Abstract
Accurate survival estimations in Ewing sarcoma are necessary to develop risk- and response adaptive treatment strategies allowing for early decision-making. We aim to develop an easy-to-use survival estimation tool from diagnosis and surgery. A retrospective study of 1314 Ewing sarcoma patients was performed. Associations between prognostic variables at diagnosis/surgery and overall survival (OS), were investigated using Kaplan-Meier and multivariate Cox models. Predictive accuracy was evaluated by cross-validation and Harrell C-statistics. Median follow-up was 7.9 years (95%CI 7.6–8.3). Independent prognostic factors at diagnosis were age, volume, primary tumor localization and disease extent. 5 risk categories (A-E) were identified with 5-year OS of 88% (86–94), 69% (64–74), 57% (50–64), 51% (42–60) and 28% (22–34) respectively. Harrell C-statistic was 0.70. Independent prognostic factors from surgery were age, volume, disease extent and histological response. In categories A-B, 5y OS increased to 92% (87–97) and 79% (71–87) respectively for 100% necrosis and decreased to 76% (67–85) and 62% (55–69) respectively for <100% necrosis. In categories C-E, 5y OS increased to 65% (55–75), 65% (52–78) and 52% (38–66) respectively for ≥90% necrosis and decreased to 38% (22–54), 11% (0–26) and 7% (0–19) respectively for <90% necrosis. We present an easy-to-use survival estimation tool from diagnosis in Ewing sarcoma based on age, volume, primary tumor localization and disease extent. Histological response is a strong additional prognostic factor for OS.
Subject terms: Bone cancer, Paediatric cancer, Sarcoma
Introduction
Ewing sarcoma (EwS) is an aggressive bone and soft-tissue tumor predominantly affecting children and young adults1. Management rapidly evolved over the last decades, leading to a multimodality approach consisting of chemotherapy, surgery and/or radiotherapy that has become the standard of care. As a result of collaborating trials overall survival (OS) improved drastically, with 10-year OS rates of 55–65% for localized disease. Survival in metastatic disease, present in 20–25% of the patients and usually affecting the lungs (70–80%) and bone/bone marrow (40–45%), is still dismal with 5-year OS varying from 20–35%2–5. In primary non-metastatic disease 30–40% of patients experience recurrence, in metastatic disease this number increases to 60–80%. Relapse is mostly systemic (71–73%), followed by combined (12–18%) and local (11–15%) relapse6,7. 5-year post-relapse survival is poor, 15–25%, with local recurrence faring better than systemic6,8,9.
Personalized medicine encompasses tailoring of treatment based on individual patient characteristics, needs and preferences to improve outcome. Accurate estimations of survival according to the individual patient’s risk profile at different time points are necessary to offer EwS patients the most appropriate treatment, balancing survival and prognosis with toxicity and quality of life. Especially in this young patient population, this balance is essential in our aim to provide the best possible care. Correct survival estimations are difficult and patients and physicians tend to be overoptimistic10. Better selection of risk groups and thereby adjusted treatment allows for early decision making, will help improve future outcomes and assists in clinical trial design.
Many studies evaluated the influence of various risk factors on survival in EwS. Only three8,11,12 described combining these prognostic factors into risk groups. All three models present shortcomings. They are based on small homogeneous cohorts, that are either not validated or did not include all relevant variables in the model. Keeping these shortcomings in mind, our aim was to develop an easy-to-use survival estimation tool for EwS. Objectives are to: (1) Identify prognostic factors for overall survival from diagnosis and surgery; (2) Develop an accurate baseline prognostic model; (3) Validate the models’ predictive accuracy; (4) Develop a second prognostic model from surgery.
Methods
This study was reviewed and approved by the Ethical Committee of the Leiden University Medical Center and granted a waiver for informed consent.
Study population
A retrospective analysis of patients (randomized and non-randomized) from the EURO-E.W.I.N.G 99 trial database was performed. As detailed in Fig. 1, from 1480 available patients, 166 were excluded due to missing data. Thus, 1314 patients were eligible for analysis at diagnosis. Following induction chemotherapy 982 patients underwent surgery of the primary tumor, 190 were excluded due to missing data, resulting in 792 patients eligible for analysis at surgery. All patients were treated according to the protocol with the aim to administer six cycles of VIDE (vincristine, ifosfamide, doxorubic, etoposide) induction chemotherapy followed by local treatment of the primary tumor. The choice of local treatment, surgery, radiotherapy or both, was left to discretion of the multidisciplinary team. After local treatment patients received maintenance therapy.
Figure 1.
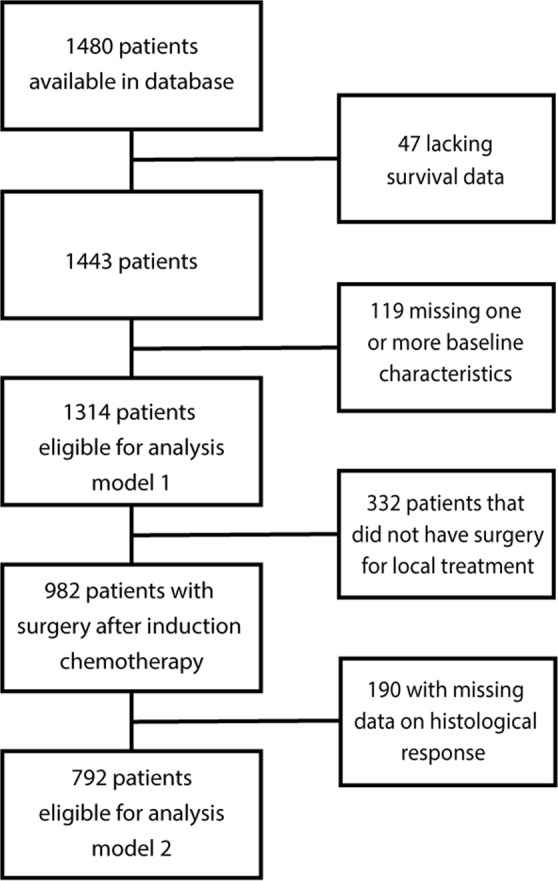
Flowchart inclusion.
Measures
For accurate risk group stratification large representative and contemporary datasets that closely reflect the target population are needed to enhance the relevance, reproducibility and generalizability of the model13–17. Cohorts often contain more variables than can reasonably be used for prediction. Therefore, the most predictive and sensible predictors should be selected. In order to provide all relevant risk factors for such a prognostic model a systematic review18 on the current known prognostic factors for overall survival (OS) and event-free survival (EFS) was performed. Based on this systematic review we selected the most predictive and sensible predictors to be included in the univariate analysis. Prognostic factors and outcome were collected prospectively. Patient characteristics included gender and age. Tumor characteristics included location, type, volume at diagnosis, skip lesions, disease extent and number of metastatic lesions. Histological response (percentage necrosis) and resection margins were assessed on the surgical specimen by local pathologists.
Statistical analysis
The outcome of interest was overall survival (OS) measured from date of diagnosis or date of surgery, until last day of follow-up or date of death. Prognostic factors were evaluated using univariate Cox regression analyses; significant prognostic factors were subsequently included into a multivariate Cox model.
Significant risk factors at diagnosis from the corresponding multivariate Cox model were used to build a stratification scheme of prognostic groups. Prognostic groups were narrowed down into risk categories based on clinical expertise. Another set of risk categories was obtained from the same multivariate Cox model based on predicted survival; a leave-one-out cross-validation framework was used to form cross-validated risk categories on predicted 5-year survival probability19. The prognostic value of the clinical risk categories was assessed by comparison with cross-validated risk categories. Details on cross-validation methodology and risk category classification are provided in Supplementary File 1. Correspondence of clinical and cross-validated risk categories was evaluated using precision and recall (Supplementary File 1). Discriminative ability of both stratification schemes was assessed using Harrell’s C-index20. Observed survival probabilities of clinical risk categories and corresponding cross-validated counterparts were compared by Kaplan-Meier estimators.
Significant risk factors at surgery from the corresponding univariate analysis were used to build a second multivariate Cox model. Associations were considered significant at a rejection level of 5%. All analyses were performed using SPSS version 23.0, R version 3.4.3, and Python 3.6.5.
Results
Baseline characteristics and treatment details of the 1314 patients at diagnosis are presented in Table 1. Median follow-up, assessed by reversed Kaplan-Meier method21, was 7.9 years (95% confidence interval (CI) 7.6–8.3 years); 531 patients died. Localized disease was present in 916 (69.7%), pulmonary metastasis alone in 182 (13.9%) and extrapulmonary metastasis with or without additional pulmonary metastasis in 216 (16.4%) patients. The 5-year OS was 73% (95%CI, 70–76%), 53% (95%CI, 45–60%) and 28% (95%CI, 22–34%) respectively.
Table 1.
Patient demographics at diagnosis.
| Characteristic | N (%) |
|---|---|
| Total | 1314 |
| Gender | |
| Male | 792 (60.3) |
| Female | 522 (39.7) |
| Age (mean, years + SD) | 16,8 (9.9) |
| Origin | |
| Osseous | 1107 (84.2) |
| Extra-osseous | 207 (15.8) |
| Primary tumor localization | |
| Extremity | 499 (38.0) |
| Upper | 108 (8.2) |
| Lower | 391 (29.8) |
| Axial | 815 (62.0) |
| Pelvic | 312 (23.7) |
| Other | 503 (38.3) |
| Volume at diagnosis | |
| <200 ml | 740 (56.3) |
| ≥200 ml | 574 (43.7) |
| Skip lesions at diagnosis | 63 (4.8) |
| Disease extent | |
| Localized | 916 (69.7) |
| Pulmonary metastasis | 182 (13.9) |
| Extrapulmonary metastasis | 216 (16.4) |
| Number of metastatic lesions | |
| One | 43 (3.3) |
| ≥2 | 355 (27.0) |
| Local treatment modality | |
| Surgery | 550 (41.9) |
| Radiotherapy | 193 (14.7) |
| Surgery + radiotherapy | 432 (32.9) |
| Pre-operative radiotherapy | 47 (3.6) |
| Post-operative radiotherapy | 385 (29.3) |
| Unknown | 139 (10.5) |
SD = standard deviation.
Continuous variables are presented by means along with corresponding standard deviation between brackets, categorical variables as a number with the percentage between brackets.
Prognostic factors at diagnosis
Univariate and multivariate Cox proportional hazard models were estimated to investigate the effect of risk factors on OS. Results are shown in Table 2. Univariate analysis showed that age, volume, primary tumor localization, skip lesions, disease extent and number of metastatic lesions are significantly associated with OS. In multivariate analysis age ≥16 years (HR 1.36; 95%CI 1.15–1.62); p < 0.001) volume ≥200 ml (HR 1.50; 95%CI 1.25–1.79; p < 0.001), pelvic location (HR 1.34; 95%CI 1.07–1.67; p = 0.015), pulmonary metastasis only (HR 1.79; 95%CI 1.42–2.27; p < 0.001), extrapulmonary metastasis with or without pulmonary metastasis (HR 3.72; 95%CI 3.02–4.56; p < 0.001) and ≥2 metastatic lesions (HR 2.80; 95%CI 2.33–3.36; p < 0.001) remained significant for OS.
Table 2.
Hazard ratio (HR) with corresponding 95% confidence interval (CI) from univariate and multivariate analysis at time of diagnosis (n = 1314).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Gender | ||||
| Female | 1 | |||
| Male | 1.12 (0.94–1.34) | 0.195 | ||
| Age | ||||
| <16 years | 1 | 1 | ||
| ≥16 years | 1.53 (1.29–1.82) | <0.001 | 1.36 (1.15–1.62) | <0.001 |
| Origin | ||||
| Osseous | 1.13 (0.89–1.45) | 0.313 | ||
| Extra-osseous | 1 | |||
| Volume | ||||
| <200 ml | 1 | 1 | ||
| ≥200 ml | 1.96 (1.65–2.33) | <0.001 | 1.50 (1.25–1.79) | <0.001 |
| Location | ||||
| Extremity | 1 | 1 | ||
| Axial (excl pelvic) | 1.17 (0.95–1.43). | 0.148 | 1.16 (0.94–1.44) | 0.178 |
| Pelvic | 1.9 (1.54–2.35) | <0.001 | 1.34 (1.07–1.67) | 0.015 |
| Skiplesions at diagnosis | ||||
| No | 1 | 1 | ||
| Yes | 1.56 (1.10–2.22) | 0.013 | 1.11 (0.76–1.60) | 0.595 |
| Disease extent | ||||
| Localized | 1 | 1 | ||
| Pulmonary metastasis | 2.05 (1.63–2.58) | <0.001 | 1.79 (1.42–2.27) | <0.001 |
| Extrapulmonary metastasis | 4.33 (3.56–5.28) | <0.001 | 3.72 (3.02–4.58) | <0.001 |
| Number of metastatic lesions | ||||
| None | 1 | 1 | ||
| One | 1.71 (1.1–2.66). | <0.001 | 1.54 (0.98–2.40) | 0.059 |
| ≥2 | 3.25 (2.73–3.87) | <0.001 | 2.80 (2.33–3.36) | <0.001 |
Baseline prognostic model
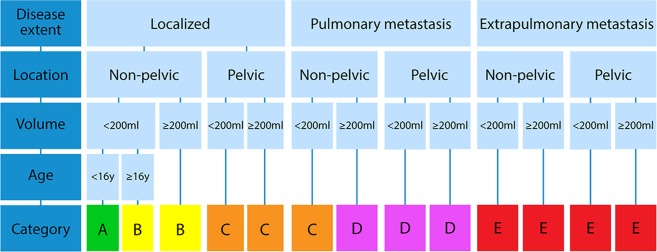
Based on the independent prognostic factors at diagnosis (age, volume, location and disease extent), 13 prognostic groups were created and 5 clinically relevant categories (A-E) were estimated. Table 3 provides a detailed description of the prognostic groups and corresponding OS at 3 and 5 years. The 5-year OS for categories A-E was 88% (95%CI 86–94), 69% (95%CI 64–74), 57% (95%CI 50–64), 51% (95%CI 42–60) and 28% (95%CI 22–34) respectively. Figure 2 presents a flowchart to stratify patients at diagnosis. Age only showed strong impact on survival in the first two prognostic groups. In the other prognostic groups survival was similar for patients aged younger than 16 and patients aged 16 and above. Age is therefor only included in the stratification scheme for the first two prognostic groups.
Table 3.
Overall survival at 3 and 5 years for each prognostic group.
| Prognostic group | Disease extent | Location | Volume | Age | N | Overall survival (95%CI) | Category | |
|---|---|---|---|---|---|---|---|---|
| 3 years | 5 years | |||||||
| 1 | Localized | Non-pelvic | <200 ml | <16 | 296 | 90% (86–94) | 88% (84–92) | A |
| 2 | Localized | Non-pelvic | <200 ml | ≥16 | 207 | 80% (75–85) | 71% (64–78) | B |
| 3 | Localized | Non-pelvic | ≥200 ml | 243 | 75% (70–80) | 67% (61–73) | B | |
| 4 | Localized | Pelvic | <200 ml | 78 | 74% (64–84) | 62% (50–74) | C | |
| 5 | Localized | Pelvic | ≥200 ml | 92 | 67% (57–77) | 53% (43–63) | C | |
| 6 | Pulmonary | Non-pelvic | <200 ml | 57 | 77% (66–88) | 58% (45–71) | C | |
| 7 | Pulmonary | Non-pelvic | ≥200 ml | 62 | 60% (48–72) | 48% (36–60) | D | |
| 8 | Pulmonary | Pelvic | <200 ml | 17 | 82% (67–95) | 76% (56–96) | D | |
| 9 | Pulmonary | Pelvic | ≥200 ml | 46 | 54% (39–69) | 45% (30–60) | D | |
| 10 | Extrapulmonary | Non-pelvic | <200 ml | 63 | 36% (24–48) | 29% (17–41) | E | |
| 11 | Extrapulmonary | Non-pelvic | ≥200 ml | 74 | 33% (22–44) | 31% (20–42) | E | |
| 12 | Extrapulmonary | Pelvic | <200 ml | 22 | 46% (25–67) | 46% (25–67) | E | |
| 13 | Extrapulmonary | Pelvic | ≥200 ml | 57 | 21% (10–32) | 17% (7–27) | E | |
Creation of 13 prognostic groups based on disease extent, tumor localization, volume and age showing overall survival (OS) with corresponding 95% confidence interval (CI) at 3 and 5 years. Last column shows the risk category based on clinical expertise (n = 1314).
Figure 2.
Flowchart for stratification of Ewing sarcoma patients at diagnosis.
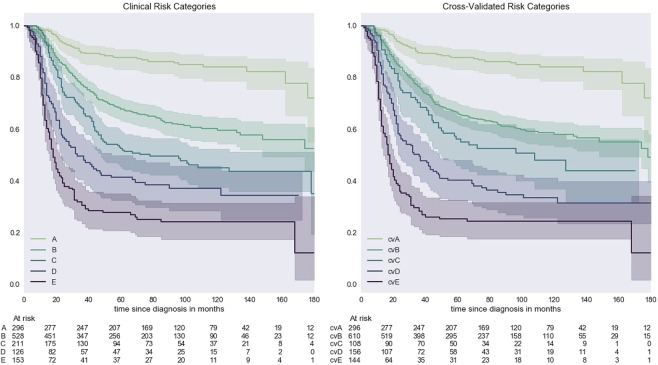
Harrell’s C-statistic was 0.70. Discriminatory ability was further evaluated using cross validation. Detailed comparisons of OS in the clinical and cross-validated risk categories at 2, 3 and 5 years are presented in Table 4. Survival probabilities do not show any difference between clinical and cross-validated risk categories. The overall agreement is very good (precision 90.26%; recall 89.57%). Figure 3 illustrates the models’ discrimination ability visualized by the spread of Kaplan-Meier estimates.
Table 4.
Overall survival at 2, 3 and 5 years for clinical and cross-validated categories.
| Category | n | 2-year OS (95%CI) | 3-year OS (95%CI) | 5-year OS (95%CI) | |||
|---|---|---|---|---|---|---|---|
| Clinical | Cross-validated | Clinical | Cross-validated | Clinical | Cross-validated | ||
| A | 296 | 93% (91–96) | 93% (91–96) | 90% (86–93) | 90% (86–83) | 88% (84–92) | 88% (84–92) |
| B | 450 | 85% (82–88) | 84% (81–87) | 77% (73–81) | 76% (73–80) | 68% (64–72) | 66% (62–70) |
| C | 227 | 74% (68–80) | 76% (68–84) | 68% (62–75) | 70% (62–79) | 52% (46–60) | 56% (47–67) |
| D | 125 | 57% (49–66) | 57% (50–66) | 50% (42–59) | 50% (42–58) | 41% (33–51) | 40% (33–49) |
| E | 216 | 39% (32–48) | 36% (29–45) | 30% (24–39) | 28% (22–37) | 28% (21–36) | 25% (19–33) |
Detailed comparison of overall survival (OS) with corresponding 95% confidence interval (CI) in each of the clinical and cross-validated risk categories at 2, 3 and 5 years (n = 1314).
Figure 3.
Kaplan-Meier curves for overall survival of clinical risk categories and cross-validated risk categories. Survival is measured in months from diagnosis. On the left the Kaplan-Meier survival curves of the clinical risk categories (A–E) based on the 13 prognostic groups. On the right the Kaplan-Meier survival curves of the cross-validated risk categories (cvA-cvE).
Prognostic factors known at time of surgery
Table 5 shows the effect of prognostic factors known at surgery in univariate and multivariate analysis. Univariate analysis showed that age, volume at diagnosis, primary tumor localization, disease extent, number of metastatic lesions, surgical margin and histological response are significantly associated with OS. In multivariate analysis age ≥16 years (HR 1,38; 95%CI 1,08–1.77; p = 0,01), pulmonary metastasis (HR 1,99; 95%CI 21.47–2,70; p < 0.001), extrapulmonary metastasis with or without pulmonary metastasis (HR 3.18; 95%CI 2.23–4.53; p < 0,001), ≥2 metastatic lesions (HR 2.53; 95%CI 1.93–3.32; p < 0,001) and histological response of 90–99% (HR 1.58; 95%CI 1.16–2.16; p = 0,04) and of <90% (HR 2.90; 95%CI 2,15–3,93; p < 0,001) remained significant prognostic factors for OS.
Table 5.
Hazard ratio (HR) with corresponding 95% confidence interval (CI) from univariate and multivariate analysis at time of surgery (n = 792).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Gender | ||||
| Female | 1 | |||
| Male | 1.08 (0.84–1.37) | 0.564 | ||
| Age | ||||
| <16 years | 1 | 1 | ||
| ≥16 years | 1.53 (1.20–1.94) | <0.001 | 1.38 (1.08–1.77) | 0.010 |
| Origin | ||||
| Osseous | 1 | |||
| Extra-osseous | 1.23 (0.87–1.74) | 0.245 | ||
| Volume | ||||
| <200 ml | 1 | 1 | ||
| ≥200 ml | 1.65 (1.30–2.09) | <0.001 | 1.29 (0.99–1.66) | 0.053 |
| Location | ||||
| Extremity | 1 | 1 | ||
| Axial (excl pelvic) | 1.09 (0.82–1.43) | 0.564 | 1.05 (0.79–1.41) | 0.735 |
| Pelvic | 1.59 (1.18–2.15) | 0.002 | 1.30 (0.94–1.79) | 0.110 |
| Disease extent | ||||
| Localized | 1 | 1 | ||
| Pulmonary metastasis | 2.09 (1.55–2.81) | <0.001 | 1.99 (1.47–2.70) | <0.001 |
| Extrapulmonary metastasis | 2.88 (2.03–4.08) | <0.001 | 3.18 (2.23–4.53) | <0.001 |
| Number of metastatic lesions | ||||
| None | 1 | 1 | ||
| One | 1.52 (0.85–2.73) | 0.159 | 1.62 (0.90–2.92) | 0.108 |
| ≥2 | 2.54 (1.96–3.29) | <0.001 | 2.53 (1.93–3.32) | <0.001 |
| Margin status | ||||
| Wide | 1 | 1 | ||
| Marginal | 1.48 (1.08–2.03) | <0.001 | 1.06 (0.76–1.47) | 0.736 |
| Intralesional | 2.43 (1.55–3.81) | <0.001 | 1.47 (0.91–2.93) | 0.120 |
| Histological response | ||||
| 100% | 1 | 1 | ||
| 90–99% | 1.66 (1.22–2.25) | <0.001 | 1.58 (1.16–2.16) | 0.004 |
| <90% | 2.86 (2.15–3.81) | <0.001 | 2.90 (2.15–3.93) | <0.001 |
| Radiotherapy | ||||
| No | 1 | |||
| Pre-operative radiotherapy | 1.19 (0.71–1.99) | 0.503 | ||
| Post-operative radiotherapy | 1.10 (0.85–1.41) | 0.478 | ||
Effect of histological response on overall survival
A multivariate Cox model with prognostic factors histological response, risk categories and an interaction term was estimated. The interaction between histological response and risk category was not significant, meaning that the effect of histological response does not vary significantly across the risk categories. The association between histological response and OS was therefore assessed by fitting a Cox model with risk category and histological response, details are presented in Table 6.
Table 6.
Cox model for overall survival from surgery.
| N | Cox model | p | |
|---|---|---|---|
| HR (95%CI) | |||
| Histological response | |||
| 100% | 360 | 1 | |
| 90–99% | 224 | 1.57 (1.15–2.12) | 0.004 |
| <90% | 208 | 3.15 (2.37–4.19) | <0.001 |
| Risk category | |||
| A | 199 | 1 | |
| B | 316 | 2.07 (1.42–3.03) | <0,001 |
| C | 135 | 3.68 (2.46–5.52) | <0,001 |
| D | 73 | 4.38 (2.64–7.28) | <0,001 |
| E | 69 | 6.23 (3.72–10.44) | <0,001 |
Hazard ratio (HR) along with 95% confidence interval (CI) (n = 792).
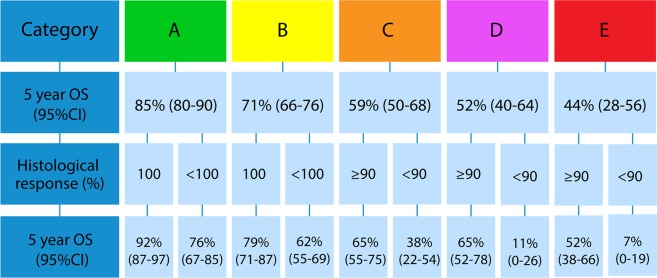
Figure 4 presents a flowchart to stratify patients at surgery based on the Cox model. For patients in category A with 100% necrosis, 5y OS increased to 92% (95%CI 87–97), but decreased to 76% (95%CI 67–85) when necrosis was <100%. For patients in category B, 5y OS increased to 79% (95%CI 71–87) when necrosis was 100% and decreased to 62% (95%CI 55–69) when necrosis was <100%. In category C, survival increased to 65% (95%CI 55–75) when necrosis was ≥90% and decreased to 38% (95%CI 22–54) when necrosis was <90%. In category D, 5y OS increased to 65% (95%CI 52–78) when necrosis was ≥90% but decreased to 11% (95%CI 0–26) when necrosis was <90%. The same pattern accounts for category E where 5y OS increases to 52% (95%CI 38–66) when necrosis was ≥90% necrosis but drastically decreases to 7% (95%CI 0–19) when necrosis was <90%.
Figure 4.
Flowchart for stratification of Ewing sarcoma patients at surgery assessed by Kaplan Meier method.
Discussion
To further improve survival in Ewing sarcoma development of risk- and response adaptive treatment strategies are necessary to allow decision making at different disease stages. Accurate survival estimations are challenging. We developed and validated an easy-to-use survival estimation tool for EwS, based on age, volume, primary tumor localization and disease extent. Furthermore, we show that during the course of treatment survival changes as more information becomes available.
The model presented is based on a cohort of 1314 EwS patients with uniformity in diagnostics and treatment and availability of all relevant prognostic factors. The provided flowcharts are easy-to-use and based on assessable variables. The 13 prognostic groups provide detailed insight in expected survival and could assist in fine-tuning individual treatment. The prognostic groups were narrowed down to 5 risk categories (A-E) based on clinical expertise. The risk categories defined on clinical criteria are consistent with cross-validated risk categories defined on predicted 5-year survival probability. The information gained after surgery offers a second time-point for multidisciplinary decision-making, at this point histological response is an strong additional prognostic factor for OS.
The prognostic significance of the variables in both models has previously been reported. Disease extent is the foundation of the model and strongest prognostic factor in this study. This is consistent with previous studies demonstrating that the presence of metastasis is a strong prognostic factor for survival22–24; patients with extrapulmonary metastasis do significantly worse than patients with pulmonary metastasis alone2,25,26. Disease extent is also used to define risk groups in previous and current European EwS trials. We also found that primary tumors in the pelvic strongly affect survival, consistent with previously published studies27. Other studies suggested an adverse effect on survival for axial localizations (including pelvic) compared to tumours in the extremities11,28–30. Volume has also been used to design EwS trails31; research shows that larger volumes are associated with poorer survival. Cut-off points at 100 ml26 and 150 ml32 have been evaluated, but 200 ml seems the most appropriate33,34 and was therefore used in this study. Age is an independent prognostic factor for survival in the current study, but only shows strong impact on outcome in two prognostic groups. Cut-of points at 1822,29,30 and 14 years35 have been evaluated. Strong evidence for a specific cut-off point is lacking. All studies consistently show that older age is associated with poorer survival. We chose 16 years as cut-off, as it is at the interface of pediatric and adult treatment. Histological response, used to tailor treatment in European EwS, is considered of high prognostic value as confirmed in this study. According to literature patients with 100% necrosis have the best survival28,32, other studies showed similar results using cut-of points at 95%36 and 90% necrosis33.
To our knowledge, only three studies described combining prognostic factors into risk groups. Rodriquez-Galindo et al.8 used Cox proportional hazards models to identify four risk groups in 220 EwS patients based on age (</≥14 years), primary tumor site (pelvic/non-pelvic) and disease extent (localized/isolated lung metastasis/extrapulmonary metastasis). Although based on a small cohort and not validated, our risk groups are similar, with the exception that we added volume to the model. Although they found that tumor size was an independent prognostic factor for survival, they did not include it in the final model. Biswas et al.11 developed a prognostic model for localized EwS based on 244 patients. Cox models were estimated showing that patients with axial tumors and elevated white blood cell count (WBC) (>11 × 109/L) had poor OS (HR 4.44 (95%CI 2.1–9.4; p < 0.001) and patients with symptoms >4 months, tumor size ≥8 cm and elevated WBC had poor event-free survival (HR 3.89 (95%CI 1.63–9.26; p = 0.002). These models were not validated and are based on a small unmixed cohort limiting its usefulness for clinical decision-making. Additionally, in the systematic review we performed before the start of the current study a consistent association between several biomarkers, such as neutrophil to lymphocyte ratio, hemoglobin and WBC count could not be found, in contrast to the model of Biswas et al.11 and another study37. Lastly Karski et al.12 derived prognostic groups from 2124 EwS patients in the Surveillance, Epidemiology, and End Results (SEER) database. Using Cox models for OS they constructed five prognostic groups: (1) Localized, <18 years, non-pelvic; (2) Localized, <18 years, pelvic or localized, ≥18 years, White/non-Hispanic; (3) Localized, ≥18 years, other ethnicities; (4) Metastatic, <18 years; (5) Metastatic, ≥18 years. Validation was performed on a cohort of 1680 EwS patients from the Children’s Oncology Group trials, which showed significantly different OS based upon this classification. Although validated, the primary model did not include all relevant variables as the SEER database lacks information on metastatic site. In addition tumor size was missing in 40% of the patients and therefore not included, limiting the strengths of the models.
Limitation of this study include the fact that the local treatment choice was left to discretion of the threatening multidisciplinary teams and might have influenced the results discussed in this article. Secondly, a good prediction model should provide accurate prediction of events by using a comprehensive dataset. In addition, the model should be relatively simple and clinically easy to use. Inaccurate estimates of future events will mislead physicians to provide insufficient treatment. On the other hand, a model with high predictability but which is complex or has too many factors will not be useful. Achieving the optimal balance between predictability and simplicity is the key to a good prediction model13–17. Cohorts often contain more variables than can reasonably be used for prediction and for sufficient power one needs at least 10 events per variable. We therefor choose to select the most predictive and sensible predictors to be included in the univariate analysis based on our systematic review18. Using a more extensive variable profile could have given useful insights, but we feel that by doing so we would lose simplicity while not improving predictability. Third, surgical margins and histological response were assessed by local pathologists and not by a reference pathologist. Differences between centers in analyzing specimens are possible. Last, the retrospective study design using data form a prospectively performed trial led to some missing data (11%), despite this, a large cohort of EwS patients was available for analysis.
Conclusion
This study presents an easy-to-use clinical tool to predict OS from diagnosis in EwS, based on age, tumor volume, tumor localization and disease extent. After surgery, the second multidisciplinary decision point, histological response is a strong additional prognostic factor for OS.
Supplementary information
Acknowledgements
We thank Stephanie Klco-Brosius and Susanne Jabar for their assistance. This study was supported by a donation from the Team Westland Foundation (to PDSD), a German Cancer Aid grant 108128 to UD; ERA-Net-TRANSCAN consortium, project number 01KT1310, and Euro Ewing Consortium EEC, project number EU-FP7 602856, both funded under the European Commission Seventh Framework Program FP7-HEALTH (http://cordis.europa.eu/) (to UD); and by David Dressler+ (to UD). This funding source had no role in the design of this study as well as any role during its execution, analyses, interpretation of the data, in the writing of the report or decision to submit the article for publication.
Author Contributions
S.E.B.: conceptualization; data curation; formal analysis; methodology; project administration; writing - original draft. C.L.: formal analysis; methodology; validation; writing – original draft, review and editing. A.J.R.: formal analysis; methodology; validation; writing – review and editing. A.R.: data curation; methodology; writing – review and editing. H.G.: supervision; writing – review and editing. M.F.: supervision; methodology, writing – review and editing. M.A.J.S.: supervision; writing – review and editing. P.D.S.D.: supervision; funding acquisition; writing – review and editing. U.D.: conceptualization, data curation; supervision; writing – review and editing.
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information Files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
S.E. Bosma and C. Lancia contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46721-8.
References
- 1.Fletcher, C. D. M., Bridge, J. A., Hogendoorn, P. C. W., Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone. 4th edition edn, (IARC, 2013).
- 2.Ladenstein R, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 3.Haeusler J, et al. The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES) Cancer. 2010;116:443–450. doi: 10.1002/cncr.24740. [DOI] [PubMed] [Google Scholar]
- 4.Grunewald TGP, et al. Ewing sarcoma. Nat Rev Dis Primers. 2018;4:5. doi: 10.1038/s41572-018-0003-x. [DOI] [PubMed] [Google Scholar]
- 5.Pappo AS, Dirksen U. Rhabdomyosarcoma, Ewing Sarcoma, and Other Round Cell Sarcomas. J Clin Oncol. 2018;36:168–179. doi: 10.1200/JCO.2017.74.7402. [DOI] [PubMed] [Google Scholar]
- 6.Stahl M, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer. 2011;57:549–553. doi: 10.1002/pbc.23040. [DOI] [PubMed] [Google Scholar]
- 7.Barker LM, Pendergrass TW, Sanders JE, Hawkins DS. Survival after recurrence of Ewing’s sarcoma family of tumors. J Clin Oncol. 2005;23:4354–4362. doi: 10.1200/JCO.2005.05.105. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Galindo C, et al. Analysis of prognostic factors in ewing sarcoma family of tumors: review of St. Jude Children’s Research Hospital studies. Cancer. 2007;110:375–384. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- 9.Bacci G, et al. Therapy and survival after recurrence of Ewing’s tumors: the Rizzoli experience in 195 patients treated with adjuvant and neoadjuvant chemotherapy from 1979 to 1997. Ann Oncol. 2003;14:1654–1659. doi: 10.1093/annonc/mdg457. [DOI] [PubMed] [Google Scholar]
- 10.Cheon S, et al. The accuracy of clinicians’ predictions of survival in advanced cancer: a review. Ann Palliat Med. 2016;5:22–29. doi: 10.3978/j.issn.2224-5820.2015.08.04. [DOI] [PubMed] [Google Scholar]
- 11.Biswas B, et al. Developing a prognostic model for localized Ewing sarcoma family of tumors: A single institutional experience of 224 cases treated with uniform chemotherapy protocol. J Surg Oncol. 2015;111:683–689. doi: 10.1002/jso.23861. [DOI] [PubMed] [Google Scholar]
- 12.Karski EE, et al. Identification of Discrete Prognostic Groups in Ewing Sarcoma. Pediatr Blood Cancer. 2016;63:47–53. doi: 10.1002/pbc.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]
- 14.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 15.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: Developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 16.Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–1931. doi: 10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosma SE, Ayu O, Fiocco M, Gelderblom H, Dijkstra PDS. Prognostic factors for survival in Ewing sarcoma: A systematic review. Surgical oncology. 2018;27:603–610. doi: 10.1016/j.suronc.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Simon RM, Subramanian J, Li MC, Menezes S. Using cross-validation to evaluate predictive accuracy of survival risk classifiers based on high-dimensional data. Brief Bioinform. 2011;12:203–214. doi: 10.1093/bib/bbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrell, F. E., Jr., Lee, K. L. & Mark, D. B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med15, 361–387, doi:10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4 (1996). [DOI] [PubMed]
- 21.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Hoang BH, Ziogas A, Zell JA. Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. 2010;116:1964–1973. doi: 10.1002/cncr.24937. [DOI] [PubMed] [Google Scholar]
- 23.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with Ewing’s sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39:189–195. doi: 10.1016/j.canep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Jawad MU, et al. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database, 1973–2005. Cancer. 2009;115:3526–3536. doi: 10.1002/cncr.24388[doi]. [DOI] [PubMed] [Google Scholar]
- 25.Takenaka S, et al. Treatment outcomes of Japanese patients with Ewing sarcoma: differences between skeletal and extraskeletal Ewing sarcoma. Japanese journal of clinical oncology. 2016;46:522–528. doi: 10.1093/jjco/hyw032. [DOI] [PubMed] [Google Scholar]
- 26.Cotterill SJ, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 27.Fizazi K, et al. Ewing’s family of tumors in adults: multivariate analysis of survival and long-term results of multimodality therapy in 182 patients. J Clin Oncol. 1998;16:3736–3743. doi: 10.1200/JCO.1998.16.12.3736. [DOI] [PubMed] [Google Scholar]
- 28.Albergo JI, et al. Ewing’s sarcoma: only patients with 100% of necrosis after chemotherapy should be classified as having a good response. Bone Joint J. 2016;98-B:1138–1144. doi: 10.1302/0301-620X.98B8.37346. [DOI] [PubMed] [Google Scholar]
- 29.Verma V, Denniston KA, Lin CJ, Lin C. A Comparison of Pediatric vs. Adult Patients with the Ewing Sarcoma Family of Tumors. Front Oncol. 2017;7:82. doi: 10.3389/fonc.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller BJ, Gao YB, Duchman KR. Does surgery or radiation provide the best overall survival in Ewing’s sarcoma? A review of the National Cancer Data Base. Journal of Surgical Oncology. 2017;116:384–390. doi: 10.1002/jso.24652. [DOI] [PubMed] [Google Scholar]
- 31.Protocol: Euro Ewing 99. European Ewing tumour Working Initiative of National Groups, Ewing Tumour Studies 1999/EE99. (Euro Ewing 99, 2006).
- 32.Bacci G, et al. Role of surgery in local treatment of Ewing’s sarcoma of the extremities in patients undergoing adjuvant and neoadjuvant chemotherapy. Oncol Rep. 2004;11:111–120. [PubMed] [Google Scholar]
- 33.Gaspar N, et al. Risk adapted chemotherapy for localised Ewing’s sarcoma of bone: the French EW93 study. Eur J Cancer. 2012;48:1376–1385. doi: 10.1016/j.ejca.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Foulon S, et al. Can postoperative radiotherapy be omitted in localised standard-risk Ewing sarcoma? An observational study of the Euro-E.W.I.N.G group. Eur J Cancer. 2016;61:128–136. doi: 10.1016/j.ejca.2016.03.075. [DOI] [PubMed] [Google Scholar]
- 35.Jenkin RD, et al. Localised Ewing sarcoma/PNET of bone–prognostic factors and international data comparison. Med Pediatr Oncol. 2002;39:586–593. doi: 10.1002/mpo.10212. [DOI] [PubMed] [Google Scholar]
- 36.Oberlin O, et al. Prognostic factors in localized Ewing’s tumours and peripheral neuroectodermal tumours: the third study of the French Society of Paediatric Oncology (EW88 study) Br J Cancer. 2001;85:1646–1654. doi: 10.1054/bjoc.2001.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li YJ, et al. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag Res. 2017;9:443–451. doi: 10.2147/CMAR.S146827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information Files).