Abstract
For seeking high enantiopurity, the previously reported thermal asymmetric catalysis is usually carried out at low temperature sometimes with limited yield, that is, the high enantiomeric excess (ee) usually at the cost of high yield. Thus, the achieving both high stereoselectivity and yield is an enormous challenge. We report herein two metal nanoparticle (M NP)-loaded and porphyrin-containing homochiral covalent organic framework (CCOF)-based composite catalysts, and their application in the thermally-driven asymmetric one-pot Henry and A3-coupling reactions. All the reactions are conducted at elevated temperatures with both excellent stereoselectivity and yield which resulted from the synergy of CCOF confinement effect and M NP catalytic activation. Notably, the needed thermal energy for the asymmetric reactions herein is derived from the photothermal conversion via porphyrin-based CCOF upon irradiation with visible light. Remarkably, the CCOF confinement effect can be effectively maintained up to 100 °C for the asymmetric one-pot Henry and A3-coupling reactions herein.
Subject terms: Heterogeneous catalysis, Metal-organic frameworks, Asymmetric catalysis
Achieving both high stereoselectivity and yield is a challenge for conventional asymmetric catalysis. Here, the authors report two metal nanoparticle-loaded and porphyrin-containing homochiral covalent organic framework-based composite catalysts that exhibit high stereoselectivity and yield in the thermally-driven asymmetric reactions.
Introduction
Photothermal conversion materials (PTCMs), which can transfer light energy into thermal energy, exhibit a variety of potential applications involved photothermal conversion process. For example, PTCMs have been successfully used in the fields of solar collection1, microcontrollers2, and especially photothermal therapy (PTT) for cancer treatment3. Among various applications, PTCMs-based photothermal chiral catalysis, especially natural sunlight triggered thermally-driven asymmetric synthesis, has attracted much less attention4.
As it is known, both yield and enantioselectivity are the crucial indicators for the chiral synthesis, and the achieving high yield with excellent enantiomeric excess (ee) is meaningful, especially for those important asymmetric organic transformations, such as asymmetric Henry and benzaldehyde–phenylacetylene–pyrrolidine A3-coupling reactions, that can transform the achiral compounds into the high value-added chiral organic chemicals. For seeking high enantiopurity, the most reported high-performance and thermally-driven asymmetric reactions, however, were generally performed at relatively low temperature, which often caused a low product yield. For example, the recently reported asymmetric Henry reaction between benzaldehyde and nitromethane was carried out in a temperature range of −78–45 °C with 28–99% yields and 17–99% ee5–25.
On the other hand, homogenous catalysis has been the main theme in asymmetric synthesis, but it often involved noble metals that cannot be efficiently recycled during the catalytic process. Therefore, the precious metal-based reusable heterogeneous asymmetric catalytic systems are imperative due to more and more serious sustainable and global environmental issues26. Up to date, however, only a few heterogeneous catalytic enantioselective Henry reactions were reported. For instance, the chiral cyclodextrin27 and aerogel-supported MgO with chiral binol28 were used to promote the benzaldehyde-nitromethane addition in high 98 (90% ee) and 95% (90% ee) yields, but they are conducted at −20 and −78 °C, respectively. In addition, a chiral MOF-catalyzed benzaldehyde-nitromethane addition at 45 °C was also reported29, and the desired product was obtained in high 95% ee albeit with only 71% yield.
In addition, the traditional asymmetric catalytic systems are normally complicated, and various additives such as cocatalysts are often coexisted30. For example, the optically active pyrrolidine-derived propargylamine generated from the homogeneous CuI-catalyzed benzaldehyde–phenylacetylene–pyrrolidine A3-coupling can be achieved in high 92% yield and 92% ee, but with the aid of the chiral acid-thiourea cocatalysts and molecular sieve at 0 °C31.
For meeting the multifaceted requirements of the thermally-driven asymmetric catalysis, we report herein two metal nanoparticle (M NP)-loaded and homochiral covalent organic framework (CCOF)-based asymmetric catalytic materials, termed as M@CCOF-CuTPP (M=Au (2), Pd (3)), which are composed of a porphyrin-derived CCOF (CCOF-CuTPP (1)) and corresponding M NP. The obtained 2 and 3 feature chiral templating, photothermal conversion and highly catalytic activity, which allow them to highly promote the thermally-driven asymmetric one-pot Henry and A3-coupling reactions under photothermal conversion conditions, respectively.
Results
Synthesis and characterization of 1–3
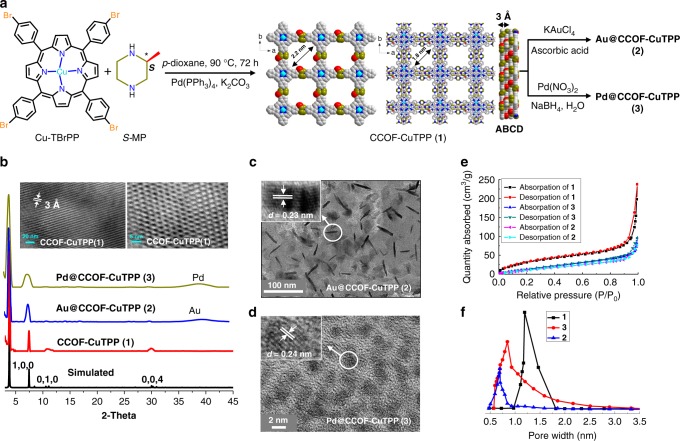
As shown in Fig. 1a, the homochiral organic framework of CCOF-CuTPP (1) was prepared by directly assembling the chiral organic component of S-(+)−2-methylpiperazine (S-MP) and photothermal conversion species of copper tetrabromophenolphthalein (Cu-TBrPP) in anhydrous p-dioxane (90 °C, 72 h) with the aid of Pd(PPh4)3 and K2CO3 in 63% yield. After filtration and successively and completely washed with hydrochloric acid, dichloromethane, ethanol, and water, 1 was obtained as purple-black crystalline powders that were insoluble in common organic solvents and water.
Fig. 1.
Synthesis and characterization of 1–3. a Synthesis of 1–3. Single 2D layer and crystal packing patterns of 1. b Simulated and measured PXRD patterns of CCOF-CuTPP (1), Au@CCOF-CuTPP (2) and Pd@CCOF-CuTPP (3). TEM images (side and top views) of 1 were inserted. c, d TEM images of 2 and 3. Their SEM images are shown in Supplementary Fig. 1. e N2 adsorption isotherms of 1–3 at 77 K. f The pore widths of 1–3 are centered at 1.20, 0.71, and 0.85 nm, respectively
Scanning electron microscopy (SEM) was used to visualize the as-synthesized 1, and its particle morphology was observed (Supplementary Fig. 1). Thermogravimetric analysis (TGA) indicated that no weight loss of 1 was observed up to ca. 350 °C (Supplementary Fig. 2). In addition, IR (Supplementary Fig. 3) and 13C CP-MAS (Supplementary Fig. 4) were also used to establish the connectivity of the COF species and the existence of Cu-TPP and S-MP moieties in 1 were directly evidenced. Notably, 1 shows good crystallinity as revealed by its powder X-ray diffraction (PXRD) pattern (Fig. 1b). The structural modeling was thus conducted with the software of Materials Studio (ver. 2018) (Supplementary Fig. 5). The most probable structure of 1 was simulated, analogous to that of 1 as a 2D staggered layered-sheets using the chiral space group of P41 with the optimized parameters of a = b = 22.073 Å and c = 12.000 Å (residuals wRp = 3.72% and Rp = 2.89%, Supplementary Table 1). As indicated in Fig. 1b, the intense PXRD peaks at 3.9, 8.3 and 29.6° displayed by 1 correspond to the (1,0,0), (0,1,0) and (0,0,4) planes, respectively. The structural modeling shows that the Cu-TPP is linked together via S-MP into a 2D layer extended in the crystallographic ab plane with square-like cavity, in which the diagonal C···C distance is ca. 2.2 nm (Fig. 1a). These layers further stack together in an ABCD-fashion to form tetragonal channels with a reduced pore size (diagonal C···C distance at ca. 1.8 nm) due to their stagger arrangement (Fig. 1a). The interlayer distance of 3.0 Å met the low-energy minimum, which is consistent with the (0,0,4) diffraction peak at 29.6°, indicating that the adjacent layers in 1 are in π-π contact. Transmission electron microscopy (TEM) images clearly revealed that 1 is a 2D layered structure (dinterlayer = 3 Å) which contains tetragonal pore with a ca. 1.8 nm diameter (Fig. 1b, inset), which is well consistent with its structural modeling analysis. The reported 2D COFs usually favored AA-32, AB-32 and ABC-stacking modes33, and the ABCD stacking mode was uncommon. 1 herein with other stacking modes, however, gave the PXRD patterns that largely deviated from the experimentally observed profile (Supplementary Fig. 5). In addition, 1 crystalized in P41 space group with ABCD stacking mode possessed the lowest total energy (221.93 kcal/mol) among other types of possible simulated space groups (P21 285.50 kcal/mol, P1 344.74 kcal/mol) with different stacking modes (Supplementary Tables 2–3), implying that the ABCD stacking mode is more energetically favored. Furthermore, it seems that the CuTBrPP monomer played an important role in the crystallization of 1. If the metal-free TBrPP instead of CuTBrPP was used to perform the COF synthesis under the same reaction conditions, the COF with the decreased crystallinity was obtained (Supplementary Fig. 5).
Au@CCOF-CuTPP (2) and Pd@CCOF-CuTPP (3) were prepared by successive solution impregnating and metal reduction steps (Fig. 1a). As shown in Fig. 1b, the framework of 1 remained intact during the M NP loading process. The diffraction of 2θ values at 39 and 40o in PXRD confirmed the existence of Au(0) in 2 and Pd(0) in 3 (Fig. 1b), which was further supported by X-ray photoelectron spectroscopy (XPS). However, no valence change of Cu(II) in 2 and 3 was detected (Supplementary Figs. 6–7)34. The uploaded Au and Pd amount, as determined by inductively coupled plasma (ICP) measurement, was up to 17.9 and 24.8 wt%, respectively. TEM showed that the Au NR (mean width and length ca. 3 and 30 nm) and Pd NP (particle size ca. 2–5 nm) were highly dispersed in the CCOF matrix, and their characteristic atomic lattice fringes (dinterplanar spacing = 0.23 nm for Au and dinterplanar spacing = 0.24 nm for Pd) were clearly observed35,36(Fig. 1c, d). In addition, the porosity difference before and after M NP loading was demonstrated by the gas adsorption-desorption measurement. As shown in Fig. 1e, N2 absorption amount of 1–3 at 77 K is 237.9, 79.4 and 94.8 cm3 g−1, respectively. Their corresponding surface areas calculated on basis of the BET model are 686 (1), 95 (2), and 101 m2 g−1 (3), respectively. The decreased N2 adsorption and the reduced surface area in 2 and 3 were obviously caused by the M NP loading. The pore size distribution curves, calculated from Barrett-Joyner-Halenda analysis, indicated that 1–3 possessed the narrow pore diameter distributions (Fig. 1f). Owing to the existence of chiral S-MP, 1–3 herein are optical active. For 1, it displayed positive Cotton effect at 450 nm and negative dichroic signal at 230 nm; for 2 and 3, they all exhibited positive Cotton effect at 445 nm and negative dichroic signals at 220 nm in their CD spectra (Supplementary Fig. 8).
Catalytic activity of 2 and 3 under photothermal conditions
The catalytic activity of Au NR loaded 2 was preliminarily examined by the one-pot asymmetric Henry reaction starting from benzyl alcohol with nitromethane. As is known, the optically active β-nitro alcohols generated from the asymmetric Henry reaction are important intermediates for the synthesis of various valuable chiral chemicals, such as bioactive natural products and pharmaceutical agents37,38.
The yield and ee for all reactions, together with the control experiments, are displayed in Table 1 (entries 1–12). Catalytic reactions were performed under various conditions, including different solvents (Table 1, entries 1–4) and catalysts with or without visible light irradiation at different temperatures (Table 1, entries 5–9). The best result was observed when the reaction was conducted in toluene/EtOH (1:1) at room temperature for 10 h (Table 1, entry 1) with 2 (1 mol% Au, lower catalyst loading would lead lower yield) upon illumination with visible light (λ > 400 nm). The nitroaldol was obtained in excellent 98% yield (TON = 98, TOF = 9.8 h−1) with excellent enantiomeric excess (98% ee). The measured reaction solution temperature was 58 °C, implying that the porphyrin-derived 2 is a good COF-based PTCM. A series of control experiments were performed to further confirm this 2-promoted one-pot asymmetric Henry reaction was a photoinduced thermally-driven one (Table 1, entries 5–6), in which 1 was responsible for not only chiral confinement and photothermal conversion, but also partial catalytic function (Table 1, entries 7–8). The partially catalytic function of 1 resulted from the CuTPP building block in 1, which was further confirmed by the catalytic test performed on the CuTBrPP monomer (Table 1, entry 9). The loaded Au NR, however, served as the dominated catalytic sites (Table 1, entry 10, Supplementary Fig. 11). In contrast, the Au@amorphous polymer generated from Cu-TBrPP, S-MP and Au NR provided a very low 8% ee and a 73 % yield (Table 1, entry 11, Supplementary Fig. 11), which demonstrated the crystalline CCOF displayed much better chiral templating and catalytic performance. It is noteworthy that this one-pot asymmetric Henry reaction could even significantly occurred under natural sunlight irradiation, and the desired nitroaldol was obtained in moderate yield (49%) with excellent stereoselectivity (96% ee) (Table 1, entry 12).
Table 1.
Optimization of the 2-catalyzed model one-pot asymmetric Henry reactiona
|
| ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | T(°C)/hv | Yield (ee) %b |
| 1 | 2 (1 mol Au %) | PhMe/EtOH | r.t./hv | 98 (98) |
| 2 | 2 (1 mol Au %) | PhMe | r.t./hv | 12 (95) |
| 3 | 2 (1 mol Au %) | EtOH | r.t./hv | 8 (92) |
| 4 | 2 (1 mol Au %) | CH3CN | r.t./hv | 14 (96) |
| 5 | 2 (1 mol Au %) | PhMe/EtOH | 58 °C/dark | 98 (91) |
| 6 | 2 (1 mol Au %) | PhMe/EtOH | r.t./dark | − (−) |
| 7 | 1 (1 mol%) | PhMe/EtOH | 58 °C/dark | 45 (94) |
| 8 | 1 (1 mol %) | PhMe/EtOH | r.t./hv | 40 (92) |
| 9 | Cu-TBrPP monomer | PhMe/EtOH | r.t./hv | 43 (−) |
| 10 | Au NR (1 mol %) | PhMe/EtOH | 58 °C/dark | 97 (−) |
| 11 | Au@amorphous polymer (1 mol% Au %) | PhMe/EtOH | r.t./hv | 73 (18) |
| 12 | 2 (1 mol Au %) | PhMe/EtOH | r.t./solar light | 49 (96) |
aReaction conditions: 2 (6.0 mg, 1 mol% Au equiv), benzyl alcohol (0.5 mmol), nitromethane (1.5 mmol), K2CO3 (1.5 mmol), PhMe/EtOH (1:1, 2 mL), 300 W xenon with a power density of 2.5 W cm−2 (λ > 400 nm), 10 h, in air
bYield was determined by the GC on HP-5 column, and ee was determined by HPLC with a Chiralcel OD-H column (90: 10 = n-hexane: isopropanol, 1.0 mL min−1, 230 nm) (Supplementary Figs. 9, 10). Because no one-pot tandem asymmetric Henry reaction starting from benzyl alcohol with nitromethane was reported so far, so only the comparison of 2 with reported asymmetric Henry reactions between benzaldehyde and nitromethane was shown in Supplementary Table 4
The first benzyl alcohol selective oxidation step was separately examined (Supplementary Figs. 12–13). The result showed that the benzyl alcohol was selectively oxidized to benzaldehyde in PhMe/EtOH (1:1) with the aid of 2 in 98% yield under the given conditions, which ensured that the whole one-pot tandem reaction proceeded smoothly. As a typical heterogeneous catalyst (Supplementary Fig. 14), 2 can be reused in this one-pot tandem Henry reaction and the yield was still up to 93% (95% ee) even after five catalytic cycles without loss its crystallinity and structural integrity (Supplementary Figs. 15–18). The leaching amount of Au in 2 is only 15.6% after five catalytic cycles which was determined by ICP-AES.
With the optimized conditions in hand, we investigated the scope of 2-catalyzed one-pot Henry reactions utilizing various substrates. As shown in Table 2, the substituted benzyl alcohols with either electron-donating or electron-withdrawing groups at different substituted positions under the optimized conditions furnished the excellent yields (93–99 %) with the excellent ee values (94–98% ee, entries 1–9). Meanwhile, the larger sized 9H-fluorene-2-methanol (5.06 × 10.66 Å, Supplementary Fig. 20) and 9-anthracenemethanol (7.13 × 8.92 Å, Supplementary Fig. 20) substrates (Table 2, entries 10–11) were unfavorable for the reaction, and the corresponding products were obtained in low yields (6–15%) but with 97% ee. As shown above, the pore size of 2 was centered at 0.71 nm, so these larger sized aromatic alcohols were not easy to enter the COF cavity, consequently, led to a low yield. On the other hand, this indicated that the one-pot asymmetric reaction should be an inner pore catalytic process. Notably, the generated products featured R configuration.
Table 2.
Scope of the 2-catalyzed one-pot asymmetric Henry reactionsa
|
| ||||
|---|---|---|---|---|
| Entry | Ar-CH2OH | Yield (%)b | ee (%)b | TON |
| 1 | PhCH2OH | 98 | 98 (R) | 98 |
| 2 | 4-Me-PhCH2OH | 97 | 98 (R) | 97 |
| 3 | 3-Me-PhCH2OH | 98 | 95 (R) | 98 |
| 4 | 4-MeO-PhCH2OH | 99 | 96 (R) | 99 |
| 5 | 3-MeO-PhCH2OH | 99 | 94 (R) | 99 |
| 6 | 4-NO2-PhCH2OH | 94 | 97 (R) | 94 |
| 7 | 3-NO2-PhCH2OH | 95 | 95 (R) | 95 |
| 8 | 4-F-PhCH2OH | 93 | 94 (R) | 93 |
| 9 | 4-Cl-PhCH2OH | 95 | 98 (R) | 95 |
| 10 | 9H-Fluorene-2-methanol | 6 | 97 (R) | 6 |
| 11 | 9-Anthracenemethanol | 15 | 97 (R) | 15 |
aReaction conditions: 2 (6 mg, 1 mol% Au equiv), aromatic alcohol (0.5 mmol), nitromethane (1.5 mmol), K2CO3 (1.5 mmol), PhMe/EtOH (1:1, 2 mL), room temperature, irradiated by 300 W xenon with a power density of 2.5 W cm−2 (λ > 400 nm), 10 h. Yields and ee are determined by GC and chiral HPLC analysis, respectively (Supplementary Fig. 19)
For demonstrating the generality of M NPs-loaded and CCOF-based asymmetric photothermal catalysis, we next evaluated the 3-catalyzed asymmetric A3-coupling (aldehyde-alkyne-amine) reaction, which is well-known a typical thermally-driven one-pot multicomponent reaction and the generated propargylamines are the important intermediates for the synthesis of various value-added nitrogen-containing compounds39,40.
Optimization of A3-coupling was based on the one-pot benzaldehyde-pyrrolidine-phenylacetylene (mole ratio, 1.0:1.0:1.5) coupling with or without visible light irradiation and heating (Table 3, entries 1–15). As shown in Table 3, various organic solvents such as p-dioxane, toluene, acetonitrile, and ethanol were utilized to perform the A3-coupling model reaction (Table 3, entries 1–4). Upon illumination by visible light (λ > 400 nm), the ideal result was obtained when the reaction was carried out in p-dioxane at room temperature for 10 h (Table 3, entries 1 and 5) in the presence of 3 with 2.1 or 3.2 mol% Pd loading. The reaction provided the pyrrolidine-derived propargylamine in 98% yield with an excellent enantiomeric excess (96% ee). The measured reaction system temperature was 45 °C under the given conditions. In addition, when the reaction was conducted in p-dioxane with a lower 1.4 mol% Pd loading, the coupling product was obtained in slightly lower 90% yield (97% ee) (Table 3, entry 6). For confirming the A3-coupling herein was a thermally-driven reaction, the reaction was carried out at 45 °C with 3 (2.1 mol% Pd) in the dark (Table 3, entry 7), and a good coupling yield (90%) with excellent 96% ee value was observed. In contrast, only 21% coupling yield (90% ee) was obtained once the reaction was performed with 3 in the dark at room temperature (Table 3, entry 8). In addition, a moderate yield (55%) with high enantioselectivity (92% ee) was observed when the reaction was conducted with 1 under visible light irradiation (Table 3, entry 9), which implied the catalytic activity coming from the synergy effect of 1 and loaded Pd NP. This was further proved by the reactions with 1 (Table 3, entry 10) and CuTBrPP (Table 3, entry 11) conducted at 45 °C in the dark and illuminated by visible light, respectively. Besides catalytic activity, the chiral templating effect of 1 was well demonstrated by the control experiments, as shown in Table 3 (entries 12 and 13). Without the aid of 1, the coupled product was isolated in 17% yield by visible light irradiation (Table 3, entry 12) or 67% yield on heating treatment (Table 3, entry 13) in the presence of only Pd NP, but without any effective enantioselectivity. It is similar to 2-catalyzed one-pot Henry reaction, this 3-catalyzed A3-coupling even significantly proceeded under natural sunlight irradiation, and the desired propargylamine was generated in moderate 45% yield with excellent 92% ee after 10 h at ambient temperature (Table 3, entry 14). Again, no desirable yield and stereoselectivity were observed when the Pd@amorphous polymer formed by Cu-TBrPP, S-MP, and Pd NP was used instead of 3 to perform the reaction (Table 3, entry 15), further demonstrated the importance of the highly crystalline CCOF in this asymmetric A3-coupling reaction.
Table 3.
Optimization of the 3-catalyzed asymmetric A3-coupling reactiona

| ||||
|---|---|---|---|---|
| Entry | Cat. | Solvent | T (°C)/hv | Yield (ee) %b |
| 1 | 3 (2.1 mol Pd %) | p-dioxane | r.t./hv | 98 (96) |
| 2 | 3 (2.1 mol Pd %) | Toluene | r.t./hv | 81 (89) |
| 3 | 3 (2.1 mol Pd %) | EtOH | r.t./hv | 58 (92) |
| 4 | 3 (2.1 mol Pd %) | CH3CN | r.t./hv | 70 (96) |
| 5 | 3 (2.8 mol Pd %) | p-dioxane | r.t./hv | 98 (96) |
| 6 | 3 (1.4 mol Pd %) | p-dioxane | r.t./hv | 90 (97) |
| 7 | 3 (2.1 mol Pd %) | p-dioxane | 45 °C/dark | 90 (96) |
| 8 | 3 (2.1 mol Pd %) | p-dioxane | r.t./dark | 21 (90) |
| 9 | 1 (1.0 mol %) | p-dioxane | r.t./hv | 55 (92) |
| 10 | 1 (1.0 mol %) | p-dioxane | 45 °C/dark | 50 (87) |
| 11 | Cu-TBrPP | p-dioxane | r.t./hv | 46 (−) |
| 12 | Pd NPs (5.0 mol %) | p-dioxane | r.t./hv | 17 (−) |
| 13 | Pd NPs (5.0 mol %) | p-dioxane | 45 °C/dark | 67 (−) |
| 14 | 3 (2.1 mol Pd %) | p-dioxane | r.t./sunlight | 45 (92) |
| 15 | Pd@amorphous polymer (2.1 mol% Pd %) | p-dioxane | r.t./hv | 78 (14) |
aReaction conditions: 3 (6.0 mg, 2.8 mol Pd% equiv; 4.5 mg, 2.1 mol Pd% equiv; 3.0 mg, 1.4 mol Pd% equiv), benzaldehyde (0.5 mmol), phenylacetylene (0.5 mmol) and pyrrolidine (0.75 mmol), solvent (2 mL), 300 W xenon with a power density of 2.5 W cm−2 (λ > 400 nm), in nitrogen, 10 h
bYield was determined by the GC on HP-5 column, and ee was determined by HPLC with a Chiralcel OJ-H column (99: 1 = n-hexane: isopropanol, 1.0 mL min−1, 254 nm) (Supplementary Figs. 21 and 22). The comparison of 3 with reported asymmetric A3-coupling reaction was shown in Supplementary Table 5. 3 herein met excellent yield and stereoselectivity, mild reaction conditions and multiple reuse
The turnover number (TON) and turnover frequency (TOF) for the model reaction are 46.7 and 4.67 h−1, respectively. The hot leaching test confirmed 3 is a typical heterogeneous catalyst (Supplementary Figs. 24–29). Additionally, 3 can be reused and the yield was still up to 91% (92% ee) even after five catalytic cycles (Supplementary Fig. 25). Also, the crystallinity and structural integrity of the reused 3 were well maintained, which was confirmed by the PXRD patterns (Supplementary Fig. 29). No significant Pd species loss in 3 was observed after five catalytic cycles (Pd 20.1 wt%, Cu 4.4 wt % based on ICP-AES). Thus, 1 is an ideal scaffold to support Pd NP for this asymmetric A3-coupling reaction.
In addition, 3 could also be applicable to various substrates with either electron-donating or electron-withdrawing groups at different substituted positions with good-to-excellent yields (68–98%) and excellent ee values (90–98% ee, Table 4 (entries 1–26), Supplementary Figs. 30–31). As shown in Table 4, most of the substrates under the optimized conditions furnished good-to-excellent yields (68–98%, entries 1–26) with excellent ee values (90–98% ee, entries 1–26). Meanwhile, the larger-sized substrates such as 9-anthraldehyde and 2-fluorenecarboxaldehyde (Table 4, entries 27–28) were unfavorable for the reaction, and the corresponding products were obtained in lower yields (37–38%) with 87–89% ee, indicating that the asymmetric A3-coupling reactions herein should be an inner pore catalytic process. Notably, benzaldehyde or the para-substituted aromatic aldehydes gave the products with S configuration (Table 4, entries 1–19), while the meta-substituted aromatic aldehydes generated the products with R configuration (Table 4, entries 20–26). Although we currently distance ourselves from any type of explanation and say forthright that we do not know why the different position substituted aromatic aldehydes preferred the different chiral configurations, it is much possible because of the different templating effect toward the para- or meta-substrates caused by the rigid chiral host-framework. When the reactions were carried out at 0 °C, the same results were obtained.
Table 4.
Scope of the 3-catalyzed asymmetric A3-coupling reactionsa
|
| ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 R4 | Yield (%)b | ee (%)b | TON |
| 1 | Ph− | Ph− | −(CH2)4− | 98 | 96 (S) | 46.7 |
| 2 | Ph− | 4-Me−Ph− | −(CH2)4− | 92 | 96 (S) | 43.8 |
| 3 | Ph− | 4-MeO−Ph− | −(CH2)4− | 90 | 94 (S) | 42.9 |
| 4 | Ph− | 4-NO2−Ph− | −(CH2)4− | 96 | 98 (S) | 45.7 |
| 5 | Ph− | 4-F−Ph− | −(CH2)4− | 97 | 98 (S) | 46.2 |
| 6 | 4-Me−Ph− | Ph− | −(CH2)4− | 94 | 95 (S) | 44.8 |
| 7 | 4-MeO−Ph− | Ph− | −(CH2)4− | 95 | 98 (S) | 45.2 |
| 8 | 4-NO2−Ph− | Ph− | −(CH2)4− | 86 | 92 (S) | 41.0 |
| 9 | 4-F−Ph− | Ph− | −(CH2)4− | 88 | 95 (S) | 41.9 |
| 10 | 4-Me−Ph− | 4-NO2−Ph− | −(CH2)4− | 95 | 98 (S) | 45.2 |
| 11 | 4-MeO−Ph− | 4-NO2−Ph− | −(CH2)4− | 95 | 98 (S) | 45.2 |
| 12 | 4-NO2−Ph− | 4-NO2−Ph− | −(CH2)4− | 83 | 95 (S) | 39.5 |
| 13 | 4-F−Ph− | 4-NO2−Ph− | −(CH2)4− | 84 | 95 (S) | 40.0 |
| 14 | 4-Me−Ph− | 4−Me−Ph− | −(CH2)4− | 86 | 93 (S) | 41.0 |
| 15 | 4-MeO−Ph− | 4-Me−Ph− | −(CH2)4− | 84 | 91 (S) | 40.0 |
| 16 | 4-NO2−Ph− | 4-Me−Ph− | −(CH2)4− | 91 | 94 (S) | 43.3 |
| 17 | 4-F−Ph− | 4-Me−Ph− | −(CH2)4− | 92 | 95 (S) | 43.8 |
| 18 | Ph− | Ph− | −(CH2)2O(CH2)2− | 68 | 90 (S) | 32.4 |
| 19 | Ph− | Ph− | −(CH2)5− | 95 | 95 (S) | 45.2 |
| 20 | 3-Me−Ph− | Ph− | −(CH2)4− | 98 | 96 (R) | 46.7 |
| 21 | 3-MeO−Ph− | Ph− | −(CH2)4− | 98 | 94 (R) | 46.7 |
| 22 | 3-NO2−Ph− | Ph− | −(CH2)4− | 86 | 95 (R) | 41.0 |
| 23 | 3-Me−Ph− | 4-Me−Ph− | −(CH2)4− | 87 | 91 (R) | 41.4 |
| 24 | 3-Me−Ph− | 4-NO2−Ph− | −(CH2)4− | 95 | 95 (R) | 45.2 |
| 25 | 3-NO2−Ph− | 4-Me−Ph− | −(CH2)4− | 94 | 93 (R) | 44.8 |
| 26 | 3-NO2−Ph− | 4-NO2−Ph− | −(CH2)4− | 86 | 94 (R) | 41.0 |
| 27 | 9-anthral | Ph− | −(CH2)4− | 37 | 87 (S) | 17.6 |
| 28 | Fluorene-2-carboxal | Ph− | −(CH2)4− | 38 | 89 (S) | 18.1 |
aReaction conditions: N2, 3 (4.5 mg, 2.1 mol% Pd), aromatic aldehyde (0.5 mmol), aromatic alkyne (0.5 mmol) and secondary amine (0.75 mmol), p-dioxane (2 mL), r.t., 300 W xenon with a power density of 2.5 W cm−2 (λ > 400 nm)
bYield and ee are determined by GC and chiral HPLC, respectively (Supplementary Figs. 30 and 31)
Photothermal behavior of 1–3
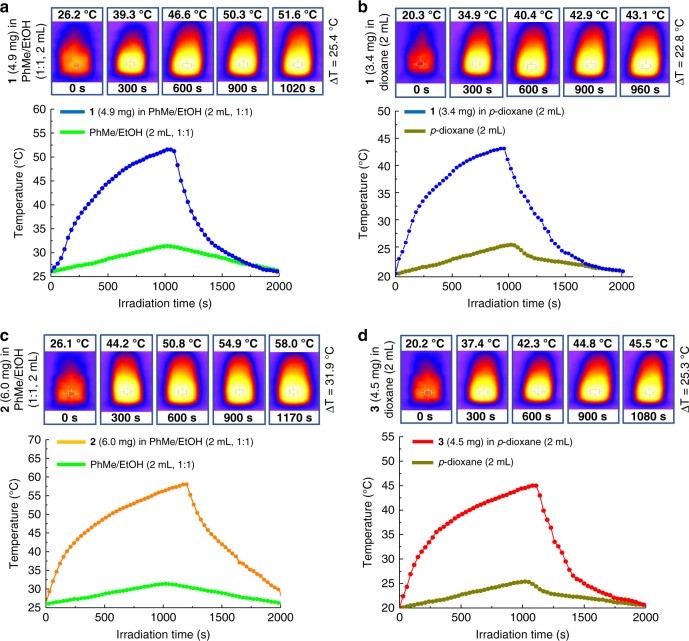
As shown above, the 2-catalyzed Henry and 3-catalyzed A3-coupling reactions herein are typical thermally-driven instead of a photoinduced reactions, and the maximum yield for either one-pot Henry reaction or A3-coupling reaction was obtained at elevated temperatures by photothermal conversion. To further evaluate the photothermal conversion of 1–3, the visible light induced temperature increase (∆T) of above reaction systems (i.e., PhMe/EtOH (1:1) and p-dioxane) in the presence of catalytic amounts of 1–3 was tested based on their absorption spectra (Supplementary Fig. 32).
As shown in Fig. 2a, b, when the PhMe/EtOH (1:1, 2 mL)-1 (4.9 mg) system was irradiated with visible light for 17 min., a significant temperature increase (∆T) of 25.4 °C was detected. For p-dioxane (2 mL)-1 (3.4 mg) system, the measured ∆T is 22.8 °C at 16 min. For 2, a 31.9 °C temperature increase was observed in PhMe/EtOH (1:1, 2 mL)−2 (6.0 mg) system upon irradiation for 19.5 min (Fig. 2c). Meanwhile, ∆T of 25.3 °C was presented when p-dioxane (2 mL)-3 (4.5 mg) system was illuminated for 18 min (Fig. 2d). As shown in Fig. 1a, the 2D porphyrin-derived CCOF stacking nature, together with the central Cu(II) ion, induced the formation of porphyrin-aggregates in 1, which enabled the obtained CCOF microparticles with effective light-to-heat conversion efficiency41. Thus, the porphyrin-based CCOF 1 herein is responsible for not only the chiral templating and partial catalytic functionality, but also the photothermal conversion. The slight difference in ∆T by 1–3 should be caused by different type and amount of the embedded M NP with different amount of 1 in different media.
Fig. 2.
Photothermal examination of 1–3. a, b Photothermal behavior of 1 in PhMe/EtOH (1:1, 2 mL) and p-dioxane (2 mL), respectively. c Photothermal effect of 2 in PhMe/EtOH (1:1, 2 mL). d Photothermal effect of 3 in p-dioxane (2 mL). Visible light source: 300 W xenon lamp, λ > 400 nm with the intensity at 2.5 W cm−2. 4.9 and 3.4 mg of 1 are equivalent to the CCOF content in 6.0 mg of 2 and 4.5 mg of 3, respectively
This interesting phenomenon inspired us to further explore the chiral templating ability of 1 at even higher temperature, which is very important factor to balance the yield and enantioselectivity in asymmetric catalysis. The one-pot Henry and A3-coupling model reactions were therefore conducted within a temperature range of 60–100 °C under the optimized conditions. The obtained results were summarized in Table 5.
Table 5.
2- and 3-catalyzed asymmetric reactions at different temperaturesa
|
| |||||
|---|---|---|---|---|---|
| T(°C) | 60 | 70 | 80 | 90 | 100 |
| Yield (%)b | 97 | 97 | 98 | 98 | 99 |
| ee (%)b | 96 | 97 | 95 | 93 | 88 |
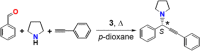
| |||||
| T(°C) | 60 | 70 | 80 | 90 | 100 |
| Yield (%)b | 97 | 97 | 98 | 98 | 99 |
| ee (%)b | 97 | 96 | 96 | 92 | 86 |
aReactions are carried out under the optimized conditions except in dark by heating
bYield and ee are determined by GC and chiral HPLC, respectively (Supplementary Fig. 33)
We are very pleased to find that the chiral confined effect of 1 could be well maintained even at a higher temperature. As shown in Table 5, 2-catalyzed model one-pot Henry reaction of benzyl alcohol with nitromethane gave the target chiral β-nitro alcohol in 97–98% yields but with an excellent 93–96% ee within 60–90 °C. At 100 °C, the reaction provided 99% yield but still with a significant stereoselectivity (88% ee). The similar result was observed in the 3-catalyzed model A3-coupling reaction of benzaldehyde-pyrrolidine-phenylacetylene. The desired pyrrolidine-derived propargylamine was generated in 97–98% yields with excellent 92–97% ee in a temperature range of 60–90 °C. Again, a good enough enantiopurity (86% ee) was obtained even at 100 °C but with an excellent 99% yield. Thus, the M@CCOF-CuTPP herein could well tolerate high-temperature condition and broke the bottleneck of high enantiopurity at the cost of yield in the thermally-driven asymmetric catalysis, especially for those low-yield asymmetric catalysis caused by the low-temperature condition. So far, we are not aware of any reported asymmetric catalysts possessing such a feature.
Discussion
As mentioned above, M@CCOF-CuTPP of 2 (M=Au) and 3 (M=Pd) are the multifunctional catalytic materials and they can highly promote thermally-driven asymmetric catalysis at the elevated temperature by photothermal conversion. First, CCOF-CuTPP (1) is a rigid homochiral host-framework42–45, and it can be a powerful chiral temple to steer the organic substrates in the specific spatial orientation within the CCOF confined space even at high temperature, consequently, provide the desired products with high enantiopurity; second, 1 contains porphyrin moiety which is a widely recognized PTCM that can readily transfer light into thermal energy46. Therefore, the obtained porphyrin-involved CCOF herein can supply the reaction needed thermal energy upon visible light irradiation; and third, 1 is highly porous and heteroatom-rich COF host which is qualified for the M NP loading and stabilization47.
Notably, the 2D porphyrin-based CCOF herein involves the connection of building blocks through a carbon–nitrogen (C–N) single bond linkage under the Pd-catalyzed reaction. This result implies that C–N bonding enables the system to proceed a thermodynamically controlled process, in which the crystallinity of an in-plane structure is handled via continued 2D chain growth and self-healing processes. It is different from the most commonly reported C=N driven COFs, the C–N single bond connected COF is chemically stable toward the reducing agent such as NaBH4, which is widely used to prepare the M NP loaded catalytic materials.
In summary, the concept of CCOF-based multifunctional integration leads the enantioselective synthesis to be economical, safe, eco-friendly, energy and source saving, more importantly, broke through the bottleneck of high enantiopurity at the cost of yield in the thermal-driven asymmetric catalysis. We think this approach to be general, effective and viable for the fabrication of many more practical multifunctional composite catalytic materials for a variety of asymmetric thermally-driven chemical transformations with both high yield and stereoselectivity.
Methods
Synthesis of CCOF-CuTPP (1)
Under nitrogen, a mixture of S-(+)-2-methylpiperazine (0.4 mmol, 40 mg), anhydrous K2CO3 (1.2 mmol, 166 mg) and Pd[P(Ph)3]4 (0.03 mmol, 33 mg) in anhydrous p-dioxane (50 mL) was stirred at room temperature. Then, the mixture was heated to 90 °C, and the TBrPP (0.2 mmol, 186 mg) in anhydrous p-dioxane (6 mL) was added dropwise. After refluxed for 3 days at 90 °C, the reaction system was allowed to stand at ambient temperature for two days. The purple-black crystalline product of 1 was collected by filtration and completely washed with HCl (1.0 M, 10 mL), water, dichloromethane, and ethanol to remove the unreacted starting precursors. Yield, 63%.
Synthesis of Au@CCOF-CuTPP (2)
1 (5 mg) was added to a CH3CN (1.5 mL) solution of KAuCl4 (5.0 mg, 0.09 mmol). The mixture was stirred for 3 h at room temperature. The resulting solid was isolated by centrifugation and washed with CH3CN and EtOH, respectively. The obtained solid was mixed with ascorbic acid (2.5 mg, 0.05 mmol) in water (1.5 mL) and stirred at room temperature for an additional 1 h to afford 2. The obtained crystalline solids were completely washed with H2O, CH3CN and EtOH and dried in air. Yield, 98%.
Synthesis of Pd@CCOF-CuTPP (3)
1 (5 mg) was added to a CH3CN (1.5 mL) solution of palladium nitrate (5.0 mg, 0.09 mmol). The mixture was stirred for 3 h at room temperature. The resulting solids were isolated by centrifugation and washed with CH3CN and EtOH. The obtained solids were mixed with NaBH4 (2 mg, 0.05 mmol) in water (1.5 mL) and stirred at room temperature for additional 1 h to afford 3. The obtained crystalline solids were completely washed with H2O, CH3CN and EtOH and dried in air. Yield, 97%.
Model one-pot asymmetric Henry reaction catalyzed by 2
A mixture of benzyl alcohol (0.5 mmol), nitromethane (1.50 mmol), K2CO3 (1.50 mmol) and 2 (6.0 mg, 1 mol% Au equiv) in PhMe/EtOH (1:1, 2 mL) was stirred under 300 W xenon lamp (λ > 400 nm with the intensity at 2.5 W cm−2, 30 cm away from the reaction vessel) irradiation for 10 h in air to afford the corresponding adducts. The yield was determined by the GC measurement on HP-5 column, and ee was determined by HPLC with a Chiralcel OD-H column (90: 10 = n-hexane: isopropanol, 1.0 mL min−1, 230 nm), respectively.
Model one-pot asymmetric A3-coupling reaction catalyzed by 3
Under nitrogen, a mixture of benzaldehyde (0.5 mmol), phenylacetylene (0.5 mmol), pyrrolidine (0.75 mmol) and 3 (4.5 mg, 2.1 mol% Pd equiv) in p-dioxane (2 mL) was stirred at room temperature under 300 W xenon lamp (λ > 400 nm with the intensity at 2.5 W cm−2, 30 cm away from the reaction vessel) irradiation for 10 h to afford the corresponding product. Yield was determined by the GC measurement on HP-5 column, and ee was determined by HPLC with a Chiralcel OJ-H column (99: 1 = n-hexane: isopropanol, 1.0 mL min−1, 254 nm), respectively.
Supplementary information
Acknowledgements
We are grateful for financial support from NSFC (Grant Nos. 21671122, 21475078, 21301109), Taishan scholar’s construction project and Changjiang scholar project at SDNU.
Author contributions
All authors contributed extensively to the work presented in this paper. Y.-B.D. and G.-J.C. conceived the research project. H.-C.M., C.-C.Z. and G.-J.C. conducted the experiments and performed the characterizations. Y.-B.D. and G.-J.C. wrote the manuscript with the input from the other authors.
Data availability
The source data supporting the findings of this study are available within the article, as well as the Supplementary Information file, or available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gong-Jun Chen, Email: gongjchen@126.com.
Yu-Bin Dong, Email: yubindong@sdnu.edu.cn.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11355-x.
References
- 1.Bogaerts WF, Lampert CM. Materials for photothermal solar energy conversion. J. Mater. Sci. 1983;18:2847–2875. doi: 10.1007/BF00700767. [DOI] [Google Scholar]
- 2.Liu GL, Kim J, Lu Y, Lee LP. Optofluidic control using photothermal nanoparticles. Nat. Mater. 2006;5:27–32. doi: 10.1038/nmat1528. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014;114:10869–10939. doi: 10.1021/cr400532z. [DOI] [PubMed] [Google Scholar]
- 4.Linic S, Christopher P, Ingram DB. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011;10:911–921. doi: 10.1038/nmat3151. [DOI] [PubMed] [Google Scholar]
- 5.Mayani VJ, et al. Heterogeneous chiral copper complexes of amino alcohol for asymmetric nitroaldol reaction. J. Org. Chem. 2010;75:6191–6195. doi: 10.1021/jo1010679. [DOI] [PubMed] [Google Scholar]
- 6.Noole A, Lippur K, Metsala A, Lopp M, Kanger T. Enantioselective Henry reaction catalyzed by cuii salt and bipiperidine. J. Org. Chem. 2010;75:1313–1316. doi: 10.1021/jo902664v. [DOI] [PubMed] [Google Scholar]
- 7.Evans DA, et al. A new copper acetate-bis(oxazoline)-catalyzed, enantioselective Henry reaction. J. Am. Chem. Soc. 2003;125:12692–12693. doi: 10.1021/ja0373871. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Deng P, Zeng Y, Xiong Y, Zhou H. anti-Selective asymmetric Henry reaction catalyzed by a heterobimetallic Cu−Sm−aminophenol sulfonamide complex. Org. Lett. 2016;18:1578–1581. doi: 10.1021/acs.orglett.6b00432. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Pérez B, Zhang Z, Gao R, Guo Z. Mining catalytic promiscuity from Thermophilic archaea: an acyl-peptide releasing enzyme from Sulfolobus tokodaii (ST0779) for nitroaldolreactions. Green. Chem. 2016;18:2753–2761. doi: 10.1039/C5GC02674A. [DOI] [Google Scholar]
- 10.Kuhbeck D, Saidulu G, Reddy KR, Dıaz DD. Critical assessment of the efficiency of chitosan biohydrogel beads as recyclable and heterogeneous organocatalyst for C–C bond formation. Green. Chem. 2012;14:378–392. doi: 10.1039/C1GC15925A. [DOI] [Google Scholar]
- 11.Lang K, Park J, Hong S. Urea/transition-metal cooperative catalyst for anti-selectiveasymmetric nitroaldol reactions. Angew. Chem. Int. Ed. 2012;51:1620–1624. doi: 10.1002/anie.201107785. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Zong H, Bian G, Yue H, Song L. Correlating the effects of the N substituent sizes of chiral 1,2-amino phosphinamide ligands on enantioselectivities in catalytic asymmetric henry reaction using physical steric parameters. J. Org. Chem. 2014;79:9455–9464. doi: 10.1021/jo500982j. [DOI] [PubMed] [Google Scholar]
- 13.Breugst M, Houk KN. Computational analysis of cyclophane-based bisthiourea-catalyzed Henry reactions. J. Org. Chem. 2014;79:6302–6309. doi: 10.1021/jo501227m. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, et al. Solvent-dependent strong asymmetric amplification in the catalytic enantioselective Henry reaction using the trans-N,N-bis-biphenyl-4-ylmethylcyclohexane-1,2-diamine-CuCl2 complex. Chem. Commun. 2015;51:7907–7910. doi: 10.1039/C5CC02302E. [DOI] [PubMed] [Google Scholar]
- 15.Kitagaki S, Uedab T, Mukai C. Planar chiral [2.2]paracyclophane-based bis(thiourea) catalyst: application to asymmetric Henry reaction. Chem. Commun. 2013;49:4030–4032. doi: 10.1039/c3cc41789a. [DOI] [PubMed] [Google Scholar]
- 16.White DJ, Shaw S. A new catalyst for the asymmetric Henry reaction: synthesis of β nitroethanols in high enantiomeric excess. Org. Lett. 2012;14:6270–6273. doi: 10.1021/ol3030023. [DOI] [PubMed] [Google Scholar]
- 17.Xiong Y, Wang F, Huang X, Wen Y, Feng X. A new copper(I)–tetrahydrosalen-catalyzed asymmetric Henry reaction and its extension to the synthesis of (S)-norphenylephrine. Chem. Eur. J. 2007;13:829–833. doi: 10.1002/chem.200601262. [DOI] [PubMed] [Google Scholar]
- 18.Scharnagel D, et al. The first modular route to core-chiral bispidine ligands and their application in enantioselective copper(II)-catalyzed Henry reactions. Chem. Eur. J. 2015;21:12488–12500. doi: 10.1002/chem.201502090. [DOI] [PubMed] [Google Scholar]
- 19.Spangler KY, Wolf C. Asymmetric copper(I)-catalyzed Henry reaction with an aminoindanol-derived bisoxazolidine ligand. Org. Lett. 2009;11:4724–4727. doi: 10.1021/ol9018612. [DOI] [PubMed] [Google Scholar]
- 20.Marcelli T, Haas RNS, Maarseveen JH, Hiemstra H. Asymmetric organocatalytic Henry reaction. Angew. Chem. Int. Ed. 2006;45:929–931. doi: 10.1002/anie.200503724. [DOI] [PubMed] [Google Scholar]
- 21.Scharnagel D, Prause F, Kaldun J, Haase RG, Breuning M. 2S,5R)-2-Methylaminomethyl-1-methyl-5-phenylpyrrolidine, a chiral diamine ligand for copper(II)-catalysed Henry reactions with superb enantiocontrol. Chem. Commun. 2014;50:6623–6625. doi: 10.1039/C4CC02429J. [DOI] [PubMed] [Google Scholar]
- 22.El-Asaad B, Métay E, karamé I, Lemaire M. Chiral N-arylated diamine-copper complexes catalyzed asymmetric Henry reaction. Mol. Catal. 2017;435:76–81. doi: 10.1016/j.mcat.2017.03.017. [DOI] [Google Scholar]
- 23.Xu F, Wang J, Liu B, Wu Q, Lin X. Enzymatic synthesis of optical pure b-nitroalcohols by combining D-aminoacylase-catalyzed nitroaldol reaction and immobilized lipase PS-catalyzed kinetic resolution. Green. Chem. 2011;13:2359–2361. doi: 10.1039/c1gc15417f. [DOI] [Google Scholar]
- 24.Kureshy RI, Das An, Khan NH, Abdi SHR, Bajaj HC. Cu(II)-macrocylic [H4]salen catalyzed asymmetric nitroaldol reaction and its application in the synthesis of α1-adrenergic receptor agonist (R)-phenylephrine. ACS Catal. 2011;1:1529–1535. doi: 10.1021/cs2004467. [DOI] [Google Scholar]
- 25.Kodama K, Sugawara K, Hirose T. Synthesis of chiral 1, 3-diamines derived from cis-2- benzamidocyclohexanecarboxylic acid and their application in the Cu-catalyzed enantioselective Henry reaction. Chem. Eur. J. 2011;17:13584–13592. doi: 10.1002/chem.201102136. [DOI] [PubMed] [Google Scholar]
- 26.Meemken F, Baiker A. Recent progress in heterogeneous asymmetric hydrogenation of C═O and C═C bonds on supported noble metal catalysts. Chem. Rev. 2017;117:11522–11569. doi: 10.1021/acs.chemrev.7b00272. [DOI] [PubMed] [Google Scholar]
- 27.Kanagaraj K, Suresh P, Pitchumani K. Per-6-amino-cyclodextrin as a reusable promoter and chiral host for enantioselective Henry reaction. Org. Lett. 2010;12:4070–4073. doi: 10.1021/ol101658n. [DOI] [PubMed] [Google Scholar]
- 28.Choudary BM, Ranganath KVS, Pal U, Kantam ML, Sreedhar B. Nanocrystalline MgO for asymmetric Henry and michael reactions. J. Am. Chem. Soc. 2005;127:13167–13171. doi: 10.1021/ja0440248. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y, Ren Y, Li J, Yue C, Jiang H. Enhanced activity and enantioselectivity of Henry reaction by the postsynthetic reduction modification for a chiral Cu(salen)-based metal-organic framework. Inorg. Chem. 2018;57:11986–11994. doi: 10.1021/acs.inorgchem.8b01551. [DOI] [PubMed] [Google Scholar]
- 30.Hong L, Sun W, Yang D, Li G, Wang R. Additive effects on asymmetric catalysis. Chem. Rev. 2016;116:4006–4123. doi: 10.1021/acs.chemrev.5b00676. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C, Seidel D. Enantioselective A3 reactions of secondary amines with a Cu(I)/acid-thiourea catalyst combination. J. Am. Chem. Soc. 2015;137:4650–4653. doi: 10.1021/jacs.5b02071. [DOI] [PubMed] [Google Scholar]
- 32.Albacete P, et al. Layer-stacking-driven fluorescence in a two-dimensional imine-linked covalent organic framework. J. Am. Chem. Soc. 2018;140:12922–12929. doi: 10.1021/jacs.8b07450. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Han X, Liu Y, Liu Y, Cui Y. Control interlayer stacking and chemical stability of two-dimensional covalent organic frameworks via steric tuning. J. Am. Chem. Soc. 2018;140:16124–16133. doi: 10.1021/jacs.8b08452. [DOI] [PubMed] [Google Scholar]
- 34.Durando M, Morrish R, Muscat AJ. Kinetics and mechanism for the reaction of hexafluoroacetylacetone with CuO in supercritical carbon dioxide. J. Am. Chem. Soc. 2008;130:16659–16668. doi: 10.1021/ja8050662. [DOI] [PubMed] [Google Scholar]
- 35.Cuenya BR, Baeck SH, Jaramillo TF, McFarland EW. Size- and support-dependent electronic and catalytic properties of Au0/Au3+ nanoparticles synthesized from block copolymer micelles. J. Am. Chem. Soc. 2003;125:12928–12934. doi: 10.1021/ja036468u. [DOI] [PubMed] [Google Scholar]
- 36.Huang XQ, Tang SH, Yang J, Tan YM, Zheng NF. Etching growth under surface confinement: an effective strategy to prepare mesocrystalline Pd nanocorolla. J. Am. Chem. Soc. 2011;133:15946–15949. doi: 10.1021/ja207788h. [DOI] [PubMed] [Google Scholar]
- 37.Rosini, G. In Comprehensive Organic Synthesis Vol. 2 (eds Trost, B. M., Fleming, I. C. & Heathcock, H.) 321 (Pergamon, New York, 1991).
- 38.Ono, N. In The Nitro Group in Organic Synthesis Ch. 3 (Wiley-VCH, Weinhem, 2001).
- 39.Peshkov VA, Pereshivko OP, Van der Eycken EV. A walk around the A3-coupling. Chem. Soc. Rev. 2012;41:3790–3807. doi: 10.1039/c2cs15356d. [DOI] [PubMed] [Google Scholar]
- 40.Zani, L., Bolm, C. Direct addition of alkynes to imines and related C=N electrophiles: a convenient access to propargylamines. Chem. Commun. 4263-4275, (2006). [DOI] [PubMed]
- 41.Zou Q, et al. Biological photothermal nanodots based on self-assembly of peptide–porphyrin conjugates for antitumor therapy. J. Am. Chem. Soc. 2017;139:1921–1927. doi: 10.1021/jacs.6b11382. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, et al. Catalytic covalent organic frameworks via pore surface engineering. Chem. Commun. 2014;50:1292–1294. doi: 10.1039/C3CC48813F. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Gao J, Jiang D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015;7:905–912. doi: 10.1038/nchem.2352. [DOI] [PubMed] [Google Scholar]
- 44.Xu H-S, Ding S-Y, An W-K, Wu H, Wang W. Constructing crystalline covalent organic frameworks from chiral building blocks. J. Am. Chem. Soc. 2016;138:11489–11492. doi: 10.1021/jacs.6b07516. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, et al. Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 2016;138:12332–12335. doi: 10.1021/jacs.6b07714. [DOI] [PubMed] [Google Scholar]
- 46.Lovell JF, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011;10:324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 47.Ma H-C, Kan J-L, Chen G-J, Chen C-X, Dong Y-B. Pd NPs-loaded homochiral covalent organic frameworks for heterogeneous asymmetric catalysis. Chem. Mater. 2017;29:6518–6524. doi: 10.1021/acs.chemmater.7b02131. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data supporting the findings of this study are available within the article, as well as the Supplementary Information file, or available from the corresponding authors on reasonable request.