Summary
Background
Patent foramen ovale (PFO) is a contributor to embolic stroke of undetermined source (ESUS). Subgroup analyses from previous studies suggest that anticoagulation could reduce recurrent stroke compared with antiplatelet therapy. We hypothesised that anticoagulant treatment with rivaroxaban, an oral factor Xa inhibitor, would reduce the risk of recurrent ischaemic stroke compared with aspirin among patients with PFO enrolled in the NAVIGATE ESUS trial.
Methods
NAVIGATE ESUS was a double-blinded, randomised, phase 3 trial done at 459 centres in 31 countries that assessed the efficacy and safety of rivaroxaban versus aspirin for secondary stroke prevention in patients with ESUS. For this prespecified subgroup analysis, cohorts with and without PFO were defined on the basis of transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE). The primary efficacy outcome was time to recurrent ischaemic stroke between treatment groups. The primary safety outcome was major bleeding, according to the criteria of the International Society of Thrombosis and Haemostasis. The primary analyses were based on the intention-to-treat population. Additionally, we did a systematic review and random-effects meta-analysis of studies in which patients with cryptogenic stroke and PFO were randomly assigned to receive anticoagulant or antiplatelet therapy.
Findings
Between Dec 23, 2014, and Sept 20, 2017, 7213 participants were enrolled and assigned to receive rivaroxaban (n=3609) or aspirin (n=3604). Patients were followed up for a mean of 11 months because of early trial termination. PFO was reported as present in 534 (7·4%) patients on the basis of either TTE or TOE. Patients with PFO assigned to receive aspirin had a recurrent ischaemic stroke rate of 4·8 events per 100 person-years compared with 2 ·6 events per 100 person-years in those treated with rivaroxaban. Among patients with known PFO, there was insufficient evidence to support a difference in risk of recurrent ischaemic stroke between rivaroxaban and aspirin (hazard ratio [HR] 0·54; 95% CI 0·22–1·36), and the risk was similar for those without known PFO (1·06; 0·84–1·33; pinteraction=0·18). The risks of major bleeding with rivaroxaban versus aspirin were similar in patients with PFO detected (HR 2·05; 95% CI 0·51–8·18) and in those without PFO detected (HR 2·82; 95% CI 1·69–4·70; pinteraction=0·68). The random-effects meta-analysis combined data from NAVIGATE ESUS with data from two previous trials (PICSS and CLOSE) and yielded a summary odds ratio of 0·48 (95% CI 0·24–0·96; p=0·04) for ischaemic stroke in favour of anticoagulation, without evidence of heterogeneity.
Interpretation
Among patients with ESUS who have PFO, anticoagulation might reduce the risk of recurrent stroke by about half, although substantial imprecision remains. Dedicated trials of anticoagulation versus antiplatelet therapy or PFO closure, or both, are warranted.
Funding
Bayer and Janssen.
Introduction
Patent foramen ovale (PFO) is a potential cause of cryptogenic stroke. Device closure of PFO in patients with ischaemic stroke has been tested in six randomised trials,1–6 with three showing significant reductions in the intention-to-treat analyses for recurrent stroke,4–6 and two meta-analyses supporting the efficacy of closure compared with medical therapy.7,8 All but one of these trials allowed anticoagulation as an option for medical therapy, and the benefit of closure was observed predominantly in comparison with antiplatelet therapy, not with anti-coagulants.9,10 Stroke related to PFO is primarily thought to be a consequence of paradoxical embolism originating as venous thrombus, and ample data indicate that anti-coagulation is superior to antiplatelet agents for prevention and treatment of venous thromboembolism.11
The six randomised trials assessing PFO closure only enrolled patients younger than 60 years.1–6 The role of PFO in older patients is less clear.12 Older patients are generally at increased risk of thrombosis, and some studies have suggested that PFO confers an increased risk of stroke in this group,13 whereas others have suggested that PFO is less likely to be related to stroke in older patients.14
We aimed to compare antithrombotic strategies in a large cohort of patients with PFO and cryptogenic ischaemic stroke. We hypothesised that patients with PFO would have a lower risk of subsequent stroke if they were randomly assigned to receive rivaroxaban rather than aspirin. The NAVIGATE ESUS trial enrolled an older population than that of the closure trials, thereby allowing analysis of the associations of age, PFO, and stroke risk, in addition to the effects of antithrombotic treatment. We also did a systematic review of the literature to synthesise the existing data across studies of anticoagulation for PFO.
Methods
Study design and patients
NAVIGATE ESUS was an international, double-blinded, randomised phase 3 trial done at 459 centres in 31 countries. NAVIGATE ESUS compared rivaroxaban to aspirin in patients with embolic stroke of undetermined source (ESUS).15 The study rationale, additional design details, and participant features have been previously published.15,16 The protocol was approved by appropriate health authorities and institutional review boards at all study sites and all patients provided written informed consent before participation.
In brief, eligible patients were those with recent ischaemic stroke (between 7 days and 6 months) confirmed by neuroimaging who met criteria for ESUS as proposed by the Cryptogenic Stroke and ESUS International Working group,17 with minor modifications.15 In brief, participants were required to have an ischaemic stroke visualised by neuroimaging that was not lacunar, documented absence of extracranial atherosclerosis causing more than 50% luminal stenosis in arteries supplying the area of ischaemia (intracranial imaging was optional, but if done, >50% stenosis excluded participation), no major-risk cardioembolic source of embolism, and no other specific cause of stroke identified. Patients had to be older than 50 years at the time of the qualifying stroke; if aged 50–59 years, they were required to have at least one additional vascular risk factor. After the qualifying stroke, at least 20 h of cardiac rhythm monitoring was required to exclude atrial fibrillation lasting longer than 6 min, although investigators could choose to monitor for a longer time according to local clinical practice standards. However, all cardiac rhythm monitoring had to be completed before randomisation (ie, implantable loop recorders excluded participation). Patients diagnosed with PFO were eligible unless there were plans for closure. Notably, trials showing efficacy of PFO closure were published 1 week before completion of enrolment in NAVIGATE ESUS, and were therefore unlikely to have had a relevant impact on recruitment into this trial.2,4,5 Exclusion criteria included a history of atrial fibrillation, severely disabling stroke (modified Rankin Scale score ≥4 at screening), the presence of, or plan to insert, an implantable electrocardiogram loop recorder, specific indication for chronic anticoagulation or for chronic antiplatelet therapy, or previous non-traumatic intracranial haemorrhage (see the protocol15 for a complete list of exclusion criteria). Patients were followed up until trial termination on Oct 5, 2017.
The NAVIGATE ESUS trial was terminated early at the recommendation of the data monitoring committee because of absence of efficacy for stroke prevention coupled with an increase in major bleeding associated with rivaroxaban.15 This prespecified subgroup analysis of the effect of antithrombotic treatments among patients with PFO was planned before completion of the trial.
Procedures
Each patient was given either rivaroxaban at a dose of 15 mg (immediate-release, film-coated tablets) plus placebo-aspirin or aspirin at a dose of 100 mg (enteric coated tablets) plus placebo-rivaroxaban; in each group, the two tablets (active drug and placebo) were taken orally once daily with food. Participants returned for study visits at 1, 6, and 12 months and then every 6 months during which there was assessment for the occurrence of safety and efficacy events, adherence, and adverse events.
Echocardiography was required for all patients before enrolment to assess for intracardiac thrombus (an exclusion criterion), but the protocol did not specify transthoracic echocardiography (TTE) or transoesophageal echocardiography (TOE), nor did it require performance or documentation of a so-called bubble (agitated saline or echocardiographic contrast media) study. For either TTE or TOE, PFO was described as present, absent, or not reported. For these analyses, we dichotomised exposure as PFO present or not present. If TOE was done and PFO was present, it was further characterised as small, large, or of uncertain size, and the presence or absence of atrial septal aneurysm was also recorded, both based on local interpretation. We therefore defined three partially overlapping analytic cohorts: patients with TTE, patients with TOE, and patients with TTE or TOE, or both, with the final cohort being used for the primary analyses. Other diagnostic testing for PFO, such as transcranial Doppler ultrasound with bubble study, was not recorded.
Outcomes
The primary efficacy outcome of NAVIGATE ESUS was time to recurrent stroke (including ischaemic, haemorrhagic, or undefined strokes) or systemic embolism.15 For this prespecified subgroup analysis, the primary efficacy outcome was time to recurrent ischaemic stroke, for consistency with other PFO trials. The primary safety outcome of the subgroup analysis was major bleeding, according to the criteria of the International Society of Thrombosis and Haemostasis.18 Potential efficacy and safety outcome events were verified by a masked adjudication process.
Search strategy and selection criteria
We also did a systematic review and meta-analysis of the literature to identify previous randomised clinical trials in which patients with cryptogenic stroke and PFO confirmed by TOE were randomly assigned to treatment with an anticoagulant or antiplatelet therapy, and the risk of recurrent ischaemic stroke was reported. We searched MEDLINE on May 17, 2018, using the following search strategy: (“stroke”[MeSH Terms] OR “stroke”[All Fields]) AND (PFO[All Fields] OR (“foramen ovale”[MeSH Terms] OR (“foramen”[All Fields] AND “ovale”[All Fields]) OR “foramen ovale”[All Fields])) AND (anticoagulation[All Fields] OR (“warfarin”[MeSH Terms] OR “warfarin”[All Fields])) AND ((“clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH Terms] OR “clinical trial”[All Fields]) OR (“random allocation”[MeSH Terms] OR (“random” [All Fields] AND “allocation” [All Fields]) OR “random allocation”[All Fields] OR “randomised”[All Fields])). We also reviewed reference lists and asked experts in the field to identify additional studies. There were no language restrictions. Two authors reviewed the search results and resolved conflicts through consensus. Summary data were extracted from each trial.
Statistical analysis
We anticipated that PFO would be detected equally in about 40% of patients who were randomly assigned into both groups, and assumed a 4% annual stroke rate on aspirin over an average of 2 years of follow-up, which would provide 80% power with an α of 0·05 to detect at least 34% lower risk of stroke with rivaroxaban. Because of early termination of the trial, fewer events were observed than anticipated.
The primary analyses were based on the intention-to-treat population. The sensitivity analysis was done in the on-treatment population.15 Time-to-recurrent ischaemic stroke between treatment groups was compared with a log-rank test, and Kaplan-Meier estimates were used to plot the cumulative incidence risk over time. Risk reduction was estimated with the Cox proportional hazards model. Comparisons by randomised treatment assignment were not adjusted for any covariates. The comparison of event rates in the PFO group versus the no PFO group were presented both unadjusted and adjusted for age and vascular risk factors. All reported p values are two-sided. We did not adjust for multiplicity in these exploratory analyses.
For the meta-analysis, we did a random-effects metaanalysis of the studies along with data from our TOE cohort. We report Mantel-Haenszel odds ratios and used the I2 to evaluate heterogeneity. We did not assess for publication bias since only three studies were included. SAS software, version 9.4, was used for the NAVIGATE analysis and Review Manager 5.3 was used for the meta-analysis.
NAVIGATE ESUS is registered with ClinicalTrials.gov, number .
Role of the funding source
The study sponsors participated in the design of the parent NAVIGATE ESUS trial along with the investigators. Two of the coauthors are employed by the sponsors. The sponsors were not otherwise involved in the design, analysis, or interpretation of this pre-specified PFO cohort subgroup analysis. The sponsors had the opportunity to review the manuscript and to provide optional suggestions, but sponsor approval was not required. The sponsors had no other role in the writing of this report nor in the decision to submit for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Dec 23, 2014, and Sept 20, 2017, 7213 patients were enrolled in NAVIGATE ESUS and assigned to receive rivaroxaban (n=3609) or aspirin ( n=3604). TTE was done in 6884 patients, TOE in 1382, and either TTE or TOE in 7210 (including both in 1056; appendix). Echocardiographic information was missing for four patients. PFO was reported as present in 534 (7·4%) patients by either TTE or TOE. PFO was detected in 313 (4·6%) patients by TTE and in 379 (27·4%) by TOE. Baseline characteristics based on TTE, TOE, or both are summarised in table 1 (baseline characteristics for the separate TTE and TOE cohorts are provided in the appendix). Patients with PFO were younger, had a lower burden of traditional vascular risk factors, and had less severe strokes than did those without PFO. Global regional differences in the detection of PFO were also observed, with higher rates of detection in the USA, Canada, and western Europe than elsewhere (appendix).
Table 1:
Baseline characteristics of patients by patent foramen ovale identified by transthoracic or transoesophageal echocardiography, or both
| PFO detected (n=534) | PFO not detected (n=6675) | |
|---|---|---|
| Age, years | 64·6 (9·2) | 67·1 (9·8) |
| Age <60 years | 162 (30%) | 1552 (23%) |
| Sex | ||
| Men | 336 (63%) | 4096 (61%) |
| Women | 198 (36%) | 2579 (39%) |
| Race | ||
| White only | 367 (69%) | 4847 (73%) |
| Black only | 4 (1%) | 107 (2%) |
| East Asian only | 102 (19%) | 1311 (20%) |
| Others (includes not reported or multiracial) | 61 (11%) | 410 (6%) |
| BMI, kg/m2 | 26·9 (5·0) | 27·3 (5·0) |
| Weight, kg | 77·0 (16·4) | 76·1 (16·5) |
| Mean estimated glomerular filtration rate, mL/min per 1·73 m2 | 78·4 (19·3) | 78·6 (20·6) |
| Medical history | ||
| Hypertension | 359 (67%) | 5222 (78%) |
| Diabetes | 96 (18%) | 1709 (26%) |
| Current tobacco use | 105 (20%) | 1377 (21%) |
| Coronary artery disease | 24 (4%) | 447 (7%) |
| Heart failure | 8 (1%) | 230 (3%) |
| Cancer | 33 (6%) | 586 (9%) |
| Previous stroke or TIA | 93 (17%) | 1168 (17%) |
| Global region | ||
| USA and Canada | 96 (18%) | 820 (12%) |
| Latin America | 30 (6%) | 716 (11%) |
| Western Europe | 270 (51%) | 2810 (42%) |
| Eastern Europe | 37 (7%) | 1081 (16%) |
| East Asia | 101 (19%) | 1248 (19%) |
| Qualifying stroke | ||
| Clinical TIA with imaging-confirmed infarction as qualifying event | 70 (13%) | 450 (7%) |
| Arterial territory of qualifying stroke | ||
| Anterior circulation | 377 (71%) | 4808 (72%) |
| Posterior circulation | 176 (33%) | 2091 (31%) |
| Location of qualifying stroke | ||
| Single location | ||
| Cerebral hemisphere with cortical involvement | 318 (60%) | 3715 (56%) |
| Cerebral hemisphere, subcortical only | 78 (15%) | 1440 (22%) |
| Brainstem only | 22 (4%) | 309 (5%) |
| Cerebellum only | 49 (9%) | 512 (8%) |
| Multiple locations | 67 (13%) | 694 (10%) |
| Chronic infarct on imaging (in addition to index stroke) | 137 (26%) | 2212 (33%) |
| Aspirin use before qualifying stroke | 79 (15%) | 1174 (18%) |
| Statin use before randomisation | 324 (61%) | 4107 (62%) |
| Treated with intravenous tPA for qualifying stroke | 121 (23%) | 1135 (17%) |
| Treated with endovascular intervention for qualifying stroke | 29 (5%) | 271 (4%) |
| NIHSS score at randomisation | 0·0 (0·0–1·0) | 1·0 (0·0−2·0) |
| NIHSS score ≤5 | 524 (98%) | 6398 (96%) |
| Modified Rankin Scale (mRS) score at randomisation | ||
| mRS 0 or 1 | 390 (73%) | 4278 (64%) |
| mRS 2 | 108 (20%) | 1563 (23%) |
| mRS ≥3 | 36 (7%) | 833 (12%) |
| MoCA score at randomisation | 26·0 (23·0–28·0) | 24·0 (21·0–27·0) |
| Time from qualifying stroke to randomisation, days | 39·5 (15·0–98·0) | 36·0 (14·0–87·0) |
| Extracranial vascular imaging completed | ||
| CT angiography | 228 (43%) | 2511 (38%) |
| Magnetic resonance angiography | 246 (46%) | 2132 (32%) |
| Carotid ultrasound | 302 (57%) | 4248 (64%) |
| Conventional angiography | 9 (2%) | 112 (2%) |
| Intracranial vascular imaging completed | 483 (90%) | 5158 (77%) |
| Duration of cardiac rhythm monitoring ≥48 h | 254 (48%) | 2179 (33%) |
Data are n (%), mean (SD), or median (IQR). PFO=patent foramen ovale. BMI=body-mass index. TIA=transient ischaemic stroke. tPA=tissue plasminogen activator. NIHSS=National Institutes of Health Stroke Scale/Score. MoCA=Montreal Cognitive Assessment.
Recurrent ischaemic stroke occurred at a rate of 3·7 events per 100 person-years among patients with PFO on TTE or TOE, or both, compared with 4·8 events per 100 person-years in those without evidence of PFO (unadjusted hazard ratio [HR] 0·80, 95% CI 0·51–1·26; p=0·33; adjusted HR 0·84 [after adjustment for age, hypertension, diabetes, coronary disease, and heart failure], 95% CI 0·53–1·32; p=0·44). In the PFO group, 14 (70%) of 20 recurrent ischemic strokes were classified as recurrent ESUS and involved the cerebral or cerebellar cortex, or both (table 2). Around 4 (20%) of 20 recurrent ischaemic strokes were potentially disabling with a modified Rankin Scale score greater than 2 at 7 days or at discharge.
Table 2:
Features of recurrent ischaemic stroke in patients assessed with transthoracic or transoesophageal echocardiography, or both
| PFO detected | PFO not detected | p value | |
|---|---|---|---|
| Patients with recurrent stroke | 20 | 295 | 0·46 |
| Topography | |||
| Deep only* | 6 (30%) | 100 (34%) | 0·44 |
| All others† | 14 (70%) | 158 (54%) | .. |
| Subtype | |||
| ESUS | 14 (70%) | 144 (49%) | 0·07 |
| Non-ESUS | 6 (30%) | 151 (51%) | .. |
| Outcome at 7 days or discharge | |||
| mRS ≤2 | 16 (80%) | 182 (65%) | 0·16 |
| mRS >2 | 4 (20%)‡ | 100 (35%) | .. |
Data are n or n (%), unless otherwise stated. PFO=patent foramen ovale. ESUS=embolic stroke of undetermined source. mRS=modified Rankin Scale score.
Subcortical only or brainstem only.
Any cortical, any cerebellum, multiple, and so on.
Of the four patients with mRS >2, three occurred on rivaroxaban and one on aspirin.
Overall, there was no difference in the risk of recurrent ischaemic stroke with rivaroxaban versus aspirin (HR 1·02; 95% CI 0·82–1·27; p=0·52). Because of early termination of the trial, the anticipated statistical power required for our analyses was not achieved and a post-hoc calculation based on the observed effects indicated only 45% power. With this caveat, some effect modification was apparent in relation to PFO (figure 1; table 3). Among patients with PFO detected by either TTE or TOE, there was insufficient evidence to support a difference in the risk of recurrent ischaemic stroke with rivaroxaban compared with aspirin (HR 0·54; 95% CI 0·22–1·36; table 3). There was no difference between rivaroxaban and aspirin for those without known PFO (HR 1·06 [95% CI 0·84–1·33]; pinteraction=0·18; table 3). We observed consistent effect sizes of rivaroxaban versus aspirin for the outcome of recurrent ESUS (appendix). We also did an on-treatment sensitivity analysis and found no difference in the results (appendix).
Figure 1: Kaplan-Meier curve for time to recurrent ischaemic stroke by treatment assignment and PFO status.
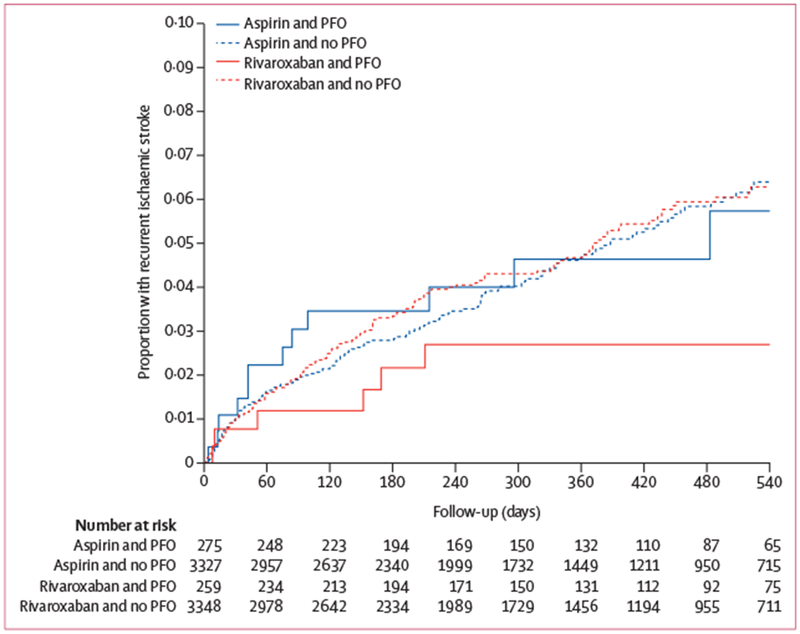
PFO=patent foramen ovale.
Table 3:
Recurrent ischaemic strokes assessed with transthoracic or transoesophageal echocardiography, or both
| Rivaroxaban group (n=3609) |
Aspirin group (n=3604) |
Hazard ratio (95% CI)* | pinteraction* | |||
|---|---|---|---|---|---|---|
| Patients | Events (event rate†) | Patients | Events (event rate†) | |||
| Overall‡ | 3607 | 159 (4·7) | 3602 | 156 (4·7) | 1·02 (0·82–1·27) | 0·86 |
| Presence of PFO (detected by TTE or TOE)‡ | ||||||
| Present | 259 | 7 (2·6) | 275 | 13 (4·8) | 0·54 (0·22–1·36) | .. |
| Absent | 3348 | 152 (4·9) | 3327 | 143 (4·6) | 1·06 (0·84–1·33) | 0·18 |
| Size of PFO§ | ||||||
| Large | 23 | 0 (0·0) | 25 | 2 (9·4) | NA | .. |
| Small | 112 | 6 (4·5) | 112 | 8 (6·6) | 0·68 (0·24–1·97) | NA |
| Arterial septal aneurysm reported§ | ||||||
| Yes | 31 | 0 (0·0) | 40 | 3 (6·7) | NA | .. |
| No | 151 | 7 (4·4) | 157 | 9 (6·0) | 0·75 (0·28–2·02) | NA |
| RoPE score¶ | ||||||
| 0–4 | 118 | 5 (4·1) | 135 | 4 (2·9) | 1·32 (0·35–4·94) | .. |
| 5–10 | 141 | 2 (1·4) | 140 | 9 (6·8) | 0·21 (0·05–0·98) | 0·07 |
| Age (years) | ||||||
| <60 | 77 | 4 (5·1) | 85 | 3 (3·8) | 1·42 (0·32–6·34) | .. |
| 60 to <70 | 103 | 2 (1·9) | 108 | 7 (6·9) | 0·29 (0·06–1·39) | .. |
| ≥70 | 79 | 1 (1·2) | 82 | 3 (3·5) | 0·34 (0·03–3·25) | 0·30 |
PFO=patent foramen ovale. TTE=transthoracic echocardiography. TOE=transoesophageal echocardiography. RoPE=risk of paradoxical embolism.
Hazard ratio (95% CI) and pinteraction not reported if hazard ratio was ≥10 or could not be computed.
Event rates reported per 100 person-years.
Among participants who reported information (presence or absence) about PFO by TTE or TOE. Four patients did not report this information and were thus excluded.
Information available only when PFO was identified with TOE.
RoPE score calculated only if PFO was present.
Given the modest number of recurrent events, we were unable to adequately assess the role of potential prognostic factors for stroke related to PFO, such as size, atrial septal aneurysm, and risk of paradoxical embolism (RoPE) score (table 3). However, an apparent divergent treatment effect of age was observed among patients with PFO, with a benefit of rivaroxaban suggested mainly among those older than 60 years.
When these analyses were repeated with TTE alone or TOE alone, or for the outcome of recurrent ESUS, the results were consistent (appendix).
Atrial fibrillation was detected during follow-up at a rate of 2·4 events per 100 person-years among patients with PFO detected by either TTE or TOE, compared with 3·7 per 100 person-years in those without PFO (HR 0·65; 95% CI 0·37-1·13, appendix), with similar rates of atrial fibrillation detection in all three cohorts. The risks of major bleeding with rivaroxaban compared with aspirin were similar in patients with PFO detected (HR 2·05; 95% CI 0·51-8·18) and in those without PFO detected (HR 2·82; 95% CI 1·69-4·70; pinteraction=0·68; appendix).
Systematic review of the literature identified 62 published studies. Only two previous trials enrolled patients with cryptogenic stroke who had PFO confirmed by TOE, did a randomised comparison of anticoagulation versus antiplatelet therapy and reported the outcome of ischaemic stroke. The PFO in Cryptogenic Stroke Study (PICSS) comprised a cohort of 98 patients with cryptogenic stroke who were randomly assigned to receive warfarin or aspirin.19 The Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) trial comprised a cohort of 361 patients who were randomly allocated to anticoagulation or antiplatelet therapy, with the choice of medication within each category left to the treating physician (336 [93%] of 361 patients on anticoagulation were given vitamin K antagonists).5 Results of these two studies, along with those of 379 patients with PFO in the TOE cohort from NAVIGATE ESUS, yielded highly concordant results and were combined in a random-effects meta-analysis. The summary odds ratio was 0·48 (95% CI 0·24-0·96; p=0·04) in favour of anticoagulation among patients with PFO, without evidence of heterogeneity (I2=0%; figure 2; appendix).
Figure 2: Forest plot of randomised comparisons of anticoagulation or antiplatelet therapy for patients with patent foramen ovale.
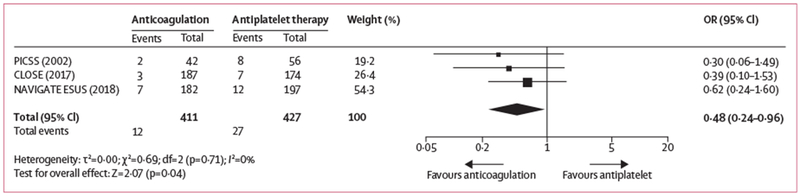
OR=odds ratio.
Discussion
Patients with ESUS and PFO who were enrolled in the NAVIGATE ESUS trial were younger and had fewer vascular risk factors than did those without an identified PFO, suggesting that these patients are a specific subset of the larger ESUS population that might be pathophysiologically distinct.14 Nevertheless, these patients had a high risk of recurrent stroke, similar to the overall ESUS population and greater than that in younger patients (<60 years) enrolled in the PFO closure trials. The rate of recurrent stroke was not significantly lower in patients with PFO receiving rivaroxaban than in those receiving aspirin, nor was there a significant interaction in treatment effect according to PFO status. Because the NAVIGATE ESUS trial was terminated early at the recommendation of the data monitoring committee, the power of this study was limited. Combined with data from previous randomised trials, although each also had limited power, our meta-analysis estimates that anticoagulation might reduce recurrent stroke in patients with PFO and ESUS by about half, although substantial imprecision remains. This result was also similar to that of meta-analyses based on non-randomised comparisons.10
Age might be a pertinent factor in the role of anticoagulants for PFO.13,20 We did not find significant treatment interaction by age, again possibly owing to limited power, but point estimates suggested a benefit in the older group. Although a possible association has been reported between the risk of atrial fibrillation and PFO,21 we did not find any such association in this cohort, suggesting that this is not the mechanism by which patients with PFO might benefit from anticoagulation. Older patients might be exposed to a higher risk of venous thromboembolism because of reduced physical activity and comorbidities, and therefore might be more likely to benefit from an anticoagulation strategy.22 The efficacy and safety of PFO closure has been shown in younger patients, and might not necessarily apply to this older group.23 A recent meta-analysis of randomised trials of percutaneous closure of PFO indicated that percutaneous closure was superior to aspirin therapy, but not superior to anticoagulation.9 Furthermore, some patients with paradoxical embolism might be at risk of future venous thromboembolism or pulmonary embolism, which would not be prevented with closure.
In the NAVIGATE ESUS trial, PFO was underdetected, particularly when TTE was used alone, because the use of a bubble study was not mandated by the protocol or recorded. Among 1382 patients who underwent TOE, PFO was identified in 370 (27%), a prevalence slightly higher than that observed in the general population24,25 and similar to that of older populations with cryptogenic stroke.12,20,26 There were notable regional differences in PFO detection by echocardiography, suggesting variations in practice in the assessment of cryptogenic stroke. These differences could be related to the availability of resources for diagnostic testing or variability in opinion about the importance of detecting PFO in this population, especially before publication of the results of the recent closure trials.
The major strength of our study is the randomised comparison of anticoagulation versus antiplatelet therapy in a prespecified subgroup of interest. Results of subgroup analyses of negative trials, even those that are prespecified, must be interpreted with caution.27,28 The NAVIGATE ESUS trial required echocardiography for all patients, but did not require a standardised approach to the diagnosis of PFO, and therefore we are likely to have underestimated the prevalence of PFO. We might also have been more likely to detect larger PFOs. Some sites might have used transcranial Doppler to detect PFO, but this information was not collected. This type of misclassification is likely to bias our results toward the null, although the effect size is similar to that of previous research in which PFO was specifically investigated.5,19 The early termination of the trial substantially truncated our planned period of follow-up and yielded a lower number of events than anticipated, reducing power to only 45%. Statistical tests for interactions typically offer limited power as well. Moreover, our meta-analysis included only three trials done over a 20-year span with relatively few events, and changes in diagnosis and treatment during this period are likely to have occurred, which might limit the validity of data pooling, although the absence of heterogeneity is reassuring.
We conclude that patients meeting criteria for ESUS and who have PFO represent an identifiable group of patients for whom further trials of anticoagulation versus antiplatelet therapy or PFO closure, or both, are warranted.
Supplementary Material
Research in context.
Evidence before this study
We searched MEDLINE up to May 17, 2018, for randomised controlled trials comparing anticoagulant therapy and antiplatelet therapy for secondary stroke prevention in patients with cryptogenic ischaemic stroke and patent foramen ovale (PFO). Several studies showed that PFO closure was superior to medical therapy for the prevention of stroke in patients aged younger than 60 years, but only two included direct randomised comparisons of anticoagulation versus antiplatelet therapy.
Added value of this study
NAVIGATE ESUS was a large, randomised phase 3 clinical trial that compared anticoagulation (with rivaroxaban) and antiplatelet therapy (with aspirin) in patients with embolic stroke of undetermined source (ESUS). The trial was terminated early because of absence of efficacy in the overall study population. The prespecified subgroup analysis investigated the treatment effect in patients with PFO. Although rivaroxaban lowered the risk of recurrent stroke compared with aspirin, the result in this study alone was not significant. When combined with previous randomised trial data, the strategy of anticoagulation reduced the risk of recurrent stroke by about half, although this estimate is based on substantial imprecision.
Implications of all the available evidence
The efficacy of anticoagulation for stroke prevention in patients with cryptogenic stroke and PFO has not been established, but existing data suggest that this strategy should be further investigated in dedicated randomised trials. Anticoagulation might be a preferred option for older patients who were not studied in previous trials or for patients who are averse to device implantation.
Acknowledgments
Declaration of interests
SFA and SB report grants from Bayer and Janssen during the conduct of the study. SDB was employed by Bayer during the conduct of the study. SJC reports grants from Bayer and Janssen during the conduct of the study; grants and personal fees from Boehringer Ingelheim, Sanofi Aventis, and Bayer; personal fees from Portola; grants from Boston Scientific, outside of the submitted work; and an institutional research grant from Bayer. ME reports grants and non-financial support from Bayer during the conduct of the study; grants and fees paid to his institution for lectures and advisory board participation from Bayer; and fees paid to his institution for lectures and advisory board participation from Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Amgen, Sanofi, Covidien, GlaxoSmithKline, Ever, and Novartis, outside of the submitted work. RGH a research contract from Bayer, during the conduct of the study, and a research contract from Bayer, outside of the submitted work. SEK reports grants from Bayer, grants and personal fees from Janssen, during the conduct of the study; personal fees from Bristol-Myers Squibb, personal fees from Boehringer Ingelheim, personal fees from Medtronic, personal fees from AbbVie, and grants from WL Gore, outside of the submitted work. PL reports grants and personal fees from Bayer and grants from PHRI, during the conduct of the study; grants and personal fees from The George Institute for Global Health; grants from FONIS CONICYT, non-financial support from Boehringer Ingelheim; non-financial support from Bayer, and grants and non-financial support from Clinica Alemana, outside of the submitted work. AL reports grants from Bayer and Janssen during the conduct of the study; and personal fees from Bayer, Bristol-Myers Squibb/Pfizer, AstraZeneca, Boehringer Ingelheim, and Reneuron, outside of the submitted work. HL reports grants from Bayer and Janssen during the conduct of the study. SRM reports grants from Bayer and Janssen during the conduct of the study; and grants from WL Gore & Associates outside of the submitted work. HM was employed by Bayer during the conduct of the study. KM reports grants from Bayer and Janssen, and personal fees from Bayer during the conduct of the study; and personal fees from Daiichi-Sankyo and non-financial support from Boehringer Ingelheim outside of the submitted work. KN reports personal fees from advisory boards for Bayer (Schweiz) AG, outside of the submitted work. VO reports grants from Bayer and Janssen during the conduct of the study. KP reports grants and personal fees from Bayer and grants from Janssen during the conduct of the study. GS reports grants from Bayer and Janssen during the conduct of the study. MS eports grants and personal fees from Bayer and Janssen during the conduct of the study; personal fees from Bristol-Myers Squibb, Boehringer Ingelheim, Portola, and Daiichi Sankyo outside of the submitted work. AS reports grants from Bayer, Janssen, and Bayer Canada, and personal fees from Bayer Canada, during the conduct of the study. JDS reports grants from Bayer and Janssen during the conduct of the study. RV reports grants from Bayer and Janssen during the conduct of the study, and grants and personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo, outside of the submitted work. BS declares no competing interests.
Footnotes
References
- 1.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366: 991–99. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med 2017; 377: 1022–32. [DOI] [PubMed] [Google Scholar]
- 3.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368: 1083–91. [DOI] [PubMed] [Google Scholar]
- 4.Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017; 377: 1033–42. [DOI] [PubMed] [Google Scholar]
- 5.Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 2017; 377: 1011–21. [DOI] [PubMed] [Google Scholar]
- 6.Lee PH, Song J-K, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO Trial. J Am Coll Cardiol 2018; 71: 2335–42. [DOI] [PubMed] [Google Scholar]
- 7.Riaz H, Khan MS, Schenone AL, Waheed AA, Khan AR, Krasuski RA. Transcatheter closure of patent foramen ovale following cryptogenic stroke: an updated meta-analysis of randomized controlled trials. Am Heart J 2018; 199: 44–50. [DOI] [PubMed] [Google Scholar]
- 8.Smer A, Salih M, Mahfood Haddad T, et al. Meta-analysis of randomized controlled trials on patent foramen ovale closure versus medical therapy for secondary prevention of cryptogenic stroke. Am J Cardiol 2018; 121: 1393–99. [DOI] [PubMed] [Google Scholar]
- 9.Kent DM, Dahabreh IJ, Ruthazer R, et al. Anticoagulant vs. antiplatelet therapy in patients with cryptogenic stroke and patent foramen ovale: an individual participant data meta-analysis. Eur Heart J 2015; 36: 2381–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saber H, Palla M, Kazemlou S, Azarpazhooh MR, Seraji-Bozorgzad N, Behrouz R. Network meta-analysis of patent foramen ovale management strategies in cryptogenic stroke. Neurology 2018; 91: e1–7. [DOI] [PubMed] [Google Scholar]
- 11.Jain A, Cifu AS. Antithrombotic therapy for venous thromboembolic disease. JAMA 2017; 317: 2008–09. [DOI] [PubMed] [Google Scholar]
- 12.Mazzucco S, Li L, Binney L, Rothwell PM. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol 2018; 17: 609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homma S, DiTullio MR, Sacco RL, Sciacca RR, Mohr JP. Age as a determinant of adverse events in medically treated cryptogenic stroke patients with patent foramen ovale. Stroke 2004; 35: 2145–49. [DOI] [PubMed] [Google Scholar]
- 14.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013; 81: 619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018; 378: 2191–201. [DOI] [PubMed] [Google Scholar]
- 16.Kasner SE, Lavados P, Sharma M, et al. Characterization of patients with embolic strokes of undetermined source in the NAVIGATE ESUS randomized trial. J Stroke Cerebrovasc Dis 2018; 27: 1673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014; 13: 429–38. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–94. [DOI] [PubMed] [Google Scholar]
- 19.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105: 2625–31. [DOI] [PubMed] [Google Scholar]
- 20.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007; 357: 2262–68. [DOI] [PubMed] [Google Scholar]
- 21.Berthet K, Lavergne T, Cohen A, et al. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke 2000; 31: 398–403. [DOI] [PubMed] [Google Scholar]
- 22.Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107: I9–16. [DOI] [PubMed] [Google Scholar]
- 23.Kiblawi FM, Sommer RJ, Levchuck SG. Transcatheter closure of patent foramen ovale in older adults. Catheter Cardiovasc Interv 2006; 68: 136–42. [DOI] [PubMed] [Google Scholar]
- 24.Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007; 49: 797–802. [DOI] [PubMed] [Google Scholar]
- 25.Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke prevention: assessment of risk in a community. Mayo Clin Proc 1999; 74: 862–69. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez CJ, Homma S, Sacco RL, et al. Race-ethnic differences in patent foramen ovale, atrial septal aneurysm, and right atrial anatomy among ischemic stroke patients. Stroke 2003; 34: 2097–102. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med 2007; 357: 2189–94. [DOI] [PubMed] [Google Scholar]
- 28.Burke JF, Sussman JB, Kent DM, Hayward RA. Three simple rules to ensure reasonably credible subgroup analyses. BMJ 2015; 351: h5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


