Abstract
Acylation of lysine residues is a common post-translational modification of cellular proteins. Here, we show that lysine succinylation, a type of acylation, occurs in human lens proteins. All of the major crystallins exhibited Nε-succinyllysine (SuccK) residues. Quantification of SuccK in human lens proteins (from donors between the ages of 20 and 73 years) by LC–MS/MS showed a range between 1.2 and 14.3 pmol/mg lens protein. The total SuccK levels were slightly reduced in aged lenses (age > 60 years) relative to young lenses (age < 30 years). Immunohistochemical analyses revealed that SuccK was present in epithelium and fiber cells. Western blotting and immunoprecipitation experiments revealed that SuccK is particularly prominent in αB-crystallin, and succinylation in vitro revealed that αB-crystallin is more prone to succinylation than αA-crystallin. Mass spectrometric analyses showed succinylation at K72, K90, K92, K166, K175, and potentially K174 in human lens aB-crystallin. We detected succinylation at K72, K82, K90, K92, K103, K121, K150, K166, K175, and potentially K174 by mass spectrometry in mildly succinylated αB-crystallin. Mild succinylation improved the chaperone activity of αB-crystallin along with minor perturbation in tertiary and quaternary structure of the protein. These observations imply that succinylation is beneficial to αB-crystallin by improving its chaperone activity with only mild conformational alterations.
Graphical abstract
The ocular lens is a unique avascular organ, the major function of which is to focus images onto the retina. It has elongated fiber cells that accumulate high concentrations of crystallin proteins during their differentiation from epithelial cells. Crystallins comprising α-, β-, and γ-crystallin constitute 90% of the total lens protein.1 Whereas β-and γ-crystallin are primarily structural proteins in the lens, α-crystallin is a functional protein, and it accounts for approximately 40% of the total proteins in human lenses.2 α-Crystallin is an oligomeric protein comprising two subunits, αA-crystallin and αB-crystallin, that associate in a 3:1 molar ratio in the human lens.3 Like all small heat shock proteins (sHSPs), both αA-crystallin and αB-crystallin possess a conserved “α-crystallin domain” and are major β-sheet proteins.4 Through their chaperone activity, they prevent aggregation of other lens proteins. In addition, several studies of human hereditary cataract and genetically altered animals have revealed that a loss in the chaperone activity of α-crystallin results in cataract formation.5–7 Based on these observations, the chaperone activity of αA-crystallin and αB-crystallin is postulated to be critical for the maintenance of lens transparency.
Several studies have shown that post-translational modifications (PTMs) of α-crystallin alter its structural and functional properties. Oxidation of α-crystallin causes conformational alterations and decreases chaperone activity,1 and deamidation perturbs its tertiary conformations, which leads to unfolding and aggregation.8 Phosphorylation of α-crystallin alters its surface charge, quaternary structure, and stability and reduces its solubility and chaperone activity.9,10 However, some studies suggest that phosphorylation improves the chaperone activity of αB-crystallin.12,13 In addition, modification of αA-crystallin with methylglyoxal has been shown to improve the chaperone activity.11
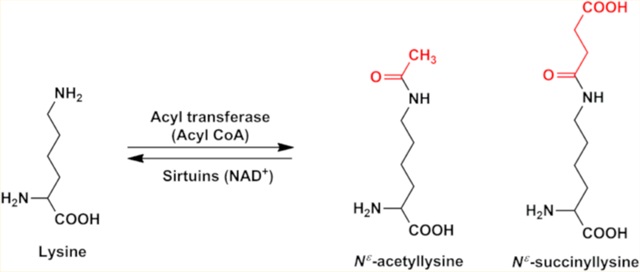
Another related PTM is acylation,12 which occurs on lysine residues in proteins. The acyl modifications of lysine residues include acetylation,13,14 propionylation,15 butyrylation,16 malo-nylation,17 crotonylation,18 and succinylation.19,20 Among all of these acyl modifications, succinylation and acetylation are found to occur predominantly in eukaryotes.21,22 Previously, our group demonstrated acetylation (Figure 1) in human lens αA-, αB-, and γ-crystallins.23–25
Figure 1.
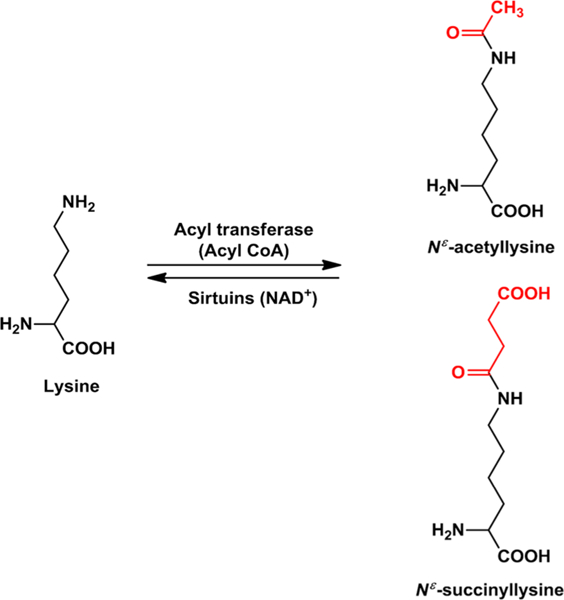
Acetylation and succinylation of proteins. The epsilon amino group of lysine residues in proteins is acylated by acetyl CoA or succinyl CoA via enzymatic (by acyl transferase) or nonenzymatic reaction. Acylation of lysine can be reversed by the NAD+-dependent sirtuins.
Our studies have revealed that acetylation of the N-terminal glycine1 and lysine2 (K2) residues in γD-crystallin promotes its aggregation by destabilizing its structure.23 We also showed that K70 and K99 in αA-crystallin and K92 and K166 in αB-crystallin are acetylated in human lenses.24,25 Furthermore, we showed that the introduction of an acetylation mimic at K92 in αB-crystallin improved its chaperone activity.24 In addition, we previously demonstrated that acetyl derivatives of the mini-chaperone peptides of αA-crystallin and αB-crystallins inhibited sodium selenite-induced lens epithelial cell apoptosis and cataract development to a slightly greater extent than the native peptides.26 These observations suggest that acetylation of α-crystallin is beneficial to the lens.
Succinylation is another acyl modification that overlaps extensively with acetylation in eukaryotes.21 It involves conversion of the positive charge on the epsilon amino group of a lysine residue to a negatively charged carboxylic acid (Figure 1). Extensive succinylation of proteins in the mitochondria and cytoplasm of cells has been reported.21 Whether lens proteins are succinylated and whether succinylation affects functions of α-crystallin have not been studied yet. We report here that succinylation occurs in all of the major lens crystallins and that αB-crystallin is more prone to succinylation than αA-crystallin. We also show that succinylation improves the chaperone activity of αB-crystallin.
EXPERIMENTAL PROCEDURES
Materials.
Insulin, citrate synthase, protease inhibitor cocktail, dithiothreitol, trinitrobenzenesulfonic acid (TNBS), 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid (bis-ANS), dipotassium salt, and succinyl coenzyme A (SuccCoA) were obtained from Sigma-Aldrich (St. Louis, MO). Nε-Acetyllysine was obtained from Chem-Impex International Inc. (Wood Dale, IL). All other chemicals were of analytical grade.
Cloning, expression, and purification of human αA and αB were performed as previously described.24,25
Western Blot Detection of SuccK in Lens Proteins.
Human lenses were obtained from Saving Sight, Kansas City, MO, and stored at –80 °C until use. Water-soluble protein (WS) and water-insoluble protein (WIS) subjected to sonication and centrifugation were prepared as previously described.25 For Western blotting, protein (30 μg) was separated on 12% SDS-PAGE, electrophoretically transferred to a nitrocellulose membrane, and probed with a SuccK antibody (diluted 1:2000, cat. no. PTM-419, PTM Biolabs, Chicago, IL). The membrane was stripped and reprobed for αB-crystallin (diluted 1:500 000, Developmental Studies Hybridoma Bank; we obtained a hybridoma culture from this vendor and purified the antibody ourselves). The secondary antibody was horseradish peroxidase (HRP)-conjugated antidonkey IgG (diluted 1:5000, Cell Signaling Technology, Danvers, MA, cat. no. 7076S). To show protein loading in the Western blots, membranes were stained with Ponceau S stain.
Immunoprecipitation to Deplete αB-Crystallin in Human Lens WS.
We immunoprecipitated αB-crystallin from 100 μg of WS protein (human 9-year-old lens) using 100 μg of the αB-crystallin monoclonal antibody and treated with Protein A-Agarose (40 μL, Santa Cruz Biotechnology), centrifuged at 1000g for 5 min and used the supernatant in Western blotting for αB-crystallin or SuccK. The sample was processed similarly but without the antibody served as a control.
Immunohistochemistry.
Paraffin-embedded human eye sections after deparafinization rehydration and high-temperature antigen retrieval were blocked with 5% normal goat serum in PBS for 1 h. Sections were incubated overnight at 4 °C with a monoclonal antibody for SuccK (diluted 1:100) followed by 1 h of incubation at 37 °C with Alexa Fluor 488 conjugated goat antimouse IgG (1:250 dilution, cat no. A11001, Life Technologies, Carlsbad, CA). Sections were mounted with DAPI/Vectashield for observations of the nuclei. Images were taken using a fluorescence microscope (Nikon Eclipse 80i, Nikon) at 20× magnification and Nikon NIS software. The background fluorescence from the secondary antibody was verified by processing sections as above but eliminating incubation with the primary antibody.
Determination of Reactive Amino Groups in α-Crystallin.
The TNBS assay was adopted as previously described.27 Briefly, αA or αB (0.2 mg/mL) was incubated with TNBS (0.02%) in 100 mM NaHCO3 buffer, pH 8, at 40 °C for 3 h. The absorbance was measured at 335 nm against a reagent blank.
Succinylation of αA, αB, and WS in Vitro.
SuccCoA was prepared at a 10 mM concentration in PBS and added to 300 μg of αB (in 50 mM phosphate buffer, pH 7.4) such that the final molar ratios of lysine residues in αB to SuccCoA reached 1:0.01, 1:0.007, 1:0.003, and 1:0.001. The pH of each mixture was adjusted to between 7.5 and 8 by adding 0.5 M NaOH, and the mixture was incubated for 3 h at 4 °C. Control samples were processed similarly except for the absence of SuccCoA. The samples were then dialyzed overnight against 50 mM phosphate buffer, pH 7.4. Succinylated αB prepared at the 1:0.001 molar ratio is referred to as Succ-αB hereafter.
To determine the relative succinylation capacity of αA and αB (each 1 mg/mL), the two proteins were succinylated under identical conditions using 0.01 mM SuccCoA and a pH between 7.5 and 8, as above. The samples were then analyzed by electrophoresis and Western blots, and the SuccK was detected using the monoclonal antibody described above diluted 1:2,000. Water-soluble 25-, 30-, and 31-year-old lenses were succinylated using 0.01 mM SuccCoA under the conditions as above and analyzed in Western blots for differential succinylation of αA-crystallin and αB-crystallin.
Q-TOF Tandem MS/MS Mass Spectrometric Identification of SuccK Residues.
Succ-αB (47 μg) and human lens proteins (5 mg) were digested using either a modified filter-aided sample preparation method,28 or the urea digest protocol provided by cell signaling technologies. Succ-αB samples were denatured, reduced, and alkylated using 4% SDS in 100 mM Tris-HCl buffer at pH 8.5, tris(2-carboxyethyl)phosphine (TCEP), and iodoacetamide, respectively. SDS was removed using an Amicon Ultra 30 kDa molecular weight cutoff spin filter by sequentially washing with 100 mM Tris-HCl, pH 8.5, containing 8 M urea followed by 2 M urea and finally with 5% trifluoroethanol (TFE) in 50 mM ammonium bicarbonate (ABC). The protein was then digested at room temperature for 4 h with a ratio of 1:50Lys-C (Wako chemicals, Osaka, Japan) to substrate and then overnight at 37 °C with a ratio of 1:40 trypsin to substrate. The resulting peptide solutions were evaporated to dryness in a Speed Vac concentrator at 45 °C. Human lens samples were digested with trypsin and C18 cleaned according to the manufacturer’s protocol provided with the SuccK Motif Kit no. 13764 (Cell Signaling Technology). Briefly, human lens protein was solubilized and denatured using a urea lysis buffer with protease inhibitors before being reduced and alkylated with dithiothreitol (DTT) and iodoacetamide. Samples were diluted to a final urea concentration of 2 M in 20 mM HEPES buffer before digesting overnight at room temperature with 1:40 trypsin to substrate on a platform rocker set to 30 rpm. The resulting peptide solution was desalted using a 0.7 mL C18 Sep-Pak column (Waters Corporation, Milford, MA), and the eluted peptides were lyophilized. An immunoprecipitation for peptides bearing a SuccK modification was performed on both digested Succ-αB and human lens samples using the SuccK Motif Kit no. 13764 according to the manufacturer’s protocol. Briefly, the beads were washed with PBS four times before incubation with the sample for 2 h at 4 °C on a shaker. After incubation, the supernatant was removed, and the beads were washed twice with ice-cold 1× IAP buffer and then three times with ice-cold water (Honeywell Inc. Burdick and Jackson, Morris Plains, NJ). The peptides were eluted by two washes with 0.15% TFA, pooled, and purified with a Pierce C18 spin column (Thermo Fisher Scientific Inc., Waltham, MA) before being evaporated to dryness in a Speed Vac concentrator at 25 °C. The peptides were resuspended in 3% acetonitrile in 0.1% formic acid for MS analysis. The enriched SuccK-bearing peptides were loaded onto a nano ProntoSIL 5u 200A C18AQ trapping column, and chromatography was performed on a 0.1 mm × 150 mm 3u 200A ProntoSIL C18AQ reverse phase nano column (nano LCMS Solutions LLC, Gold River, CA) using a nano-Advance UPLC (Bruker Daltonics, Inc., Billerica, MA). The mobile phase consisted of water + 0.1% formic acid (A) and acetonitrile +0.1% formic acid (B). Peptides of Succ-αB were separated at a flow rate of 800 nL/min using a gradient of 2–50% B over 30 min. Peptides from human lens proteins were separated at a flow rate of 800 nL/min using a gradient of 5–50% B for 60 min followed by a column wash at 90% B for 5 min. Data were collected on an Impact HD Q-TOF equipped with a captive spray source (Bruker Daltonics Inc., Billerica, MA) operated using intensity-dependent CID MS/MS. Proteinscape software (Bruker Daltonics Inc.) was used to submit the data to Mascot v.2.4 for database searching. The parent ion mass tolerance was 10 ppm. Peptides were searched against the Homo sapiens database on SwissProt allowing up to 4 missed tryptic cleavages with variable carbamidomethyl (C), deamidated (NQ), oxidation (M), and succinyl (K) modifications. Peptides with a minimum ion score of 20 were accepted.29,30
Synthesis of N2-(terf-Butoxycarbonyl)-N6-(3-carboxypropanoyl)lysine.
Isolation of Nε-Sucdnyl-Nα-boc-L-Lysine.
A total of 246 mg (1 mmol)of Nα-boc-L-lysine was dissolved in 5 mL of water. The pH was adjusted to 10 by adding 10 M sodium hydroxide solution. To this solution, 100 mg (1 mmol) of succinic anhydride dissolved in 1 mL of 1,4-dioxane was added in steps of 100 μL. Five min after every addition, the pH was adjusted to 10 before adding more succinic anhydride. The solution was diluted with 10 mL of water and lyophilized. The crude product was suspended in 2 mL of water/acetonitrile (1:1 v/v) and fractionated by flash chromatography (silica gel, ethyl acetate +1% formic acid (v/v)). Fractions were checked by TLC (silica gel, ethyl acetate + 1% formic acid (v/v), ninhydrin staining), and fractions showing a spot with Rf = 0.4 were pooled. Evaporation of the solvent yielded a white powder [100 mg (0.3 mmol), yield = 30%]. 1H NMR (500 MHz, D2O): δ 1.42 (m, 2H), 1.44 (s, 9H), 1.54 (m, 2H), 1.71 (m, 1H), 1.84 (m, 1H), 2.53 (t, J = 6.7 Hz, 2H), 2.67 (t, J = 6.8 Hz, 2h), 3.20 (t, J = 6.8 Hz, 2H), 4.08 (t, J = 7.1 Hz, 1H). 13C NMR (125 MHz, D2O): δ 24.8, 28.7, 29.7, 30.8, 40.4, 41.5, 44.0, 58.4, 83.9, 160.0, 177.2, 179.4, 179.5.
Isolation of SuccK.
A total of 80 mg (0.23 mmol) of Nε-Succinyl-Nα-boc-L-lysine was deprotected with 500 μL of 3 M hydrochloric acid for 30 min at room temperature. Afterward, the solution was neutralized and fractionated by flash chromatography (RP C18, 5% acetonitrile + 0.1% trifluoroacetic acid (v/v)). Fractions were checked by TLC (RP C18, 5% acetonitrile + 0.1% trifluoroacetic acid (v/v), ninhydrin staining), and fractions showing a spot with Rf = 0.9 were pooled and lyophilized. The SuccK was obtained as a white powder (55 mg (0.22 mmol), yield = 96%). 1H NMR (500 MHz, D2O): δ 1.44 (m, 2H), 1.56 (m, 2H), 1.91 (m, 1H), 1.97 (m, 1H), 2.52 (t, J = 7.3 Hz, 2H), 2.66 (t, J = 7.4 Hz, 2H), 3.20 (t, J = 7.0 Hz, 2H), 4.01 (t, J = 7.4 Hz, 1H). 13C NMR (125 MHz, D2O): δ 24.1, 31.9, 32.9, 40.2, 41.3, 42.6, 56.4, 175.2, 177.3, 179.5.
LC–MS/MS Quantification of AcK and SuccK.
Normal lenses from human donors were obtained from Saving Sight (Kansas City, MO) and stored at –80 °C until used. Decapsulated lenses were weighed and homogenized in 1 mL of argon-saturated PBS containing 1 mM EDTA. Three hundred microliters of the homogenate was dialyzed against 20 mM phosphate buffer, pH 7.4, and lyophilized. Enzymatic digestion of lens proteins was performed as described previously with some minor modifications.31 Five hundred micrograms of freeze-dried protein was dissolved in 150 μL of PBS, and one small crystal of thymol was added. To this solution, 0.1 units of Pronase E (two additions), 0.3 units ofleucine aminopeptidase, and 0.3 units of carboxypeptidase Y were added stepwise at 24 h intervals for 96 h. Finally, the sample was filtered through a 3 kDa molecular weight cutoff filter (VWR, Radnor, PA). To determine protein digestion efficiency, we compared the Nε-carboxymethyllysine (CML) levels in the enzyme digests with those in acid hydrolysates of the same sample and considered CML levels in acid-hydrolyzed samples to represent 100% efficiency of hydrolysis, as described previously.31 Briefly, 500 μg of protein powder was hydrolyzed with 6 M HCl at 110 °C for 24 h. The samples were dried in a Speed Vac to remove the HCl and then dissolved in water.
Sample aliquots were diluted with water prior to UPLC-MS2 analysis. Chromatographic analyses were carried out on a Waters ACQUITY UPLC system (Milford, MA) connected to a Sciex 4500 QTrap mass spectrometer (Redwood City, CA). Chromatographic separations were carried out on an ACQUITY HSS T3 column (100 mm × 2.1 mm, 1.8 μm, Waters, Milford, MA) connected to a guard column using a flow rate of 0.6 mL/min. Water (solvent A) and 80% acetonitrile in water (solvent B, v/v) were used as eluents. To both solvents, 0.12% heptafluorobutyric acid (v/v) was added. Analyses were performed at a column temperature of 40 °C using a gradient elution: 2% B (0–2.2 min) to 8% B (3.3 min) to 34% B (7.6 min) to 100% B (7.8 to 9.5 min). The column was equilibrated at 2% B for 2.5 min prior to the next analysis. Detection of SuccK (tR = 4.4 min), AcK (tR = 3.9 min), and CML (tR = 2.3 min) was achieved by using multiple reaction monitoring. The ion source was run under the following conditions: temperature, 650 °C; ion spray voltage, 2.5 kV; curtain gas, 35 mL/min; nebulizer gas, 65 mL/min; heating gas, 70 mL/min. The declustering potential for SuccK was set to 30 V and for AcK and CML to 40 V. The MRM parameters were as follows: Q1 → Q3 [m/z], collision energy [eV], cell exit potential [V]. SuccK quantifier, 247.0 →84.0, 38, 11; qualifier 1, 247.0 → 184.0,19, 15; qualifier 2, 247.0 → 129.9, 22,10. AcK quantifier, 189.2 → 126.1,18, 12; qualifier 1, 189.2 → 84.2, 31, 5; qualifier 2, 189.2 → 143.1, 14, 10. CML quantifier, 205.1 → 130.2, 17, 11; qualifier 1, 205.1 → 84.1, 25, 13; qualifier 2, 205.1 → 56.1, 50, 10. Quantitation was performed based on the standard addition method.
To confirm the identity of SuccK in digested lens proteins, a full product ion scan of isolated SuccK was recorded. Therefore, free SuccK was isolated from digested lens proteins by UPLC. In detail, lens proteins were enzymatically digested according to the protocol described above. Aliquots of this digest were injected into the UPLC system using the aforementioned parameters. The effluent containing free SuccK (from 3.5 to 4 min) was collected. The effluents from multiple runs were combined, dried in a speed vac, and finally dissolved in a small volume of water. An aliquot of this solution was injected into the UPLC-MS2 system. Instead of MRM transitions, a full product ion scan of the parent ion m/z = 247 (SuccK, [M + H]+) was recorded using the ion source parameters mentioned above with the collision energy for the fragmentation of the parent ion set to 25 eV. A standard spectrum of SuccK was recorded under the same conditions using a 1 μM solution of the reference compound (preparation described above).
Chaperone Activity Assays.
The chaperone activity of αB and Succ-αB was determined using two client proteins, insulin and citrate synthase (CS), using a UV spectrophotometer (V630 Biospectrophotometer, JASCO, Easton, MD, total volume of assay = 500 μL) or a Spectramax 190 (Molecular Devices, San Jose, CA, total volume of assay = 200 μL). Percent protection by the chaperone was calculated by taking the OD at 60 min.
-
(i)
For the DTT-induced aggregation assay of insulin, insulin at 0.2 mg/mL was incubated in the presence or absence of αB or Succ-αB (0.1 mg/mL) at 25 °C in 50 mM phosphate buffer, pH 7.4. The aggregation of insulin was initiated by adding DTT (20 mM). Light scattering was measured at 400 nm for 1 h in a kinetic mode.
-
(ii)
For the thermal aggregation of CS, CS at 0.12 mg/mL in 40 mM HEPES buffer, pH 7.4, was incubated with or without αB or Succ-αB (0.12 mg/mL) at 43 °C for 1 h in a spectrophotometer. Light scattering was monitored at 400 nm in a kinetic mode for 1 h.
Separation of the αB-or Succ-αB-CS Complex by Gel Filtration.
CS (0.35 mg/mL) and αB (or Succ-αB) (1.0 mg/mL) were incubated in 40 mM HEPES buffer, pH 7.4, at 43 °C for 1 h, and the mixture was centrifuged at 10 000g for 10 min. The supernatant was collected and used for the separation of the αB-or Succ-αB-CS complex. αB-CS and Succ-αB-CS complexes were separated by FPLC (NGC Chromatography System, Bio-Rad, Hercules, CA), which was equipped with an Enrich SEC-650 analytical gel filtration column (10 mm × 300 mm, 24 mL). Protein (0.65 mg of protein in 500 μL) was injected onto the column after equilibration with PBS. The column was eluted in PBS at a flow rate of 0.7 mL/min. The absorbance of the column effluent was monitored at 280 nm. αB-Crystallin and CS were detected in the eluted fractions by SDS-PAGE. We conducted this experiment in triplicate.
Surface Hydrophobicity and Tryptophan Fluorescence.
Surface hydrophobicity was measured by incubating αB or Succ-αB (0.05 mg/mL) with bis-ANS (10 μM) in 50 mM phosphate buffer, pH 7.4, at 25 °C for 1 h. The fluorescence emission spectra were recorded as previously described.32 Intrinsic tryptophan and phenylalanine fluorescence of αB (0.1 mg/mL) in 50 mM phosphate buffer, pH 7.4, was recorded as previously described.32
Circular Dichroism (CD) Measurements.
Far UV-CD and near UV-CD measurements were performed using a spectro-polarimeter (J 600 CD Spectrometer, JASCO) at 25 °C, as previously described.33
Dynamic Light Scattering Study.
The hydrodynamic diameters of αB and Succ-αB were measured at 25 °C using dynamic light scattering (Zetasizer Nano S, Malvern instruments, U.K.) as previously described.33 Protein solutions (1 mg/mL) in 50 mM phosphate buffer, pH 7.4, were passed through a 0.22 μm membrane filter prior to these measurements. Each number recorded is an average of 36 scans.
Statistical Analysis.
The data are the means ± SD from the number of experimental replicates indicated in the figure legends. Statistical differences between the groups were analyzed by Student’s t-test using Graphpad Prism 6 software. Ap value ≤ 0.05 was considered statistically significant.
RESULTS
Spatial Distribution of Succinylated Proteins in Human Lens.
Paraffin-embedded human eye sections (lens shown) were incubated with a SuccK antibody followed by an Alexa Fluor 488 conjugated secondary antibody. We found a strong immunoreactivity for SuccK (green) in the anterior epithelial cells and mild immunoreactivity in fiber cells (Figure 2).
Figure 2.
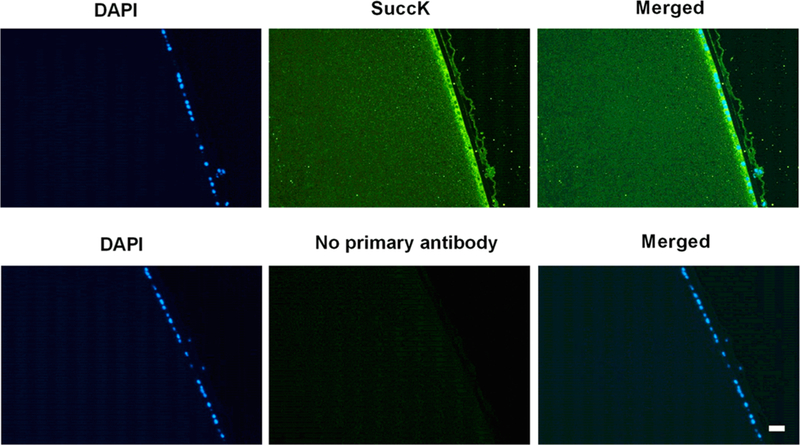
SuccK-modified proteins are present in the human lens. Immunohistochemical analyses of a human lens (age: 20 years) transverse section showed a strong immunoreactivity for SuccK (green) in the anterior epithelium. The cortical region also showed immunoreactivity, but the intensity was less than that in the epithelium. Omission of the primary antibody resulted in no significant immunoreactivity (negative control, bottom three panels). The nucleus of the epithelial cells was stained with DAPI (blue). The scale bar =100 μm.
Effect of Age on the SuccK Content of Human Lens Proteins.
Figure 3A,B shows the results from the Western blot analyses of the WS and WIS fractions. A strong immunoreactivity was observed at approximately 20 kDa in all lenses from both the WS (Figure 3A) and WIS fractions (Figure 3B), suggesting succinylation of crystallins. However, there was no clear trend of an increase or a decrease in the SuccK levels with advancing age of the lenses. We also detected succinylation in other proteins although their levels were lower than crystallins.
Figure 3.
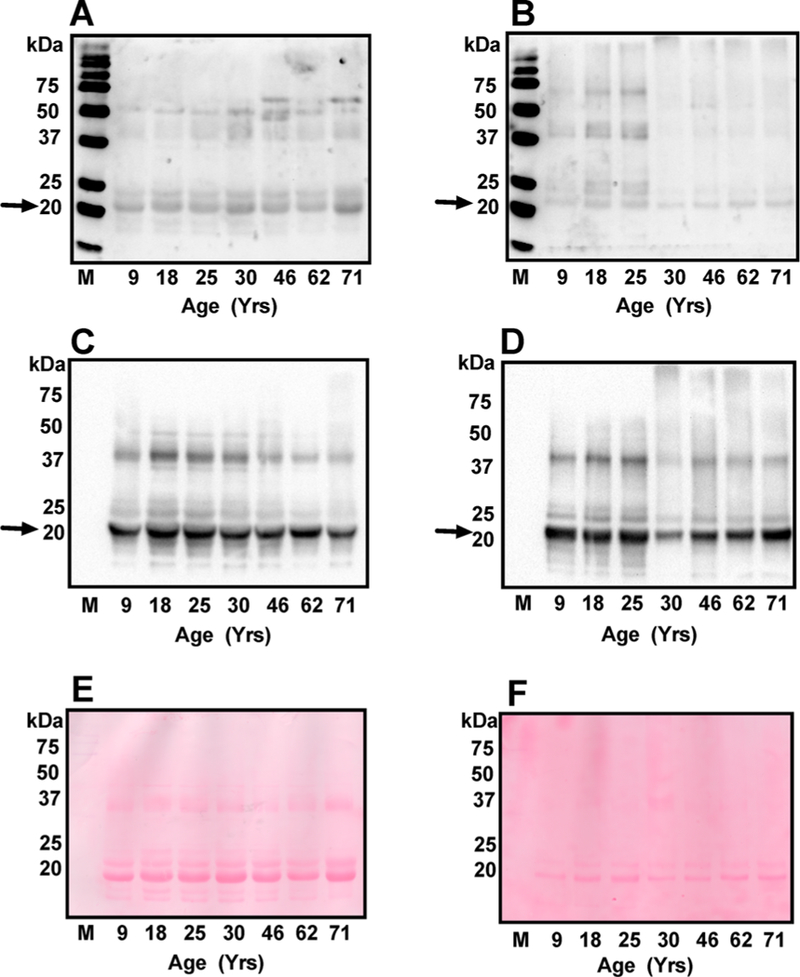
The effect of age on SuccK-modified proteins in the human lens. Western blotting of the WS (A) and WIS (B) (30 μg protein from each) was performed using a monoclonal antibody against SuccK. The membranes were reprobed with a monoclonal antibody against αB-crystallin (C and D). Comparison of the blots in panels A and C as well as B and D suggests that αB-crystallin (indicated by arrows) is the major SuccK-bearing protein in the lens. M, molecular weight markers. Ponceau S staining of the membranes (E and F, respectively) showing equal protein loading.
αB-Crystallin Is the Major SuccK-Modified Protein in the Human Lens.
Strong immunoreactivity in a protein just above 20 kDa suggested αB-crystallin as the modified protein. When the membranes from the above experiment were reprobed with an αB-crystallin antibody, the αB-crystallin and the major SuccK-bearing protein overlapped both in the WS (Figure 3C) and the WIS (Figure 3D) fractions, suggesting that aB-crystallin is the major SuccK-modified protein in the lens. Ponceau S staining of these membranes was performed to show equal protein loading (Figure 3E,F).
To further verify that αB-crystallin is the major SuccK-bearing protein in the lens, the WS of three young lenses (25, 30, and 31 years of age) were succinylated using 0.01 mM SuccCoA and probed in Western blots for succinylation in αA-crystallin and αB-crystallins. The αA-crystallin (slightly below 20 kDa) (Figure 4A,D) and αB-crystallin (slightly above 20 kDa) (Figure 4B,E) were separated well on the gel. At the concentration of SuccCoA used, there was hardly any SuccK detected in αA, but a considerable amount was detected in αB-crystallin in all lenses (Figure 4C). Faint immunoreactivity was seen in unmodified proteins at the α-crystallin region. It should be noted here that we used 10 μg of protein for this experiment, unlike 30 μg for Figure 3, which could be a reason for the less apparent αB-crystallin band. To further confirm that αB-crystallin is the major SuccK-bearing protein in the lens, we immunoprecipi-tated αB-crystallin from the WS of a 9-year-old lens and probed the remaining supernatant for αB-crystallin and SuccK. We found that depletion of αB-crystallin (Figure 4F) significantly reduced immunoreaction in the protein just above the 20 kDa region (Figure 4G), confirming that αB-crystallin is the major SuccK-bearing protein.
Figure 4.
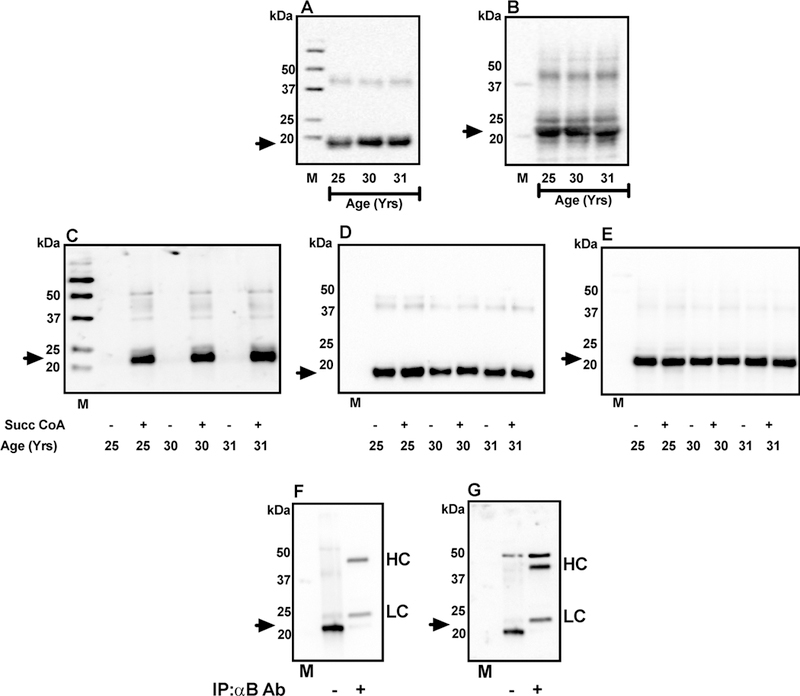
αB-Crystallin is the dominant succinylated protein in the lens. WS from 25, 30, and 31 old human lenses (10 μg of protein from each) was Western blotted using a polyclonal αA-crystallin antibody (1:2000 dilution, A) or a monoclonal αB-crystallin antibody (1:500 000 dilution, B). The arrows indicate the position of αA-crystallin and αB-crystallin in panels A and B, respectively. Western blot for unsuccinylated and succinylated WS (with 0.01 mM SuccCoA) is shown in panel C and the corresponding Westerns for αA-crystallin and αB-crystallin are shown in panels D and E. Preferential succinylation of αB-crystallin was further confirmed by an immunoprecipitation experiment. One hundred μg of human lens WS(from a 9-year-old donor) was treated with 100 μg of αB-crystallin antibody followed by 40 μL of Protein A-Agarose. After centrifugation, the supernatant was used for Western blotting. Western blot for αB-crystallin is shown in panel F and the same membrane reprobed for SuccK, which is shown in panel G. The arrows indicate the position of αB-crystallin and SuccK in panels F and G, respectively. Ab, antibody; HC, heavy chain of the free antibody; LC, light chain of the free antibody; M, molecular weight markers.
We then verified the relative propensity of αA and αB for succinylation by first measuring the reactive amino groups followed by succinylation of the two proteins under identical conditions. The TNBS assay showed that recombinant human αB-crystallin (αB) had significantly more reactive amino groups (~2-fold) than recombinant human αA-crystallin (αA) (Figure 5A). The Western blot results showed that αB was more prone to succinylation than αA (Figure 5B). There were two additional protein bands at a higher molecular weight; the identity of those is not known. Coomassie staining of the gel confirmed equal loading of proteins (Figure 5C). There were some minor proteins in the high molecular weight region that were succinylated but were not visible in the Coomassie stained gel. Densitometric scans of the monomeric protein revealed that αB had ~4-fold more SuccK than αA (Figure 5D). In summary, these experiments demonstrated that αB is more prone to succinylation than αA.
Figure 5.
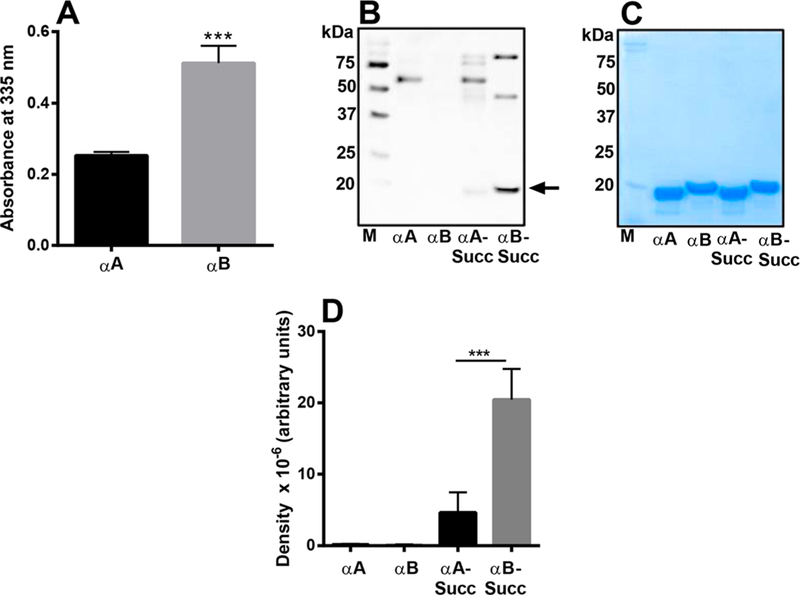
Comparison of reactive amino groups and SuccK levels in αA and αB succinylated in vitro. The TNBS assay determined the reactive amino groups in αA and αB (A). A Western blot of unsuccinylated and succinylated αA and αB (with 0.01 mM SuccCoA) was performed using a SuccK antibody (B). Coomassie blue staining of the gel (shown are after protein transfer) showed equal loading of the proteins in the samples (C). A densitometric plot for the Western blot is shown in panel D. The bar graphs indicate the means ± SD of triplicate measurements. M, molecular weight markers. ***p < 0.0005.
Q-TOF Tandem MS/MS Mass Spectrometric Identification of SuccK Sites in Human Lens Proteins.
Mass spectrometric analyses of the WS fraction from 23-and 73-year-old human lenses indicated that K72, K90, K92, K166, K175, and potentially K174 of αB-crystallin were succinylated (Table 1). Based on the mass spectra data, SuccK at K174 could not be localized but is a potential modification site. It should be noted that succinylated K72 was identified only in the 23-year-old lens. The 73-year-old lens had an additional SuccK-modified lysine residue at K92. Mass spectrometry also revealed SuccK in αA, β-, and γ-crystallins (Table S1). These data confirm that succinylation occurs in all of the crystallins in the human lens.
Table 1.
Q-TOF Tandem MS/MS Mass Spectrometric Identification of SuccK Residues in αB-Crystallin of Human Lenses and in αB-Crystallin Succinylated in Vitro (Immunoprecipitated Using a SuccK Antibody)a
| peptides | SuccK site |
in vitro mass (da) |
human lens age 23 mass (da) |
human lens age 73 mass (da) |
theoretical mass (da) |
mass error inv, 23, 73 (ppm) |
|---|---|---|---|---|---|---|
| LEKDRFSVNLDVK | K72 | 1661.8569 | 1661.8656 | NF | 1661.8625 | −3.37, 1.87, NF |
| FSVNLDVKHFSPEELKVK | K82 | 2215.1728 | NF | NF | 2215.1525 | 9.16, NF, NF |
| HFSPEELKVK | K90 | 1312.6558 | 1312.6686 | 1312.6728 | 1312.6663 | −8.00, 1.75, 4.95 |
| VKVLGDVIEVHGK | K92 | 1491.8217 | NF | 1491.8304 | 1491.8297 | −5.36, NF, 0.27 |
| VLGDVIEVHGKHEER | K103 | 1815.9040 | NF | NF | 1815.9115 | −4.13, NF, NF |
| KYRIPADVDPLTITSSLSSDGVLTVNGPR | K121 | 3170.6451 | NF | NF | 3170.6459 | −0.25, NF, NF |
| KQVSGPER | K150 | 999.4992 | NF | NF | 999.4985 | 0.70, NF, NF |
| TIPITREEKPAVTAAPK | K166 | 1921.0455 | 1921.0533 | 1921.0470 | 1921.0520 | −3.38, 0.68, −2.60 |
| TIPITREEKPAVTAAPKK | K174 or 175 | 2049.1569 | NF | 2049.1456 | 2049.1470 | 4.83, NF, −0.68 |
| EEKPAVTAAPKK | K174 or 175 | 1367.7201 | 1367.7348 | 1367.7237 | 1367.7296 | −6.95, 3.80, −4.31 |
| TIPITREEKPAVTAAPKK | K175 | 2049.1551 | NF | NF | 2049.1470 | 3.95, NF, NF |
| EEKPAVTAAPKK | K175 | 1367.7334 | NF | 1367.7282 | 1367.7296 | 2.78, NF, −1.02 |
NF = no spectrum matched for a specific peptide sequence and SuccK modification. K’s shown in bold are succinylated residues. K174 or K175 denotes an ambiguous site identification and either lysine could be succinylated. See Figure S1A–L for the MS/MS spectra associated with each peptide.
LC–MS/MS Quantification of SuccK and N ε-Acetylly-sine (AcK) in Human Lens Proteins.
SuccK and AcK were quantified in protein digests of 23 human donor lenses. The age of those lenses ranged from 20 to 73 years. For unambiguous identification, the product ion mass spectra of an authentic SuccK standard and a lens workup are shown in Figure 6A,B. Due to matrix interferences, SuccK had to be isolated from enzymatically digested lens proteins. Consequently, a clean product ion mass spectrum was obtained that was identical to the mass spectrum observed for the standard. The levels of SuccK and AcK in human lens proteins in relation to the age of individual lenses are shown in Figure 6C,D. The SuccK levels ranged from 1.2 to 5.3 pmol/mg protein. One 37-year-old lens showed a much higher level (14.3 pmol/mg protein), and it was considered an outlier; the levels in other lenses showed a weak but significant negative correlation with the lens age. The AcK levels, in comparison, were significantly higher than the SuccK levels; the levels ranged from 1610 to 6200 pmol/mg protein, but there was no significant correlation with age. Together, these data suggest that succinylation is a relatively minor modification compared with acetylation in lens proteins. However, even at those levels, succinylation impacts the function of αB-crystallin (see below).
Figure 6.
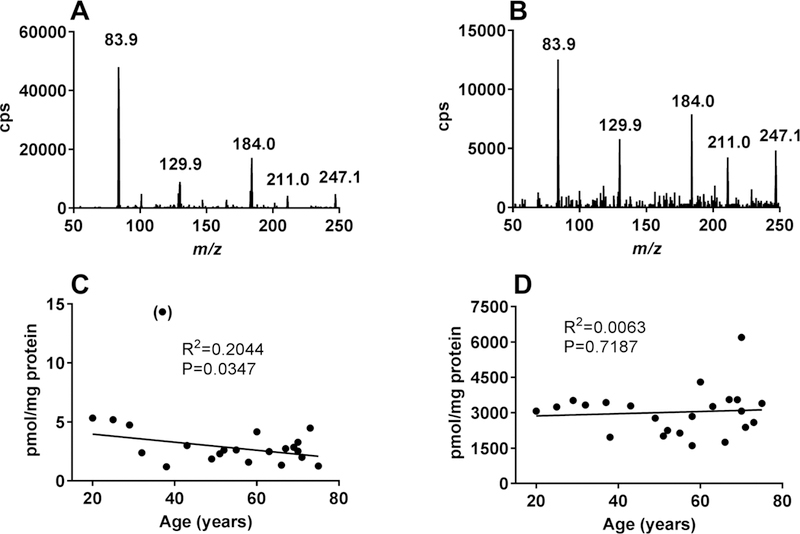
LC–MS/MS quantification of SuccK and AcKin human lens proteins. A product ion scan of a 1 μM authentic SuccK (m/z = 247.1) standard is shown in panel A. A product ion scan of a human lens protein workup is shown in panel B. The levels of SuccK in the enzyme digested human lens protein as a function of age is shown in panel C; the data point in parentheses was excluded from the statistical analysis. The levels of AcK in the enzyme digested human lens protein as a function of age is shown in panel D.
Succinylation in Vitro of αB to Levels Found in the Crystallins of Human Lenses.
To understand the implications of succinylation on αB-crystallin, it was necessary to succinylate αB-crystallin to an extent similar to what is found in human lenses. Quantification of αB in human lens WS fraction was performed by Western blotting. Densitometric analysis of the Western blot revealed that 20 μg of WS contained approximately 2 μg of αB (Figure 7A). Recombinant αB was then succinylated with various concentrations of SuccCoA to match SuccK levels to those in αB in WS fraction of human lenses. We used 2 μg of the succinylated αB (Succ-αB) and 20 μg of lens WS for Western blot analysis to compare the SuccK levels in human lens αB and recombinant αB. Western blots in Figure 7B demonstrated similar levels of αB in human lens samples and the recombinant nonsuccinylated or succinylated proteins. Western blot in Figure 7C showed that at a molar ratio of 1:0.001 (the lowest SuccCoA concentration used) the SuccK level in in vitro succinylated αB was comparable to that found in the in vivo succinylated αB-crystallin in human lenses. To further confirm that αB was succinylated at a 1:0.001 ratio, we digested the sample with proteolytic enzymes (as above), immunoprecipitated SuccK-bearing peptides and analyzed these SuccK-enriched peptide by mass spectrometry. We found K72, K82, K90, K92, K103, K121, K150, K166, and K175 (and potentially K174) to be succinylated (Table 1).Apointto note here is that succinylated αB-crystallin in human lenses showed a discordant migration in SDS-PAGE when compared to recombinant αB-crystallin. This could be due to additional modification in the protein.
Figure 7.
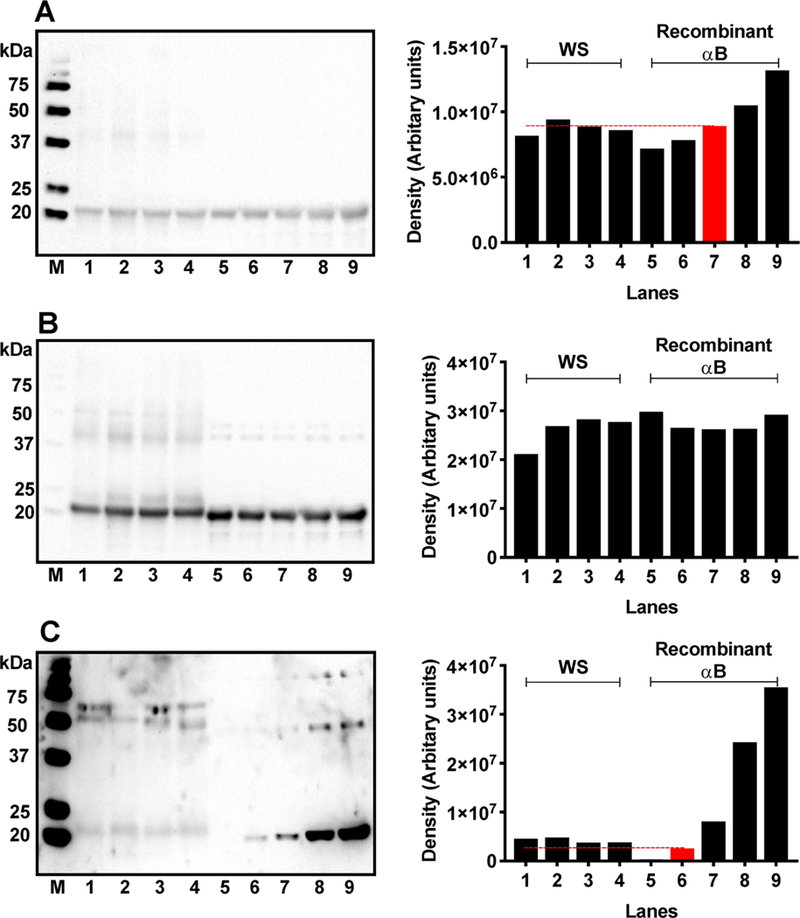
Succinylation of αB in vitro compared to the level of succinylation of αB-crystallin in human lenses. Quantitative comparison of αB levels in the WS from 9-, 23-, 46,-and 71-year-old lenses with the recombinant αB (A). M, molecular weight markers; lanes 1–4, 20 μg of WS from 9-, 23-, 46-, and 71-year-old lenses; lanes 5–9, recombinant αB at 1,1.5,2,2.5, and 3 μg of protein. Densitometric analysis in the right panel shows that 20 μg of WS contains ~2 μg of αB (red bar). Recombinant αB was then succinylated in vitro with increasing concentrations of SuccCoA and then compared with succinylated αB in human lenses. Western blot of WS and succinylated recombinant αB was performed and probed with a monoclonal antibody against αB-crystallin (B) and then reprobed for SuccK (C). The succinylation of αB in vitro was performed using various molar concentrations of SuccCoA relative to the lysine content in αB (see below). The red bar indicates succinylation of αB to an almost similar extent to that of αB-crystallin in human lenses. M, molecular weight markers; lanes 1–4, WS from 9-, 23-, 46-, and 71-year-old lenses; 5, recombinant αB control; 6–9, succinylated recombinant αB generated at αB/SuccCoA molar ratios of 1:0.001, 1:0.003, 1:0.007, and 1:0.01, respectively. Right panels are the densitometric analysis of the respective Western Blots in the left panels. The dashed line in panel C indicates the density of Succ-αB (red) (1:0.001) relative to the succinylated αB-crystallin in human lenses.
Succinylation of αB Enhances Its Chaperone Activity.
Even though the 1:0.001 Succ-αB had slightly lower amounts of SuccK than αB-crystallin in human lenses, we used this preparation in all subsequent experiments to confirm that the structural and functional changes observed in recombinant αB succinylated in vitro are relevant to human lenses. We also investigated how succinylation alters the structure and chaperone activity of αB-crystallin.
In the chemically induced aggregation assay, αB inhibited insulin aggregation by 19.9%. However, at a similar protein concentration, the Succ-αB inhibited the aggregation of insulin by 56.8% (Figure 8A,B). Similarly, in the citrate synthase (CS) thermal aggregation assay, αB inhibited the aggregation by 18%, whereas Succ-αB inhibited the aggregation by 35% (Figure 8C,D). In both the aggregation assays, when succinylation in αB was increased to 1:0.01 (M/M), the chaperone activity against insulin and CS aggregation was further increased to ~73% and ~59% (Figure 8A–D). These results imply that mild succinylation of αB (1:0.001 M/M, lysine residues in αB/succinyl CoA) at levels comparable to those found in vivo improves its chaperone activity. Even greater succinylation of αB (1:0.01 M/M, lysine residues in αB/succinyl CoA) is beneficial and causes further improvement in its chaperone function.
Figure 8.
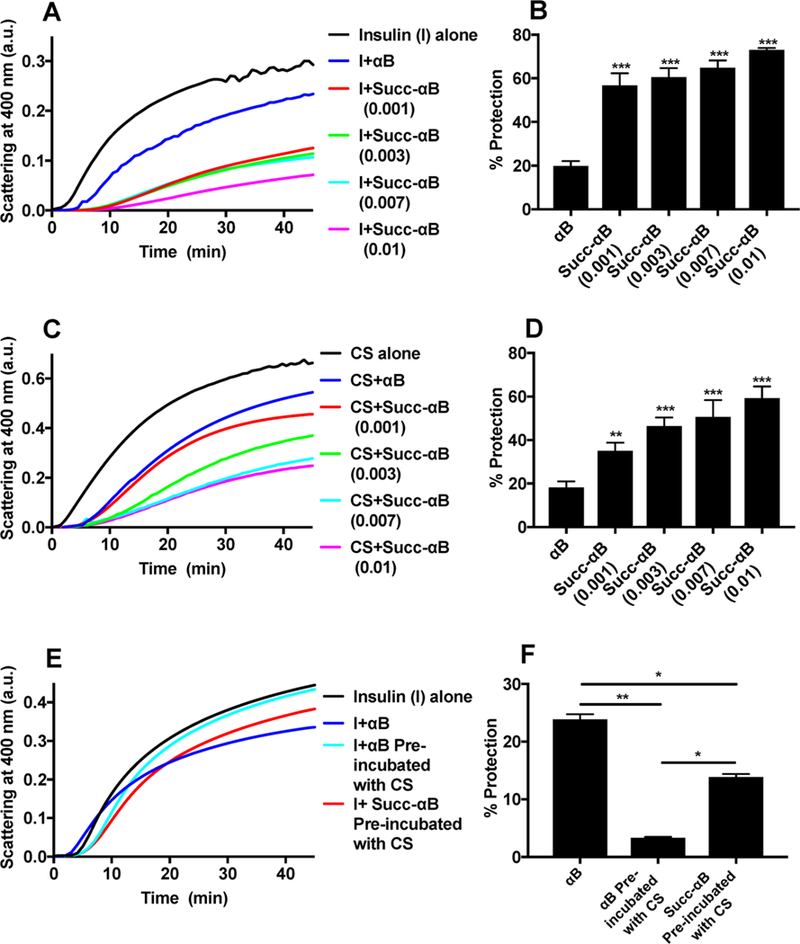
The effect of succinylation on the chaperone activity of αB. DTT-induced aggregation assay of insulin at 25 °C (A) and heat-induced aggregation assay of CS at 43 °C (C) in the absence and presence of αB and succinylated αB (generated at αB/SuccCoA molar ratios of 1:0.001, 1:0.003, 1:0.007, and 1:0.01). Total volume of assay mixture = 500 μL. Percent protection by αB and succinylated αB against insulin aggregation (B) and CS aggregation (D). DTT-induced aggregation assay of insulin in the absence or presence of αB and Succ-αB precomplexed with CS (E). Percent protection by αB and Succ-αB (generated at αB/SuccCoA molar ratios of 1:0.001) precomplexed with CS against insulin aggregation (F). The bar graphs indicate the means ± SD of triplicate measurements. *p < 0.05, **p < 0.005, ***p < 0.0005.
To establish that the improved chaperone activity in Succ-αB was due to the exposure of additional client protein binding sites, αB or Succ-αB (0.12 mg/mL) was first incubated with CS (0.12 mg/mL) at 43 °C for 2 h. This was done to ensure that all of the CS binding sites in αB were occupied. The assay mixture was then centrifuged at 10 000g for 15 min to remove the aggregated CS, and the supernatant containing the soluble complex of αB and CS was used in the insulin aggregation assay. Figure 8E,F shows that αB preincubated with CS lost its ability to prevent insulin aggregation, indicating that CS and insulin bound to the same site in αB. This was not the case for Succ-αB; Succ-αB was able to inhibit insulin aggregation by 13.8%. To ascertain that insulin has no affinity toward CS or CS itself does not aggregate in the presence of DTT, we monitored DTT-induced aggregation of insulin in the absence or presence of CS. Figure S3 clearly demonstrates that CS does not aggregate in the presence of DTT. Together, these observations suggest that succinylation exposes additional client protein binding sites in αB.
To determine whether the improved chaperone activity of αB was due to just the succinyl moiety in lysine residues, we investigated effects of succinylation in BSA and ADH. Succinylation of BSA and ADH was performed at a ratio of lysine/Succ CoA of 1:0.001 and 1:01 (M/M) and under conditions similar to those used for αB. Western blots in Figure S4 demonstrate succinylation of both BSA and ADH. We used DTT-induced aggregation of insulin and thermal aggregation of CS to test effects of succinylation in BSA and ADH. Figure S5 shows that succinylation of BSA and ADH had no effect on the aggregation of either insulin or CS. These results confirm that SuccK modification by itself does not bestow chaperone activity in proteins and strengthen our hypothesis that succinylation exposes additional client protein binding sites in αB, which makes it a better chaperone.
To further confirm the presence of additional client binding sites, we subjected αB-CS and Succ-αB-CS incubation mixtures to gel filtration. Figure 9 shows the elution profiles. Both peak 1 and peak 2 showed αB and CS in SDS-PAGE (Figure 10A), indicating they contained complexes. It is likely peak 2 also had the free form of αB (or Succ-αB). We analyzed the chaperone activity in both peaks. The components in peak 1 did not show chaperone activity against insulin aggregation (Figure 10B), but rather they promoted aggregation of insulin. Components in peak 1 did not aggregate when incubated in the presence of DTT (Figure 10B) in the absence of insulin or when incubated with insulin in the absence of DTT (Figure 10C). These results demonstrate that protein aggregation observed in the chaperone assay was due to aggregation of insulin. In contrast to peak 1, components in peak 2 prevented insulin aggregation; the chaperone activity of peak 2 obtained the from Succ-αB-CS mixture was higher (18.9% protection) than that from the αB-CS mixture (6.3% protection) (Figure 10B,D,E). Together, these data suggest that succinylation exposes additional client binding sites in αB.
Figure 9.
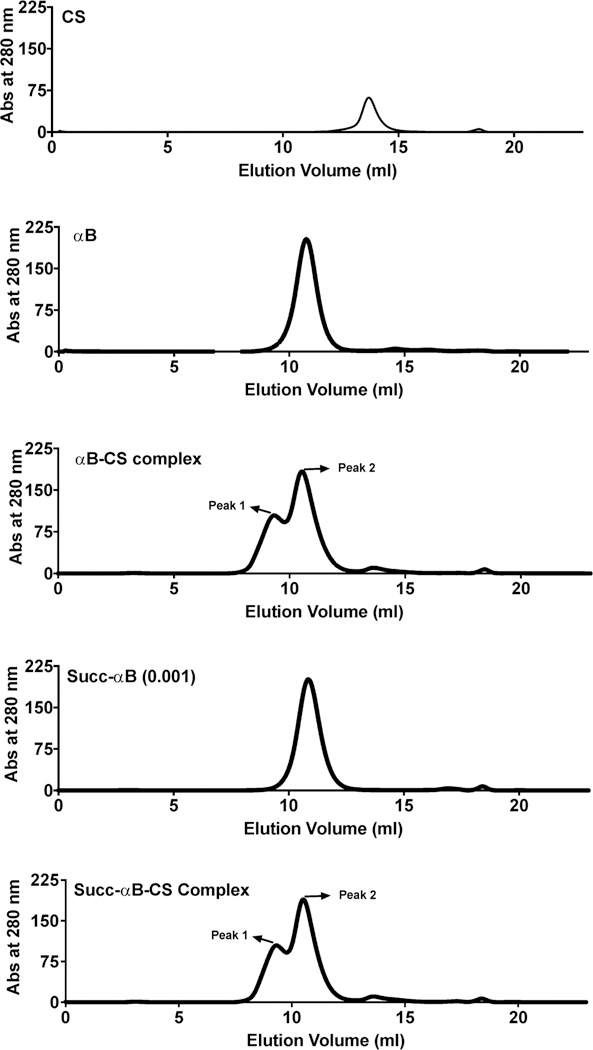
Isolation of the αB-CS complex by gel filtration. An Enrich SEC-650 analytical gel filtration column (10 mm × 300 mm, 24 mL) was used for separation of proteins. The column was equilibrated in PBS and eluted in the same buffer at a flow rate of 0.7 mL/min. The elution profiles for CS (0.35 mg/mL), αB (1 mg/mL), αB-CS complex, Succ-αB (1 mg/mL), and Succ-αB-CS complex are shown.
Figure 10.
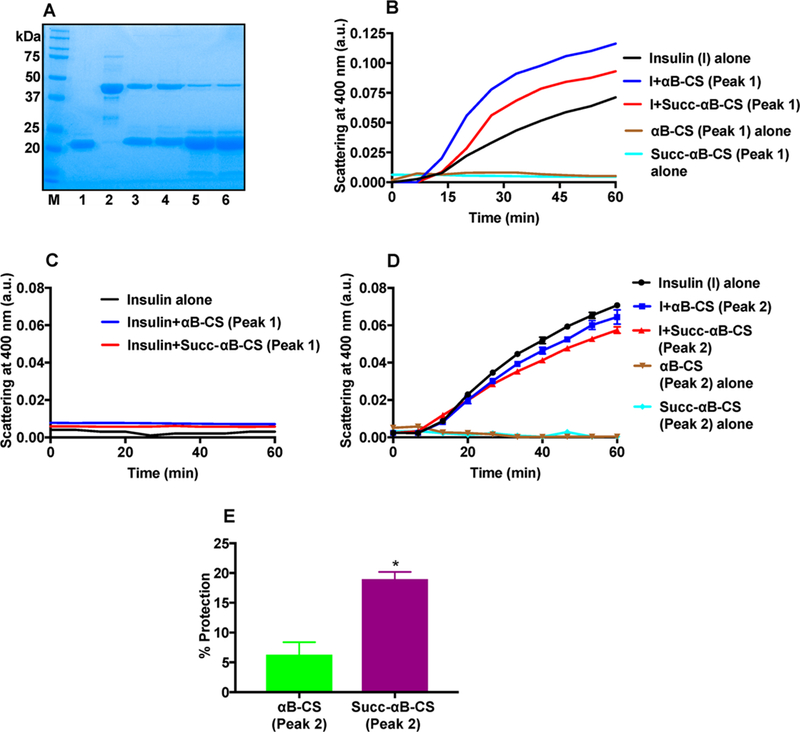
Isolation of the αB-CS complex and determination of chaperone activity. SDS-PAGE (12% gel) stained with Coomassie of FPLC-isolated αB-CS and Succ-αB-CS complexes (A). 1, αB-crystallin; 2, CS; 3, αB-CS (peak 1); 4, Succ-αB-CS (peak 1); 5, αB-CS (peak2); 6, Succ-αB-CS (peak 2). DTT-induced aggregation of insulin in the presence or absence of complexes from peak 1 (B) and peak 2 (D). Insulin incubated as above (without DTT) in the presence or absence of the αB-CS or Succ-αB-CS complex did not show aggregation (C). Total volume of assay mixture = 200 μL. In the chaperone assay in panel C, mean ± SD values at 6.6 min intervals are shown. The assay was performed in 50 mM phosphate buffer, pH 7.4, at 25 °C. We used equal concentrations of αB (determined by SDS-PAGE) from each peak in these assays. Percent protection against insulin aggregation by αB-CS and Succ-αB-CS complexes in peak 2 is shown in panel E. The bar graphs indicate the means ± SD of three independent experiments. *p < 0.05.
The Structure of Succ-αB Is Mildly Perturbed.
To ascertain whether the improvement in the chaperone activity of Succ-αB was accompanied by any alteration in its conformation, we analyzed the protein for secondary, tertiary, and quaternary structures. The far UV-CD spectra of αB and Succ-αB (0.001) or 1:0.01 succinylated αB were similar (Figure 11A), indicating that the secondary structure of αB was unaltered upon succinylation. The near UV-CD results in Figure 11B confirmed mild perturbation in the tertiary conformation of αB upon succinylation. These results suggest that succinylation mildly alters the tertiary structure of αB. The tryptophan (Figure 11C) and phenylalanine fluorescence (Figure 11D) spectra showed alterations in the intensity by ~5 and ~13%, respectively, in Succ-αB relative to αB. In 0.01 succinylated αB, similar trends in alterations of tryptophan and phenylalanine fluorescence were observed. In the dynamic light scattering (DLS) experiment, αB showed a hydrodynamic diameter of ~19.26 nm, which corresponded to a molecular weight of 674 kDa (Figure 11E). However, Succ-αB and 0.01 succinylated αB (succinylated recombinant αB generated at a αB/SuccCoA molar ratio of 1:0.01) showed a reduced hydrodynamic diameter of ~16.34 and ~16.16 nm that corresponded to a molecular weight of 458 and 447 kDa. Thus, succinylation of αB appears to cause a mild dissociation of the oligomeric assembly of the protein. Additionally, the surface hydrophobicity, measured using bis-ANS, was ~5–6% higher in Succ-αB or 0.01 succinylated αB than αB (Figure 11F). However, we did not observe a direct relationship between the chaperone activity and bis-ANS binding (Figure S6), similar to observations in other studies.33–35
Figure 11.
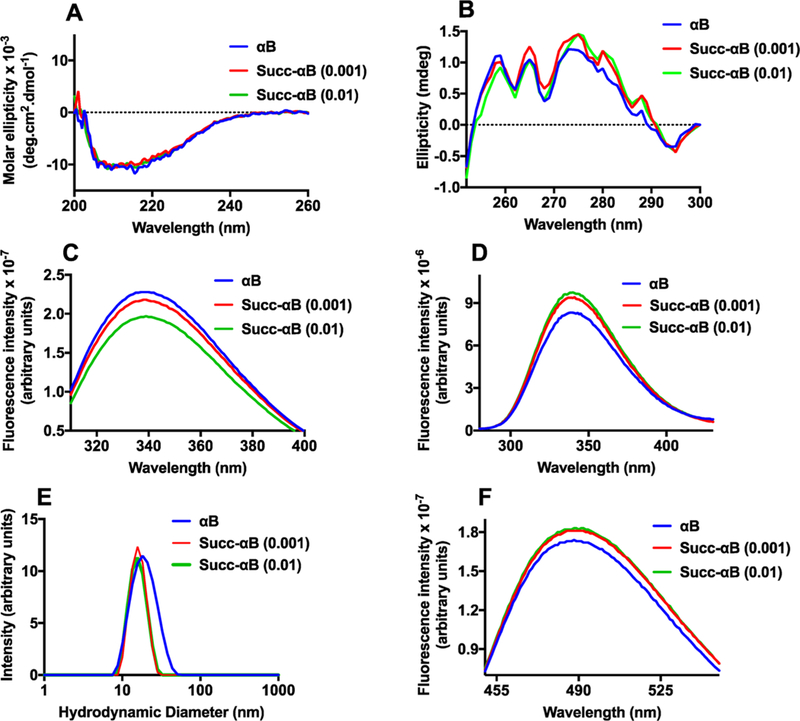
The effect of succinylation on the conformation of αB. Far UV-CD spectra (A) and near UV-CD spectra of αB and Succ-αB (B). Tryptophan fluorescence spectra at the excitation wavelength of 295 nm (C). Phenylalanine fluorescence spectra at the excitation wavelength of 257 nm (D). The excitation and emission slit widths for the tryptophan and phenylalanine fluorescence spectra were 5 nm each. The protein concentration for both fluorescence spectra was 0.1 mg/mL in 50 mM phosphate buffer, pH 7.4. The intensity particle size distribution spectra (E) of αB and Succ-αB αB (1 mg/mL) were recorded using dynamic light scattering at 25 °C in 50 mM phosphate buffer, pH 7.5. The spectra shown are the averages of 36 scans. The surface hydrophobicity of αB and succinylated αB was measured using bis-ANS (F); the excitation wavelength used was 390 nm.
DISCUSSION
The purpose of this study was to determine (1) whether succinylation of lysine residues occurs in human lens proteins, (2) the effect of age on succinylation in lens proteins, (3) whether succinylation occurs in α-crystallin, and (4) the effect of succinylation on the structure and chaperone activity of αB-crystallin.
Our study clearly established that succinylation occurs in both the WS and the WIS of human lenses and that it occurs at very early age. The LC–MS/MS results indicated that the extent of succinylation in lens proteins slightly decreased with advancing age, but such a trend was not detected by Western blotting. The fact that Western blotting is less quantitative than LC–MS/MS could be a reason for the discrepancy. Theoretical analyses using the “SuccFind” software36 predicted that K72 and K92 are most likely to be succinylated in αB. Our observation of SuccK at K72 and K92 agreed with the predicted sites. However, we also found SuccK at K90, K166, K175, and possibly K174. Some of these lysine residues are also acetylation sites as observed in our previous study,25 reiterating the notion that acetylation and succinylation sites overlap in proteins. Previous studies that have shown acetylation and succinylation to overlap in many proteins21,37 support this view.
Whether succinylation is enzymatic or nonenzymatic in the human lens is unclear. Succinylation from SuccCoA has been shown to occur nonenzymatically and is favored by a slightly alkaline pH,38 a condition likely to be present in mitochondria; however, it is also known to occur, albeit at a much slower rate, at neutral pH,38 a condition present in the cytosol of cells. Succinylation, similar to other acylations, is a two-way modification in that it can be reversed by protein deacylases. Deacylation in proteins other than histones mainly occurs through sirtuins, which are NAD+-dependent deacylases.39,40 Whether sirtuins, especially sirtuin5, which is known to desuccinylate proteins,41,42 has any role in the regulation of the succinylation levels in human lens proteins remains to be established.
Immunohistochemical experiments with human lenses showed that succinylation occurs in epithelial and fiber cells. Mass spectrometric measurements showed a gradual reduction in the SuccK content in aging lenses. These observations suggest that succinylation of proteins occurs in epithelial cells and that, as they are differentiated into fiber, proteins carrying SuccK are retained in fiber cells. A gradual reduction of SuccK content in aging lenses and reduced immunoreactivity in the cortical region (compared to the epithelium) suggest the possibility that, at least to some extent, succinylation may be reversed.
The MS analysis revealed SuccK in all of the major crystallins and several other proteins in the lens. However, αB-crystallin was found to be the major succinylated protein in the lens. Why is αB-crystallin more susceptible to succinylation than other crystallins? The difference between αA-crystallin and αB-crystallin is more understandable than the differences between αB-crystallin and other crystallins. First, the lysine content in αB is higher than αA; the former contains 10 and the latter 7 lysine residues per protein molecule. Second, the TNBS assay showed that the surface-exposed lysine content is higher in αB than αA. This is not surprising because lysine is a hydrophilic amino acid and tends to be surface-exposed in proteins. Furthermore, the reversal of succinylation, if it occurs, may be inefficient in αB-crystallin compared to other crystallins in the lens. It is also possible that aB-crystallin, unlike other crystallins, is stress-inducible; preferential succinylation could be a strategy to enhance its chaperone activity for better protection of lens cells.
The chaperone activity of αB-crystallin has been considered to be important for preventing protein aggregation in aging lenses.43 To study the effect of succinylation on the structure and function of αB in the physiological context, we succinylated the αB to levels relevant to human lenses. To accomplish this, we incubated αB with SuccCoA (corresponding to a 1:0.001 molar ratio of lysine in αB to succinyl CoA) at a pH between 7.5 and 8.0. The αB-crystallin in human lenses migrated slightly slower than the recombinant αB. Other PTMs that can occur in αB-crystallin early in life, including acetylation,44 could be the reason for this difference.
The Succ-αB we chose for further studies was an order of magnitude less succinylated than the αB-crystallin in human lenses. Even at this level of succinylation, αB showed improvement in the chaperone activity. The improvement in the chaperone activity with 1:0.001 molar ratio of lysine residues (in αB) to succinyl CoA translates into modification of one lysine residue for every 1000 lysine residues. With 10 lysine residues in each αB subunit, this would mean that 1 out of every 100 αB subunits are modified. Since each αB homooligomer contains about 20 subunits, 1 in 5 αB homooligomer would therefore be expected to contain a succinylation modification. Further work is needed to understand how this extremely low medication improved the chaperone activity of αB. The improvement in the chaperone activity of Succ-αB was accompanied by a mild increase in its surface hydrophobicity. Whether the mildly increased surface hydrophobicity was the reason for the improvement in the chaperone activity is unclear, as many studies failed to establish a clear relationship between surface hydrophobicity and chaperone activity in α-crystallin.45,46 However, our observation that CS saturated Succ-αB was still able to prevent insulin aggregation, unlike αB, pointing to the exposure of additional chaperone sites, which could have resulted in a higher hydrophobicity and chaperone activity of Succ-αB. Despite the improvement in the chaperone activity, the secondary structure was unchanged. Only very subtle changes were detected in the tertiary or quaternary structure. Despite Succ-αB displaying a higher chaperone activity when compared to αB, the results in Figure 9 indicated that the client protein binding is similar between Succ-αB and αB. It is possible that Succ-αB has a higher affinity to unfolding protein, such as, CS compared to the affinity of αB. In addition, recent studies have shown transient interactions of αB with client proteins.47,48 It is therefore possible that the transient interaction of Succ-αB with CS is higher than that of αB.
A large number of studies have demonstrated a link between the chaperone activity and oligomeric assembly of α-crystallin.49–51 It has been shown that dissociation of the quaternary structure of α-crystallin results in an enhanced chaperone activity.49 Analogous to these observations, succinylation of αB caused a mild dissociation of the oligomeric assembly of the protein. The protein quaternary structure analyzed by DLS suggested that mild succinylation reduced the oligomeric size by approximately 216 kDa or ~11 subunits. Future studies could shed light on the reason for this reduction in oligomeric size. The sequence 155PERTIPITREEK166 in αB-crystallin is considered to be the subunit–subunit interaction site.52 This sequence also possesses the “IXI” motif that links with the β4-β8 groove of neighboring dimers to form a higher order oligomeric assembly in αB.52 Any mutation in this sequence results in its altered oligomeric assembly.53 Therefore, the succinylation at K166, which we observed in Succ-αB, might have weakened the subunit–subunit interaction, triggered a mild dissociation of the oligomeric assembly, and exposed additional hydrophobic sites.
In summary, the present study demonstrated that succinylation of human lens αB-crystallin occurs early in life and continues to be present at an advanced age in the human lens. Our study showed that succinylation improves the chaperone activity of αB-crystallin. Furthermore, it is tempting to speculate that succinylation could inhibit proteolytic cleavage of proteins and modification by glycation (by competing for the same lysine residues). Thus, succinylation could be an adaptive, beneficial modification to prevent protein damage in aging human lenses and help the lens remain transparent. It is possible that succinylation of αB-crystallin in the heart, kidney, and skeletal muscle may have a similar effect on its chaperone activity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants EY028836 (R.H.N.) and AA022146 (K.S.F.) and a challenge grant from RPB to the Department of Ophthalmology, University of Colorado. We thank Dr. Johanna Rankenberg and Dr. Mi-hyun Nam for critical reading of the manuscript. The authors acknowledge the Skaggs School of Pharmacy and Pharmaceutical Sciences Mass Spectrometry Core Facility, which is supported by the Colorado Clinical and Translational Sciences Institute UL-1-RRO25780, for assistance with sample analysis.
ABBREVIATIONS
- SuccK
Nε-succinyllysine
- AcK
Nε-acetyllysine
- SuccCoA
succinyl CoA
- αA
recombinant human αA-crystallin
- αB
recombinant human αB-crystallin
- Succ-αB
αB-crystallin succinylated with 1:0.001 M/M of lysine residues in αB-crystallin to succinyl CoA
- WS
water-soluble lens protein
- WIS
sonicated and centrifuged water-insoluble lens protein
- sHSPs
small heat shock proteins
- PTMs
post-translational modifications
- CML
Nε-carboxymethyllysine
- DLS
dynamic light scattering
- IP
immunoprecipitation
- CS
citrate synthase
- DTT
dithiothreitol
- TNBS
trinitrobenzenesulfonic acid
- bis-ANS
4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid, dipotassium salt
- TCEP
tris(2-carboxyethyl)phosphine
- TFE
trifluoroethanol
- ABC
ammonium bicarbonate
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bio-chem.8b01053.
Q-TOF tandem MS/MS mass spectrometric identification of SuccK residues in αA, β, and γ-crystallins and other proteins from human lenses (immunoprecipitated using SuccK antibody), MS/MS spectra of SuccK-bearing peptides from in vitro succinylated αB and αB from WS of human lenses (23Y and 73Y), MS/MS spectra of SuccK-bearing peptides from WS of human lens proteins (23Y and 73Y), effect of CS on the aggregation of insulin, succinylation of BSA and ADH, succinylation does not alter the inhibitory capacity of BSA and ADH against insulin and CS aggregation, and the relationship between the chaperone activity and surface hydrophobicity of succinylated αB-crystallin in (A) insulin aggregation and (B) CS aggregation assays (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Michael R, and Bron AJ (2011) The ageing lens and cataract: a model of normal and pathological ageing. Philos. Trans. R. Soc., B 366, 1278–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Bloemendal H, and Hockwin O (1982) Lens proteins. CRC Crit. Rev. Biochem. 12, 1–38. [DOI] [PubMed] [Google Scholar]
- (3).Andley UP (2008) The lens epithelium: focus on the expression and function of the alpha-Crystallin chaperones. Int. J. Biochem. Cell Biol. 40, 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Groenen PJ, Merck KB, de Jong WW, and Bloemendal H (1994) Structure and modifications of the junior chaperone alpha-Crystallin. From lens transparency to molecular pathology. Eur. J. Biochem. 225, 1–19. [DOI] [PubMed] [Google Scholar]
- (5).Andley UP, Hamilton PD, Ravi N, and Weihl CC (2011) A knock-in mouse model for the R120G mutation of alphaB-Crystallin recapitulates human hereditary myopathy and cataracts. PLoS One 6, No. e17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Singh D, Raman B, Ramakrishna T, and Rao Ch M (2006) The cataract-causing mutation G98R in human alphaA-Crystallin leads to folding defects and loss of chaperone activity. Mol. Vis. 12, 1372–1379. [PubMed] [Google Scholar]
- (7).Perng MD, Muchowski PJ, van Den IP, Wu GJ, Hutcheson AM, Clark JI, and Quinlan R A. (1999) The cardiomyopathy and lens cataract mutation in alphaB-Crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J. Biol. Chem. 274, 33235–33243. [DOI] [PubMed] [Google Scholar]
- (8).Gupta R, and Srivastava OP (2004) Deamidation affects structural and functional properties of human alphaA-Crystallin and its oligomerization with alphaB-Crystallin. J. Biol. Chem. 279, 44258–44269. [DOI] [PubMed] [Google Scholar]
- (9).MacRae TH (2000) Structure and function of small heat shock/alpha-Crystallin proteins: established concepts and emerging ideas. Cell. Mol. Life Sci 57, 899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kamei A, Hamaguchi T, Matsuura N, and Masuda K (2001) Does post-translational modification influence chaperone-like activity of alpha-Crystallin? I. Study on phosphorylation. Biol. Pharm. Bull. 24, 96–99. [DOI] [PubMed] [Google Scholar]
- (11).Nagaraj R, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, and Padival AK (2003) Enhancement of chaperone function of alpha-Crystallin by methyl-glyoxal modification. Biochemistry 42, 10746–10755. [DOI] [PubMed] [Google Scholar]
- (12).Choudhary C, Weinert BT, Nishida Y, Verdin E, and Mann M (2014) The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550. [DOI] [PubMed] [Google Scholar]
- (13).Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, and Guan KL (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, Liu CF, Grishin NV, and Zhao Y (2009) Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol. Cell. Proteomics 8, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cheng Z, Tang Y, Chen Y, Kim S, Liu H, Li SS, Gu W, and Zhao Y (2009) Molecular characterization of propionyllysines in non-histone proteins. Mol. Cell. Proteomics 8, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chen Y, Sprung R, Tang Y, Ball H, Sangras B, Kim SC, Falck JR, Peng J, Gu W, and Zhao Y (2007) Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell. Proteomics 6, 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, and Zhao Y (2011) The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell. Proteomics 10, M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, and Zhao Y (2011) Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y, Boeke JD, and Zhao Y (2012) Lysine succinylation and lysine malonylation in histones. Mol. Cell. Proteomics 11, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zhang Z, Tan M, Xie Z, Dai L, Chen Y, and Zhao Y (2011) Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 7, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Weinert BT, Scholz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, and Choudhary C (2013) Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 4, 842–851. [DOI] [PubMed] [Google Scholar]
- (22).Kosono S, Tamura M, Suzuki S, Kawamura Y, Yoshida A, Nishiyama M, and Yoshida M (2015) Changes in the Acetylome and Succinylome of Bacillus subtilis in Response to Carbon Source. PLoS 0ne 10, No. e0131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).DiMauro MA, Nandi SK, Raghavan CT, Kar RK, Wang B, Bhunia A, Nagaraj R H., and Biswas, A. (2014) Acetylation of Gly1 and Lys2 promotes aggregation of human gammaD-Crystallin. Biochemistry 53, 7269–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Nahomi RB, Huang R, Nandi SK, Wang B, Padmanabha S, Santhoshkumar P, Filipek S, Biswas A, and Nagaraj RH (2013) Acetylation of lysine 92 improves the chaperone and anti-apoptotic activities of human alphaB-Crystallin. Biochemistry 52, 8126–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, and Biswas A (2012) Acetylation of alphaA-Crystallin in the human lens: effects on structure and chaperone function. Biochim. Biophys. Actay Mol. Basis Dis. 1822, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, and Nagaraj R H. (2013) Chaperone peptides of alpha-Crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J. Biol. Chem. 288, 13022–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Garcia-Castineiras S, and Miranda-Rivera MN (1983) Loss of free amino groups in the water-insoluble fraction of nuclear senile cataracts. Invest. Ophthalmol. Vis. Sci. 24, 1181–1187. [PubMed] [Google Scholar]
- (28).Wisniewski JR, Zougman A, Nagaraj N, and Mann M (2009) Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362. [DOI] [PubMed] [Google Scholar]
- (29).Fritz KS, Green MF, Petersen DR, and Hirschey MD (2013) Ethanol metabolism modifies hepatic protein acylation in mice. PLoS One 8, No. e75868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Harris PS, Roy SR, Coughlan C, Orlicky DJ, Liang Y, Shearn CT, Roede JR, and Fritz KS (2015) Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney. Redox Biol. 6, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Smuda M, Henning C, Raghavan CT, Johar K, Vasavada AR, Nagaraj RH, and Glomb MA (2015) Comprehensive analysis of maillard protein modifications in human lenses: effect of age and cataract. Biochemistry 54, 2500–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nandi SK, Rehna EA, Panda AK, Shiburaj S, Dharmalingam K, and Biswas A (2013) A S52P mutation in the ‘alpha-Crystallin domain’ of Mycobacterium leprae HSP18 reduces its oligomeric size and chaperone function. FEBS J. 280, 5994–6009. [DOI] [PubMed] [Google Scholar]
- (33).Nandi SK, Panda AK, Chakraborty A, Sinha Ray S, and Biswas A (2015) Role of Subunit Exchange and Electrostatic Interactions on the Chaperone Activity of Mycobacterium leprae HSP18. PLoS One 10, No. e0129734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Reddy GB, Das KP, Petrash JM, and Surewicz WK (2000) Temperature-dependent chaperone activity and structural properties of human alphaA-and alphaB-crystallins. J. Biol. Chem. 275, 4565–4570. [DOI] [PubMed] [Google Scholar]
- (35).Fu X, and Chang Z (2004) Temperature-dependent subunit exchange and chaperone-like activities of Hsp16.3, a small heat shock protein from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 316, 291–299. [DOI] [PubMed] [Google Scholar]
- (36).Xu HD, Shi SP, Wen PP, and Qiu JD (2015) SuccFind: a novel succinylation sites online prediction tool via enhanced characteristic strategy. Bioinformatics 31, 3748–3750. [DOI] [PubMed] [Google Scholar]
- (37).Pan J, Chen R, Li C, Li W, and Ye Z (2015) Global Analysis ofProtein Lysine Succinylation Profiles and Their Overlap with Lysine Acetylation in the Marine Bacterium Vibrio parahemolyticus. J. Proteome Res. 14, 4309–4318. [DOI] [PubMed] [Google Scholar]
- (38).Wagner GR, and Payne RM (2013) Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 288, 29036–29045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Houtkooper RH, Pirinen E, and Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Chang HC, and Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 25, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, Uppala R, Fitch M, Riiff T, Zhu L, Zhou J, Mulhern D, Stevens RD, Ilkayeva OR, Newgard CB, Jacobson MP, Hellerstein M, Goetzman ES, Gibson BW, and Verdin E (2013) SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 18, 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, Chen W, Zhang S, Weiss RS, Locasale JW, Auwerx J, and Lin H (2016) Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc. Natl. Acad. Sci. U.S.A. 113,4320–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Horwitz J (1992) Alpha-Crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci U. S. A. 89, 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Nahomi RB, Oya-Ito T, and Nagaraj RH (2013) The combined effect ofacetylation and glycation on the chaperone and anti-apoptotic functions of human alpha-Crystallin. Biochim. Biophys. Acta, Mol. BasisDis. 1832, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Bhattacharyya J, Srinivas V, and Sharma KK (2002) Evaluation of hydrophobicity versus chaperonelike activity of bovine alphaA-and alphaB-Crystallin. J. Protein Chem. 21, 65–71. [DOI] [PubMed] [Google Scholar]
- (46).Kumar MS, Kapoor M, Sinha S, and Reddy GB (2005) Insights into hydrophobicity and the chaperone-like function of alphaA-and alphaB-crystallins: an isothermal titration calorimetric study. J. Biol. Chem. 280, 21726–21730. [DOI] [PubMed] [Google Scholar]
- (47).Delbecq SP, and Klevit RE. (2018) HSPB5 engages multiple states of a destabilized client to enhance chaperone activity in a stress-dependent manner. J. Biol. Chem., jbc.RA118.003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Cox D, Selig E, Griffin MD, Carver JA, and Ecroyd H (2016) Small Heat-shock Proteins Prevent alpha-Synuclein Aggregation via Transient Interactions and Their Efficacy Is Affected by the Rate of Aggregation. J. Biol. Chem. 291, 22618–22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Santhoshkumar P, Murugesan R, and Sharma KK (2009) Deletion of (54)FLRAPSWF(61) residues decreases the oligomeric size and enhances the chaperone function of alphaB-Crystallin. Biochemistry 48, 5066–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Simon S, Michiel M, Skouri-Panet F, Lechaire JP, Vicart P, and Tardieu A (2007) Residue R120 is essential for the quaternary structure and functional integrity of human alphaB-Crystallin. Biochemistry 46, 9605–9614. [DOI] [PubMed] [Google Scholar]
- (51).Kumar LV, and Rao CM (2000) Domain swapping in human alpha A and alpha B crystallins affects oligomerization and enhances chaperone-like activity. J. Biol. Chem. 275, 22009–22013. [DOI] [PubMed] [Google Scholar]
- (52).Ghosh JG, and Clark JI (2005) Insights into the domains required for dimerization and assembly of human alphaB Crystallin. Protein Sci. 14, 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Treweek TM, Ecroyd H, Williams DM, Meehan S, Carver JA, and Walker MJ (2007) Site-directed mutations in the C-terminal extension of human alphaB-Crystallin affect chaperone function and block amyloid fibril formation. PLoS One 2, No. e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


