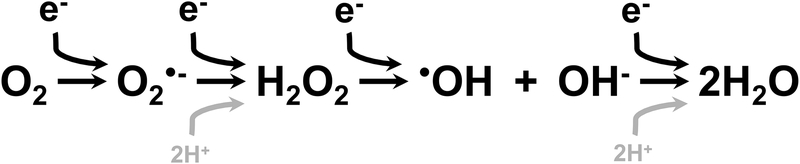
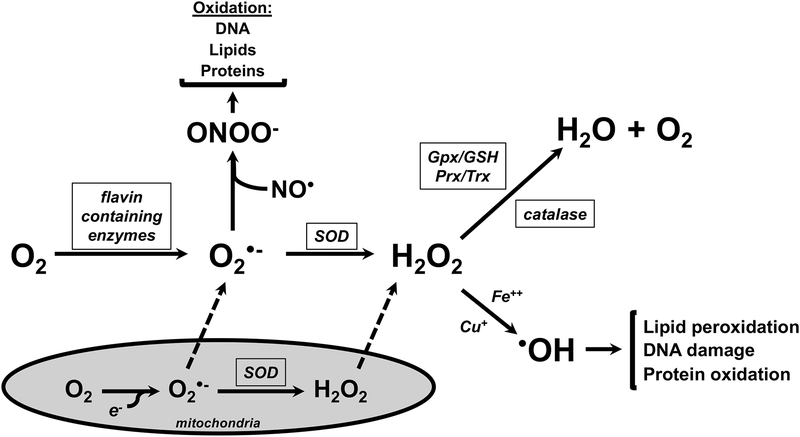
Diatomic O2 is highly reactive due to an unpaired electron in its outer orbital and requires 4 electrons for complete reduction (Figure 2). O2 is also the primary cellular metabolic fuel for aerobic metabolism.10 Under normal conditions, the reactive O2 species (ROS) generated in the process of the four electron reduction of O2 to H2O are quickly reduced (Figure 3).11 ROS generated during cellular metabolism include superoxide (O2•) and hydrogen peroxide (H2 O2)10,11. Additional oxidants including peroxinitrite (ONOO−), generated from the non-enzymatic reaction between O2•− and nitric oxide (NO•), and hydroxyl radical (•OH), generated from the reaction between H2O2 and iron (Fe++) or copper (Cu+), are primarily formed in situations in which endogenous antioxidant systems are unable to sufficiently provide electrons for reductive processes. Though the primary focus of this review is O2 toxicity, it is important to understand that excessive ROS generation in preterm infants come from a variety of sources including ischemia/reperfusion, infection, inflammation, mitochondrial respiratory chain, free iron and Fenton reaction, and hyperoxia.12–14 The generation of ROS can lead to the disruption of normal physiologic events.15 The extent of the effects of ROS on physiology depends upon specific molecular interactions, cellular locations, and timing of exposure.15
Figure 2.
Four electron reduction of O2 to H20 with intermediate generation of reactive oxygen species including superoxide (O2•-), hydrogen peroxide (H2O2), and hydroxyl radical (•OH).
Figure 3.
Effects of reactions of ROS generated by O2 metabolism in the absence of adequate detoxification. Nitric oxide (NO•) can react with O2•- to form peroxinitrite (ONOO−), which oxidized DNA, lipids and proteins. H2O2 can react with Fe++ and/or Cu+ to cause lipid peroxidation, DNA damage, and protein oxidation.
The effects of ROS contribute to quantifiable cellular, tissue, and organ damage that underlie many of the morbidities of prematurity.12 These damaging processes occur in both the placenta and the developing fetus.13 Though premature infants that develop prematurity-related morbidities are usually exposed to only the least required amount of supplemental O2 postnatally, they exhibit marked evidence of oxidant stress.6,12,14 There is evidence that excessive ROS production contributes to retinopathy of prematurity, bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia, necrotizing enterocolitis, kidney damage, and hemolysis.13,16,17 Pathophysiologically, many diseases of prematurity likely represent a convergence between injury and ROS-induced alterations in development, probably leading to increases in susceptibility to chronic diseases in adulthood, and perhaps more rapid aging as well.18
The appreciation of ROS as something other than a negative entity has grown in the last 20 years. Indeed, a number of cellular processes are actively modulated via ROS production. ROS serve as cell signaling molecules for normal biologic processes.15 For example, NADPH oxidases (NOXs) produce O2•− and/or H2O2 in tightly regulated and highly specific intracellular events.19 As such, these processes are governed by transcription factors that are influenced by the redox environment of the tissue, cell, or subcellular compartment in which they are expressed. Changes in electron flux through these pathways, whether it be through reduction of O2 or through nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Nox) influence signaling. Nox-dependent ROS production, influences developmental programming by acting upon redox sensitive transcription factors including hypoxia inducible factors (HIFs) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB). Dysregulation of HIFs and NFkB have been linked to one another and to negative outcomes in prematurely born infants.8,20 Nox isoforms contribute to signaling during lung development and injury and their function influences pulmonary airway and vascular cell phenotypes, including proliferation, hypertrophy, and apoptosis.19 Oxidative stress is also associated with altered nitric oxide (NO) signaling in which ROS and reactive nitrogen species (RNS) production are increased and bioavailable NO is decreased.21
Antioxidant Systems
Antioxidants are substances that inhibit or prevent oxidation of a substrate. Highly conserved antioxidant systems have developed to rapidly and robustly respond to alterations in cellular and subcellular redox perturbations. In the context of the previously mentioned four electron reduction of O2, antioxidant systems serve as electron donors as illustrated in Figure 3.11 Antioxidants that protect against and repair O2-mediated injury include flavin containing enzymes, superoxide dismutases (SOD), the glutathione (GSH) and thioredoxin (Trx) systems, heme oxygenases, and small molecular weight antioxidants.1,11,22 Antioxidant capacity is lower in preterm newborns than in term infants.14,17
Birth itself represents an oxidative challenge. In the days preceding full gestation, antioxidant systems are upregulated and non-enzymatic antioxidants cross the placenta in increasing amounts.9 These developmental changes provide for the transition from the relative hypoxia of intrauterine development to the oxygen-rich extrauterine environment. Furthermore, endogenous antioxidant production is upregulated immediately prior to birth in term infants and is further upregulated upon exposure to atmospheric O2. Remembering that development occurs in a hypoxic environment in utero (~20–25 Torr), exposure to even room air constitutes “hyperoxia” for the prematurely born neonate. Premature infants are at a distinct disadvantage for many reasons since they do not receive maternal antioxidants prior to delivery, have impaired ability to induce endogenous antioxidants prior to birth, and are unable to further induce endogenous antioxidant responses following delivery.5,9 Though much has been outlined regarding associations between oxidative damage and neonatal morbidities, significant gaps in knowledge still exist regarding the role of oxidative injury in the pathogenesis of neonatal diseases.12
Therapeutic strategies to mitigate ROS-induced diseases in premature infants have included both enzymatic and non-enzymatic antioxidant preparations.5 Though logically based upon the idea of “antioxidant imbalance”, studies in animal models and in preterm infants have yielded mixed results.5,15 Cysteine is a precursor of glutathione (GSH), the most abundant intracellular antioxidant in the body. Cysteine chloride supplementation in parenteral nutrition improved nitrogen balance in preterm infants; however, increased metabolic acidosis was also reported. N-acetylcysteine has shown promising results in preclinical models by acting as a precursor for de novo GSH synthesis. Unfortunately, routine N-acetylcysteine supplementation was not found to be effective in improving respiratory outcomes extremely low birth weight infants.23
One of the most promising catalytic antioxidants to undergo extensive clinical investigation in the prevention of BPD was superoxide dismutase (SOD). Though incidence of wheezing was lower in SOD-treated infants, a Cochrane metanalysis indicated there is insufficient evidence to draw firm conclusions about the efficacy of SOD in preventing chronic lung disease of prematurity; however, it appears to be well tolerated and has no serious adverse effects.24 Post-hoc analyses of the data from infants with ROP in this trial indicated that severity above stage 2 was present in 42% of placebo-treated infants versus 25% of SOD-treated infants suggesting that SOD may possibly reduce the risk of developing ROP.25
O2 toxicity-related sequelae of birth
Macromolecular oxidation
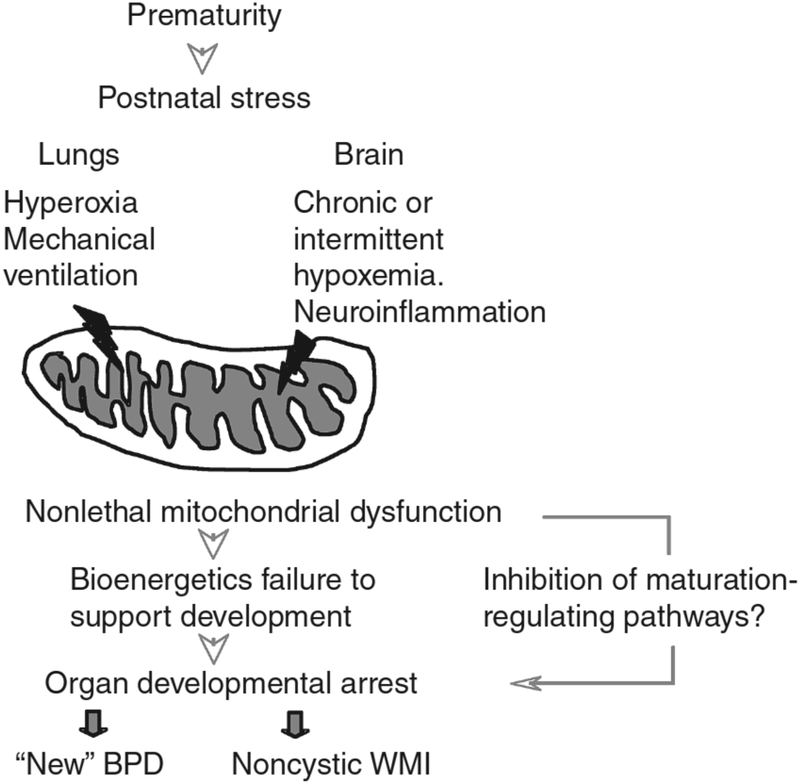
Generally speaking, similar pathophysiological mechanisms contribute to oxygen toxicity-related morbidities in infants. As described above, ROS generated from metabolism, ischemia/reperfusion, infection, hyperoxia, and inflammation, when present in excess amounts, result in detectable byproducts of oxidation. These byproducts are highlighted in Figure 4. Though nonspecific, the detectability of these byproducts has enabled associations between O2 toxicity and neonatal pathology including bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), and periventricular leukomalacia (PVL).13,16
Figure 4.
Mechanisms by which perinatal mitochondrial oxidant stress contributes to white matter injury and lung injury in preterm infants.
From Ten VS. Mitochondrial dysfunction in alveolar and white matter developmental failure in premature infants. Pediatr Res. 2017;81(2):286–292; with permission.
Glutathione (GSH) is the most abundant intracellular antioxidant in the body and cycles between thiol (GSH) and disulfide (GSSG) species. The GSH redox ratio (GSH/GSSG) is often used as a noninvasive measure of in vivo redox status. A significant negative correlation was reported between the arterio-alveolar oxygen and blood glutathione redox ratio with improved oxygenation inversely associated with decreased GSH/GSSG ratio.26 Further, associations between BPD, lipid hydro-peroxide (LOOH) and GSH concentrations in bronchoalveolar lavage fluid (BALF) levels have suggested that early LOOH level increases in preterm infants developing BPD suggest that lung biochemical monitoring of sick infants might be possible and that BPD could be predicted early by evaluating biomarkers.27 Extremely preterm infants have low GSH levels that impair their ability to detoxify ascorbylperoxides (AscOOH), an oxidant commonly found in parenteral nutrition. Higher first-week urinary AscOOH levels are associated with an increased incidence of BPD or death.28
White matter in the brains of premature infants is vulnerable to oxidative damage due to delayed expression of SOD, catalase, and GSH peroxidase enzymes.29 Isoprostanes are a quantifiable marker of ROS-mediated tissue injury and concentrations of F2-isoprostane in preterm lesions are similar to those measured in moderately severe cerebral cortical hypoxic–ischemic lesions in term infants.29 Diffuse white matter injury (WMI) involves maturation-dependent vulnerability of the oligodendrocyte (OL) lineage with selective degeneration of late oligodendrocyte progenitors (preOLs) triggered by oxidative stress and other insults.29 Oxidative damage triggers cell death in preterm human white matter and the magnitude of oxidative damage is comparable to that sustained in the cerebral cortex after severe perinatal asphyxia.29
Redox-dependent alterations in cell signaling
As presented earlier, there has been increasing recognition of O2 toxicity as an alteration in redox-dependent cellular and subcellular function. When viewed from this perspective, even subtle changes in redox balance can have persistent effects on organogenesis, tissue repair, and cellular function. As an example, multiple growth factors and signaling cascades play important roles in normal lung vascular development.30,31 One of the most extensively studied endothelial growth factors is vascular endothelial growth factor (VEGF). VEGF, a potent endothelial cell mitogen produced by type 2 alveolar epithelial cells (AECs), is significantly involved in alveolar development and its expression is regulated by hypoxia-inducible factors (HIF)32–34 Numerous studies in newborn animal models have demonstrated the importance of normal VEGF signaling to lung alveolar development.35–41 Premature delivery has deleterious effects on the O2-dependent biological processes that mediate lung development; in particular, the HIF/VEGF pathways.8
NFkB regulates angiogenesis by acting upstream of HIF/VEGF.20 Direct effects of ROS on signaling pathways include redox-sensitive transcription factors—e.g. HIF, Nrf-2, and NF-kB—as well as indirect effects through inactivation of NO-based signaling.15 For example, NF-kB is a direct regulator of VEGF-receptor-2, in the neonatal pulmonary vasculature.42 Similar to BPD, altered HIF/VEGF signaling also mechanistically contributes to retinopathy of prematurity. O2 toxicity can directly damage pulmonary parenchyma and vessels.43 Treatment with iNO can enhance additional ROS formation in the form of ONOO− leading to NO depletion and enhanced arterial pulmonary vascular constriction.43
O2-mediated activation of Nox enzymes modulate angiogenesis or apoptotic pathways in the retina and contribute to the pathophysiology of ROP. The magnitude of Nox activation from O2 fluctuations is associated with the degree of ROP.44 VEGF-induced VEGFR2 alters the interaction between Nox and p-VEGFR2 suggesting that NOX4 may be a target to alter ROS generation to modulate VEGFR2 signaling and reduce ROP.45 Patients with BPD frequently display alterations in pulmonary vascular remodeling and tone which manifest as pulmonary hypertension (PH).46 ROS and NO signaling pathways are disrupted in PH as evidenced by increased Nox expression, uncoupling of endothelial NO synthase, and reduced mitochondrial number and function.21
Redox effects in the mitochondria
More than 90% of ATP in mammalian cells is produced by oxidative phosphorylation through the action of mitochondrial ATP synthase.47 Mitochondrial bioenergetic dysfunction has been proposed as a cause of altered organ development in premature infants (Figure 4).48 Mitochondria are now thought of as one of the cell’s most sophisticated and dynamic responsive sensing systems.49 Specific signatures of mitochondrial dysfunction that are associated with disease pathogenesis and/or progression are increasingly recognized as being important.49 Though the specific pathways that regulate alveolar and white matter development are different in premature infants, both postnatal pulmonary and white matter development are dependent on proper mitochondrial function.48,50 At birth, both the lungs and brains of premature infants are structurally and functionally immature, and growth also requires substantial energy.48 Mitochondrial dysfunction is increasingly appreciated as a key pathological feature in the development of lung disease.49,50
Mitochondria govern the response to altered O2 tension and mitochondrial quality control.51 Premature neonates exhibit lower mitochondrial functional capacity, likely due to maturational delays in critical mitochondrial complexes and increased degradation of mitochondrial proteins.47 Though the role of mitochondrial processes in diseases of prematurity is complex, recent evidence suggests that mitochondria offer potential for novel diagnostics and therapeutics in lung diseases.49 Vascular endothelial mitochondrial function at birth was recently demonstrated to be a potential biomarker for BPD susceptibility in preterm infants.50 In this study, mitochondrial dysfunction in human-derived vascular endothelial cells isolated from umbilical cords at the time of birth strongly predicted the risk of poor pulmonary outcomes.50 In vitro, hyperoxia causes reduced oxygen consumption, increased uncoupling, and altered insulin secretion in human beta cells. Using ultradeep sequencing, Kleeberger and colleagues identified mtDNA sequence variation and differences in heteroplasmy between inbred mouse strains that associate with pulmonary phenotypes upon hyperoxic exposure in neonatal mice.52 The effects of these differences on mitochondrial function is an area of active investigation for the Kleeberger group. Ballinger and colleagues recently demonstrated that differences in mitochondrial bioenergetics and mtDNA damage associated with maternal ancestry may contribute to endothelial dysfunction and vascular disease.53 Collectively, these data highlight the need for a greater understanding of the impacts of mitochondrial dynamics, mitochondrial metabolism, mtDNA sequence variability, and mitochondrial protein expression in the context of neonatal diseases.49
Gaps in knowledge
Effects of genetics on redox biology in the neonate
O2 toxicity alters developmental pathways through a variety of mechanisms.54 Similarly, differential responses to O2 toxicity are also influenced by genetics in individual patients. This includes ROS production, antioxidant responses, and genetics of underlying developmental pathways. VEGF and endothelial nitric oxide synthase (eNOS) haplotypes are associated with differential effects of O2 on the development of RDS, BPD, IVH, and ROP in a population of 342 <29 week neonates.55 Collectively, the data indicated that haplotypes of VEGF and eNOS genes may also independently affect birth weight and gestational age, and act as protecting or risk markers for prematurity complications.55
With respect to antioxidants, genetic polymorphisms of SOD and catalase were recently demonstrated to influence the incidence of morbidities in premature infants.43 Genetic variations in antioxidant enzymes may contribute to the pathogenesis of redox-mediated prematurity complications. In an investigation of a cohort of 451 <30 week infants, a single-nucleotide polymorphism related to the Nox family altered the susceptibility to oxidative stress-related complications of prematurity including RDS, BPD, and ROP.56 Furthermore, it has been estimated that the effects of gestational age and the duration of supplemental O2 administration may account for up to 70% of the variance in ROP susceptibility.57
In general, SNPs of antioxidant enzymes have been poorly studied.43,58 With respect to GSH metabolism during the neonatal period, levels of oxidative stress markers in boys are greater when compared to girls. This discrepancy is likely due to alterations in estrogen metabolism which promotes the activation of glutathione metabolism.59 Thus, it is possible that considerations regarding sex must be factored into nutritionally focused antioxidant therapies that target GSH metabolism.59 After adjustment for epidemiological confounders, sequence variants of NAD(P)H quinone oxidoreductase-1 and nuclear factor, erythroid derived 2, like 2 (Nrf2) SNPs were associated with BPD and severe BPD, respectively.60 Additional study of genetic polymorphisms could help identify high risk populations, who would benefit from targeted antioxidant strategies.43
Enhancing endogenous antioxidant responses
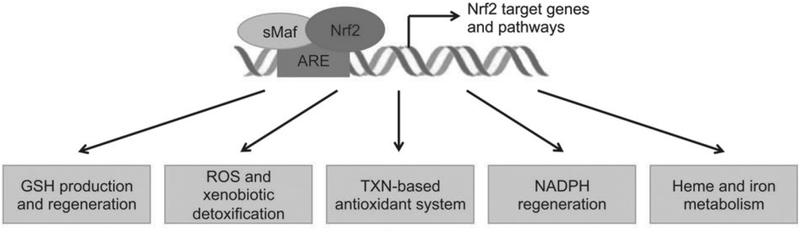
Nrf2 is a transcription factor that coordinates the basal expression of and inducible activation of antioxidant and xenobiotic genes. For a comprehensive overview of Nrf2 and associated processes, the reader is directed to the excellent review by Tonelli et al (Figure 5).61 Briefly, Nrf2 regulates de novo GSH synthesis, NADPH production, as well as autophagy, stem cell activation, and the unfolded protein response.61 O2 is a potent Nrf2 stimulus and, based upon the availability of binding partners, competition or cooperation with other activators and repressors, and crosstalk with other signaling pathways, Nrf2 epigenetically alters target gene promoters.61 Nrf2 is currently being investigated as a potential therapeutic target to enhance endogenous antioxidant responses to attenuate the impacts of O2 toxicity on the premature infant.
Figure 5.
The Nrf2 system. Nrf2 activation elicits enhanced de novo GSH synthesis, detoxification of ROS and xenobiotics, enhancement of the thioredoxin (TXN) antioxidant system, regeneration of NAPDH, and heme metabolism.
Adapted from Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745; with permission.
Trace elements including copper, zinc, iron, and selenium (Se) are essential for normal antioxidant enzyme function. Preterm infants have well documented perinatal deficiencies in Se, as recently reviewed by our group.62 Data indicate that trace mineral supplementation could optimize total antioxidant capacity.63 Though Se supplementation was associated with a reduction in sepsis in preterm infants, it did not improve survival, reduce BPD, or reduce ROP incidence.64 Using BPD models, the Kleeberger group has used bioinformatics to identify novel Nrf2-dependently modulated genes that regulate downstream targets in order to screen for chemicals or drugs that modulate expression. These types of approaches could help lead to the identification of new Nrf2 modulating therapies to prevent morbidities of prematurity.65 There is much interest in understanding the intersection between trace mineral status on the efficacy of Nrf2 modulating therapies in diseases of prematurity.66
Methodologically, analyses of oxidative stress biomarkers have not translated into routine clinical practice due to lack of automation and cost.67 Additionally, the lack of specificity, especially as it relates to redox regulated developmental processes, creates significant technical challenges economic difficulties constitutes a challenge for the immediate future since accurate evaluation of oxidative stress would contribute to improve the quality of care of our neonatal patients.67 New techniques such as surface enhanced Raman spectroscopy may improve the ability to measure oxidative stress biomarkers using low sample volumes and in real-time.67,68
Oxygen toxicity: beyond the balance
It is very clear that ROS have important regulatory and signaling roles in the newborn. Thus, antioxidant manipulation is likely to have implications for redox sensitive developmental pathways that guide proper organogenesis.16 Given our evolving understanding of oxidative stress in the neonate, future research must include evaluations of the prognostic and therapeutic value of oxidative stress biomarkers and antioxidants in premature infants.12 The lack of enhanced induction of antioxidants by O2 in preterm infants highlights the need to better understand the mechanisms responsible for differential responses and burden of disease in this highly vulnerable population.9 We are also currently unable to determine which infants are likely to achieve maximal benefit from therapies that replace antioxidants or enhance endogenous antioxidant responses.16
NFkB has a major role in lung and brain development suggesting that therapeutic strategies to selectively block or enhance discrete components of this pathway may hold promise in preventing or treating diseases of prematurity.20,42 It is also possible that preservation of mitochondrial function or prevention of mitochondrial dysfunction may be a novel strategy to prevent morbidities in prematurely born infants.48 Enhancement of NO signaling and prevention of eNOS uncoupling by Nox inhibition could help prevent mitochondrial dysfunction and/or restore mitochondrial function.21 Finally, use of high-throughput evaluation of mitochondrial biology of HUVEC or peripheral blood mononuclear cells may help modify therapeutic strategies to decrease risk for adverse outcomes in susceptible infants.50
KEY POINTS.
Oxidative stress has traditionally been presented as an “imbalance” between oxidants and antioxidants but the situation is far more complex.
Neonatal O2 toxicity has been primarily characterized by macromolecular indices of damage that are nonspecific and are inadequate to capture dynamic biochemical processes.
In premature infants, the fetal to neonatal transition occurs during a period of marked susceptibility to oxidative stressors due to deficits in antioxidant defenses and impaired endogenous antioxidant response activation.
The molecular effects of O2 on subcellular compartments and developmental pathways are poorly understood.
State-of-the-art redox biology techniques will enable more robust understanding of the global impact of O2 toxicity in preterm neonates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have no conflicts to declare
Literature Cited
- 1.Asikainen TM, White CW. Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy. Antioxid Redox Signal. 2004;6(1):155–167. [DOI] [PubMed] [Google Scholar]
- 2.Welty SE. Is there a role for antioxidant therapy in bronchopulmonary dysplasia? J Nutr. 2001;131(3):947S–950S. [DOI] [PubMed] [Google Scholar]
- 3.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. [DOI] [PubMed] [Google Scholar]
- 4.Baydas G, Karatas F, Gursu MF, et al. Antioxidant vitamin levels in term and preterm infants and their relation to maternal vitamin status. Archives of medical research. 2002;33(3):276–280. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Davis JM. Future applications of antioxidants in premature infants. Current opinion in pediatrics. 2011;23(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CV, Hansen TN, Martin NE, McMicken HW, Elliott SJ. Oxidant stress responses in premature infants during exposure to hyperoxia. Pediatr Res. 1993;34(3):360–365. [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Mehta T, Auld PA, et al. Glutathione metabolism in newborns: evidence for glutathione deficiency in plasma, bronchoalveolar lavage fluid, and lymphocytes in prematures. Pediatr Pulmonol. 1995;20(3):160–166. [DOI] [PubMed] [Google Scholar]
- 8.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175(10):978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010;15(4):191–195. [DOI] [PubMed] [Google Scholar]
- 10.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50(4–5):279–289. [DOI] [PubMed] [Google Scholar]
- 11.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. [DOI] [PubMed] [Google Scholar]
- 12.Ozsurekci Y, Aykac K. Oxidative Stress Related Diseases in Newborns. Oxidative medicine and cellular longevity. 2016;2016:2768365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrone S, Santacroce A, Longini M, Proietti F, Bazzini F, Buonocore G. The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside. Oxidative medicine and cellular longevity. 2018;2018:7483062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrone S, Bracciali C, Di Virgilio N, Buonocore G. Oxygen Use in Neonatal Care: A Two-edged Sword. Front Pediatr. 2016;4:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66(2):121–127. [DOI] [PubMed] [Google Scholar]
- 16.Jankov RP, Negus A, Tanswell AK. Antioxidants as therapy in the newborn: some words of caution. Pediatr Res. 2001;50(6):681–687. [DOI] [PubMed] [Google Scholar]
- 17.Aceti A, Beghetti I, Martini S, Faldella G, Corvaglia L. Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk. Oxidative medicine and cellular longevity. 2018;2018:7397659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meiners S, Hilgendorff A. Early injury of the neonatal lung contributes to premature lung aging: a hypothesis. Mol Cell Pediatr. 2016;3(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harijith A, Natarajan V, Fu P. The Role of Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Lung Architecture Remodeling. Antioxidants (Basel). 2017;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvira CM. Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions. Birth Defects Res A Clin Mol Teratol. 2014;100(3):202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med. 2012;52(9):1970–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asikainen TM, White CW. Antioxidant defenses in the preterm lung: role for hypoxia-inducible factors in BPD? Toxicol Appl Pharmacol. 2005;203(2):177–188. [DOI] [PubMed] [Google Scholar]
- 23.Soghier LM, Brion LP. Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst Rev. 2006(4):CD004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suresh GK, Davis JM, Soll RF. Superoxide dismutase for preventing chronic lung disease in mechanically ventilated preterm infants. Cochrane Database Syst Rev. 2001(1):CD001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parad RB, Allred EN, Rosenfeld WN, Davis JM. Reduction of retinopathy of prematurity in extremely low gestational age newborns treated with recombinant human Cu/Zn superoxide dismutase. Neonatology. 2012;102(2):139–144. [DOI] [PubMed] [Google Scholar]
- 26.Nemeth I, Boda D. Blood glutathione redox ratio as a parameter of oxidative stress in premature infants with IRDS. Free Radic Biol Med. 1994;16(3):347–353. [DOI] [PubMed] [Google Scholar]
- 27.Fabiano A, Gavilanes AW, Zimmermann LJ, et al. The development of lung biochemical monitoring can play a key role in the early prediction of bronchopulmonary dysplasia. Acta Paediatr. 2016;105(5):535–541. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed I, Elremaly W, Rouleau T, Lavoie JC. Ascorbylperoxide Contaminating Parenteral Nutrition Is Associated With Bronchopulmonary Dysplasia or Death in Extremely Preterm Infants. JPEN J Parenter Enteral Nutr. 2017;41(6):1023–1029. [DOI] [PubMed] [Google Scholar]
- 29.Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134(3):331–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar VH, Ryan RM. Growth factors in the fetal and neonatal lung. Front Biosci. 2004;9:464–480. [DOI] [PubMed] [Google Scholar]
- 31.D’Angio CT, Maniscalco WM. The role of vascular growth factors in hyperoxia-induced injury to the developing lung. Front Biosci. 2002;7:d1609–1623. [DOI] [PubMed] [Google Scholar]
- 32.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L167–178. [DOI] [PubMed] [Google Scholar]
- 33.Choi KS, Bae MK, Jeong JW, Moon HE, Kim KW. Hypoxia-induced angiogenesis during carcinogenesis. J Biochem Mol Biol. 2003;36(1):120–127. [DOI] [PubMed] [Google Scholar]
- 34.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. [DOI] [PubMed] [Google Scholar]
- 35.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L1068–1078. [DOI] [PubMed] [Google Scholar]
- 36.Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L600–607. [DOI] [PubMed] [Google Scholar]
- 37.Galambos C, Ng YS, Ali A, et al. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol. 2002;27(2):194–203. [DOI] [PubMed] [Google Scholar]
- 38.Gerber HP, Hillan KJ, Ryan AM, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. [DOI] [PubMed] [Google Scholar]
- 39.Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112(16):2477–2486. [DOI] [PubMed] [Google Scholar]
- 40.Kunig AM, Balasubramaniam V, Markham NE, et al. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L529–535. [DOI] [PubMed] [Google Scholar]
- 41.Maniscalco WM, Watkins RH, D’Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol. 1997;16(5):557–567. [DOI] [PubMed] [Google Scholar]
- 42.Iosef C, Alastalo TP, Hou Y, et al. Inhibiting NF-kappaB in the developing lung disrupts angiogenesis and alveolarization. Am J Physiol Lung Cell Mol Physiol. 2012;302(10):L1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dani C, Poggi C. The role of genetic polymorphisms in antioxidant enzymes and potential antioxidant therapies in neonatal lung disease. Antioxid Redox Signal. 2014;21(13):1863–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Investigative ophthalmology & visual science. 2008;49(4):1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Yang Z, Jiang Y, Hartnett ME. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol Vis. 2014;20:231–241. [PMC free article] [PubMed] [Google Scholar]
- 46.Alvira CM. Aberrant Pulmonary Vascular Growth and Remodeling in Bronchopulmonary Dysplasia. Front Med (Lausanne). 2016;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honzik T, Wenchich L, Bohm M, et al. Activities of respiratory chain complexes and pyruvate dehydrogenase in isolated muscle mitochondria in premature neonates. Early Hum Dev. 2008;84(4):269–276. [DOI] [PubMed] [Google Scholar]
- 48.Ten VS. Mitochondrial dysfunction in alveolar and white matter developmental failure in premature infants. Pediatr Res. 2017;81(2):286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cloonan SM, Choi AM. Mitochondria in lung disease. The Journal of clinical investigation. 2016;126(3):809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kandasamy J, Olave N, Ballinger SW, Ambalavanan N. Vascular Endothelial Mitochondrial Function Predicts Death or Pulmonary Outcomes in Preterm Infants. Am J Respir Crit Care Med. 2017;196(8):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schumacker PT, Gillespie MN, Nakahira K, et al. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol. 2014;306(11):L962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vellers HK, Association SR of Basal Mitochondrial Function Assessments and Mitochondrial DNA Sequence, Heteroplasmy, and Copy Number in Mice. Am J Respir Crit Care Med 2018;197(A2905). [Google Scholar]
- 53.Krzywanski DM, Moellering DR, Westbrook DG, et al. Endothelial Cell Bioenergetics and Mitochondrial DNA Damage Differ in Humans Having African or West Eurasian Maternal Ancestry. Circ Cardiovasc Genet. 2016;9(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saugstad OD, Sejersted Y, Solberg R, Wollen EJ, Bjoras M. Oxygenation of the newborn: a molecular approach. Neonatology. 2012;101(4):315–325. [DOI] [PubMed] [Google Scholar]
- 55.Poggi C, Giusti B, Gozzini E, et al. Genetic Contributions to the Development of Complications in Preterm Newborns. PLoS One. 2015;10(7):e0131741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huizing MJ, Cavallaro G, Moonen RM, et al. Is the C242T Polymorphism of the CYBA Gene Linked with Oxidative Stress-Associated Complications of Prematurity? Antioxid Redox Signal. 2017;27(17):1432–1438. [DOI] [PubMed] [Google Scholar]
- 57.van Wijngaarden P, Brereton HM, Coster DJ, Williams KA. Hereditary influences in oxygen-induced retinopathy in the rat. Doc Ophthalmol. 2010;120(1):87–97. [DOI] [PubMed] [Google Scholar]
- 58.Cuna A, George L, Sampath V. Genetic predisposition to necrotizing enterocolitis in premature infants: Current knowledge, challenges, and future directions. Semin Fetal Neonatal Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavoie JC, Tremblay A. Sex-Specificity of Oxidative Stress in Newborns Leading to a Personalized Antioxidant Nutritive Strategy. Antioxidants (Basel). 2018;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva DM, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2015;309(11):L1239–1272. [DOI] [PubMed] [Google Scholar]
- 61.Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tindell R, Tipple T. Selenium: implications for outcomes in extremely preterm infants. J Perinatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perrone S, Tataranno ML, Buonocore G. Oxidative stress and bronchopulmonary dysplasia. J Clin Neonatol. 2012;1(3):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev. 2003(4):CD003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho HY, Wang X, Li J, Bell DA, Kleeberger SR. Potential therapeutic targets in Nrf2-dependent protection against neonatal respiratory distress disease predicted by cDNA microarray analysis and bioinformatics tools. Curr Opin Toxicol. 2016;1:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tindell R, Wall SB, Li Q, et al. Selenium supplementation of lung epithelial cells enhances nuclear factor E2-related factor 2 (Nrf2) activation following thioredoxin reductase inhibition. Redox Biol. 2018;19:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres-Cuevas I, Parra-Llorca A, Sanchez-Illana A, et al. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017;12:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panikkanvalappil SR, James M, Hira SM, et al. Hyperoxia Induces Intracellular Acidification in Neonatal Mouse Lung Fibroblasts: Real-Time Investigation Using Plasmonically Enhanced Raman Spectroscopy. J Am Chem Soc. 2016;138(11):3779–3788. [DOI] [PubMed] [Google Scholar]