Abstract
BACKGROUND AND PURPOSE
Atrial fibrillation (AF) is associated with dementia independent of clinical stroke. The mechanisms underlying this association remain unclear. In a community-based cohort, the ARIC study, we evaluated: (1) the longitudinal association of incident AF and (2) the cross-sectional association of prevalent AF with brain MRI abnormalities.
METHODS
The longitudinal analysis included 963 participants (mean age, 73±4.4 years, 62% women, 51% black) without prevalent stroke or AF who underwent a brain MRI in 1993–1995 and a second MRI in 2004–2006 (mean, 10.6±0.8 years). Outcomes included subclinical cerebral infarctions (SCI), sulcal size, ventricular size, and, for the cross-sectional analysis, white matter hyperintensity (WMH) volume and total brain volume (TBV).
RESULTS
In the longitudinal analysis, 29 (3.0%) participants developed AF after the first brain MRI. Those who developed AF had higher odds of increase in SCIs (OR, 3.08; 95% CI, 1.39–6.83), worsening sulcal grade (OR, 3.56; 95% CI, 1.04–12.2), and worsening ventricular grade (OR, 9.34; 95% CI 1.24–70.2). In cross-sectional analysis, of 969 participants, 35 (3.6%) had prevalent AF at the time of the 2004–2006 MRI scan. Those with AF had greater odds of higher sulcal (OR, 3.9; 95% CI, 1.7–9.1) and ventricular grade (OR, 2.4; 95% CI, 1.0–5.7) after multivariable adjustment, and no difference in WMH or TBV.
CONCLUSION
AF is independently associated with increase in SCI and worsening sulcal and ventricular grade—morphological changes associated with aging and dementia. More research is needed to define the mechanisms underlying AF-related neurodegeneration.
Keywords: Atrial fibrillation, Arrhythmias, Magnetic Resonance Imaging (MRI), Cognitive Impairment, Cerebrovascular Disease/Stroke
INTRODUCTION
Atrial fibrillation (AF) is associated with cognitive decline and dementia independent of clinical stroke.1–7 The exact mechanisms have not been completely elucidated, but may involve subclinical cerebral infarctions (SCI), cerebral microinfarctions, chronic cerebral hypoperfusion, inflammation, and shared vascular risk factors such as hypertension and diabetes. Brain MRI studies have provided some insights into the relationship between brain morphological changes and impaired cognitive function. Increased white mater hyperintensitites (WMH), brain atrophy and volume loss, and SCI are clinically linked to several markers of cognitive decline in community-based populations.8–10
Existing research on AF and brain morphological changes is limited, as analyses were either cross-sectional or had limited follow-up. A 2013 cross-sectional analysis of a community-based cohort from Iceland found AF to be associated with lower total brain volume.11 However, a more recent cross-sectional analysis of the community-based Framingham Heart Study Offspring Cohort did not find AF to be significantly associated with lower total brain volume after adjustment for vascular risk factors.12 Furthermore, in a longitudinal analysis among a subset of participants who underwent repeat brain MRI 6.5 years apart there was no association between prevalent AF and change in regional or total brain volumes.12
Additional prospective studies are needed in order to enhance understanding of the association between AF and the development of brain MRI abnormalities. Also, existing research is limited, as it has been conducted predominantly in white populations. Thus, in the present study we evaluated the association between incident AF and longitudinal change in brain morphology over 10 years as assessed by brain MRI in the Atherosclerosis Risk in Communities (ARIC) study, a prospective mostly biracial community-based cohort study. We hypothesized that in longitudinal analysis, incident AF will be associated with more SCIs and worsening sulcal and ventricular grades. Additionally, in a cross-sectional analysis, we hypothesized that prevalent AF will be associated with higher odds of presence of SCI, higher sulcal or ventricular grade, greater WMH, and lower total brain volume (TBV).
METHODS
Study Population
The ARIC study13 is a prospective community-based cohort that originally enrolled 15,792 men and women aged 45 to 64 years from 4 communities in the United States (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD) between 1987 and 1989. After the baseline visit in 1987–1989 (visit 1), participants returned in 1990–1993 (visit 2), 1993–1995 (visit 3), 1996–1999 (visit 4), and in 2004–2006 a subset took part in the ancillary brain MRI visit. The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure in accordance with ARIC policies. Data is maintained by ARIC through the University of North Carolina Collaborative Studies Coordinating Center. Further information is available on the ARIC website.
Herein we present two analyses; one which is prospective and evaluates change in brain MRI markers between visit 3 (1993–95) and at the subsequent ancillary brain MRI follow up visit (2004–06), and another which is a cross-sectional analysis of participants who underwent brain MRIs in 2004–2006. Only participants from two of the four ARIC sites – Forsyth County, NC and Jackson, MS – were invited to participate in the brain MRI scans, and are included in the present analysis. Participants were exclusively African American in Jackson and a mix of African Americans and whites in Forsyth County.
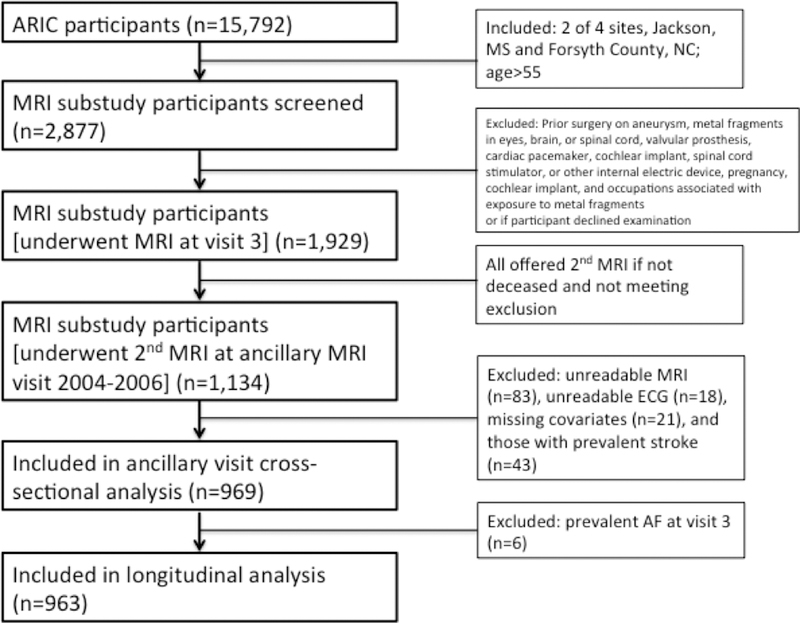
In regards to the longitudinal analysis, during visit 3, all participants aged >55 years (n=2877) at the Forsyth County, NC and Jackson, MS sites were screened for eligibility and invited to participate in the ancillary study, and 1,929 participants completed brain MRI scans.14 Then, in 2004–2006 all 1,929 participants were invited for a repeat brain MRI, and 1,134 returned for imaging. Of the 1,134 patients who underwent both brain MRIs, we excluded participants with unreadable MRI (n=83), unreadable ECG (n=18), missing covariates (n=21), prevalent stroke (n=43), and prevalent AF at visit 3 (n=6). After exclusions, 963 participants remained in the sample for longitudinal analysis, of whom 29 participants (3.0%) developed incident AF. For the longitudinal analysis, we only considered incident AF, and not prevalent AF to avoid prevalence-incidence (Neyman) bias. A flow chart is provided in Figure 1. The mean time (SD) between MRIs was 10.6 (0.84) years.
Figure 1.
Flow of Study Participants
For the cross-sectional analysis, of the 1,134 participants who underwent the brain MRI in 2004–2006, we excluded participants with unreadable MRI (n=83), unreadable ECG (n=18), missing covariates (n=21), and prevalent stroke (n=43). After exclusions, 969 participants remained in the sample for cross-sectional analysis, of whom 35 participants (3.6%) had prevalent AF. We chose the 2004–2006 population for the cross-sectional analysis due to the higher prevalence of AF in 2004–2006 and brain volume measures were not available at visit 3. For the analysis in 2004–2006, we considered prevalent AF cases since it was a cross-sectional analysis.
The ARIC study was approved by the institutional review boards at each participating center, and written informed consent was obtained from all participants.
Ascertainment of AF
The details of AF ascertainment have been previously published.15 Briefly, AF cases were identified from study visit ECGs and by review of hospital discharge records. At each study exam, a standard supine 12-lead resting ECG was recorded with a MAC PC Personal Cardiograph [Marquette Electronics, Milwaukee, Wisconsin, USA] and transmitted to the ARIC ECG Reading Center [EPICARE Center, Wake Forest School of Medicine, Winston Salem, NC] for automatic coding. A cardiologist visually confirmed all AF cases automatically detected from the study ECG. Information on hospitalizations during follow-up was obtained from annual follow-up calls and surveillance of local hospitals, with hospital discharge diagnoses codes collected by trained abstractors. AF during follow-up was defined as International Classification of Disease 9th revision, Clinical Modification [ICD-9-CM] 427.31 or 427.32 diagnosis codes. AF cases detected in the same hospitalization with open cardiac surgery were not considered AF in the analysis.
Brain MRI
The details of brain MRI acquisition and interpretation have been previously published.16, 17 Briefly, all scans were performed on 1.5 T magnets and included axial 5-mm contiguous T1, T2, and proton density-weighted images. The visit 3 [1993–1995] brain MRIs were obtained on GE or Picker scanners, and were interpreted at the ARIC MRI Reading Center at Johns Hopkins Medical Institutions. The ancillary visit [2004–2006] MRI scans were performed on GE scanners, and were interpreted at the University of Washington by neuroradiologists who were trained by one of the readers involved in the baseline study. The neuroradiologists interpreting the ancillary study were tested on a sample of the earlier scans, which verified an acceptable (>85%) agreement within one grade for the sulci, and ventricles, and presence or absence of infarcts.
As previously published18, automated brain and WMH volumes were determined from axial fluid-attenuated inversion recovery images for the 2004–2006 visit scans. An automated algorithm was used to segment each of the axial fluid-attenuated inversion recovery images into voxels assigned to 1 of 3 categories based on signal intensity: normal brain, cerebrospinal fluid, or WMH. The WMH maps were manually edited to exclude infarcts and other lesions. The mean absolute error and test-retest coefficient of variation for this method were 6.6% and 1.4%, respectively, for WMH volume. Total intracranial volume was manually measured from T1-weighted sagittal images. Volumetric measurements of WMH were standardized to an intracranial volume of 1500 cm3. Fully quantitative WMH volumes were not possible from visit 3 scans; therefore, WMH volume and TBV were not available from the visit 3 scans and these variables were not included in the longitudinal analysis.
Infarcts were defined based on signal characteristics on T1, T2, and proton density images: bright on T2 and proton density and dark on T1 images. Infarcts were counted only if they were >3 mm in maximum diameter. All scans were subjected to double-reads for infarcts scoring, and scans with discrepancies in numbers of infarcts between readers were read a third time for adjudication. A reliability exercise using 104 randomly selected cases was carried out, and interrater agreement was 89%.
For each visit MRI, Axial T1-weighted images were used for assessment of ventricular grade and sulcal grade using a 0 to 9 scale developed and validated by the Cardiovascular Health Study with 9 being the largest and most abnormal.19 The reliability coefficients for 26 pairs of readings by 2 independent neuroradiologists for ventricular grade = 0.87 and sulcal grade = 0.63.20
Covariates
We assessed covariates including age, race, sex, study center, education, occupation, cigarette smoking, body mass index, hypertension, diabetes mellitus, prevalent coronary heart disease, prevalent congestive heart failure, and anticoagulant use. Education was defined as less than high school; high school, high school equivalent, or vocational school; or college, graduate, or professional school, occupation included 9 categories, and cigarette smoking status was defined as current, former, or never. Body mass index (kg/m2) was recorded. Hypertension was defined as use of anti-hypertensive medication, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg, Diabetes mellitus was defined as a self-reported physician’s diagnosis of diabetes mellitus, use of hypoglycemic medications, nonfasting serum glucose levels ≥200 mg/dL, or fasting serum glucose level ≥126 mg/dL. Prevalent congestive heart failure was defined as the reported use of medications to treat heart failure in the previous 2 weeks or the presence of heart failure according to Gothenburg criteria.21 Prevalent coronary heart disease was defined as physician-diagnosed coronary heart disease or the presence of a previous myocardial infarction by ECG, plus adjudicated cases. Ascertainment of clinical stroke was conducted by computerized algorithm and physician adjudication based on annual telephone interviews, field center examinations, surveillance of the ARIC Study community hospitals for all cohort members’ hospitalizations, and the review of death certificates, physician questionnaires, coroner/medical examiner reports, and informant interviews. Anticoagulant medication use was participant-reported at study visits and on annual telephone interviews.
Statistical Analysis
We conducted two analyses, one longitudinal and one cross-sectional. For the longitudinal analysis, regression models were constructed based on incident AF status specifically, based on participants ascertained to have a new diagnosis of AF after the visit 3 MRI. Participants with prevalent AF at the time of the visit 3 MRI were excluded from the longitudinal analysis. Age-, race-, and sex-adjusted logistic regression models were fit for worsening sulcal grade, worsening ventricular grade, and increase in number of subclinical cerebral infarctions [model 1]. Additional multivariate regression models were adjusted for study center, education, occupation, cigarette smoking, body mass index, hypertension, diabetes mellitus, prevalent coronary heart disease, prevalent congestive heart failure, and anticoagulant use [model 2]. Worsening sulcal grade and ventricular grade were defined as change from a particular grade on the initial brain MRI to at least one grade worse (e.g.,. from grade 4 to 5) on the second MRI.
For the cross-sectional analysis, regression models were constructed based on prevalent AF status in 2004–2006; therefore, this population included those participants with incident AF between visit 3 and 2004–2006 as well as participants with prevalent AF at the time of the visit 3 scan. Linear regression models for TBV and WMH volume were standardized to TIV 1500 cm3, and logistic regression models for sulcal grade ≥ 4, ventricular grade ≥ 4, and presence of SCI were adjusted for variables as described for models 1 and 2.
To determine whether brain morphological changes are mediated by SCIs, we conducted a secondary analysis by excluding participants with SCIs on brain MRI scans in both the longitudinal and cross-sectional analyses. Analyses were performed using Statistical Analysis Systems software version 9.2 (SAS Institute, Cary, NC).
RESULTS
Study Population
Baseline characteristics, which are based on the cross-sectional study population from the 2004–2006 visit, are shown in Table 1. Of 969 participants included in the cross-sectional analysis, 35 (3.6%) had prevalent AF by 2004–06. Mean age was 73 ± 4.4 years, 62% were women, and 51% were black. Participants with AF had higher prevalence of cardiovascular risk factors and conditions including smoking, diabetes mellitus, hypertension, coronary heart disease, and heart failure.
Table 1:
Baseline Characteristics of Study Participants, ARIC, 2004–2006
| Developed Atrial Fibrillation by 2004–06 | ||
|---|---|---|
| No (N=934) | Yes (N=35) | |
| Age, mean (SD) | 72.7 (4.4) | 74.5 (4.3) |
| Female | 62.3 | 40.0 |
| Black race | 51.2 | 40.0 |
| Education | ||
| Less than High School | 21.2 | 28.6 |
| High School or College | 35.6 | 25.7 |
| Graduate school | 43.3 | 45.7 |
| Cigarette Smoking | ||
| Current | 7.3 | 14.3 |
| Former | 36.0 | 51.4 |
| Never | 56.8 | 34.3 |
| Body Mass Index (kg/m2), mean (SD) | 28.6 (5.3) | 28.8 (5.5) |
| Diabetes Mellitus | 23.7 | 37.1 |
| Hypertension | 68.8 | 82.9 |
| Prevalent Coronary Heart Disease | 4.2 | 14.3 |
| Prevalent Congestive Heart Failure | 2.5 | 31.4 |
| Warfarin Use | 1.7 | 5.7 |
All values are percent unless otherwise noted.
kg/m2= kilogram per meter squared, SD=Standard Deviation
Longitudinal analysis
The associations of AF with longitudinal change in brain MRI findings are shown in Table 2. This analysis included only incident AF after visit 3 and excluded participants with prevalent AF at visit 3. In Model 2, compared with participants who did not develop AF, those who developed AF had higher odds of worsening sulcal grade (OR, 3.56; 95% CI, 1.04–12.2), worsening ventricular grade (OR, 9.34; 95% CI, 1.24–70.2), and increase in SCIs (OR, 3.08; 95% CI, 1.39–6.83). There were no significant interactions with respect to sex and race.
Table 2.
Longitudinal Association of Incident Atrial Fibrillation (AF) with Brain MRI Abnormalities in the ARIC Ancillary MRI Substudy, 1993–2006
| Brain MRI Variable | No AF (n-934) | AF (n=29) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| N (%) | N (%) | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Worsening Sulcal Grade | 634 (68%) | 26 (90%) | 3.65 (1.08–12.3) | 0.04 | 3.56 (1.04–12.2) | 0.04 |
| Worsening Ventricular Grade | 691 (74%) | 28 (97%) | 8.61 (1.16–63.7) | 0.04 | 9.34 (1.24–70.2) | 0.03 |
| Increase in Subclinical Cerebral Infarctions | 172 (18%) | 12 (41%) | 2.92 (1.36–6.28) | 0.006 | 3.08 (1.39–6.83) | 0.006 |
Logistic regression was used for dichotomous outcomes to calculate odds ratios.
Model 1: Adjusted for age, race and sex
Model 2: Adjusted for age, race, sex, center, education, occupation, cigarette smoking, body mass index, hypertension, diabetes, prevalent coronary heart disease, prevalent congestive heart failure, anticoagulant medication use
MRI=Magnetic Resonance Imaging, SD= Standard Deviation, cm3= Cubic Centimeters
In the secondary analysis, excluding participants with SCIs resulted in a substantial reduction in the number of participants with incident AF. Nevertheless, we observed that incident AF was associated with greater than 2-fold higher odds of worsening sulcal grade, albeit with wide CI (Supplemental Table I). We were not able to estimate the odds of worsening ventricular grade since all participants with AF had worsening ventricular grade.
Cross-sectional analysis
The cross-sectional associations of AF with brain MRI findings are shown in Table 3. In Model 2, compared to participants without AF, those with AF had greater odds of higher sulcal grade (OR, 3.9; 95% CI, 1.7–9.1) and greater odds of higher ventricular grade (OR, 2.4; 95% CI, 1.0–5.7). When sulcal and ventricular grade were assessed as ordinal variables, AF was similarly found to be associated with higher sulcal and ventricular grade. AF was not associated with WMH or TBV. There were no significant interactions with respect to sex and race.
Table 3:
Cross-Sectional Association of Prevalent Atrial Fibrillation (AF) with Brain MRI Abnormalities in the ARIC Ancillary MRI Substudy, 2004–2006
| Brain MRI Variable | No AF (n=934) | AF (n=35) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Difference (95% CI) | P | Difference (95% CI) | P | |
| White Matter Hyperintensity Volume, cm3 | 13.1 (11.8) | 14.3 (11.3) | 0.0 (−3.9–3.9) | 0.99 | −1.5 (−5.6–2.5) | 0.45 |
| Total Brain Volume, cm3* | 1048 (108) | 1022 (100) | −17.1 (−34.2–0.12) | 0.05 | −13.7 (− 31.7–4.2) | 0.13 |
| N (%) | N (%) | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Sulcal Grade ≥4 | 324 (35%) | 26 (75%) | 4.2 (1.9–9.2) | 0.0004 | 3.9 (1.7–9.1) | 0.002 |
| Ventricular Grade ≥4 | 441 (48%) | 26 (75%) | 2.3 (1.1–5.1) | 0.04 | 2.4 (1.0–5.7) | 0.04 |
| Presence of Subclinical Cerebral Infarctions | 212 (23%) | 15 (43%) | 2.5 (1.2–5.0) | 0.01 | 1.8 (0.9–3.8) | 0.12 |
Linear regression was used for continuous outcomes to calculate differences, and logistic regression for dichotomous outcomes to calculate odds ratios.
Model 1: Adjusted for age, race and sex
Model 2: Adjusted for age, race, sex, center, education, occupation, cigarette smoking, body mass index, hypertension, diabetes, prevalent coronary heart disease, prevalent congestive heart failure, anticoagulant medication use
Additionally adjusted for intracranial volume.
MRI=Magnetic Resonance Imaging, SD= Standard Deviation, cm3= Cubic Centimeter
In the secondary analysis, excluding participants with SCIs substantially reduced the number of participants with prevalent AF. Nonetheless, we observed that prevalent AF was associated with more than 2-fold greater odds of higher sulcal and ventricular grade in Model 2, albeit with wide CI (Supplemental Table II).
DISCUSSION
In a population-based cohort, we found that participants who developed AF had adverse longitudinal changes in brain morphology on MRI. Compared with participants who did not develop AF, those who developed AF had an increase in SCIs on longitudinal brain MRIs. Additionally, participants who developed AF were also at higher risk of worsened ventricular grade and worsened sulcal grade; changes that are also associated with advanced aging and dementia. These associations remained significant after adjusting for age, sex, and cardiovascular risk factors. In cross-sectional analysis, we observed an association between AF and higher ventricular and sulcal grades. Importantly, even after excluding participants with SCIs in both longitudinal and cross-sectional analyses, we observed associations with worse sulcal and ventricular grade, albeit the associations were attenuated. Notably, there was no association with WMH volume or with TBV. Finally, we did not observe any significant sex- or race-based interactions with AF. Collectively, our findings suggest that other than the known association between AF and SCI, there are additional associations with adverse changes in brain architecture, implicating other mechanisms beyond clinical stroke and SCI in explaining the relationship of AF to cognitive decline and dementia.
Two previous studies on this topic warrant closer examination. First, a 2013 cross sectional analysis of over 4000 participants in the AGES-Reykjavik study found that participants with AF had lower total brain volume than those without AF.11 This was an older (mean age 76±5 years) and more homogeneous community-dwelling population than our cohort. The association was stronger with persistent and permanent AF than with paroxysmal AF and with increased time from first diagnosis of disease. By contrast, a more recent 2016 cross-sectional analysis in the Framingham Heart Study Offspring Cohort12 and 2 other studies2, 22 did not find an association between AF and lower total brain volume after adjustment for vascular risk factors. In the Framingham study, the investigators also analyzed prevalent AF at the initial visit and longitudinal change in MRI with a second brain MRI at least one year after the first (mean time interval 6.5±1.3 years) and found no statistically significant change in total or regional brain volume when adjusted for vascular risk.
Our study builds on these prior analyses by evaluating longitudinal brain MRI changes over a longer follow-up period of 10 years to further characterize the association between AF and brain morphology. To the best of our knowledge, this is the first time that an association between AF and longitudinal worsening of ventricular and sulcal grade has been described. Also, although SCI in patients with AF has been previously described—notably in the Veterans Affairs Stroke Prevention in Nonrheumatic AF study23, and a more contemporary meta-analysis of 11 CT and MRI studies24 which showed a two-fold higher presence of SCI in patients with AF than those without—our study builds on prior evidence by describing a 3-fold increased risk of gain of SCI lesions over 10 years among participants with AF compared to those without. Similar to the Framingham and other studies2, 12, 22, in our study, we did not observe an association between AF and TBV in a more racially and geographically diverse participant population making these findings more generalizable.
The association between AF and the multiple brain MRI abnormalities in our study substantiates the notion that several mechanisms may contribute to development of dementia and cognitive decline in patients with AF independent of clinical stoke. A prior analysis from the ARIC study25 suggests that SCI are an important mediator for the association between AF and cognitive decline independent of clinical stroke. In that analysis, incident AF was only associated with cognitive decline, as measured by decline in the digit symbol substitution test, in the subgroup of AF participants with SCI and not in those without SCI. The additional findings in our current study of enlargement of brain ventricles and widening and effacement of cortical sulci with increased intracranial free/cerebrospinal fluid space, mirrors findings in patients with Alzheimer’s dementia.26 Possible mechanisms for these findings range from cerebral microinfarctions to global hypoperfusion. Microinfarctions, visible on histological examination or diffusion weighted MRI, but not seen on gross examination or conventional MRI, could be the result of microscopic cardioembolic phenomenon originating in the left atrium or from cerebral small vessel injury in the context of inflammation and/or shared multiple vascular risk factors.27, 28 Regarding the cerebral hypoperfusion hypothesis, there are limited data showing that cerebral blood flow (CBF) is lower in patients with AF. In one series, CBF increased the day after successful electrical cardioversion to sinus rhythm and continued to increase at 30 days.29 In a contemporary series, 17 patients with persistent AF demonstrated brain hypoperfusion by SPECT imaging, especially in areas critical for memory, and, after pacemaker implantation and atrioventricular node ablation leading to regularization of ventricular rate, improved perfusion was evident in all patients.30 Notably, WMH, which are attributed to small vessel disease and global cerebral hypoperfusion, and are associated with cognitive decline and dementia, were not found to be associated with AF in our study. Overall, it seems likely that a complex interplay leads to cerebral injury through various mechanisms affecting the cerebral macro and microenvironment.
The strengths of this community-based prospective cohort study includes the long follow-up, inclusion of non-white participants, and extensive measurement of covariates. There are several limitations. The number of participants with AF was small. The ascertainment method for AF in ARIC may have missed paroxysmal AF that was managed exclusively in the outpatient setting. However, the incidence rates of AF in ARIC are similar with other population-based studies.31–33 Also, as noted previously25, ARIC participants who failed to complete the 11 years of observation (ARIC visit 3 through 2004–2006) because of death, an inability to undergo MRI, or refusal had greater burden of disease (e.g., diabetes mellitus, hypertension) and worse baseline MRI findings than those who completed follow up. Therefore, participants who completed the longitudinal assessments described here comprised the healthier subset of the original cohort. Because we are unable to classify AF type (paroxysmal, persistent, permanent) or AF burden accurately in the ARIC study, we did not assess the relationship between AF type or AF burden and brain morphological changes. Also, information on warfarin use for the study visits and the annual follow-up phone calls may not be complete. Other limitations relating to the brain MRI include inability to measure longitudinal change in WMH volume and TBV, due to lack of volumetric data from the visit 3 MRI.
CONCLUSION
In this large community-based cohort of blacks and whites, incident AF was longitudinally associated with adverse changes in brain MRI including worsened sulcal grade, worsened ventricular grade, and increase in SCI. AF was not associated with WMH volume or TBV in cross-sectional analysis. While our findings advance our knowledge regarding AF-associated brain morphological changes, further research is needed to elucidate the mechanisms underlying AF-associated brain morphological changes, which ultimately lead to cognitive decline and dementia.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
SOURCES OF FUNDING: The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
DISCLOSURES: Dr. Chen is also supported by the National Heart, Lung, and Blood Institute grants R01 HL141288 and R01 HL126637. Dr. Alonso is also supported by American Heart Association grant 16EIA26410001. The authors report no relevant financial disclosures.
REFERENCES
- 1.Kilander L, Andrén B, Nyman H, Lind L, Boberg M, Lithell H. Atrial fibrillation is an independent determinant of low cognitive function. A Cross-Sectional Study in Elderly Men. 1998;29:1816–1820 [DOI] [PubMed] [Google Scholar]
- 2.Knecht S, Oelschläger C, Duning T, Lohmann H, Albers J, Stehling C, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. European Heart Journal. 2008;29:2125–2132 [DOI] [PubMed] [Google Scholar]
- 3.Ott A, Breteler MMB, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. 1997;28:316–321 [DOI] [PubMed] [Google Scholar]
- 4.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and alzheimer’s dementia. Heart Rhythm. 2010;7:433–437 [DOI] [PubMed] [Google Scholar]
- 5.de Bruijn RG, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurology. 2015;72:1288–1294 [DOI] [PubMed] [Google Scholar]
- 6.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: A meta-analysis. Annals of internal medicine. 2013;158:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LY, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agarwal SK, et al. Association of atrial fibrillation with cognitive decline and dementia over 20 years: The aric‐ncs (atherosclerosis risk in communities neurocognitive study). Journal of the American Heart Association. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: The framingham heart study. Archives of Neurology. 2006;63:246–250 [DOI] [PubMed] [Google Scholar]
- 9.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine. 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 10.Rusinek H, Santi SD, Frid D, Tsui W-H, Tarshish CY, Convit A, et al. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal mr imaging study of normal aging. Radiology. 2003;229:691–696 [DOI] [PubMed] [Google Scholar]
- 11.Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44:1020–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piers RJ, Nishtala A, Preis SR, DeCarli C, Wolf PA, Benjamin EJ, et al. Association between atrial fibrillation and volumetric magnetic resonance imaging brain measures: Framingham offspring study. Heart Rhythm. 2016;13:2020–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The-ARIC-Investigators. The atherosclerosis risk in communities (aric) study: Design and objectives. American Journal of Epidemiology. 1989;129:687–702 [PubMed] [Google Scholar]
- 14.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. 1996;27:2262–2270 [DOI] [PubMed] [Google Scholar]
- 15.Soliman EZ, Lopez F, O’Neal WT, Chen LY, Bengtson L, Zhang Z-M, et al. Atrial fibrillation and risk of st-segment elevation versus non-st segment elevation myocardial infarction: The atherosclerosis risk in communities (aric) study. Circulation. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopman DSMP AD MBChB, PhD, MSc, MPH; Catellier DJ DrPH; Coker LH PhD; Shibata DK MD; Sharrett AR MD, PhD; Mosley Jr. PhD. Vascular risk factors and longitudinal changes on brain mri: The aric study. Neurology. 2011;76:1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, et al. A method for using mr to evaluate the effects of cardiovascular disease on the brain: The cardiovascular health study. American Journal of Neuroradiology. 1994;15:1625–1633 [PMC free article] [PubMed] [Google Scholar]
- 18.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, et al. Blood pressure and white matter disease progression in a biethnic cohort: The atherosclerosis risk in communities (aric) study. Stroke; a journal of cerebral circulation. 2010;41:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue NC, Arnold AM, W T Longstreth J, Elster AD, Jungreis CA, O’Leary DH, et al. Sulcal, ventricular, and white matter changes at mr imaging in the aging brain: Data from the cardiovascular health study. Radiology. 1997;202:33–39 [DOI] [PubMed] [Google Scholar]
- 20.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain mri: The aric study. Neurology. 2011;76:1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksson H, Caidaul K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, et al. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical-epidemiological use: The study of men born in 1913. European Heart Journal. 1987;8:1007–1014 [DOI] [PubMed] [Google Scholar]
- 22.Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The cardiovascular health study. Stroke. 1994;25:318–327 [DOI] [PubMed] [Google Scholar]
- 23.Ezekowitz MD, James KE, Nazarian SM, Davenport J, Broderick JP, Gupta SR, et al. Silent cerebral infarction in patients with nonrheumatic atrial fibrillation. Circulation. 1995;92:2178–2182 [DOI] [PubMed] [Google Scholar]
- 24.Kalantarian S, Ay H, Gollub RL, et al. Association between atrial fibrillation and silent cerebral infarctions: A systematic review and meta-analysis. Annals of Internal Medicine. 2014;161:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L, et al. Atrial fibrillation and cognitive decline–the role of subclinical cerebral infarcts: The aric study. Stroke. 2014;45:2568–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikram MA, Vrooman HA, Vernooij MW, Heijer Td, Hofman A, WJ Niessen, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiology of Aging. 2010;31:378–386 [DOI] [PubMed] [Google Scholar]
- 27.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: The invisible lesions. The Lancet Neurology. 2012;11:272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangen KJ, Nation DA, Delano-Wood L, Weissberger GH, Hansen LA, Galasko DR, et al. Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed alzheimer’s disease. Alzheimer’s & Dementia. 2015;11:394–403.e391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen P, Kastrup J, Videbæk R, Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. Journal of Cerebral Blood Flow & Metabolism. 1989;9:422–425 [DOI] [PubMed] [Google Scholar]
- 30.Efimova I, Efimova N, Chernov V, Popov S, Lishmanov Y. Ablation and pacing: Improving brain perfusion and cognitive function in patients with atrial fibrillation and uncontrolled ventricular rates. Pacing and Clinical Electrophysiology. 2012;35:320–326 [DOI] [PubMed] [Google Scholar]
- 31.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: The framingham heart study. JAMA. 1994;271:840–844 [PubMed] [Google Scholar]
- 32.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461 [DOI] [PubMed] [Google Scholar]
- 33.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.