Abstract
Fragile X Syndrome (FXS) is a leading genetic cause of autism and intellectual disabilities. The Fmr1 knockout (KO) mouse is a commonly studied pre-clinical model of FXS. Adult male Fmr1 KO mice produce fewer ultrasonic vocalizations (USVs) during mating, suggestive of abnormal social communication. Minocycline treatment for 2 months from birth alleviates a number of FXS phenotypes in mice, including USV call rate deficits. In the current study, we investigated if treatment initiated past the early developmental period would be effective, given that in many cases, individuals with FXS are treated during later developmental periods. Wildtype (WT) and Fmr1 KO mice were treated with minocycline between postnatal day (P) 30 and P58. Mating-related US Vs were then recorded from these mice between P75 and P90 and analyzed for call rate, duration, bandwidth, and peak frequency. Untreated Fmr1 KO mice call at a significantly reduced rate compared to untreated WT mice. After minocycline treatment from 1–2 months of age, WT and Fmr1 KO mice exhibited similar call rates, due to an increase in calling in the latter group. Minocycline is thought to be effective in reducing FXS symptoms by lowering matrix-metalloproteinase-9 (MMP-9) levels. To determine whether abnormal MMP-9 levels underlie USV deficits, we characterized USVs in Fmr1 KO mice which were heterozygous for MMP-9 (MMP-9+/−/Fmr1 KO). The MMP-9+/−/Fmr1 KO mice were between P75 and P90 at the time of recording. MMP-9+/−/Fmr1 KO mice exhibited significantly increased USV call rates, at times even exceeding WT rates. Taken together, these results suggest that minocycline may reverse USV call rate deficits in Fmr1 KO mice through attenuation of MMP-9 levels. These data suggest targeting MMP-9, even in late development, may reduce FXS symptoms.
Keywords: Autism, Fragile X Syndrome, Minocycline, MMP-9, Ultrasonic Vocalization, Social Communication
1. Introduction
Fragile X Syndrome (FXS) is a leading genetic cause of intellectual and social communication disabilities and autism in humans, affecting 1 in 8000 females and 1 in 4000 males (Murray et al., 1997; Hagerman, 2008). FXS is caused by an expansion of CGG trinucleotide repeats in the 5’ untranslated region of the fragile X mental retardation (Fmr1) gene. This leads to a reduction or loss of fragile X mental retardation protein (FMRP, Pieretti et al., 1991), which is involved in normal synaptic development and plasticity (Darnell et al., 2011, Huber et al., 2011). FXS phenotypes in humans include a wide range of learning disabilities, cognitive impairment, hyperactivity, anxiety, and language deficits such as repetition of sounds and words and articulation difficulties (Hagerman et al., 2009; Hanson et al., 1986; Largo et al., 1985). The Fmr1 knockout (KO) mouse recapitulates various FXS-like phenotypes including learning deficits, anxiety, and sensory hypersensitivity (Bakker et al., 1994; McNaughton et al., 2008). Fmr1 KO mice also show abnormal social interactions including reduced ultrasonic vocalizations (USV) during mating interactions (Rotschafer et al., 2012).
Minocycline is an FDA-approved antibiotic that has recently been tested as a potential treatment for FXS (Paribello et al. 2010; Utari et al. 2010; Schneider et al., 2013). Eight weeks of oral administration of minocycline in FXS patients is associated with significant improvements in Aberrant Behavior Checklist-Community Edition Irritability Subscale scores. These subscales measure various problem behaviors within 5 domains, including lethargy, hyperactivity, stereotypy, and inappropriate speech (Paribello at al. 2010). Minocycline also improved language and behavioral functions in children with FXS based on caretaker reports (Utari et al., 2010). A randomized, double blind, placebo-controlled trial of children and adolescents (age 3.5–16 years) with up to 3 months of minocycline treatment showed improvement in the Clinical Global Impression Scale (Leigh et al., 2013). At the physiological level, minocycline treatment in humans with FXS reverses habituation deficits in sound evoked electrophysiological responses (Schneider et al., 2013). There is also evidence for reversal of various behavioral phenotypes in the Fmr1 KO mouse with minocycline treatment (Dansie et al., 2013). Minocycline treatment from birth to 2 months restores USV call rates of Fmr1 KO mice to WT levels (Rotschafer et al., 2012). In addition, KO mice treated for a month beginning a week after birth show less anxiety in the elevated plus maze and more exploratory behavior than untreated KO mice (Bilousova et al., 2009).
FXS is a neurodevelopmental disorder, but current clinical trials of drugs are conducted in adolescents and young adults. Few studies have compared treatments across different developmental ages in terms of efficacy in reversing symptoms. Dansie et al., (2013) showed that minocycline treatment for 4 or 8 weeks reverses hyperactivity and anxiety-like behaviors in Fmr1 KO mice, and beneficial effects were long lasting when treatment occurred during early development (Dansie et al., 2013). In our previous study, Fmr1 KO mice treated with minocycline from birth to 2 months restored USV call rate deficits to WT levels (Rotschafer et al., 2012). However, it remains unclear if the effectiveness was due to modification of vocalization-related circuits during a critical developmental period, or if late developmental treatment is sufficient to reverse USV call rates. Therefore, the first aim of this study was to determine if minocycline treatment in 1–2 month old mice would reverse USV call rate deficits. In order to test this, we analyzed mating related US Vs produced by Fmr1 KO mice that were administered minocycline between P30 and P58 (1–2 months of age). The mice were tested between P75 and P90 (2.5 – 3 months of age). In addition to call rates, we characterized properties of individual calls including duration, spectral bandwidth, and peak frequency to gain insight into whether syllable structure is abnormal in the Fmr1 KO mice.
Minocycline acts on multiple mechanisms in the brain including microglia function and apoptosis pathways (Yong et al., 2004). In addition, minocycline may reduce FXS symptoms through inhibition of matrix metalloproteinase-9 (MMP-9), an enzyme involved in cleavage of extracellular matrix components (Reinhard et al., 2015), including specialized assemblies found around inhibitory interneurons called perineuronal nets (Wen et al., 2018a,b). MMP-9 translation is regulated by FMRP and MMP-9 mRNA is a known FMRP target (Janusz et al., 2013). Indeed, MMP-9 levels are elevated in FXS (Dziembowska et al., 2013; Gkogkas et al., 2014; Sidhu et al., 2014; Lovelace, et al., 2016), and minocycline administration lowers plasma and brain MMP-9 levels in both humans and mice (Bilousova et al., 2009, Dziembowska et al., 2013). Genetic loss or reduction of MMP-9 in Fmr1 KO mice restores both structural and functional deficits associated with FXS, including dendritic spine abnormalities in adult hippocampus (Sidhu et al., 2014), impaired perineuronal net (PNN) formation around parvalbumin interneurons in the auditory cortex (Wen et al., 2018a), and auditory evoked potential habituation deficits in KO mice (Lovelace et al., 2016). As minocycline may restore FXS-related deficits through attenuation of MMP-9 levels (Siller and Broadie, 2011), the second aim of this study was to determine if genetic reduction of MMP-9 in Fmr1 KO mice would restore USV call rates to WT levels. In order to test this, we analyzed USVs in in 2.5–3 month old Fmr1 KO mice which were heterozygous for MMP-9 (MMP-9 +/−/Fmr1 KO).
2. Methods
2.1. Mice
Breeding pairs of FVB.Cg–Mmp–9tm1Tvu/J FVB.129P2-Pde6b+Tyrc-chFmr1tm1Cgr/J (Jax 004624; Fmr1 KO) and their congenic controls FVB.129P2-Pde6b+Tyrc-ch/AntJ controls (Jax 002848; WT) obtained from Jackson laboratories were housed in an accredited vivarium with 12 h light/dark cycle. The FVB.Cg-Mmp-9tm1Tvu/J mice were backcrossed, in-house, with Fmr1 KO or WT mice for at least five generations. Genetic reduction in MMP-9 levels was achieved by deleting only 1 allele of the Mmp9 gene in Mmp9 +/−/Fmr1 KO mice (Wen et al., 2018a). The MMP-9 +/−/Fmr1 KO mice (henceforth, ‘HET’ mice) were housed in the same vivarium room as all other mice used in this study. Neither the minocycline treated nor the Mmp9 +/−/Fmr1 KO mice show any overt deficits in behavior or appearance. At the time of USV recording, all mice were between 2.5 – 3 months of age. As minocycline treatment did not need to be administered in the HET mice, these mice were weaned into separate cages until they reached 2.5–3 months of age for USV recordings. All studies were performed in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee.
2.2. Minocycline administration
WT and Fmr1 KO mice were housed in separate cages. Minocycline (30 mg/kg) was dissolved in drinking water of WT and Fmr1 KO mice and provided every day for 28 days starting at P30. Water consumption was tracked daily by determining water levels with a graduated cylinder prior to adding fresh water. Previous studies have shown that this method of minocycline administration leads to detectable concentrations of minocycline in mouse blood (Lee et al., 2006). After minocycline treatment, USVs were recorded from male WT and Fmr1 KO mice between P75 and P90. None of the female partner mice received minocycline treatment. One of the major purposes of the present study was to compare our findings with data from Rotschafer et al., (2012) in which WT and Fmr1 KO mice were treated with minocycline from birth to 2 months of age, after which USVs were recorded ~3 months of age. To stay consistent with that protocol, and to identify if treatment between just 1 and 2 months of age (young adult) would have the same effect, we recorded USVs at 2.5 to 3 months age in this study.
2.3. Recording mouse vocalizations
The main goal of this study was to record USVs from mice in a social interaction task. We chose courtship behavior because the USVs generated in this context have been well characterized (Egnor et al., 2016) and are likely less influenced by developmental experience (Mahrt et al., 2013). Male mice produce stereotyped USVs when placed with a receptive female (Scattoni et al., 2009). We did not use female urine as a stimulus (Holy and Guo, 2005; Hodges et al., 2017) or record vocalizations after the female was removed (Belagodu et al., 2016) because these do not constitute the same dyadic social interactions as observed in courtship. In addition, because one of the goals of the study was to compare with our previous results in terms of optimal treatment windows with minocycline, we used similar methods with dyadic mixed-sex pairing. Females can produce acoustically similar calls as males in complex social interactions (e.g., 2 males, 2 females). In dyadic mixed-sex interactions, Warburton et al. (1989) concluded based on laryngeal nerve damage that males were the primary emitters of USVs. Nevertheless, to reduce the potential impact of genotype on female vocalizations in our study, we only used female Fmr1 KO mice as the partner mouse in all tests.
To record USV calls, virgin male WT or Fmr1 KO mice were placed in a 50.8 × 40.6 × 20.3 cm plastic recording chamber within a soundproof room (Gretch-Ken Industries Inc.) and allowed to habituate for 5 minutes. The female mouse was not in the chamber during this habituation period; it was only the test mouse for the first 5 minutes. Estrus was induced in virgin female Fmr1 KO mice 36 hours before pairing with a male, and then placed inside the recording chamber with WT or Fmr1 KO male mice for 10 minutes. USVs were recorded during this 10 minutes of pairing. Estrus was induced by i.p. injection of 0.06 mL of 0.6 mg/mL estradiol benzoate solution 36 hours prior to mating and 0.02 mL of 6 mg/mL progesterone 4 hours prior to mating (McGill, 1962). USVs were recorded with a full spectrum Petterssen D1000x (Pettersson Elektronik AB, Upsala, Sweden) bat detector (250 kHz sampling rate) placed 22 cm above the enclosure. This detector has been mostly used to record bat echolocation calls, with a few studies using it to record vocalizations of other mammals including genetic mouse models (Berger et al., 2012, Mervis et al., 2012, Kessler et al., 2014). The detector gain was consistent across pairings. USV calls were recorded when the following virgin male mice were paired with virgin female untreated Fmr1 KO mice: untreated WT (WT, n=7), untreated Fmr1 KO (KO, n=6), minocycline-treated WT (MTWT, n=6), minocycline-treated Fmr1 KO (MTKO, n=8), and MMP-9+/−/Fmr1 KO (HET, n=10). Once mice were paired one time for USV recording, they were not used again in this study.
2.4. Vocalization analysis
USVs were analyzed using Avisoft SASLab Pro software. Audio recordings were first cut to 1 minute segments. A bandpass filter from 30–100 kHz was used to remove all background noise outside the frequency range of interest. Thus only USVs were analyzed. A pulse train analysis was applied to capture and tag the calls that crossed a particular intensity threshold. The tag associated with each call was its respective chronological order number in the set of calls per minute. The pulse train analysis refers to the software function used to automatically extract the duration, bandwidth, and peak frequency information from each call that met the threshold for tagging. A threshold was individually set for each 1-minute segment of the sound file and placed in such a way that it captured all of the calls recorded in that time frame. Calls were then tagged and manually inspected. Tags were kept for calls which matched the known shapes of mouse USVs. Tags were removed if sounds that were not calls (background noise) were detected. These procedures resulted in the deletion of <3% of all tagged sounds. Due to the unique intensity threshold placed on each segment and subsequent manual inspection to delete background noise, it is likely that all USV calls recorded were detected as such.
Calls were then analyzed for call rate (USVs/minute), individual call duration (measured as the time between the beginning and end of each individual USV), bandwidth (the range of frequencies within each USV), and peak frequency (highest frequency value reached by each particular USV). Statistical analyses were conducted using SigmaPlot software and the tests used are mentioned alongside description of results below.
3. Results
3.1. Minocycline treatment from 1–2 months reverses USV call rate deficit in Fmr1 KO mice
Mating-elicited USVs were recorded from adult WT and Fmr1 KO mice between 2.5 and 3 months of age. Four groups of mice were compared in a 2 genotype (WT, Fmr1 KO) × 2 treatment (regular water, MT (minocycline-treated) water) design (WT, Fmr1 KO, MTWT and MTKO). The major goals of this analysis were to determine whether the vocalization properties of adult Fmr1 KO mice were different than WT mice, and to determine if minocycline treatment between 1–2 months of age corrected such deficits.
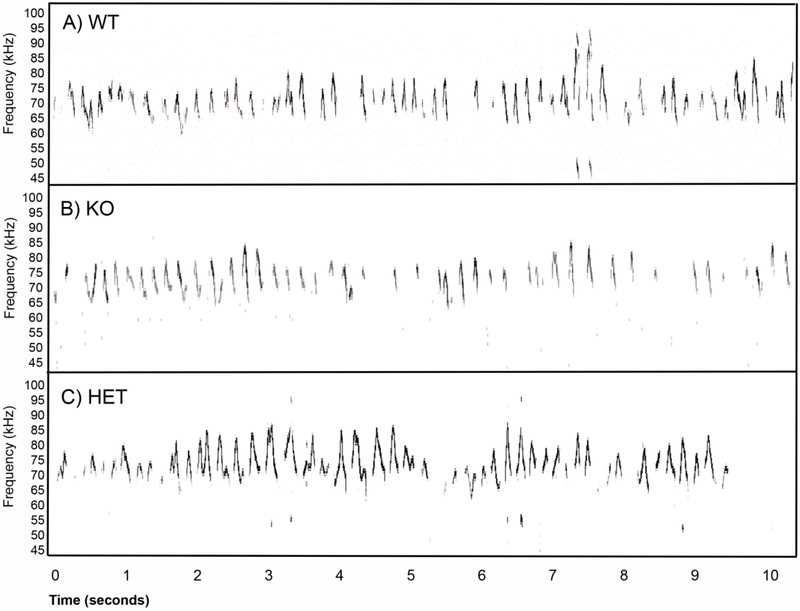
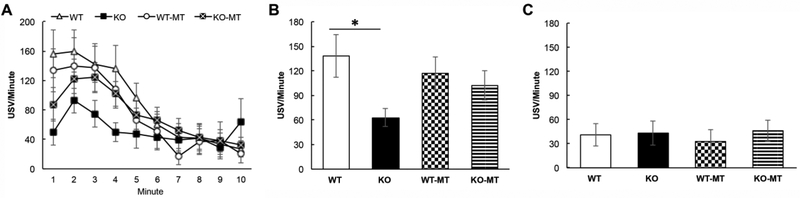
Figure 1 shows example USVs recorded from a WT (A), Fmr1 KO (B), and HET (C) mouse. These snippets are illustrative of the main finding that Fmr1 KO mice call at a reduced rate compared to WT mice, similar to our previous findings (Rotschafer et al., 2012). Figure 2A shows the average minute-by-minute changes in call rate across the 4 groups of mice over the course of a 10-minute courtship period. All groups showed a decline in call rate between approximately the first and second half of the mating window. During the first 5 minutes, where most calling occurred, Fmr1 KO mice produced significantly lower call rates compared to WT mice. When the average call rate over the first 5 minutes was compared (Fig. 2B), a 2-way ANOVA showed an effect of genotype (F(l,23)=5.02, p=0.035), but no effect of treatment (F(l,23)=0.20, p=0.70) and no significant treatment x genotype interactions (F(l,23)=2.25, p=0.15). Tukey post-hoc pairwise comparison for the untreated groups revealed a WT vs. Fmr1 KO difference (p=0.016), but no difference between MT treated WT and Fmr1 KO groups (p=0.60).
Figure 1).
Example spectrograms of ultrasonic vocalizations recorded in a mating context from a male WT (A), a male Fmr1 KO (B), and a male HET (C) mouse. In all three examples, the females were untreated, virgin and estrous induced Fmr1 KO mice. These example 10 second snippets were chosen to illustrate the point that Fmr1 KO mice produce calls at a reduced rate.
Figure 2). Minocycline treatment (MT) between P30-P58 increases calling rate in Fmr1 KO mice to WT levels.
A) Minute-by-minute mean (+/− s.e.) USV call rate during the course of 10 minute courtship interactions in a 2 genotypes (WT, Fmr1 KO) x 2 treatments (regular water, MT (minocycline-treated) water) design. The graph shows that the calling rates tend to decrease over time in all 4 groups of mice. Over the first 5 minutes, WT mice call at a higher rate (calls/minute) than the Fmr1 KO mice. The MT mice (both genotypes) tend to call at intermediate rates. B) To quantify genotype and treatment effects, the mean calling rates during the first 5 minutes were compared in the 2 genotypes x 2 treatments design. For the first 5 minutes, a 2-way ANOVA showed a genotype effect F(l,23)= 5.02, p=0.035, but no significant effect of treatment (p=0.70) and no significant treatment x genotype interaction (p=0.10). Tukey post-hoc pairwise comparison revealed a WT vs. Fmr1 KO difference in the untreated group (*p=0.016), but not a difference in the MT treated group (p=0.60). C) For the second 5 minutes of pairing, a 2-way ANOVA showed no genotype effect F(l,23)= 0.63, p=0.44, no effect of treatment (p=0.69), and no significant treatment x genotype interaction (p=0.67).
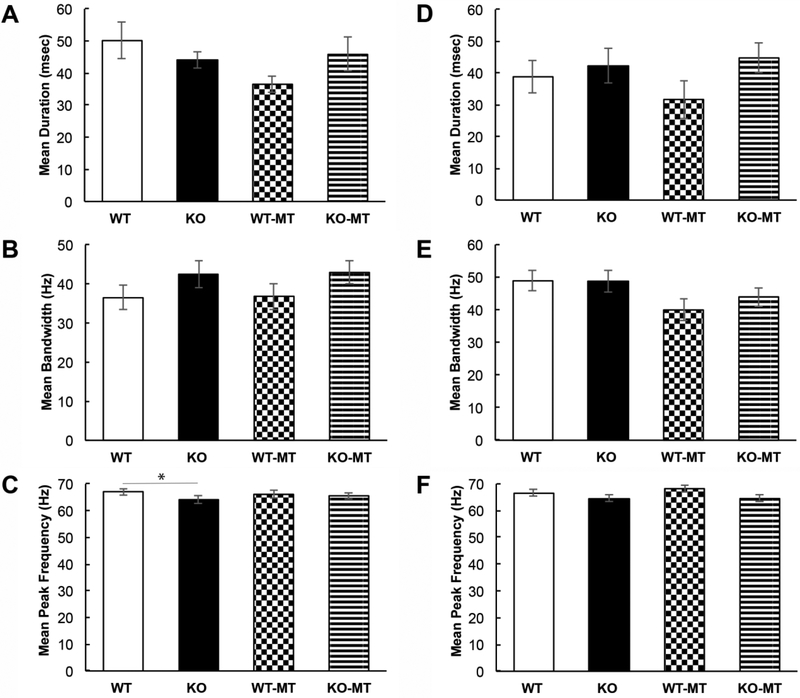
To determine if the untreated Fmr1 KO mice called at a significantly lower rate than the MT treated Fmr1 KO mice, we performed a 2-way ANOVA (time in minutes and treatment as factors) and found a significant effect of treatment (F(l,60) = 9.13, p=0.004), but no significant effect of time (F(4,60)=1.95, p=0.11) or treatment x time interactions (F(4,60) = 0.17, p= 0.96).. A similar analysis of WT and MT treated WT mice showed no difference for treatment (F(l,55=1.37, p=0.25), time (F(4,55)= 1.88, p= 0.13) or treatment x time interactions (F(4,55)= 0.06, p= 0.99). This indicates that during the first 5 minutes of dyadic pairing the untreated Fmr1 KO mice called at a significantly lower rate than untreated WT mice, and minocycline treatment in the 1–2 month age range is sufficient to increase USV call rate in Fmr1 KO mice to levels similar to WT mice. In addition, minocycline treatment did not affect call rate in WT mice demonstrating that the effects of minocycline are specific to the Fmr1 KO mice. There were no differences in average duration and bandwidth of individual calls in the first five minutes (2-way ANOVA: average duration: genotype effect: F(l,23)=0.12, p=0.74; treatment effect: F(l,23)=1.59, p=0.22; interaction: F(l,23)=2.74, p=0.11; average bandwidth: genotype effect: F(l,23)= 3.54, p=0.073; treatment effect: F(l,23)=0.019, p=0.89; interaction: F(l,23)=0.0005, p=0.98), but there was a trending genotype difference in average peak frequency (2-way ANOVA: average peak frequency: genotype effect: F(l,23)= 4.41, p=0.05; treatment effect: F(l,23)=0.34, p=0.57; interaction: F(l,23)=0.03, p=0.86). Bonferroni post-hoc pairwise comparisons for genotype revealed a WT vs. Fmr1 KO difference (p=0.05), but no difference between MT treated WT and Fmr1 KO groups (p=0.18) (Fig. 3A-C). With the exception of a trend for decreased average call peak frequency in KO mice, the call properties were mostly similar between genotypes and treatments.
Figure 3). Spectrotemporal properties of USV calls are not grossly affected by minocycline treatment during the first 5 minutes of dyadic interactions.
A) Average duration of individual USV calls during the first 5 minutes showed no significant difference between the groups (2-way ANOVA, p=0.74). B) Average USV bandwidth during the first 5 minutes showed no significant difference between the groups (2-way ANOVA, p=0.073). C) Average USV peak frequency during the first 5 minutes showed a trending decrease in Fmr1 KO mice (2-way ANOVA, p=0.05). (D-F) Same data as in (A-C) during the second half (5 mins) of recording window when the mice called at a reduced rate.
Although the second 5 minutes of dyadic interactions produced significantly reduced calling, we examined potential genotype or treatment effects during this time window. The average call rate over the second 5 minutes was compared using a 2-way ANOVA (Fig. 2C), but no significant effects were observed for genotype (F(l,23)=0.63, p=0.44), treatment (F(l,23)=0.16, p=0.69), or treatment x genotype interaction (F(l,23)=0.18, p=0.67). No differences were observed in any of the average spectrotemporal properties of the calls during the second five minutes (2-way ANOVA: average duration: genotype effect: F(l,22)=3.47, p=0.08; treatment effect: F(l,22)=0.65, p=0.43; interaction: F(l,22)=0.68, p=0.42; average bandwidth: genotype effect: F(l,22)=0.31, p=0.58; treatment effect: F(l,22)=3.86, p=0.06; interaction: F(l,22)=0.35, p=0.56; average peak frequency: genotype effect: F(l,22)=3.58, p=0.072; treatment effect: F(l,22)=0.31, p=0.59; interaction: F(l,22)=0.36, p=0.55) (Fig. 3D-F).
3.2. Genetic reduction of MMP-9 in Fmr1 KO mice restores USV call rates to WT levels
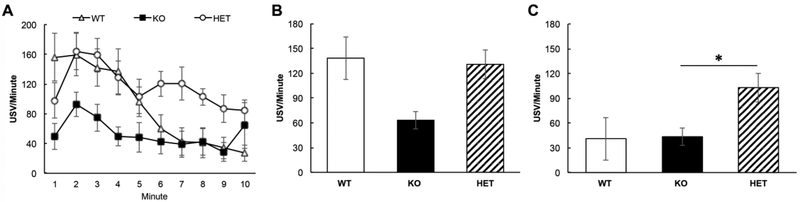
To determine if abnormal MMP-9 levels in the Fmr1 KO mouse may be a mechanism leading to reduced USV call rate, we recorded calls from MMP-9 +/−/Fmr1 KO mice, which are Fmr1 KO mice heterozygous for MMP-9 (henceforth referred to as HET mice). Figure 4A shows the average minute-by-minute dynamics of call rates across the 3 groups of mice (WT, Fmr1 KO, and HET). The WT and Fmr1 KO mice data are the same as shown in Figure 2. A qualitative examination of Fig. 4A shows that HET mice called at a rate similar to the WT mice and higher than the Fmr1 KO mice over the first 5 minutes of the mating window. Interestingly, the HET mice appear to sustain the calling rate even during the second half of the mating window. These observations were confirmed by a 2-way ANOVA (time in minutes and genotype as factors) which showed a main effect of genotype (F(2,200)=21.64, p<0.001), an effect of time (F(9,200)= 5.60, p<0.001) but no time x genotype interactions (F(18,200)= 1.45, p= 0.11). Tukey post-hoc tests showed significant differences in call rates between WT and KO mice (p<0.01), KO and HET mice (P<0.001) and WT and HET mice (p<0.01). This suggests that the genetic reduction of MMP-9 in Fmr1 KO mice restored call rates to WT levels. The observed difference in call rate between WT and HET mice is attributed to enhanced calling in the latter group throughout the mating window.
Figure 4). Genetic reduction of MMP-9 in Fmr1 KO (HET) mice increases USV calling rate to WT levels.
A) Minute by minute changes in average USV call rate during a 10 minute courtship window. The WT and Fmr1 KO data are the same as shown in Figure 2. Over the first 5 minutes, the HET mice showed call rates similar to the WT mice, and generally more calls than the Fmr1 KO mice. Interestingly, the HET mice continued to call at a relatively higher rate even during the last 5 minutes compared to the other genotypes. A 2-way ANOVA (genotype and time) (F(2,200)=21.64, p<0.001) shows that the Fmr1 KO mice exhibit significantly lower call rates compared to WT mice (Tukey test of main effects WT vs. KO **p<0.01) and genetic reduction of MMP-9 in HET mice restored call rates to WT levels (KO vs. HET ***p<0.001; WT vs. HET *p<0.05). The WT vs. HET difference is likely carried by the enhanced calling in the latter group throughout the recording. B) Analysis of just the first 5 minutes shows a genotype difference, likely carried by reduced call rate in the Fmr1 KO mice (1-way ANOVA: F(2,20)=3.74, p=0.042; Tukey test of main effects WT vs. KO p=0.07, HET vs. KO p=0.08, WT vs. HET p=1.00). C) Analysis of the second 5 minutes shows a genotype difference (One Way ANOVA: F(2,20)=5.89, p=0.01 : Tukey test of main effects HET vs. KO p=0.033).
Reduced call rates in KO mice and restoration of calls with MMP-9 reduction is also evident from analysis of average call rates during the first (Fig. 4B) and second half of the mating window (Fig. 4C). During the first 5 minutes of mating, KO mice exhibited fewer calls than WT or HET mice (One-way ANOVA: F(2,20)=3.74, p=0.04; Tukey test of main effects WT vs. KO p=0.07, HET vs. KO p=0.08, WT vs. HET p=1.00). In contrast, during the second five 5 minutes of mating, WT and KO call rates were comparably low, while HET mice maintained a high call rate (One-way ANOVA: F(2,20)=5.89, p=0.01 : Tukey test of main effects WT vs. KO p=1.00, HET vs. KO p=0.033, HET vs. WT p=0.02.
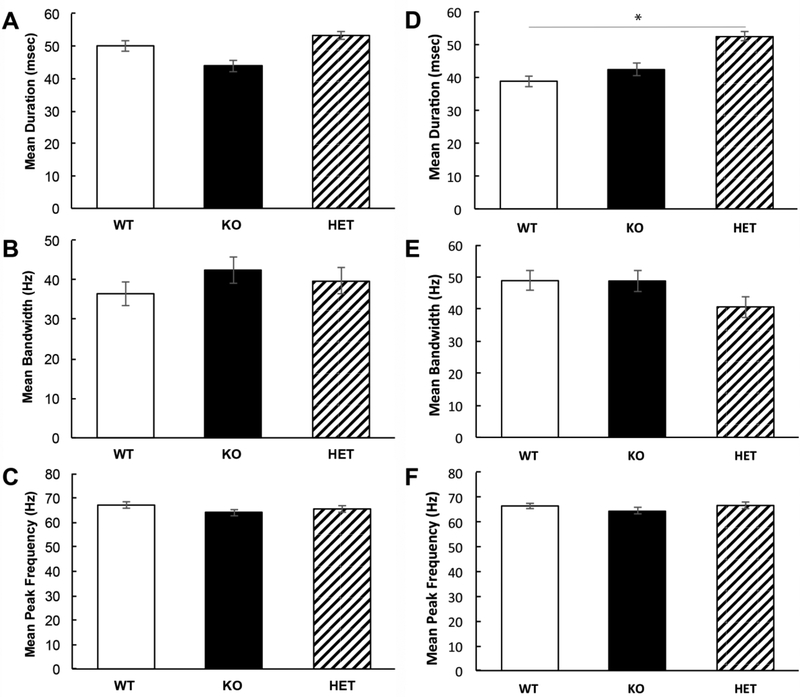
There were no differences in the spectrotemporal properties of the calls in the first 5 minutes of courtship interactions (One-way ANOVA: average duration: F(2,20)= 1.25, p=0.31; average bandwidth: F(2,20)=0.75, p=0.49; average peak frequency: F(2,20)= 0.98, p=0.39) (Fig. 5). In the second five minutes, there was a significant increase in average call duration between WT and HET mice (1-way ANOVA: F(2,20)=4.32, p=0.03, Tukey test of main effects HET vs. WT p=0.03, KO vs. HET p=0.15, KO vs. WT p=0.80), but no difference in bandwidth (F(2,20)= 2.09, p=0.15) or peak frequency (F(2,20)=0.68, p=0.52). The average difference in call duration during the second 5 minutes may stem from the fact the WT and Fmr1 KO mice produce far fewer calls compared to HET mice during that time. Taken together, these data indicate that genetic reduction of MMP-9 in Fmr1 KO mice increases call rates. These findings suggest that enhanced MMP-9 may contribute to reduced calling rates in Fmr1 KO mice.
Figure 5). Call properties were not different in HET mice compared to WT and Fmr1 KO mice during the first 5 minutes of dyadic interactions.
A) Average duration of USV calls during the first 5 minutes was comparable across genotypes (1-way ANOVA, p=0.31). B) Average USV bandwidth during the first 5 minutes was comparable across genotypes (1-way ANOVA, p=0.49). C) Average USV call peak frequency during the first 5 minutes was comparable across genotypes (1-way ANOVA, p=0.39). Same data as in (A-C) during the second half (5 mins) of recording window.
4. Discussion
In this study, we found that adult Fmr1 KO mice produce USVs at a significantly reduced rate compared to WT mice during dyadic mixed-sex courtship interactions. Minocycline treatment between 1 and 2 months of age results in an increase in USV call rate in the Fmr1 KO mice, without affecting WT mice. These data indicate that treatment with minocycline during young adult ages is sufficient to increase call rates in the ~3 month old Fmr1 KO mice, similar to the treatment from birth (Rotschafer et al., 2012). The third major and novel finding of this study is that genetic reduction of MMP-9 in Fmr1 KO mice results in increased USV call rates. Aside from an increase in average duration of calls in the HET mice and a trending difference in the average peak frequency of calls between the untreated WT and Fmr1 KO mice, there were no major differences in spectrotemporal properties of calls across groups. Together these data show that Fmr1 KO mice produce social vocalizations at a reduced rate, which is reversed with minocycline treatment, even if administered late in development. Reduction of MMP-9 in the Fmr1 KO mice causes an overall increase in calling, sometimes even more than WT levels, generating motivation to more closely examine function of MMP-9 in USV generation in WT mice in the future. The similar effects of minocycline treatment and MMP-9 manipulation on call rates suggest that one pathway of minocycline action may be through reduction of MMP-9 activity.
Minocycline has beneficial effects in both humans with FXS and in animal models. Schneider et al. (2013) showed that ~3 month minocycline treatment (placebo controlled) of young (mean age 10.5 years) children with FXS caused a reduction in amplitude of auditory event-related potentials (ERP), as well as improved ERP amplitude habituation in response to repeated sound presentation. The enhanced ERP amplitude and reduced habituation may reflect increased synchronization of neural signals in the cortex (Goncalves et al., 2013), and possible neural correlates of hypersensitivity to sounds in FXS. An open label study of minocycline in older FXS individuals (ages 13–32) indicated improvement in the aberrant behavior checklist-community edition (ABC-C) irritability subscale, the clinical global improvement scale (CGI), and the visual analog scale for behavior (VAS) (Paribello et al., 2010). In the drosophila model of FXS, Siller and Broadie (2011) demonstrated that minocycline normalized synaptic structure in multiple brain regions. In Fmr1 KO mice, Bilousova et al. (2009) showed that minocycline treatment promoted dendritic spine maturation in hippocampal neurons and reduced anxiety-like behaviors. Chronic treatment with minocycline reversed deficits in novel-mouse social interaction and novel-object recognition tests in Fmr1 KO mice (Yau et al., 2016, 2018). Minocycline treatment of Fmr1 KO mice during early development caused long lasting amelioration of anxiety-like behaviors (Dansie et al., 2013). Minocycline administration also reduced the number and severity of audiogenic seizures in Fmr1 KO mice. Interestingly, Dansie et al., (2013) showed that treatment in adult mice also caused behavioral changes, but these benefits were transient and were lost as soon as treatment was stopped. Our data show that minocycline administered during adolescent and young adult ages (P30-P58) in mice is sufficient to reverse USV call rate deficits in Fmr1 KO mice, even when these mice were tested 15–30 days after cessation of minocycline administration. Thus outcomes on the elevated plus maze and open field behavioral tests show long-term benefits only with early developmental minocycline treatment. But, adult treatment is sufficient to reverse USV calling deficits in a sustained manner (at least 15–30 days past the last injection). This indicates that different outcome measures are affected differently by early versus late treatments and should be taken into account during drug treatment design and outcome measure evaluation for effective preclinical and clinical tests. Currently, however there is very little published data on the longevity of drug effects in FXS, or any autism spectrum disorder, to compare young versus adult treatments.
The beneficial effects of minocycline may occur through inhibition of apoptotic cell death, reduced activation and proliferation of microglia and reduced inflammation (Chen et al., 2000; Tikka et al., 2001, Yong et al., 2004). Another well-characterized effect of minocycline is reduction of MMP-9 levels and activity (Kim and Suh, 2009). MMP-9 is an endopeptidase involved in cleavage of extracellular matrix, and is a target of FMRP-based translational control (Janusz et al., 2013). Indeed, MMP-9 levels are increased in FXS, in both humans and in mouse models (Sidhu et al., 2014; Gkogkas et al., 2014). Minocycline treatment causes reduction of MMP-9 levels in FXS (Dziembowska et al., 2013). We have previously shown that reduction or removal of MMP-9 in the Fmr1 KO mice alleviates auditory cortex hyperexcitability, and corrects abnormal ERP habituation (Lovelace et al., 2016; Wen et al., 2018a). The suggestion that minocycline acts via MMP inhibition is supported by Siller and Broadie (2011) who showed that removal of MMP-1 from the drosophila FXS model normalized synaptic architecture in a manner similar to minocycline treatment. Our current data show that genetic reduction of MMP-9 in the Fmr1 KO mice reverses USV call rate deficits similar to minocycline treatment. Although these data do not eliminate any other potential targets of minocycline action, they do suggest that the recovery of USV call rate with minocycline may occur through reduction of MMP-9. Indeed, in WT mice, MMP-9 levels are typically higher in the brain during early development, and decrease into adulthood. In contrast, in the few regions of the Fmr1 KO mice that have been examined, MMP-9 levels continue to stay high into adulthood (Bilousova et al., 2009; Sidhu, et al. 2014; Gkogkas et al. 2014; Lovelace et al. 2016; Wen et al. 2018a).Therefore, the specific effect of minocycline on Fmr1 KO, but not WT, mice may occur through reduction of excessive MMP-9 levels without affecting normal MMP-9 levels in WT mice.
USVs produced by males in a courtship context and by pups when separated from their mothers have stereotypical elements that make them useful as potential biomarkers of social communication deficits in pre-clinical models of genetic disorders (Silverman et al., 2010). Indeed, a number of studies have shown altered USV call rates and/or properties in models of autism spectrum disorders. USVs are also sensitive to treatments, another property that makes them useful as biomarkers. In the Fmr1 KO mouse, a number of studies have examined USV properties in both adults and pups. Roy et al. (2012) recorded vocalizations from P8 pups for 3 minutes after isolation from their mothers. They found no differences in the number of calls, but found that a specific type of call (‘flat’) was emitted with increased carrier frequency in the Fmr1 KO pups. They also found a decrease in the percentage of downward calls relative to total calls in these mice and an increase in the average bandwidth of calls in the Fmr1 KO pups. There were no genotype differences in the average duration of calls. Lai et al. (2013) analyzed pup calls at three time points in development (P4, P7 and P10) and found a transient increase in the number of calls made by Fmr1 KO pups at P7. Although these studies are not directly comparable to the adult courtship context recordings of our study, these data nevertheless point to significant differences in US Vs in Fmr1 KO mice compared to WT from a very early age. Whether these differences in calls result in different maternal care remain unknown. Belagodu et al., (2016) recorded from adult mice and in contrast to our data, they did not find a difference in call rate, but found that the Fmr1 KO mice produced a higher proportion of specific syllables compared to WT mice. While strain differences (FVB vs. C57bl/6) may partly explain the alternate findings, the main difference between the two studies is that Belagodu et al., (2016) recorded vocalizations after the female mouse was removed. Therefore, the mice are likely calling under different motivational contexts across the two studies.
One potential drawback of our approach is that females may make some of the calls recorded. While female mice can produce USVs when presented with another female (Moles et al., 2007; Portfors, 2007), female mice do not readily vocalize during dyadic courtship sessions (Warburton et al., 1989). When presented with laryngeal-nerve transected males, no calls were detected (Warburton et al., 1989), leading to the conclusion that males are the primary producers of USVs in adult courtship mixed-sex pairs. More recently, Neunuebel et al., (2015) used microphone array based localization to show that females and males produce acoustically similar USVs in complex social interactions (2 males and 2 females). Taken together, these studies suggest that USV calling depends critically on the social context, with females potentially contributing to the calls recorded with increasingly more complex social grouping. We minimized the possible influence of genotype differences in female song production by using only Fmr1 KO females in dyadic courtship interactions. However, we cannot exclude the possibility that female mice produced vocalizations and their call rate changed depending on the male genotype. Future studies will test Fmr1 KO mice using the localization system similar to that developed by Neunuebel et al., (2015). Genetic reduction of MMP-9 in the Fmr1 KO mice results in an increase in calling USV calling rate. This suggests that MMP-9 may be involved in the development and/or functioning of vocalization circuitry in mice. Future studies should determine if genetically reducing MMP-9 in WT mice would also affect USV calling rate. It remains unclear where along the vocalization pathway MMP-9 reduction may increase vocal output in Fmr1 KO mice. Regions in the vocal production pathway in the mouse include the anterior cingulate cortex, motor cortex, periaqueductal gray and pontine reticular formation (Jürgens et al., 2009). Future studies will examine MMP-9 levels in these regions in Fmr1 KO mice to identify specific circuit deficits. Comparisons of studies across ages in the Fmr1 KO mice indicate that USV calls are different compared to WT mice. In adult mice engaged in dyadic mixed-sex courtship interactions, Fmr1 KO mice call at a reduced rate with no differences in basic spectrotemporal call properties. Minocycline treatment between 1 −2 months of age is sufficient to reverse the call rate deficits suggesting, 1) that USVs have the potential to serve as outcome measures in pre-clinical models of autism and 2) treatment in late development is sufficient to reverse USV deficits. Future studies with specific inhibitors of MMP-9 that do not impact apoptosis or microglia function are warranted to develop treatments that specifically impact pathways affected by reduction of FMRP.
Highlights.
Adult Fmr1 KO mice show reduced USV call rate when compared to WT mice.
Minocycline treatment from 1–2 months reverses call rate deficit in Fmr1 KO mice.
Genetic reduction of MMP-9 in Fmr1 KO mice restores USV call rates to WT levels.
Targeting MMP-9 in humans with FXS may alleviate symptoms.
Acknowledgments
We thank the members of the Razak lab for comments on the manuscript.
Funding
The work was supported by a UCR Undergraduate Education STEM grant to MAT and the National Institutes of Health [U54 HD082008] to IME, DKB and KAR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, 1994. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X consortium. Cell, 78(1), pp. 23–33. [PubMed] [Google Scholar]
- Belagodu A, Galvez R, Johnson A, 2016. Characterization of Ultrasonic Vocalization of Fragile X Mice. Behav. Brain Res, 310, pp.76–83. [DOI] [PubMed] [Google Scholar]
- Berger A, Tran AH, Dida J, Minkin S, Gerard NP, Yeomans J and Paige CJ, 2012. Diminished pheromone-induced sexual behavior in neurokinin-1 receptor deficient (TACR1−/−) mice. Genes, Brain and Behavior, 11(5), pp.568–576. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM, 2009. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet, 46, pp. 94–102. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM and Hobbs W, 2000. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med, 6(7), pp.797. [DOI] [PubMed] [Google Scholar]
- Dansie LE, Phommahaxay K, Okusanya AG, Uwadia J, Huang M, Rotschafer SE, Razak KA, Ethell DW, Ethell IM, 2013. Long-lasting effects of minocycline on behavior in young but not adult Fragile X mice. Neuroscience, 246, pp. 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Meie A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW and Licatalosi DD, 2011. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell, 146(2), pp.247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Pretto DI, Janusz A, Kaczmarek L, Leigh MJ, Gabriel N, Durbin-Johnson B, Hagerman RJ and Tassone F, 2013. High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am. J.Med. Genet. Part A, 161(8), pp. 1897–1903. [DOI] [PubMed] [Google Scholar]
- Egnor SR and Seagraves KM, 2016. The contribution of ultrasonic vocalizations to mouse courtship. Curr. Opin. Neurobiol, 38, pp .1–5. [DOI] [PubMed] [Google Scholar]
- Gkogkas CG, Khoutorsky A, Cao R, Jafarnejad SM, Prager-Khoutorsky M, Giannakas N, Kaminari A, Fragkouli A, Nader K, Price TJ and Konicek BW, 2014. Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell Rep, 9(5), pp. 1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, 2008. The fragile X prevalence paradox J. Med. Genet, 45, pp. 498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M, 2009. Advances in the treatment of fragile X syndrome. Pediatrics, 123, pp. 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson DM, Jackson AW, Hagerman RJ, 1986. Speech disturbances (cluttering) in mildly impaired males with the Martin-Bell/fragile X syndrome. Am. J. Med. Genet, 23, pp. 195–206. [DOI] [PubMed] [Google Scholar]
- Hodges SL, Nolan SO, Reynolds CD and Lugo JN, 2017. Spectral and temporal properties of calls reveal deficits in ultrasonic vocalizations of adult Fmr1 knockout mice. Behav. Brain Res, 332, pp.50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE and Guo Z, 2005. Ultrasonic songs of male mice. PLoS Biol, 3(12), pp. 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST and Bear MF, 2002. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sei. U.S.A, 99(11), pp.7746–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz A, Milek J, Perycz M, Pacini L, Bagni C, Kaczmarek L, Dziembowska M, 2013. The fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses.J. Neurosci, 33(46), pp. 18234–18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U, 2009. The neural control of vocalization in mammals: a review. J. Voice, 23(1), pp. 1–10. [DOI] [PubMed] [Google Scholar]
- Kim HS, Suh YH, 2009. Minocycline and neurodegenerative diseases Behav. Brain Res, 196, pp. 168–179. [DOI] [PubMed] [Google Scholar]
- Kessler SE, Radespiel U, Hasiniaina AI, Leliveld LM, Nash LT and Zimmermann E, 2014. Modeling the origins of mammalian sociality: moderate evidence for matrilineal signatures in mouse lemur vocalizations. Frontiers in zoology, 11 (l), p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JKY, Sobala M, Zhou L, Doering L, Faure P, Foster J, 2013. Temporal and Spectral Differences in the Ultrasonic Vocalizations of Fragile X Knock Out Mice During Postnatal Development. Behav. Brain Res, 259, pp. 119–130. [DOI] [PubMed] [Google Scholar]
- Largo RH, Schinzel A, 1985. Developmental and behavioral disturbances in 13 boys with fragile X syndrome. Eur. J. Pediatr, 143, pp. 269–275. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Yao JS, Huang Y, Zhai W, Liu W, Guglielmo BJ, Lin E, Yang GY, Young WL, 2006. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J. Cereb. Blood Flow Metab, 26, pp. 1157– 1164. [DOI] [PubMed] [Google Scholar]
- Leigh MJS, Nguyen DV, Mu Y, Winarni TI, Schneider A, Chechi T, Polussa J, Doucet P, Tassone F, Rivera SM and Hessl D, 2013. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. Journal of developmental and behavioral pediatrics: JDBP, 34(3), p. 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace JW, Wen TH, Reinhard S, Hsu MS, Sidhu H, Ethell IM, Binder DK, Razak KA, 2016. Metalloproteinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of fragile X syndrome. Neurobiol Dis, 89, pp. 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrt EJ, Perkel DJ, Tong L, Rubel EW and Portfors CV, 2013. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J. Neuroscience, 33, pp.5573–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Dida J, Lam E, Crawford-Zelli NA, Young EJ, Henderson DR, Onay T, Morris CA, Woodruff-Borden J, Yeomans J and Osborne LR, 2012. Duplication of GTF2I results in separation anxiety in mice and humans. The American Journal of Human Genetics, 90(6), pp. 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE, 1962. Sexual behavior in three inbred strains of mice. Behaviour, 19, pp. 341–350. [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ, 2008. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav. Neurosci, 122, pp. 293–300. [DOI] [PubMed] [Google Scholar]
- Moles A, Costantini F, Garbugino L, Zanettini C and D’amato FR, 2007. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav. Brain Res, 182(2), pp. 223–230. [DOI] [PubMed] [Google Scholar]
- Murray J, Cuckle H, Taylor G and Hewison J, 1997. Screening for fragile X syndrome. Health Technol. Assess, 1(4), pp. i–iv. [PubMed] [Google Scholar]
- Neunuebel JP, Taylor AL, Arthur BJ and Egnor SR, 2015. Female mice ultrasonically interact with males during courtship displays. Elife, 4, p.e06203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM, Ethell DW, 2010. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol, 10, pp. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, et al. 1991. Absence of expression of the FMR-1 gene in Fragile X syndrome. Cell, 66, pp. 817–822. [DOI] [PubMed] [Google Scholar]
- Portfors CV, 2007. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sei, 46, pp. 28–34. [PubMed] [Google Scholar]
- Reinhard SM, Razak K and Ethell IM, 2015. A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci, 9,pp. 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA, 2012. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X syndrome. Brain Res, 1439, pp. 7–14. [DOI] [PubMed] [Google Scholar]
- Roy S, Watkins N, and Heck D, 2012. Comprehensive Analysis of Ultrasonic Vocalizations in a Mouse Model of Fragile X Syndrome Reveals Limited, Call Type Specific Deficits. PLoS One, 7(9): e44816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J and Ricceri L, 2009. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev, 33(4), pp. 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Leigh MJ, Adams P, Nanakul R, Chechi T, Olichney J, 2013. Electrocortical changes associated with minocycline treatment in fragile X syndrome. J. Psychopharmacol, 27, pp. 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM, 2014. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J. Neurosci, 34(30), pp. 9867–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller SS & Broadie K, 2011. Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis. Model Mech, 4, pp. 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C and Crawley JN, 2010. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci, 11(7), pp. 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinänen R and Koistinaho J, 2001. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci, 21(8), pp. 2580– 2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utari A, Chonchaiya W, Rivera SM, Schneider A, Hagerman RJ, Faradz SM, Ethell IM, Nguyen DV, 2010. Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am. J. Intellect. Dev. Disabil, 115, pp. 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton VL, Sales GD, Milligan SR, 1989. The emission and elicitation of mouse ultrasonic vocalizations: the effects of age, sex and gonadal status. Physiol. Behav, 45, pp. 41–47. [DOI] [PubMed] [Google Scholar]
- Wen TH, Afroz S, Reinhard SM, Palacios AR, Tapia K, Binder DK, Razak KA, Ethell IM, 2018a. Genetic Reduction of Matrix Metalloproteinase-9 Promotes Formation of Perineuronal Nets Around Parvalbumin-Expressing Intemeurons and Normalizes Auditory Cortex Responses in Developing Fmr1 Knock-Out Mice. Cereb. Cortex, 28(11), pp. 3951–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TH, Binder DK, Ethell IM and Razak KA, 2018b. The perineuronal ‘safety’net? Perineuronal net abnormalities in neurological disorders. Frontiers in Molecular Neuroscience, 11, p.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau SY, Bettio L, Vetrici M, Truesdell A, Chiu C, Chiu J, Truesdell E, Christie BR, 2018. Chronic minocycline treatment improves hippocampal neuronal structure, NMD A receptor function, and memory processing in Fmr1 knockout mice. Neurobiol. Dis, 113, pp. 11–22. [DOI] [PubMed] [Google Scholar]
- Yau SY, Chiu C, Vetrici M and Christie BR, 2016. Chronic minocycline treatment improves social recognition memory in adult male Fmr1 knockout mice. Behav. Brain Res, 312, pp. 77–83. [DOI] [PubMed] [Google Scholar]
- Yong VW, Wells J, Giuliani F, Casha S, Power C and Metz LM, 2004. The promise of minocycline in neurology. The Lancet Neurology, 3(12), pp. 744–751. [DOI] [PubMed] [Google Scholar]