Abstract
Background:
Stillbirth, defined as fetal death ≥20 weeks’ gestation, is associated with poor fetal growth and is often attributed to placental abnormalities, which are also associated with poor fetal growth. Evaluating inter-relationships between placental abnormalities, poor fetal growth, and stillbirth may improve our understanding of the underlying mechanisms for some causes of stillbirth.
Objective:
Our primary objective was to determine whether poor fetal growth, operationalized as small for gestational age (SGA), mediates the relationship between placental abnormalities and stillbirth.
Methods:
We used data from the Stillbirth Collaborative Research Network study, a population-based case-control study conducted from 2006-2008. Our analysis included 266 stillbirths and 1,135 live births. We evaluated associations of stillbirth with five types of placental characteristics (developmental disorders, maternal and fetal inflammatory responses, and maternal and fetal circulatory disorders) and examined mediation of these relationships by SGA. We also assessed exposure-mediator interaction. Models were adjusted for maternal age, race/ethnicity, education, body mass index, parity, and smoking status.
Results:
All five placental abnormalities were more prevalent in cases than controls. After adjustment for potential confounders, maternal inflammatory response (odds ratio [OR]: 2.58; 95% confidence interval [CI]: 1.77, 3.75), maternal circulatory disorders (OR: 4.14; 95% CI: 2.93, 5.84), and fetal circulatory disorders (OR: 4.58; 95% CI: 3.11, 6.74) were strongly associated with stillbirth, and the relationships did not appear to be mediated by SGA status. Associations for developmental disorders and fetal inflammatory response diverged for SGA and non-SGA births, and strong associations were only observed when SGA was not present.
Conclusions:
Fetal growth did not mediate the relationships between placental abnormalities and stillbirth. The relationships of stillbirth with maternal and fetal circulatory disorders and maternal inflammatory response appear to be independent of poor fetal growth, while developmental disorders and fetal inflammatory response likely interact with fetal growth to affect stillbirth risk.
Keywords: Placenta, infant, small for gestational age, fetal growth retardation, stillbirth
Background
In the United States, roughly 1 in 160 pregnancies end in stillbirth, defined as fetal death at or after 20 weeks’ gestation.1 Many placental abnormalities are more common in stillbirths compared to live births, although this may differ by gestational age.2 Additionally, while a potential cause of death is never identified for many stillbirths, one systematic review reported that 11.2-64.9% of stillbirths are attributable to placental abnormalities.3 Identification of placental abnormalities is often based on postnatal placental assessment, which is a key component of stillbirth evaluation.4 However, evaluating placental function during pregnancy, including the use of biomarkers to assess placental function, has proven only marginally useful for reducing the risk of stillbirth.5
Placental abnormalities have also been associated with abnormal fetal growth. Inadequate placental function may prevent the placenta from meeting the needs of the fetus.6 Additionally, poor fetal growth is associated with increased risk of stillbirth.7 In evaluating twenty-five placental abnormalities, Bukowski et al. (2017) reported that ten were related to both stillbirth and fetal growth, five were related to stillbirth but not fetal growth, one was related to fetal growth but not stillbirth, and nine were not related to stillbirth or fetal growth.8 While poor fetal growth has been reported as a risk factor for stillbirth, not every growth restricted fetus is stillborn.9
We sought to build on this work by estimating stillbirth risk in relation to placental abnormalities. Given the role of the placenta in regulating fetal growth, we also evaluated whether these associations were mediated by poor fetal growth. Placental abnormalities of interest included indicators of developmental disorders, maternal inflammatory response, fetal inflammatory response, maternal circulatory disorders, and fetal circulatory disorders.
Methods
Case-control selection
The Stillbirth Collaborative Research Network (SCRN) study was a multicenter, ethnically, racially, and geographically diverse case-control study of stillbirth. Enrollment occurred between March 2006 and September 2008 from 59 hospitals representing five catchment areas of the United States: Rhode Island and counties in Massachusetts, Georgia, Texas, and Utah. The final study included 663 women with a stillbirth (cases) and 1,932 women with a live birth (controls) selected through a stratified random method.10 Details of the study design, including sampling methods, are available.10
Women had the option to consent to a placental examination, which was conducted by trained perinatal pathologists using a standardized, study-specific protocol.11 Pathologists also participated in centralized workshops to ensure consistent and standardized placental assessment. Of the 663 women with a stillbirth, 98.6% consented to a placental examination.11 Among the 573 singleton stillbirths, the placental examination was completed and considered adequate in 483 (Figure 1). Similarly, of the 1,932 women with a live birth, 93.4% consented to a placental examination.11 The placental examination was completed and considered adequate for 1,135 (Figure 1). The most common reason for an incomplete placental examination was the placenta being discarded before collection.11 Information on covariates, including maternal, pregnancy, and neonatal characteristics, was obtained from medical chart abstraction and maternal interview.
Figure 1.
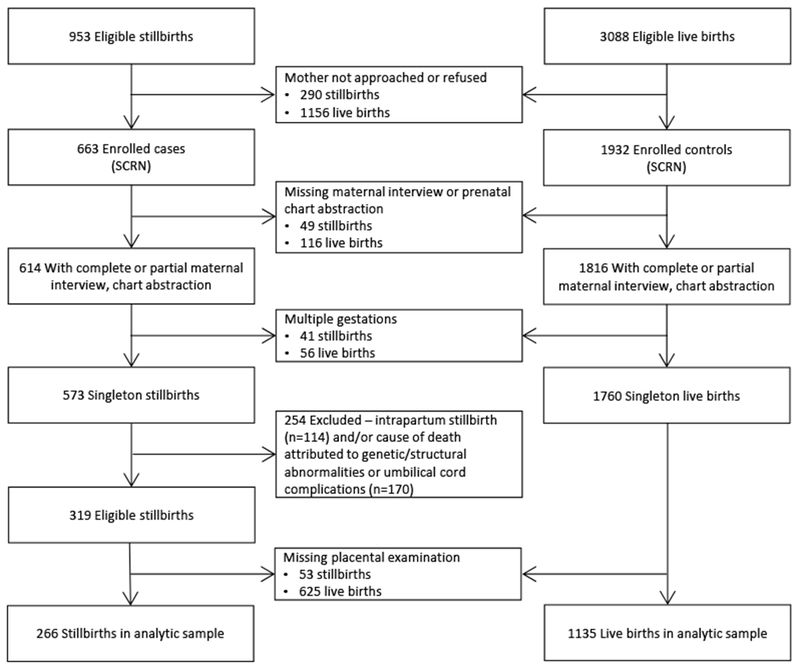
Stillbirth Collaborative Research Network study (2006-2008) enrollment and inclusion.
Our secondary analysis was restricted to singleton stillbirths and live births with a complete placental examination (Figure 1). Additionally, we excluded intrapartum stillbirths and stillbirths with a cause of death attributed to genetic/structural abnormalities or umbilical cord complications, as these stillbirths are likely unrelated to the mechanisms of interest.12, 13 Our analytic sample included 266 stillbirths and 1,135 live births. Data weights were calculated to account for differential consent for enrolment, study design characteristics (oversampling of certain groups), and availability of the placental examination. Weights for the availability of the placental examination incorporated clinic site, induction, time trend, delivery time, weekend delivery, and mode of delivery. Additional information on data weights are also published.10
Exposure
We classified placental characteristics into five groups: developmental disorders, maternal inflammatory response, fetal inflammatory response, maternal circulatory disorders, and fetal circulatory disorders (see Table 1 for definitions of these groups). Placental group variables were then dichotomized based on presence of one or more characteristics within each group. Placentas could have characteristics for more than one group and each placental group variable was analyzed in a separate model. We approximated poor fetal growth using small for gestational age (SGA), defined as a birthweight for gestational age < 10th percentile. SGA was determined from adapting individualized norms using estimated fetal weight developed by Bukowski et al., which adjust for pregnancy characteristics.14 For stillbirths, SGA status was determined using estimated gestational age at fetal death to partially account for potential misclassification of SGA status.15, 16 Additional details on the modifications made to the individualized norms in this study have been published.15
Table 1.
Placental characteristics of singleton stillbirths and live births from the Stillbirth Collaborative Research Network Study (2006-2008) using the weighted sample.
| Characteristic | Stillbirths %w | Live births %w |
|---|---|---|
| N=266 | N=1135 | |
| Nw=262 | Nw=915 | |
| Developmental Disorders | ||
| Any Developmental Disorder | 20.3 | 11.0 |
| Umbilical cord | ||
| Single umbilical artery | 5.2 | 1.8 |
| Velamentous insertion | 3.2 | 1.1 |
| Furcate insertion | 1.9 | 3.6 |
| Placental Membranes | ||
| Circummarginate insertion | 1.0 | 0.5 |
| Circumvallate insertion | 0.7 | 1.0 |
| Fetal villous capillaries | ||
| Terminal villous immaturity (diffuse) | 8.3 | 2.4 |
| Terminal villous hypoplasia (diffuse) | 3.5 | 1.9 |
| Inflammatory Disorders | ||
| Any Maternal inflammatory response | 34.7 | 15.6 |
| Acute chorioamnionitis - placental membranes | 30.9 | 11.9 |
| Acute chorioamnionitis - chorionic plate | 24.0 | 11.9 |
| Any Fetal inflammatory response | 13.9 | 8.5 |
| Acute funisitis | 11.6 | 3.4 |
| Acute umbilical cord arteritis (≥1 artery) | 4.2 | 1.8 |
| Acute umbilical cord phlebitis | 5.4 | 3.0 |
| Chorionic plate acute vasculitis | 7.2 | 4.9 |
| Circulatory Disorders | ||
| Any Maternal circulatory disorder | 58.8 | 29.6 |
| Retroplacental hematoma | 27.5 | 4.2 |
| Parenchymal infarction | 29.6 | 15.8 |
| Intraparenchymal thrombus | 18.4 | 13.6 |
| Perivillous, intervillous fibrin, fibrinoid deposition | 7.9 | 1.5 |
| Any Fetal circulatory disorder | 41.5 | 14.5 |
| Fetal vascular thrombi in the chorionic plate | 24.4 | 7.3 |
| Avascular villi | 19.4 | 6.9 |
| Placental edema | 5.3 | 1.1 |
Abbreviations: Nw – weighted sample size; %w – weighted percent
Outcome
Our primary outcome was stillbirth, defined as fetal death ≥20 weeks’ gestation.10 Stillbirths were identified based on APGAR scores of zero at one and five minutes after delivery or no signs of life by direct observation.
Statistical analysis
We conducted a mediation analysis using logistic models to evaluate the total association of stillbirth with placental characteristics, the indirect effect of this association that operates through fetal growth (mediation), and the direct effect that operates independent of fetal growth (eFigure 1). We also estimated the proportion of the total effect that is due to mediation. Mediation models additionally allowed for interaction between placental abnormalities and fetal growth (exposure-mediator interaction). Mediation models were operationalized using a SAS macro developed by Valeri and VanderWeele (with minor modifications to accommodate complex sample design weights) that uses a counterfactual approach to mediation analyses, allows for exposure-mediator interaction, and supports dichotomous mediators and outcomes.17 To satisfy assumptions for mediation analyses, models were adjusted for potential exposure-outcome, exposure-mediator, and mediator-outcome confounders identified using the directed acyclic graph approach. Potential confounders included race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), maternal education (0-11, 12, ≥13 years), maternal age (<20, 20-34, 35-39, ≥40 years), body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), parity (nulliparous, multiparous), and smoking status (0, <10, ≥10 cigarettes per day in the three months prior to pregnancy), as these characteristics may be related to placental morphology, fetal growth, and birthweight. We did not control for placental weight, as this may be on the causal pathway. We performed all analyses using SAS version 9.4 (SAS Institute INC., Cary, North Carolina) and SUDAAN version 11.0 (Research Triangle Institute, Research Triangle Park, North Carolina).18
Missing data
Of the 319 eligible cases and 1,760 eligible controls, placentas were not collected for 16.6% and 35.5%, respectively (Figure 1). Sample weights were calculated to account for characteristics related to missing placental pathology data.10, 11 Outcome data were available for all participants, 1.1% were missing data on fetal growth data (birthweight percentile), and 1.5% were excluded for missing information on ≥1 covariate.
Sensitivity analyses
For the primary analysis, we calculated E-values to assess the strength of an unmeasured confounder necessary to explain away the observed associations, as well as the strength of an unmeasured confounder necessary to result in the confidence interval crossing the null.19, 20 In addition, we conducted four sensitivity analyses. First, we controlled for maternal conditions (chronic hypertension/preeclampsia and pre-gestational diabetes/gestational diabetes) since these may affect placental development, fetal growth, and stillbirth. Due to uncertainty regarding direction of the relationships, these maternal conditions were not included in the primary analysis. Second, we defined SGA as birthweight <5th percentile to partially account for potential misclassification of stillbirths as SGA due to decline in birthweight after fetal death. Third, we restricted controls (live births) to term births since preterm live births do not reflect healthy ongoing pregnancies. Fourth, we controlled for the other types of placental pathology in each model to account for potential interrelationships.
Ethics approval
The study was approved by Institutional Review Boards at each clinical site, and all participants gave written informed consent.
Results
Descriptive characteristics
Placental markers of developmental disorders, maternal inflammatory markers, fetal inflammatory markers, maternal circulatory disorders, and fetal circulatory disorders were all more prevalent among stillbirths compared to live births based on presence of one or more characteristic (Table 1). Stillbirths in this analysis had a higher prevalence of SGA (41.2%) and preterm birth (<37 weeks’ gestation; 81.7%) compared to live births (15.0% and 9.0%, respectively) (Table 2). Women with live births were more likely to be non-Hispanic white (44.1%), married (61.2%), and to have a body mass index within the normal range (50.9%) than women with stillbirths (32.5%, 45.5%, and 39.7%, respectively).
Table 2.
Descriptive characteristics of singleton stillbirths and live births from the Stillbirth Collaborative Research Network Study (2006-2008) using the weighted sample.
| Characteristic | Stillbirths %w | Live births %w |
|---|---|---|
| N=266 | N=1135 | |
| Nw=262 | Nw=915 | |
| Adjusted birth weight percentilea | ||
| <5th percentile | 33.9 | 9.0 |
| 5th-10th percentile | 7.3 | 6.0 |
| 10th-25th percentile | 11.6 | 15.0 |
| 25th-50th percentile | 15.7 | 23.7 |
| 50th-75th percentile | 8.2 | 21.2 |
| 75th-90th percentile | 8.1 | 11.7 |
| 90th-95th percentile | 4.6 | 5.6 |
| 95th-100th percentile | 10.5 | 7.8 |
| Gestational Age | ||
| <20 completed weeks | 1.3 | 0.0 |
| 20-23 completed weeks | 29.4 | 0.3 |
| 24-27 completed weeks | 14.3 | 0.4 |
| 28-31 completed weeks | 15.9 | 0.7 |
| 32-36 completed weeks | 20.7 | 7.6 |
| ≥37 completed weeks | 18.3 | 91.0 |
| Maternal Age, years | ||
| <20 | 15.5 | 10.4 |
| 20 – 34 | 70.5 | 76.1 |
| 35 – 39 | 12.3 | 11.0 |
| ≥40 | 1.7 | 2.5 |
| Maternal Race/Ethnicity | ||
| Non-Hispanic white | 32.5 | 44.1 |
| Non-Hispanic black | 22.4 | 11.7 |
| Hispanic | 38.9 | 37.1 |
| Other | 6.1 | 7.1 |
| Maternal Education | ||
| 0 – 11 (none/primary/some secondary) | 25.3 | 18.4 |
| 12 (completed secondary) | 30.8 | 27.2 |
| ≥13 (college) | 43.8 | 54.4 |
| Marital Status | ||
| Not married or cohabitating | 27.2 | 15.7 |
| Cohabitating | 27.3 | 23.1 |
| Married | 45.5 | 61.2 |
| Maternal BMI | ||
| <18.5 | 2.6 | 2.8 |
| 18.5 – 24.9 | 39.7 | 50.9 |
| 25 – 29.9 | 25.7 | 22.3 |
| 30 – 34.9 | 18.0 | 12.7 |
| ≥35 | 14.0 | 11.2 |
| Pre-gestational diabetes/gestational diabetes | 9.0 | 10.1 |
| Chronic hypertension/preeclampsia | 12.0 | 9.2 |
| Insurance | ||
| No insurance | 7.1 | 4.3 |
| Any public/private insurance | 58.3 | 49.8 |
| Veterans Affairs/commercial health ins/health maintenance organization | 34.6 | 45.9 |
| Maternal Smoking Statusb | ||
| Did not smoke | 80.3 | 87.3 |
| < 10 | 10.2 | 6.5 |
| ≥ 10 | 9.5 | 6.2 |
| Alcohol Usec | ||
| Did not drink | 59.6 | 58.8 |
| Drank, no binging | 18.6 | 23.3 |
| Binged | 21.8 | 17.8 |
| Illicit Drug Used | ||
| Never used drugs | 68.7 | 71.7 |
| Ever used drugs w/o addiction | 26.9 | 26.2 |
| Ever used drugs w/ addiction | 4.4 | 2.0 |
Abbreviations: BMI – body mass index; Nw – weighted sample size; %w – weighted percent
Average number of cigarettes during three months prior to pregnancy, self-reported
Alcohol consumption during three months prior to pregnancy, self-reported
Lifetime drug use, self-reported
Analytic results
In adjusted models, presence of one or more developmental disorder (odds ratio [OR]: 1.94; 95% confidence interval [CI]: 1.29, 2.94), maternal inflammatory response (OR: 2.58; 95% CI: 1.77, 3.75), maternal circulatory disorder (OR: 4.14; 95% CI: 2.93, 5.84), and fetal circulatory disorder (OR: 4.58; 95% CI: 3.11, 6.74) were associated with increased odds of stillbirth (Table 3). Associations between placental characteristics and stillbirth operating through poor fetal growth (natural indirect effects, eFigure 1) were weak, with ORs ranging from 0.99 to 1.15 and proportions mediated ranging from −3.0% to 16.7%. Thus, associations between placental characteristics and stillbirth not operating through poor fetal growth (natural direct effects) were similar to the total effects.
Table 3.
Results examining mediation of the relationship between placental abnormalities and stillbirth by small for gestational age status using the Stillbirth Collaborative Research Network Study (2006–2008).
| Total Effect | Natural Direct Effect | Natural Indirect Effect | Controlled Direct Effect | % Mediated | ||
|---|---|---|---|---|---|---|
| SGAa | Not SGAa | |||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| ≥ 1 Developmental disorderb,c | 1.94 (1.29, 2.94) | 1.84 (1.23, 2.77) | 1.05 (0.95, 1.17) | 1.03 (0.49, 2.18) | 2.40 (1.49, 3.87)d | 9.9 |
| ≥ 1 Maternal inflammatory responseb,c | 2.58 (1.77, 3.75) e | 2.52 (1.77, 3.59) | 1.02 (0.91, 1.15) | 2.10 (1.12, 3.92) | 2.80 (1.83, 4.28) | 3.2 |
| ≥ 1 Fetal inflammatory responseb,c | 1.52 (0.95, 2.45) | 1.53 (0.96, 2.45) | 0.99 (0.96, 1.03) | 0.70 (0.27, 1.83) | 2.14 (1.26, 3.65) f | −3.0 |
| ≥ 1 Maternal circulatory disorderb,c | 4.14 (2.93, 5.84) g | 3.85 (2.78, 5.32) | 1.07 (0.96, 1.21) | 4.24 (2.40, 7.52) | 3.65 (2.49, 5.36) | 8.6 |
| ≥ 1 Fetal circulatory disorderb,c | 4.58 (3.11, 6.74) h | 4.00 (2.84, 5.62) | 1.15 (0.97, 1.35) | 4.63 (2.53, 8.48) | 3.68 (2.45, 5.54) | 16.7 |
Abbreviations: CI – confidence interval; OR – odds ratio; SGA – small for gestational age
Reference is 0 characteristics of the respective disorder group
Model contains each placental abnormality group modeled individually, adjusted for race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), maternal education (0-11, 12, ≥13 years), maternal age (<20, 20-34, 35-39, ≥40 years), body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), parity (nulliparous, multiparous), and smoking (did not smoke, <10, ≥10 cigarettes during three months prior to pregnancy)
E-value19, 20: 4.2 (minimum strength of the association of the unmeasured confounder that would explain away the observed association), E-value for confidence interval (minimum strength of the association of the confounder that would result in a confidence interval including the null value of 1.0): 2.3
E-value for estimate: 4.6; E-value for confidence interval: 2.9
E-value for estimate: 3.7; E-value for confidence interval: 1.8
E-value for estimate: 7.8; E-value for confidence interval: 5.3
E-value for estimate: 8.6; E-value for confidence interval: 5.7
The direct effect of developmental disorders and fetal inflammatory response on stillbirth diverged depending on whether the fetus was SGA (controlled direct effects), supporting exposure-mediator interaction. Stronger associations were observed among non-SGA births compared to SGA births. Associations of stillbirth with maternal inflammatory response, maternal circulatory disorders, and fetal circulatory disorders remained strong regardless of SGA status. E-values for the strength of confounding necessary to explain the observed associations ranged from 3.7 to 8.6. Results were consistent in sensitivity analyses controlling for maternal conditions (Table 4), using the 5th percentile as the cutoff for SGA (Table 5), restricting controls to term live births (eTable 1), and adjusting each model for the other types of placental pathology (eTable 2).
Table 4.
Sensitivity analysis examining mediation of the relationship between placental abnormalities and stillbirth by small for gestational age status controlling for maternal pregnancy complications using the Stillbirth Collaborative Research Network Study (2006-2008).
| Total Effect | Natural Direct Effect | Natural Indirect Effect | Controlled Direct Effect | % Mediated | ||
|---|---|---|---|---|---|---|
| SGAa | Not SGAa | |||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| ≥ 1 Developmental disorderb,c | 2.02 (1.34, 3.06) | 1.92 (1.25, 2.96) | 1.05 (0.95, 1.17) | 0.96 (0.45, 2.03) | 2.59 (1.60, 4.12) | 9.4 |
| ≥ 1 Maternal inflammatory responseb,c | 2.59 (1.76, 3.82) | 2.48 (1.73, 3.56) | 1.05 (0.92, 1.19) | 2.19 (1.15, 4.15) | 2.66 (1.73, 4.10) | 7.7 |
| ≥ 1 Fetal inflammatory responseb,c | 1.41 (0.86, 2.32) | 1.42 (0.87, 2.32) | 0.99 (0.94, 1.05) | 0.72 (0.27, 1.92) | 1.92 (1.10, 3.37) | −3.5 |
| ≥ 1 Maternal circulatory disorderb,c | 4.18 (2.92, 5.97) | 3.96 (2.83, 5.52) | 1.06 (0.93, 1.20) | 4.56 (2.54, 8.20) | 3.64 (2.47, 5.38) | 7.4 |
| ≥ 1 Fetal circulatory disorderb,c | 4.48 (3.03, 6.63) | 3.86 (2.73, 5.45) | 1.16 (0.98, 1.38) | 4.27 (2.32, 7.86) | 3.65 (2.41, 5.54) | 17.8 |
Abbreviations: CI – confidence interval; OR – odds ratio; SGA – small for gestational age
Reference is 0 characteristics of the respective disorder group
Model contains each placental abnormality group modeled individually, adjusted for race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), maternal education (0-11, 12, ≥13 years), maternal age (<20, 20-34, 35-39, ≥40 years), body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), parity (nulliparous, multiparous), smoking (did not smoke, <10, ≥10 cigarettes during three months prior to pregnancy), chronic hypertension/preeclampsia, and pre-gestational diabetes/gestational diabetes
Table 5.
Sensitivity analysis examining mediation of the relationship between placental abnormalities and stillbirth by small for gestational age status defined as birthweight <5th percentile using the Stillbirth Collaborative Research Network Study (2006-2008).
| Total Effect | Natural Direct Effect | Natural Indirect Effect | Controlled Direct Effect | % Mediated | ||
|---|---|---|---|---|---|---|
| SGAa | Not SGAa | |||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| ≥ 1 Developmental disorderb,c | 1.96 (1.30, 2.96) | 1.85 (1.23, 2.78) | 1.06 (0.94, 1.19) | 0.77 (0.33, 1.84) | 2.38 (1.51, 3.74) | 11.6 |
| ≥ 1 Maternal inflammatory responseb,c | 2.60 (1.78, 3.81) | 2.60 (1.82, 3.71) | 1.00 (0.88, 1.14) | 2.11 (0.99, 4.49) | 2.84 (1.91, 4.23) | 0.0 |
| ≥ 1 Fetal inflammatory responseb,c | 1.53 (0.95, 2.46) | 1.53 (0.95, 2.46) | 1.00 (0.97, 1.03) | 0.56 (0.18, 1.78) | 2.04 (1.23, 3.40) | 0.0 |
| ≥ 1 Maternal circulatory disorderb,c | 4.20 (2.95, 5.96) | 3.72 (2.69, 5.14) | 1.13 (0.99, 1.28) | 3.63 (1.85, 7.11) | 3.74 (2.61, 5.38) | 15.1 |
| ≥ 1 Fetal circulatory disorderb,c | 4.48 (2.93, 6.83) | 4.54 (3.10, 6.65) | 0.99 (0.81, 1.21) | 6.45 (2.95, 14.11) | 3.80 (2.59, 5.59) | −1.3 |
Abbreviations: CI – confidence interval; OR – odds ratio; SGA – small for gestational age
Reference is 0 characteristics of the respective disorder group
Model contains each placental abnormality group modeled individually, adjusted for race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), maternal education (0-11, 12, ≥13 years), maternal age (<20, 20-34, 35-39, ≥40 years), body mass index (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), parity (nulliparous, multiparous), and smoking (did not smoke, <10, ≥10 cigarettes during three months prior to pregnancy)
Comment
Principal findings
Overall, we did not find that poor fetal growth, approximated by SGA, mediates the relationship between placental abnormalities and stillbirth. We observed consistent and strong associations of stillbirth with maternal and fetal circulatory disorders and maternal inflammatory response regardless of SGA status. These results suggest that indicators of maternal and fetal circulatory disorders and maternal inflammatory response in the placenta may affect the risk of stillbirth through a mechanism that does not influence fetal growth. The associations between stillbirth and both developmental disorders and fetal inflammatory response appeared to depend on whether or not the infant was SGA, suggesting that placental indicators of these conditions may operate through a mechanism influenced by (or that influences) fetal growth. Further, the E-values suggest that an unmeasured or unknown confounder would need to be strongly associated with both the exposure and the outcome, independent of the included confounders, in order to explain the observed associations.
Strengths of the study
Strengths of the SCRN study include recruitment of a large and diverse sample and the use of a thorough and standardized placental examination conducted by trained pathologists. Analytic strengths include the use of a data weight to account for differences in those who did not consent to a placental examination and for whom the examination was incomplete. We also evaluated the strength of unmeasured confounding necessary to explain the observed associations and conducted four sensitivity analyses that yielded consistent results.
Limitations of the data
One limitation of our analysis is potential selection bias due to consent to and availability of placental examination. Placental examinations were incomplete for a larger proportion of live births as compared to stillbirths (Figure 1).11 However, characteristics related to completion of a placental examination, including study site, induction, mode of delivery, year of study, time of day of delivery, and weekend delivery, were incorporated into the data weight as an attempt to account for these differences.
Potential exposure and mediator misclassification is also limitation of this analysis. Pathologists conducted fetal autopsies and thus were not masked to the outcome of the pregnancy when conducting the placental examination, which could have affected their reporting. Similarly, defining SGA as <10th percentile may misclassify some infants. However, when we conducted a sensitivity analysis using the 5th percentile as a cutoff, the results were similar. SGA misclassification could also be differential. Stillbirths may experience a reduction in weight between fetal death and delivery,21 though results were consistent when stillbirths were restricted to those delivered within one week of fetal death (results not shown).
Another important limitation of our work is the assumption that, within a given group (e.g., developmental disorders), all placental characteristics would affect stillbirth risk in a similar manner. Individual indicators likely reflect differing mechanisms and grouping the indicators may mask associations with some individual indicators. However, we were underpowered to evaluate individual indicators due to the low prevalence of some indicators. Nonetheless, the observed summary associations would likely be biased towards the null relative to the strongest of those factors. Additionally, the inclusion of preterm births in the live birth group is a limitation of our work. Preterm live births are different from term live births and do not reflect healthy ongoing pregnancies at a given gestational age. Placental abnormalities, including fetal inflammatory markers, are also associated with preterm birth.22, 23 To account for this, we conducted a sensitivity analysis restricting controls to term births and results were consistent. Similarly, we were unable stratify by gestational age to evaluate the potential impact of gestational age at fetal death on the association between placental abnormalities and stillbirth due to concerns regarding the comparison of preterm live births and stillbirths and due to the small number of term stillbirths.
Interpretation
Consistent with our previous work, we found that placental abnormalities are associated with stillbirth, but the inter-relationships with poor fetal growth vary. Bukowski et al. (2017) identified five patterns of relationships between placental abnormalities, stillbirth, and fetal growth abnormalities. Characteristics related to maternal inflammatory response and maternal and fetal circulatory disorders were associated with stillbirth but not fetal growth abnormalities.8 This is consistent with our finding that the maternal inflammatory response and maternal and fetal circulatory disorders were associated with stillbirth independent of poor fetal growth. The current analyses extend our prior work by estimating the strength of the associations between various placental abnormalities and stillbirth and by evaluating mediation by poor fetal growth. Further, our analyses suggest that although these placental characteristics are associated with both fetal growth and stillbirth, these characteristics are unlikely to affect the risk of stillbirth through poor fetal growth. This might explain the difficulties that others note in preventing stillbirth by examining placental function.5
The weaker association between the fetal inflammatory response and stillbirth may reflect the nature of the fetal inflammatory response. The fetal inflammatory response is often related to, and preceded by, the maternal inflammatory response and may reflect a more severe condition.24 Thus, infants with evidence of a fetal inflammatory response past 20 weeks may have had to survive a significant inflammatory insult and survival bias could explain this apparent paradox. SGA fetuses with fetal inflammation may have a substantially increased risk of fetal death prior to 20 weeks’ gestation, which may explain the protective (though non-significant) association in SGA infants. Inflammation may also lead to stillbirth and preterm live birth via pathways independent of fetal growth or placental function. Similarly, survival bias may explain the null association between developmental disorders and stillbirth among SGA infants, as the double insult of developmental disorders and poor fetal growth may result in fetal death prior to 20 weeks’ gestation.
Conclusions
Our results extend earlier findings of the inter-relationships between placental abnormalities, fetal growth, and stillbirth by highlighting the importance of placental abnormalities to stillbirth and by conducting novel analyses suggesting that the association between stillbirth and a variety of placental abnormalities is not mediated by poor fetal growth. We observed that maternal circulatory disorders, fetal circulatory disorders, and the maternal inflammatory response operate independently of poor fetal growth. However, the risk of stillbirth related to developmental disorders and the fetal inflammatory response may be linked to fetal growth. This is an important finding, given reported associations of placental abnormalities with stillbirth and the role of the placenta in regulating fetal growth. In conclusion, our results support different placental mechanisms related to stillbirth and poor fetal growth. Future studies should build on this by examining fetal losses at all gestational ages to reduce the impact of survival bias.
Supplementary Material
Social Media Quote.
While placental abnormalities have been implicated in both poor fetal growth and stillbirth, and poor fetal growth is associated with stillbirth, our results indicate that poor fetal growth does not mediate the relationship between placental abnormalities and stillbirth.
Synopsis.
Study question:
Does poor fetal growth mediate the relationship between placental abnormalities and stillbirth?
What’s already known:
Both placental abnormalities and poor fetal growth are associated with stillbirth. Further, placental abnormalities are associated with poor fetal growth.
What this study adds:
We evaluated inter-relationships between placental abnormalities, poor fetal growth, and risk of stillbirth and found that poor fetal growth does not mediate the relationship between placental abnormalities and stillbirth.
Acknowledgements
We acknowledge the contribution of the Stillbirth Collaborative Research Network. We also acknowledge the members of the National Institute of Child Health and Human Development Scientific Advisory and Safety Monitoring Board for their review of the study protocol, materials, and progress, as well as all of the other physicians, study coordinators, and research nurses in the Stillbirth Collaborative Research Network.
The following institutions, clinical site investigators, and staff are part of the Stillbirth Collaborative Research Network: University of Texas Health Science Center at San Antonio: Dr. Donald J. Dudley, Dr. Deborah Conway, Josefine Heim-Hall, Karen Aufdemorte, and Angela Rodriguez; University of Utah School of Medicine: Dr. Robert M. Silver, Dr. Michael W. Varner, and Kristi Nelson; Emory University School of Medicine and Rollins School of Public Health: Dr. Carol J. Hogue, Dr. Barbara J. Stoll, Janice Daniels Tinsley, Dr. Bahig Shehata, and Dr. Carlos Abromowsky; Brown University: Dr. Donald Coustan, Dr. Halit Pinar, Dr. Marshall Carpenter, and Susan Kubaska; University of Texas Medical Branch at Galveston: Dr. George R. Saade, Dr. Radek Bukowski, Jennifer Lee Rollins, Dr. Hal Hawkins, and Elena Sbrana; RTI International: Dr. Corette B. Parker, Dr. Matthew A. Koch, Vanessa R. Thorsten, Holly Franklin, and Pinliang Chen; Pregnancy and Perinatology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development: Dr. Marian Willinger and Dr. Uma M. Reddy; Columbia University Medical Center: Dr. Robert L. Goldenberg.
The following comprise the Stillbirth Collaborative Research Network Writing Group: Dr. Carol J. Hogue (Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia); Dr. Robert L. Goldenberg (Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, New York); Dr. Radek Bukowski and Dr. George R. Saade (Department of Obstetrics and Gynecology, University of Texas Medical Branch at Galveston, Galveston, Texas); Dr. Barbara J. Stoll (McGovern Medical School, University of Texas Health Science Center, Houston, Texas); Dr. Marshall Carpenter, Dr. Donald Coustan, and Dr. Halit Pinar (Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Brown University School of Medicine, Providence, Rhode Island); Dr. Deborah Conway (Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Health Science Center at San Antonio, San Antonio, Texas); Dr. Donald J. Dudley (Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Virginia, Charlottesville, Virginia); Dr. Robert M. Silver and Dr. Michael W. Varner (Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Utah School of Medicine, and Maternal Fetal Medicine Unit, Intermountain Healthcare, Salt Lake City, Utah); Dr. Uma M. Reddy and Dr. Marian Willinger (Pregnancy and Perinatology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland); and Dr. Matthew A. Koch and Dr. Corette B. Parker (Statistics and Epidemiology Unit, Health Sciences Division, RTI International, Research Triangle Park, North Carolina).
Funding
A.A.F. was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant F31HD092025).
Footnotes
Conflict of Interest
None.
References
- 1.MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep 2012; 60:1–22. [PubMed] [Google Scholar]
- 2.Pinar H, Goldenberg RL, Koch MA, Heim-Hall J, Hawkins HK, Shehata B, et al. Placental findings in singleton stillbirths. Obstet Gynecol 2014; 123:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ptacek I, Sebire NJ, Man JA, Brownbill P, Heazell AE. Systematic review of placental pathology reported in association with stillbirth. Placenta. 2014; 35:552–562. [DOI] [PubMed] [Google Scholar]
- 4.Korteweg FJ, Erwich JJ, Timmer A, van der Meer J, Ravise JM, Veeger NJ, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol 2012; 206:53 e51–53 e12. [DOI] [PubMed] [Google Scholar]
- 5.Heazell AE, Whitworth M, Duley L, Thornton JG. Use of biochemical tests of placental function for improving pregnancy outcome. Cochrane Database Syst Rev 2015:CD011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadler TW. Chapter 8: Third Month to Birth: The fetus and Placenta In: Langman’s Medical Embryology. 13th edition ed. Philadelphia: Wolters Kluwer, 2015; pp. 105–125. [Google Scholar]
- 7.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis Lancet. 2011; 377:1331–1340. [DOI] [PubMed] [Google Scholar]
- 8.Bukowski R, Hansen NI, Pinar H, Willinger M, Reddy UM, Parker CB, et al. Altered fetal growth, placental abnormalities, and stillbirth. PLoS One. 2017; 12:e0182874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver RM. Examining the link between placental pathology, growth restriction, and stillbirth. Best Pract Res Clin Obstet Gynaecol 2018; 49:89–102. [DOI] [PubMed] [Google Scholar]
- 10.Parker CB, Hogue CJ, Koch MA, Willinger M, Reddy UM, Thorsten VR, et al. Stillbirth Collaborative Research Network: design, methods and recruitment experience. Paediatr Perinat Epidemiol 2011; 25:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Shehata B, Thorsten VR, et al. The Stillbirth Collaborative Research Network (SCRN) placental and umbilical cord examination protocol. Am J Perinatol 2011; 28:781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stillbirth Collaborative Research Network Writing G. Causes of death among stillbirths. JAMA. 2011; 306:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley DJ, Goldenberg R, Conway D, Silver RM, Saade GR, Varner MW, et al. A new system for determining the causes of stillbirth. Obstet Gynecol 2010; 116:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bukowski R, Uchida T, Smith GC, Malone FD, Ball RH, Nyberg DA, et al. Individualized norms of optimal fetal growth: fetal growth potential. Obstet Gynecol 2008; 111:1065–1076. [DOI] [PubMed] [Google Scholar]
- 15.Bukowski R, Hansen NI, Willinger M, Reddy UM, Parker CB, Pinar H, et al. Fetal growth and risk of stillbirth: a population-based case-control study. PLoS Med 2014; 11:e1001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conway DL, Hansen NI, Dudley DJ, Parker CB, Reddy UM, Silver RM, et al. An algorithm for the estimation of gestational age at the time of fetal death. Paediatr Perinat Epidemiol 2013; 27:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013; 18:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SUDAAN Language Manual, Volumes 1 and 2, Release 11.0. Research Triangle Park, NC: Research Triangle Institute; 2012. [Google Scholar]
- 19.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017; 167:268–274. [DOI] [PubMed] [Google Scholar]
- 20.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Website and R package for computing E-values. Epidemiol 2018; 29:e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chard T Does the fetus lose weight in utero following fetal death: a study in preterm infants. BJOG. 2001; 108:1113–1115. [DOI] [PubMed] [Google Scholar]
- 22.Morgan TK. Role of the Placenta in Preterm Birth: A Review. Am J Perinatol 2016; 33:258–266. [DOI] [PubMed] [Google Scholar]
- 23.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015; 213:S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol 2012; 67:287–294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


