Abstract
The proline-type organocatalysts has been efficiently employed to catalyze a wide range of asymmetric transformations; however, there are still many synthetically useful and challenging transformations that remain unachievable in an asymmetric fashion. Herein, a chiral bifunctional organocatalyst with a spirocyclic pyrrolidine backbone-derived containing fluoro-alkyl and aryl sulfonamide functionalities, are designed, prepared, and examined in the asymmetric Mannich/acylation/Wittig reaction sequence of 3,4-dihydro-β-carboline with acetaldehyde, acyl halides, and Wittig reagents. As a result, the spirocyclic pyrrolidine trifluoromethanesulfonamide catalyst can facilitate this versatile sequence as demonstrated by 18 examples displaying excellent enantioselectivity (up to 94% ee), as well as moderate to good yields (up to 54% over 3 steps). As a practical application, the asymmetric total synthesis of naucleofficine I (1a) and II (1b) in ten steps have been accomplished.
Subject terms: Asymmetric catalysis, Organocatalysis, Synthetic chemistry methodology, Natural product synthesis
Natural products often contain complex N-fused polycyclic structures with multiple substituents and stereocentres. Here, the authors developed a bifunctional organocatalyst that is instrumental in obtaining such structures and applied it to the total synthesis of naucleofficine I and II in 6 steps.
Introduction
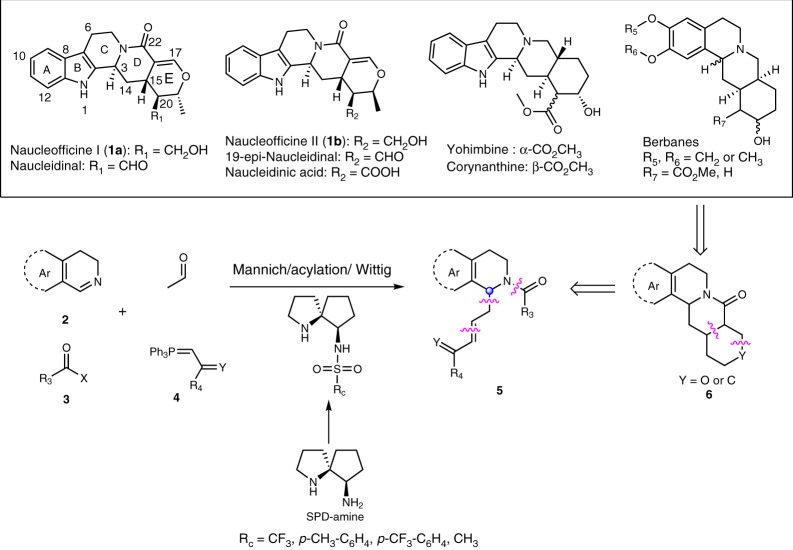
The metal-free organocatalysis based on the dual activation of amino acids and their analogs has been displaying versatile utilities in the synthesis of natural products and pharmaceuticals1–5. The proline-type organocatalysts represent a large class of useful bifunctional catalytic systems, which have facilitated a wide range of asymmetric reactions of carbonyls, imines, and their variants, including aldol, Michael addition, Diels–Alder cycloaddition, Mannich reaction, and so on6–12. Despite these advances, there are still many inspiring transformations that remain unachievable in asymmetric synthesis. Therefore, development of more and reliable bifunctional organocatalysts is of high demand for effecting these transformations. Aimed at the asymmetric synthesis of biologically important natural products and drug molecules, our current efforts have been focused on developing novel and effective catalysis systems to enable synthetically challenging transformations so as to explore the concise synthetic approaches. As indicated in Fig. 1, at least four kinds of different natural products, the naucleofficine I (1a) and II (1b), yohimbine, and berbanes, contain the common complex N-fused bicyclic structures 6 with multi-substituents and stereocenters. Compounds 1a and 1b are isolated from Nauclea genus and exhibit antibacterial and antiviral biological activities13,14. They represent the characteristic structures of a number of monoterpenoid indole alkaloids and have not been synthesized so far, although some approaches to their analogs have been reported by employing free radical cyclization and the Pictet–Spengler reaction as key steps15–18.
Fig. 1.
Design of asymmetric synthesis of monoterpenoid alkaloids. Retro-synthesis involves Diels–Alder reaction and Mannich/acylation/Wittig sequent reaction
In previous reports, the the spirocyclic pyrrolidine (SPD) backbone-derived organocatalysts have displayed high enantioselectivity in several reactions, possibly due to the rigid chiral environment of quaternary carbon stereocenter and spirocylic scaffold, and the flexibility of the lone electron pair of the pyrrolidine moiety19–21. In this regard, it is hypothesized that attachment of the strong acidic fluoroalkylsulphonamide motif to a sterically hindered SPD backbone would generate a new bifunctional catalyst, which would exhibit stronger acidity and superior enantio-induction ability compared with proline but still maintain the active pyrrolidine amine. Furthermore, the BINOL-phosphonyl TfNH catalyst (Table 1, Cat 8) exhibits much stronger acidity than BINOL-phosphoric acid alone, due to its stability of counter anion and strong electron-accepting property of Tf group22–25. Herein, we present our research results on the asymmetric Mannich/acylation/Wittig sequence to efficiently assemble the tetrahydro-β-carboline 5, which allows us to accomplish the total synthesis of naucleofficine I (1a) and naucleofficine II (1b) in a concise way.
Table 1.
Optimization of the Mannich/Acylation/Wittig reaction conditionsa

| ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst | T [ºC] | Additive | t [h] | Yield [%]b | ee [%]c |
| 1 | Cat 1 | 0 | - | 8 | 21 | 51 |
| 2 | Cat 1 | 0 | PhCO2H | 8 | 8 | −1.1 |
| 3 | Cat 1 | 0 | PTS | 8 | 12 | −57 |
| 4 | Cat 1 | 0 | Et 3 N | 4 | 51 | 91 |
| 5 | Cat 1 | 0 | DMAP | 20 | 29 | 87 |
| 6 | Cat 1 | 0 | DBU | 20 | 47 | 86 |
| 7 | Cat 1 | −5 | Et3N | 10 | 48 | 88 |
| 8 | Cat 1 | −10 | Et3N | 10 | 45 | 89 |
| 9 | Cat 2 | 0 | Et3N | 48 | 7 | 3 |
| 10 | Cat 3 | 0 | Et3N | 48 | 12 | 8 |
| 11 | Cat 4 | 0 | Et3N | 48 | <5 | 32 |
| 12 | Cat 5 | 0 | Et3N | 48 | <5 | 18 |
| 13 | Cat 6 | 0 | Et3N | 48 | <5 | 14 |
| 14 | Cat 7 | 0 | Et3N | 48 | NDd | NDd |
| 15 | Cat 8 | 0 | Et3N | 30 | 15 | 20 |
| 16 | Cat 9 | 0 | Et3N | 6 | 26 | 68 |
| 17 | Cat 10 | 0 | Et3N | 48 | 20 | 69 |
| 18 | Cat 11 | 0 | Et3N | 48 | <5 | 67 |
| 19 | Cat 12 | 0 | Et3N | 48 | 6 | 20 |
| 20 | Cat 13 | 0 | Et3N | 48 | 9 | 29 |
a Unless otherwise noted, all reactions were carried with 2a (0.1 mmol), acetaldehyde (0.3 mmol), catalyst (0.02 mmol), and additive (0.05 mmol), in 0.5 mL DCE and 0.5 mL H2O; after the reaction is complete, the solution was evaporated, then DCM (1 mL), K2CO3 (0.2 mmol), acyl halide 3 (0.15 mmol), and Wittig reagent 4 (0.2 mmol) was added sequentially to the reaction system
b Isolated yield over three steps
c Determined by HPLC analysis
d No reaction detectable
DBU 1,8-diazabicyclo[5.4.0]undec-7-ene, DMAP 4-dimethylaminopyridine, PTS p-toluenesulfonic acid, DCE 1,2-dichloroethane, DCM dichloromethane
Bold values represent the optimal condition
Results
Design plan
The synthesis of natural products in Fig. 1 could be simplified to establishment of the single C3 stereocenter and installation of two unsaturated chains in 5. In this work, we focus on the synthesis of the alkaloids 1a and 1b, our retro-synthesis involves alternatively a late-stage O-hetero-Diels–Alder cycloaddition to construct the D/E rings with multi-functional groups and stereogenic centers in one step from the key intermediate 5 (Fig. 1). Therefore, we envision a versatile Mannich/acylation/Wittig sequence would enable the construction of 5 from commercially available 3,4-dihydro-β-carboline 2, acetaldehyde, acyl halides, and corresponding Wittig reagents. To achieve this catalytic asymmetric sequence, however, some challenges exist, including the following: (1) the starting N-aliphatic imine is relatively inactive compared with generally used active Boc-26,27, Ts-28,29, aryl-imine species30,31, and Cbz-32, which requires a stronger acidity to activate the C=N bond via hydrogen bonding; (2) the active acetaldehyde33–39 could easily form byproducts via aldol condensation; and (3) asymmetric Mannich reaction of 3,4-dihydro-β-carboline and aldehydes is a challenging work, which has not been reported up to now, maybe due to the instability of the resulting amino aldehyde.
Reaction condition optimization
Initially, four catalysts Cat 1–4 with substituents possessing different electronic properties were prepared from (S,S)-SPD-amine (for details, see Supplementary Note 2). Imine 2a was selected as a model substrate to screen the optimal condition of the Mannich sequence with acetaldehyde, acetyl bromide, and methyl(triphenylphosphoranylidene)-acetate. Fortunately, the expected Mannich sequence could take place in the presence of Cat 1 in DCE/H2O at 0 °C, affording mainly the desired product 5a albeit with the poor yield (21% over three steps) and moderate enantiomeric excesses (ee) (51%) (Table 1, entry 1). Encouraged by this result, the effect of additives was then investigated (for details, see Supplementary Table 1). The presence of a basic additive was found to be crucial to improve the reaction outcome, with Et3N giving the best result (entry 4), the main byproducts were the decomposition of the amino aldehyde in the second step of acylation and the byproducts of aldol condensation have not been detected. In contrast, the acid additives gave poor results and reversed the chirality of the resulting products (entries 2–3 vs. 4–6). Subsequently, decreasing the reaction temperature led to slightly lower yield and ee (entries 7–8). Further examination of the analogous Cat 2–4 revealed that the TfNH modified Cat 1 provided a much better result than Cat 2–4 with the weaker and stronger electron withdrawing property of tolylsulfonyl, p-(triflouromethyl)-benzenesulfonyl, and methylsulfonyl, respectively (entries 9–11 vs. 4). In addition, other types of catalysts with quite different levels of reactivity and/or stereo-effect from Cat 1 were subjected to the reaction and compared with Cat 1 under the same condition as entry 4. These include the following: (1) Cat 5, Cat 13, which does not have an acidic functional group; (2) Cat 6, which has a protecting group of pyrrolidine amine; (3) Cat 8, which has a single, stronger acidity and generally identified excellent enatio-induction of BINOL framework; and (4) Cat 9–125,40–44, which have similar reactivity but quite different stereo-effect of a linear derivative. Unfortunately, all of these catalysts gave poor results (entries 12–18). Particularly, Cat 7 failed to promote this sequence and only the starting material was recovered (entry 14). These results indicated that Cat 1 exhibited both excellent reactivity and high enantio-induction ability toward the studied transformation.
Substrate scope
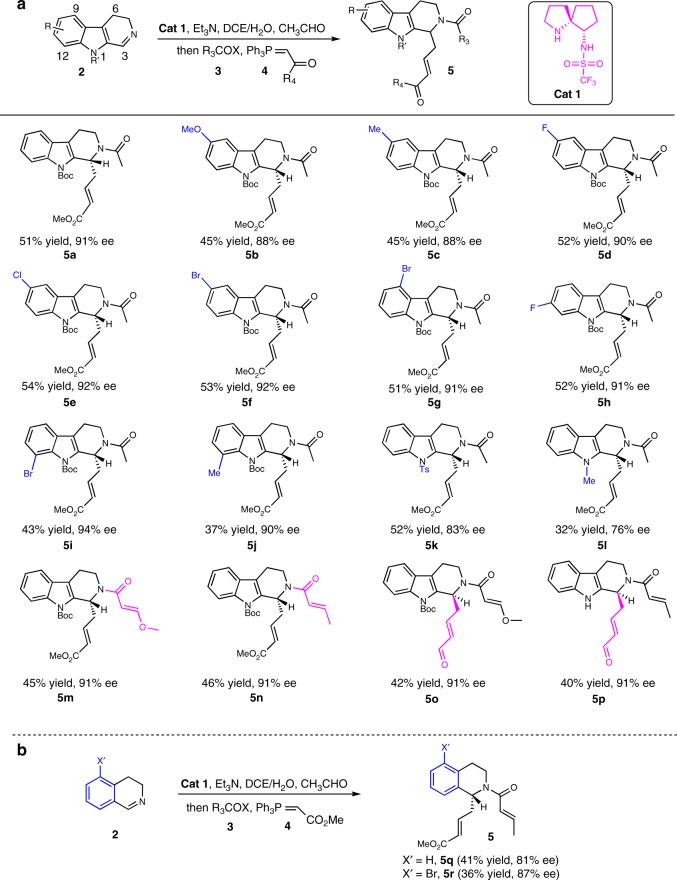
Subsequently, the optimal condition of entry 4 (the bold values in table 1) was employed for substrate expansion. First, a variety of substituted 3,4-dihydro-β-carbolines 2b–2j with a variety of substituents on the indole rings were examined and all reacted smoothly to afford the desired products 5b–5j (Fig. 2a)45–49. In detail, the EWG (electron-withdrawing group) substitutions with F, Cl, and Br at C9–C11 of the indole ring generally gave high ee (90–92%) and good yields (51–54% over three steps) (5d–5h), whereas the EDG (electron-donating group) substitutions gave slightly lower ee (88% ee) and yields (45%) (5b and 5c). Interestingly, the C12 substitutions with either EWG (5i) or EDG (5j) both gave high ee (94% and 90% ee) but merely moderate yields (43% and 37%). Moreover, substitutions at the indole nitrogen atom with the EWG (5k) or EDG (5l) resulted in similarly lower ee (83% and 76%) and varied yields (52% and 32%). In addition, variation of acyl halides could also generate high ee (both 91% ee) and moderate yields (45% and 46%) (5m and 5n), respectively. Moreover, changing the Wittig reagents gave moderate yields (42% and 40%) and high ee (both 91% ee) (5o and 5p), which were the key intermediates for the synthesis of natural products 1a/1b. Notably, two 3,4-dihydro-isoquinoline substrates were explored for the catalytic Mannich/acylation/Wittig sequence, which also proved to be effective, generating the corresponding products 5q and 5r with good ee (81% and 87%) and moderate yields (41% and 36%), respectively (Fig. 2b). As a result, the explored Cat 1 could facilitate well the expected asymmetric Mannich sequence with a wide substrate toleration in good to high efficiency in most cases.
Fig. 2.
The substrate scope of the Mannich/Acylation/Wittig reaction. Reaction condition: 2a (0.1 mmol), acetaldehyde (0.3 mmol), (S,S)-Cat 1 (0.02 mmol), and Et3N (0.05 mmol), in 0.5 mL DCE and 0.5 mL H2O, then acyl halide 3 (0.15 mmol) and Wittig reagent 4 (0.2 mmol). Isolated yield over three steps. Enantiomeric excess determined by HPLC analysis. a The scope of 3,4-dihydro-β-carboline substrates. b The scope of 3,4-dihydro-isoquinoline substrates
Asymmetric total synthesis of naucleofficine I and II
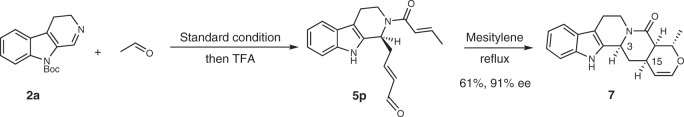
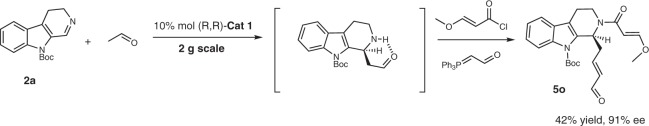
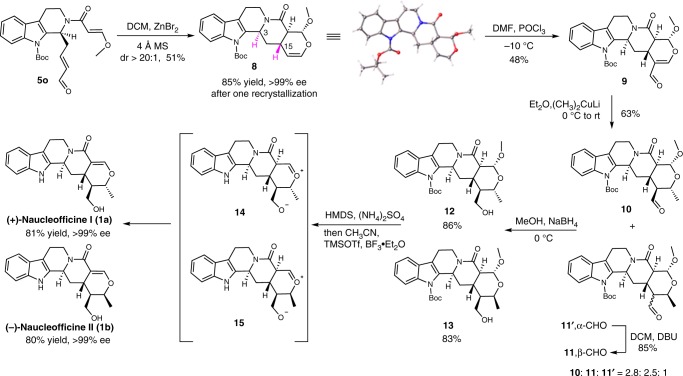
To demonstrate the utility of this sequence, the asymmetric total synthesis of 1a/1b were carried out efficiently from intermediates 5o. First, 5p underwent an O-hetero-Diels–Alder cycloaddition under reflux in mesitylene to produce the advanced pentacyclic intermediate 7 with mainly cis-configuration of C3 and C15 (Fig. 3)50. Next, we turned to the asymmetric total synthesis of the natural alkaloids 1a and 1b from intermediate 5o, which was readily prepared on the gram-scale from 2a using 10 mol % (R,R)-Cat 1 in 42% yield and 91% ee (Fig. 4). After extensive screening of the reaction conditions (for details, see Supplementary Table 2), ZnBr2 was found to enable the expected O-hetero-Diels–Alder cyclization of 5o, efficiently affording the pentacyclic intermediate 8 with four desired stereocenters in 51% yield and >20:1 dr (Fig. 5), and there were amount of natural products that owned trans-configuration of C3 and C15 had not been synthesized. One-recrystallization of 8 in MeOH/Et2O enhanced the enantio-purity to >99% ee (85% yield) and its absolute configuration was confirmed by X-ray crystallographic analysis (for details, see Supplementary Table 6). However, a thermal cyclization of 5o in mesitylene under refluxing temperature resulted mainly in the formation of undesired C15-epimer. Hydroformylation of 8 with POCl3/DMF afforded aldehyde 9 in 48% yield, which was treated with freshly prepared Me2CuLi to provide the separable products 10, 11, and 11′ (2.8: 2.5: 1) with 63% total yield. Fortunately, isomer 11′ could be readily converted to 11 with 85% yield when stirred in DBU (1,8-diazabicyclo[5.4.0]undec-7-ene)/DCM. Subsequent reduction of 10 with NaBH4 furnished 12 with 86% yield. After extensive investigations51,52, protection of the hydroxyl group of 12 with hexamethyl disilylamine at refluxing temperature gave a silyl ether, which was subjected to elimination of methoxy group and removal of Boc with BF3·Et2O and TMSOTf in CH3CN to obtain acetal 14, and then the double bond isomerization to give 1a with 81% yield, so we developed an efficient method to construct the double bond of these natural products. In the same way, 1b was also synthesized separately from 11. The spectra data of synthetic (+)-1a and (−)-1b are consistent with literature13, and the ee of (+)-1a and (−)-1b are >99% determined by chiral high-performance liquid chromatography (HPLC).
Fig. 3.
Further transformation. Thermocatalytic hetero-Diels–Alder reaction mainly obtained the cis-product of C3 and C15
Fig. 4.
Gram-scale synthesis of 5o. Reactions were performed with 10% mol (R,R)-Cat 1
Fig. 5.
Asymmetric total synthesis of naucleofficine I and II. The key steps include O-hetero-Diels–Alder cycloaddition, hydroformylation, and double bond isomerization
Discussion
In summary, a chiral bifunctional organocatalyst has been successfully developed, which is a good assembly of strong acid and sterically hindered SPD framework, and thus exhibits high reactivity as well as excellent stereo-induction ability toward the asymmetric Mannich reaction of 3,4-dihydro-β-carboline with a broad substrate toleration. Furthermore, synthetic utility of this Mannich sequence has been exemplified through the asymmetric total synthesis of naucleofficine I (1a) and II (1b) in a concise way. Further expanded studies on asymmetric catalysis with our bifunctional organocatalysts toward other transformations and synthesis of other natural products are underway.
Methods
General information
All reactions requiring anhydrous conditions were carried out under argon atmosphere using oven-dried glassware (130 °C), which was cooled under argon. All solvents were purified and dried by standard techniques,and distilled prior to use. All reactions under standard conditions were monitored by thin-layer chromatography (TLC) on gel F254 plates. The products were purified by flash column chromatography on silica gel (200~300 mesh) or neutral alumina (200~300 mesh). 1H NMR, 13C NMR, and 19F NMR spectra were obtained on Bruker AM-400, JEOL JNM-ECS-400, or Varian Mercury-600. Chemical shifts (δ) were reported in p.p.m. relative to residual solvent signals (CDCl3: 7.26 p.p.m. for 1H NMR, 77.0 p.p.m. for 13C NMR; DMSO-d6: 2.50 p.p.m. for 1H NMR, 39.5 p.p.m. for 13C NMR; CD3OD: 3.31 p.p.m. for 1H NMR, 49.0 p.p.m. for 13C NMR). The following abbreviations were used to indicate the multiplicity in NMR spectra: s, singlet; d, doublet; t, triplet; m, multiplet. High-resolution mass spectral analysis data were measured on the Bruker ApexII with ESI resource. Infrared spectra were recorded on Nicolet FT-170SX spectrometer. Melting points were measured on a melting point apparatus and were uncorrected. The ee of the products were determined by HPLC analysis. X-ray diffraction data were collected on Agilent SuperNova Eos diffractometer. Optical rotations were detected on RUDOLPH A21202-J APTV/GW.
General procedure for the Mannich sequent reaction
To a solution of substituted 3,4-dihydro-β-carboline 2 (0.1 mmol), Cat 1 (5.4 mg, 0.02 mmol) in DCE/H2O (1 mL, v/v 1: 1) were added Et3N (7 µL, 0.05 mmol) and aldehyde (16 µL, 0.3 mmol) at 0 °C. The mixture was stirred at this temperature until the substrate disappeared via TLC detection. Then the resulting solution was concentrated in vacuo. Then 1 mL DCM, K2CO3 (27.6 mg, 0.2 mmol), and acetyl bromide 3a (10 µL, 0.12 mmol) were added at 0 °C sequentially. About 30 min later, 4a (66.8 mg, 0.2 mmol) was added to the reaction at room temperature and reacted at the same temperature for 12 h. The mixture was then quenched with water and the mixture was extracted with DCM. Then the combined organic phase was washed with saturated brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified through column chromatography on neutral alumina to give substrates 5.
Supplementary information
Acknowledgements
We thank the National Natural Science Foundation of China (Nos. 21502080, 21772071, 21871117, 21772076), National Science and Technology Major project of the Ministry of Science and Technology of China (2018ZX09711001-005-002), and the ‘111’ Program of MOE for their financial support.
Author contributions
Y.-H.Y. performed all of experiments and prepared the supplementary information. X.H. and X.G. prepared materials for the synthesis of naucleofficine I and II. X.H. prepared substrates for substrate expansion. F.-P.Z. synthesized Cat 1. J.-M.T. helped the design of Mannich sequent reaction. Y.-Q.T., F.-M.Z., X.-M.Z. and S.-H.W. directed the project and discussed the results. Y.-Q.T. wrote the manuscript.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (Cat 1: CCDC 1875257, 8: CCDC 1875256). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Alakesh Bisai, Takashi Ohshima and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11382-8.
References
- 1.Ishikawa H, Suzuki T, Hayashi Y. High-yielding synthesis of the anti-influenza neuramidase inhibitor (–)-Oseltamivir by three “One-Pot” operations. Angew. Chem. Int. Ed. 2009;48:1304–1307. doi: 10.1002/anie.200804883. [DOI] [PubMed] [Google Scholar]
- 2.Jones SB, Simmons B, Mastracchio A, MacMillan DW. Collective synthesis of natural productsby means of organocascade catalysis. Nature. 2011;475:183–188. doi: 10.1038/nature10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X-Y, et al. Asymmetric organocatalytic direct aldol reactions of ketones with α-keto acids and their application to the synthesis of 2-hydroxy-γ-butyrolactones. J. Org. Chem. 2007;72:9905–9913. doi: 10.1021/jo701868t. [DOI] [PubMed] [Google Scholar]
- 4.Torii H, Nakadai M, Ishihara K, Saito S, Yamamoto H. Asymmetric direct aldol reaction assisted by water and a proline‐derived tetrazole catalyst. Angew. Chem. Int. Ed. 2004;43:1983–1986. doi: 10.1002/anie.200352724. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht L, Jiang H, Jørgensen KA. Hydrogen‐bonding in aminocatalysis: from proline and beyond. Chem. Eur. J. 2014;20:358–368. doi: 10.1002/chem.201303982. [DOI] [PubMed] [Google Scholar]
- 6.Hajos ZG, Parrish DR. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974;39:1615–1621. doi: 10.1021/jo00925a003. [DOI] [Google Scholar]
- 7.List B, Lerner RA, Barbas CF. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000;122:2395–2396. doi: 10.1021/ja994280y. [DOI] [Google Scholar]
- 8.List B. The direct catalytic asymmetric three-component Mannich reaction. J. Am. Chem. Soc. 2000;122:9336–9337. doi: 10.1021/ja001923x. [DOI] [Google Scholar]
- 9.Allemann C, Gordillo R, Clemente FR, Cheong PH-Y, Houk KN. Theory of asymmetric organocatalysis of aldol and related reactions: rationalizations and predictions. Acc. Chem. Res. 2004;37:558–596. doi: 10.1021/ar0300524. [DOI] [PubMed] [Google Scholar]
- 10.Notz W, Tanaka F, Barbas CF., III Enamine-based organocatalysis with proline and diamines: the development of direct catalytic asymmetric aldol, Mannich, Michael, and Diels−Alder reactions. Acc. Chem. Res. 2004;37:580–591. doi: 10.1021/ar0300468. [DOI] [PubMed] [Google Scholar]
- 11.Gruttadauria M, Giacalone F, Noto R. Supported proline and proline-derivatives as recyclable organocatalysts. Chem. Soc. Rev. 2008;37:1666–1688. doi: 10.1039/b800704g. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Dwivedi N. Proline catalyzed α-aminoxylation reaction in the synthesis of biologically active compounds. Acc. Chem. Res. 2013;46:289–299. doi: 10.1021/ar300135u. [DOI] [PubMed] [Google Scholar]
- 13.Wang H-Y, et al. Three new isomeric indole alkaloids from Nauclea officinalis. Chem. Biodivers. 2015;12:1256–1262. doi: 10.1002/cbdv.201400289. [DOI] [PubMed] [Google Scholar]
- 14.Boucherle B, et al. Nauclea latifolia: biological activity and alkaloid phytochemistry of a West African tree. Nat. Prod. Rep. 2016;33:1034–1043. doi: 10.1039/C6NP00039H. [DOI] [PubMed] [Google Scholar]
- 15.Mergott DJ, Zuend SJ, Jacobsen EN. Catalytic asymmetric total synthesis of (+)-Yohimbine. Org. Lett. 2008;10:745–748. doi: 10.1021/ol702781q. [DOI] [PubMed] [Google Scholar]
- 16.Lebold TP, et al. A divergent approach to the synthesis of the yohimbinoid alkaloids venenatine and alstovenine. Nat. Chem. 2013;5:126–131. doi: 10.1038/nchem.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younai A, Zeng BS, Meltzer HY, Scheidt KA. Enantioselective syntheses of heteroyohimbine natural products: a unified approach through cooperative catalysis. Angew. Chem. Int. Ed. 2015;54:6900–6904. doi: 10.1002/anie.201502011. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, et al. A radical cascade enabling collective syntheses of natural products. Chem. 2017;2:803–816. doi: 10.1016/j.chempr.2017.04.007. [DOI] [Google Scholar]
- 19.Tian J-M, et al. The design of a spiro-pyrrolidine organocatalyst and its application to catalytic asymmetric Michael addition for the construction of all-carbon quaternary centers. Chem. Commun. 2015;51:9979–9982. doi: 10.1039/C5CC02765A. [DOI] [PubMed] [Google Scholar]
- 20.Tian J-M, et al. Catalytic asymmetric cascade using spiro-pyrrolidine organocatalyst: efficient construction of hydrophenanthridine derivatives. Org. Lett. 2017;19:6618–6621. doi: 10.1021/acs.orglett.7b03330. [DOI] [PubMed] [Google Scholar]
- 21.Chen S-K, et al. Organo-cation catalyzed asymmetric homo/heterodialkylation of bisoxindoles: construction of vicinal all-carbon quaternary stereocenters and total synthesis of (−)-chimonanthidine. J. Am. Chem. Soc. 2018;140:10099–10103. doi: 10.1021/jacs.8b05386. [DOI] [PubMed] [Google Scholar]
- 22.Nakashima D, Yamamoto H. Design of chiral N-triflyl phosphoramide as a strong chiral Brønsted acid and its application to asymmetric Diels−Alder reaction. J. Am. Chem. Soc. 2006;128:9626–9627. doi: 10.1021/ja062508t. [DOI] [PubMed] [Google Scholar]
- 23.Han Z-Y, et al. Hybrid metal/organo relay catalysis enables enynes to be latent dienes for asymmetric Diels–Alder reaction. J. Am. Chem. Soc. 2012;134:6532–6535. doi: 10.1021/ja3007148. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, van Gemmeren M, List B. Asymmetric disulfonimide‐catalyzed synthesis of δ‐amino‐β‐ketoester derivatives by vinylogous Mukaiyama–Mannich reactions. Angew. Chem. Int. Ed. 2014;53:13592–13595. doi: 10.1002/anie.201407532. [DOI] [PubMed] [Google Scholar]
- 25.Zhou F, Yamamoto H. A disulfonimide catalyst for highly enantioselective Mukaiyama–Mannich reaction. Org. Lett. 2016;18:4974–4977. doi: 10.1021/acs.orglett.6b02262. [DOI] [PubMed] [Google Scholar]
- 26.Trost BM, Saget T, Hung CI. Direct catalytic asymmetric Mannich reactions for the construction of quaternary carbon stereocenters. J. Am. Chem. Soc. 2016;138:3659–3662. doi: 10.1021/jacs.6b01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai J, et al. Chiral primary amine catalysis for asymmetric Mannich reactions of aldehydes with ketimines: stereoselectivity and reactivity. Angew. Chem. Int. Ed. 2017;56:12697–12701. doi: 10.1002/anie.201706304. [DOI] [PubMed] [Google Scholar]
- 28.Qiao B, Huang YJ, Nie J, Ma J-A. Highly regio-, diastereo-, and enantioselective Mannich reaction of allylic ketones and cyclic ketimines: access to chiral Benzosultam. Org. Lett. 2015;17:4608–4611. doi: 10.1021/acs.orglett.5b02351. [DOI] [PubMed] [Google Scholar]
- 29.Arrayas RG, Carretero JC. Catalytic asymmetric direct Mannich reaction: a powerful tool for the synthesis of α,β-diamino acids. Chem. Soc. Rev. 2009;38:1940–1948. doi: 10.1039/b820303b. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Schaus SE. Asymmetric Petasis Borono‐Mannich allylation reactions catalyzed by chiral biphenols. Angew. Chem. Int. Ed. 2017;56:1544–1548. doi: 10.1002/anie.201611332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verkade JM, van Hemert LJ, Quaedflieg PJ, Rutjes FP. Organocatalysed asymmetric Mannich reactions. Chem. Soc. Rev. 2008;37:29–41. doi: 10.1039/B713885G. [DOI] [PubMed] [Google Scholar]
- 32.Trost BM, Gnanamani E, Tracy JS, Kalnmals CA. Zn-ProPhenol catalyzed enantio- and diastereoselective direct vinylogous Mannich reactions between α,β- and β,γ-butenolides and aldimines. J. Am. Chem. Soc. 2017;139:18198–18201. doi: 10.1021/jacs.7b11361. [DOI] [PubMed] [Google Scholar]
- 33.Yang JW, Chandler C, Stadler M, Kampen D, List B. Proline-catalysed Mannich reactions of acetaldehyde. Nature. 2008;452:453–455. doi: 10.1038/nature06740. [DOI] [PubMed] [Google Scholar]
- 34.García-García P, Ladépêche A, Halder R, List B. Catalytic Asymmetric Michael reactions of acetaldehyde. Angew. Chem., Int. Ed. 2008;47:4719–4721. doi: 10.1002/anie.200800847. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi Y, Itoh T, Aratake S, Ishikawa H. A diarylprolinol in an asymmetric, catalytic, and direct crossed‐aldol reaction of acetaldehyde. Angew. Chem. Int. Ed. 2008;47:2082–2084. doi: 10.1002/anie.200704870. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi Y, Itoh T, Ohkubo M, Ishikawa H. Asymmetric Michael reaction of acetaldehyde catalyzed by diphenylprolinol silyl ether. Angew. Chem. Int. Ed. 2008;47:4722–4724. doi: 10.1002/anie.200801130. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi Y, et al. Direct organocatalytic Mannich reaction of acetaldehyde: an improved catalyst and mechanistic insight from a computational study. Angew. Chem. Int. Ed. 2008;47:9053–9058. doi: 10.1002/anie.200802073. [DOI] [PubMed] [Google Scholar]
- 38.Kim SM, Kim YS, Kim DW, Rios R, Yang JW. Acetaldehyde: a small organic molecule with big impact on organocatalytic reactions. Chem. Eur. J. 2016;22:22 14–2234. doi: 10.1002/chem.201503960. [DOI] [PubMed] [Google Scholar]
- 39.Itoh T, Ishikawa H, Hayashi Y. Asymmetric aldol reaction of acetaldehyde and isatin derivatives for the total syntheses of ent-Convolutamydine E and CPC-1 and a half fragment of Madindoline A and B. Org. Lett. 2009;11:3854–3857. doi: 10.1021/ol901432a. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Wang J, Li H. Direct, highly enantioselective pyrrolidine sulfonamide catalyzed Michael addition of aldehydes to nitrostyrenes. Angew. Chem. Int. Ed. 2005;44:1369–1371. doi: 10.1002/anie.200461959. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, et al. Enantio‐ and diastereoselective Michael addition reactions of unmodified aldehydes and ketones with nitroolefins catalyzed by a pyrrolidine sulfonamide. Chem. Eur. J. 2006;12:4321–4332. doi: 10.1002/chem.200600115. [DOI] [PubMed] [Google Scholar]
- 42.Zu L, Wang J, Li H, Wang W. A recyclable fluorous (S)-pyrrolidine sulfonamide promoted direct, highly enantioselective Michael addition of ketones and aldehydes to nitroolefins in water. Org. Lett. 2006;8:3077–3079. doi: 10.1021/ol061053+. [DOI] [PubMed] [Google Scholar]
- 43.Cao C-L, Sun X-L, Kang Y-B, Tang Y. Enantioselective formal [3+3] annulation for the direct construction of bicyclic skeletons with four stereogenic centers. Org. Lett. 2007;9:4151–4154. doi: 10.1021/ol701669b. [DOI] [PubMed] [Google Scholar]
- 44.Miura T, et al. Direct asymmetric aldol reaction with recyclable fluorous organocatalyst. Org. Lett. 2010;12:1620–1623. doi: 10.1021/ol1003719. [DOI] [PubMed] [Google Scholar]
- 45.Itoh T, et al. Preparation of both enantiomers of 1-allyl-1,2,3,4-tetrahydro-β-carboline using allyltin reagents and a chiral auxiliary derived from L-proline. Tetrahedron. 2001;57:7277–7289. doi: 10.1016/S0040-4020(01)00684-6. [DOI] [Google Scholar]
- 46.Itoh T, Yokoya M, Miyauchi K, Nagata K, Ohsawa A. Proline-catalyzed asymmetric addition reaction of 9-Tosyl-3,4-dihydro-β-carboline with ketones. Org. Lett. 2003;5:4301–4304. doi: 10.1021/ol030103x. [DOI] [PubMed] [Google Scholar]
- 47.Rajapaksa NS, McGowan MA, Rienzo M, Jacobsen EN. Enantioselective total synthesis of (+)-Reserpine. Org. Lett. 2013;15:706–709. doi: 10.1021/ol400046n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lalonde MP, McGowan MA, Rajapaksa NS, Jacobsen EN. Enantioselective formal Aza-Diels–Alder reactions of enones with cyclic imines catalyzed by primary aminothioureas. J. Am. Chem. Soc. 2013;135:1891–1894. doi: 10.1021/ja310718f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eschenbrenner-Lux V, Kuchler P, Ziegler S, Kumar K, Waldmann H. An Enantioselective inverse‐electron‐demand imino Diels–Alder reaction. Angew. Chem. Int. Ed. 2014;53:2134–2137. doi: 10.1002/anie.201309022. [DOI] [PubMed] [Google Scholar]
- 50.Martin SF, Hunter JE, Benage B, Geraci LS, Mortimore M. Unified strategy for synthesis of indole and 2-oxindole alkaloids. J. Am. Chem. Soc. 1991;113:6161–6171. doi: 10.1021/ja00016a036. [DOI] [Google Scholar]
- 51.Yuan C, Hollingsworth RI. A short and efficient synthesis of 1,5-anhydro-d-glucitol 6-phosphate. Tetrahedron Lett. 2011;52:5421–5423. doi: 10.1016/j.tetlet.2011.07.082. [DOI] [Google Scholar]
- 52.Yoo SJ, Kim HO, Lim YH, Kim JM, Jeong LS. Synthesis of novel (2R,4R)- and (2S,4S)-iso dideoxynucleosides with exocyclic methylene as potential antiviral agents. Bioorg. Med. Chem. 2002;10:215–226. doi: 10.1016/S0968-0896(01)00266-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (Cat 1: CCDC 1875257, 8: CCDC 1875256). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.