The goals for treatment of eosinophilic esophagitis (EoE) are improvements in symptoms and esophageal eosinophilic inflammation with the ideal endpoint complete resolution of the latter.1 Once a diagnosis of proton pump inhibitor (PPI)-nonresponsive EoE is confirmed, treatment options include pharmacologic agents and/or dietary elimination. If pharmacologic therapy is chosen, topical CSs are effective and considered first line. Although these medications are currently not Food and Drug Administration approved for EoE, the 2 commonly used options are swallowed aerosolized FP and OVB. Systemic CSs (ie, prednisolone and methylprednisolone) may be useful if topical steroids are not effective or in patients who require rapid improvement in symptoms.
This article discusses the use of topical and systemic CSs for induction of remission and as maintenance treatment of pediatric EoE. The risks and benefits of these agents are outlined and some important and clinically relevant questions discussed.
TOPICAL CORTICOSTEROIDS FOR INDUCTION
In 1998, Faubion and colleagues2 described 4 children with eosinophilic inflammation isolated to the esophagus who improved clinically and histologically by swallowing aerosolized CSs (FP and beclomethasone) from an inhaler without use of a spacer. Over time, FP has become the topical CS used most often in EoE, although other agents are also used (discussed later). We will review prospective and randomized studies involving topical steroids used in pediatric eosinophilic esophagitis (Table 1). Adult studies are discussed elsewhere in this issue.
Table 1.
Topical steroids in pediatric eosinophilic esophagitis, prospective and randomized controlled trials
| Study | Type of Study |
Control Group (n) |
Histologic Criteria |
Drug (n) |
Dose (mg) | Length of Treatment |
Primary Outcomes |
Drug Efficacya (%) |
Control Group Response (%) |
Other Outcomes | Adverse Events |
Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teitelbaum et al, 2002 | Prospective | NA | >15 eos/hpf, superficial layering, and/or eosinophil microabscesses | FP (13) | 2–4 yo: 88 BID 5–10 yo: 220 BID 2:11 yo: 440 BID |
8 wk | Clinical improvement/resolution of symptoms | 100 | NA | 70% Still had abnormal endoscopy (loss of vascular pattern, thickened longitudinal folds), but improved histology | 18% With esophageal candidiasis, 9% (n 5 1) symptomatic | 8-wk PPI trial before diagnosis. Normal 24-h continuous pH monitoring; 9 of the patients who responded clinically to FP had failed allergy testing–based diet restriction. |
| Konikoff et al, 2006 | Randomized, double-blind, placebo-controlled | Placebo (15) | >24 eos/hpf in any x400 HPF and epithelial hyperplasia | FP (21) | 440 BID | 3 mo | Complete response: <1 eos/hpf | 50 | 9 | All FP responders: resolved distal furrowing, epithelial hyperplasia, and vomiting | Incidental esophageal candidiasis in 9% of FP pts (1/11) | Prior acid suppression therapy was not necessary for diagnosis. FP response higher in nonallergic individuals. FP response negatively correlates with patient age, height, and weight. |
| Partial response: 1–24 eos/hpf | 15 | 9 | ||||||||||
| Schaefer et al, 2008 | Randomized, comparator controlled | Prednisone 1 mg/kg/d (40) | 2:15 eos/hpf with negative pH probe studies | FP (40) | 1–10 yo: 220 QID 11–18 yo: 440 QID |
4-wk Induction | Complete histologic resolution | 50 | 81 | 97% FP group had resolution of symptoms. 100% of prednisone group had resolution of symptoms. | Incidental esophageal candidiasis in 15% of FP patients; hyperphagia, weight gain in 40% of prednisone patients. | Symptom relapse in 44% of FP patients, 45% of prednisone 12 wk after treatment stopped. |
| Improvement in biopsy grade (score based on basal cell zone % and # eos/hpf) | 94 | 94 | ||||||||||
| Dohil et al, 2010 | Randomized, double-blind, placebo-controlled | Placebo (9) | Peak eos/hpf 2:20 | OVB (15) | <5 ft Tall:1000/d 2:5 ft Tall:2000/d |
3 mo | Responders: <6 eos/hpf | 87 | 0 | Endoscopy score improved more in OVB vs placebo. Symptom score improved in OVB but not placebo group. | Oral candidiasis that responded to nystatin. Serum cortisol unchanged | All patients received PPI during drug period. <10 yo: Lansoprazole 15 mg BID; 2:10 yo: lansoprazole 30 mg BID. Placebo and PPI did not improve eosinophilia at any level. |
| Partial responders: 7–19 eos/hpf | 6.7 | 11 | ||||||||||
| Nonresponders 2:20 eos/hpf | 6.7 | 89 | ||||||||||
| Boldorini et al, 2013 | Prospective | NA | >15 eos/hpf | FP (34) | 750 TID | 6 wk | Responders: :s6 eos/hpf | 74 | NA | All children had symptomatic improvement irrespective of histologic results. Responders had more severe inflammation (higher median peak eos/hpf, higher likelihood of eosinophilic microabscesses, and peak mast cells/HPF). | No adverse events seen | All children were nonresponders to PPI or 24-h pH monitoring was negative for gastroesophageal reflux. 4 Children had celiac disease, 3 were responders 1 was not. Age, weight, and height, did not affect response. |
| Borderline: 7–20 eos/hpf | 0 | |||||||||||
| Nonresponders: >20 eos/hpf | 26 |
Fluticasone
In 2002, a prospective study using swallowed FP in children cited its ease of administration, low systemic absorption, and rapid first-pass metabolism by the liver to limit systemic side effects.3 These children had symptoms of esophageal dysfunction (ie, chest pain, food impaction, dysphagia, feeding refusal, and vomiting), eosinophilic esophageal infiltration, normal 24-hour continuous monitoring of intraesophageal pH (pH probe), and lack of clinical response to an 8-week trial of PPI. FP dosing was age dependent, with a maximum of 880 mg/d divided twice daily. Four patients had no food allergens identified by history, radioimmunosorbent assay, or skin prick testing and were started directly on swallowed FP. Eleven patients were started on dietary restriction and nutritional counseling based on abnormal allergy testing or history; however, none of these patients had clinical improvement and 9 were subsequently treated with swallowed FP. All 13 patients who received FP had resolution of their presenting symptoms, and all 11 patients with post-treatment endoscopy showed improvement in histology with similar decreases in eosinophilia in proximal and distal esophageal biopsies.
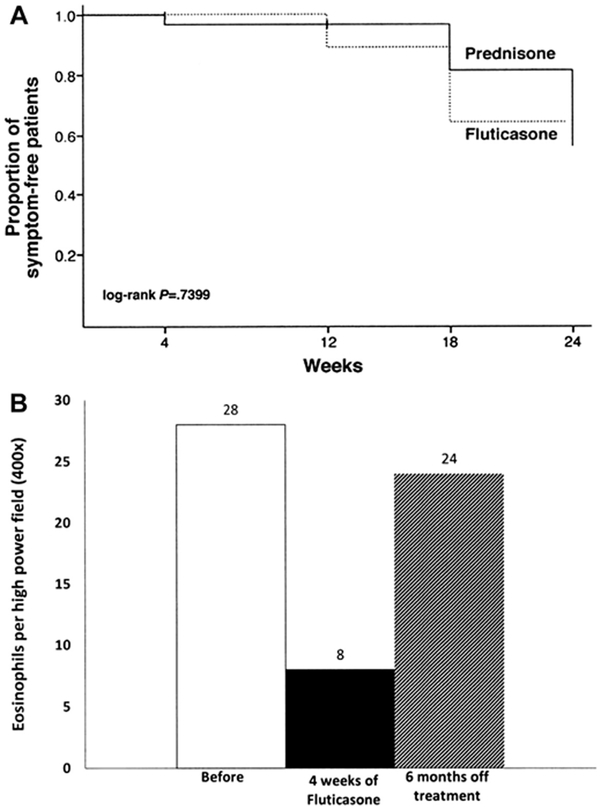
A subsequent randomized, double-blind, placebo-controlled trial in children showed that 50% of FP-treated patients achieved complete histologic remission (≤1 eosinophil [EOS] per high-power field [HPF]) with a standard dose, regardless of patient age and/or size, of 880 mg/d divided twice daily.4 Patient factors predictive of histologic resolution in this study included shorter stature and younger age. Unlike the previous study, proximal esophageal biopsies were more improved than those from the distal esophagus. Another randomized controlled trial comparing swallowed FP to oral prednisone (880–1760 mg/d based on age and 1 mg/kg/d to a maximum of 30 mg twice daily, respectively) showed complete histologic resolution in 50% of patients in FP group versus 81% in prednisone group at week 4; partial improvement in histologic grade was recorded in 94% of patients in both groups.5 As expected, symptomatic improvement was seen more often compared with histologic reversal; 97.2% of FP patients and 100% of prednisone patients had resolution of presenting symptoms with therapy although symptoms recurred in approximately 45% of patients 12 weeks after treatment was stopped (Fig. 1A).
Fig. 1.
(A) Proportion of symptom-free patients with prednisone and swallowed fluticasone. Patients received induction dose × 4 weeks, were weaned over 8 weeks, and were clinically monitored for next 12 weeks. (B) Recurrence of esophageal eosinophilia after withdrawal of swallowed fluticasone (220 mg twice daily). (From [A] Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol 2008;6:165–73, with permission; and [B] Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005;3:1202, with permission.)
A recent prospective Italian study in children using a higher dose of FP (2250 mg/d) for 6 weeks reported higher likelihood (73.5%) in reaching post-treatment peak esophageal eosinophils of less than 6 eos/hpf and suggested that more severe esophageal inflammation (higher median peak eos/hpf, presence of eosinophilic abscesses, and peak mast cells/HPF) was associated with higher response rate to FP treatment.6 Age and height did not affect response in this study.
Improvement in incidental gastric eosinophilic inflammation (≥10 eos/hpf) in patients otherwise similar to EoE patients was noted with FP.7 Therefore, mild gastric eosinophilia should not exclude FP as a possible therapeutic option for esophageal eosinophilia.
The results of the first double-blind, randomized, placebo-controlled trial of FP (1760 mg/d) in children and adults are awaited.8 Further studies are needed to determine ideal dosing regimen but current recommendations are listed in Table 2.
Table 2.
Dosing regimens for fluticasone propionate and oral viscous budesonide in pediatric eosinophilic esophagitis
| Medication | Age (y) | Drug Formulation |
Induction Dose |
Weaning Dose | Instructions |
|---|---|---|---|---|---|
| FP | 1–10 | 110 mg/puff | 2 Puffs 4 times/d | 2 Puffs 3 times/d × 3 wk, 2 puffs 2 times/d × 3 wk, 1 puff 2 times/d × 2 wk |
|
| 11–18 | 220 mg/puff | Same as above with 220 mg/puff inhaler | Same as above with 220 mg/puff inhaler | ||
| OVB | 1–10 | 0.5 mg/2 mL budesonide respules | 1 mg Daily |
|
|
| 11–18 | 0.5 mg/2 mL budesonide respules | 2 mg Daily |
Sucralose (5 g) 5 5 packets or 10 teaspoons.
Budesonide
Budesonide is another topical steroid with proved efficacy for EoE. OVB was initially developed to help patients who were developmentally unable to perform the puff and swallow technique required for FP. The first studies to evaluate its efficacy mixed aqueous budesonide (0.5 mg/2 mL suspension, Budesonide Respules [Pulmicort], Astra-Zeneca, Wilmington, DE) with sucralose (see Table 2 for recipe) to create a thickened slurry. A randomized, double-blind, placebo-controlled trial in children showed significant improvement in symptoms, endoscopic findings, and esophageal eosinophilia compared with placebo.9 Patients less than 1.5 meters (5 feet) tall received 1 mg daily; patients greater than or equal to 5 feet tall received 2 mg daily for 3 months. Patients in both groups also received twice-daily lansoprazole (15 mg twice daily if less than 10 years old and 30 mg twice daily if greater than 10 years old). Peak eosinophil counts in the OVB group improved from 66.7 to 4.8 eos/hpf, with significant reductions in proximal, mid-, and distal esophageal eosinophilia.
No studies to date have compared FP and OVB in children.
Ciclesonide
Two small case series report a total of 8 children treated with ciclesonide, a topical CS also used in asthma, allergic rhinitis, and allergic conjunctivitis.10,11 Six of the 8 patients showed histologic improvement; the 2 who did not respond had previous poor response to OVB as well. In asthma, inhaled ciclesonide seems to have similar effectiveness compared with inhaled FP and nebulized budesonide.12 Larger randomized, drug-controlled studies are needed to see if this is the case in EoE.
TOPICAL CORTICOSTEROIDS FOR MAINTENANCE THERAPY
EoE is considered a chronic immune-mediated disease, yet long-term management has not been defined. Need for maintenance therapy is underscored by the observations that 45% of children had recurrence of symptoms within 12 weeks of discontinuing CS therapy5 and esophageal eosinophilia recurred in a majority of patients13 after 6 months off therapy (see Fig. 1). Straumann and colleagues14 prospectively evaluated a maintenance regimen with swallowed nebulized budesonide (0.5 mg/d), in adolescents and adults after successful remission with 15 days of nebulized budesonide (2 mg/d). Patients placed on maintenance therapy of nebulized budesonide (0.5 mg/d) had increased eosinophil load compared with at remission/end of induction (31.8 to 0.4 eos/hpf, respectively). This increase was less pronounced when compared with patients placed on placebo after remission/end of induction (65.0 to 0.7 eos/hpf, respectively). Symptom scores were stable with maintenance therapy but increased with placebo. This study not only highlighted a shorter induction time of 15 days but also a newer mode of delivery (ie, via a nebulizer). Further studies with higher maintenance dose are needed to evaluate for efficacy and long-term adverse effects.
Maintenance therapy studies have not been done with FP or OVB or in children.
OTHER DELIVERY METHODS
Fluticasone
A tablet form (1.5 mg and 3 mg) of fluticasone is currently undergoing phase 1/2a trials in adolescents and adults.15
Budesonide
The current OVB formulation contains 10 mg sucralose per 1 mg budesonide to create an 8-mL slurry.16 Concerns about taste, cost, and potential adverse effects of sucralose have made many patients and parents wary of OVB.17 Some patients may use applesauce or other palatable food products that patients are not allergic to, although efficacy with these alternate vehicles has not been studied. At the authors’ institution, 1 to 2 tablespoons of applesauce are allowed to be mixed with 2 respules (0.5 mg/2 mL) of budesonide. Hait and colleagues18 found that 13 of 14 patients who added a hypoallergenic, amino acid–based semisolid (Neocate Nutra, Nutricia, Gaithersburg, MD) to their budesonide respules improved with post-treatment eosinophil counts less than 15 eos/hpf and continue to find results that are at least comparable to OVB with improved patient compliance (Eitan Rubinstein, personal communication, 2013).
Also in the works is a non–sucralose-based oral budesonide suspension (OBS) currently being studied in adolescents and adults. A prospective, randomized, double-blind, placebo-controlled study comparing 2 doses of OBS and placebo in children ages 2 to 18 years found panesophageal endoscopic and histologic dose-related responses.19 Histologic response (peak 6 eos/hpf) was seen in 94% of patients in the high-dose OBS group (1.4 mg twice daily for 2–9 years old and 2 mg twice daily for 10–18 years old) versus 54% of patients in the medium-dose group (1.4 mg daily for 2–9 years old and 2 mg daily for 10–18 years old) and 5.6% in the placebo arm. This higher response in the high-dose group suggests a possible need to increase OVB dosing regimens to a higher dose of 4 mg/d in patients previously thought to fail budesonide therapy.
SYSTEMIC CORTICOSTEROIDS
Oral prednisone was the first pharmacologic agent shown effective in treating EoE20 but can have systemic adverse effects in 40% of patients.5 Although complete histologic resolution is more likely with prednisone compared with FP, the symptom improvement and long-term disease remissions were similar to those with FP. With the newer therapies available, systemic prednisone is now reserved for urgent situations where topical CS may not be as rapidly effective. Intravenous methylprednisolone may be considered in situations where patients are not tolerating anything by mouth.
MARKERS OF RESPONSE
The mechanism of action of topical steroids in EoE is still unknown. In randomized trials in children, 50% to 94% of children with EoE have partial to complete response to FP or OVB treatment.4,5,9 Interpretation of published data is challenging for a variety of reasons, including varying definitions of response, type of CS, CS formulation, mode of delivery, total daily dose, number of doses per day, and adjustment of dose for clinical factors, such as age and height (discussed later). Currently, predicting who will or will not respond to CSs is not possible, but some studies have identified possible mechanisms of nonresponsiveness in these patients.
Caldwell and colleagues21 provided evidence that topical CSs directly affect esophageal epithelial gene expression in vivo. They identified 32 transcripts altered by FP treatment in responders compared with those with untreated EoE and normal healthy controls. One of the genes, FK506 binding protein 51 (FKBP51), a known steroid-induced gene in respiratory epithelial cells and lymphocytes, was increased in FP responders and found to act as a negative regulator of FP action. In vitro, increased baseline FKBP51 levels correlated with a decreased ability of glucocorticoid to repress interleukin 13–mediated eotaxin-3 promoter activity and may suggest a mechanism for steroid nonresponsiveness.
Responders to OVB (defined as patients who had <7 eos/hpf after therapy) show a decrease in lamina propria fibrosis score, esophageal fibrosis mediators (transforming growth factor b1 [TGF-b1] and phosphorylated Smad2/3), epithelial edema, and vascular cell adhesion molecule 1–positive vessels not seen in nonresponders oruntreated patients.22 This study also suggested that genetic polymorphisms in the TGF-b1 promoter may be predictive of CS responsiveness.
Medication delivery method could affect histologic response; a recent adult study showed higher mucosal medication contact time and improved eosinophil counts with OVB versus the nebulized budesonide method.23 Potential noninvasive markers for topical steroid therapy response include serum eosinophil cationic protein and serum eosinophil-derived neurotoxin.24,25
BENEFITS
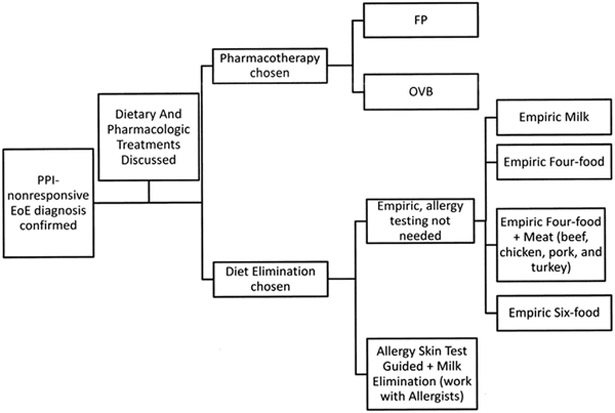
A major benefit to patients of treatment with topical CS, in addition to improving their EoE, is not having to implement dietary modifications. As demonstrated in the recently validated PedsQL EoE Module, patients on restricted diets (and their parents) reported lower quality-of-life scores, with the largest gaps concerning food, eating, and food feelings.26 Therefore, optimizing current topical CS therapy and developing other medical therapies are important in maintaining good quality of life for these patients. Nevertheless, a variety of elimination diets are also recommended as first-line therapy for EoE; practice at the authors’ institution is shown in Fig. 2. An adult study has shown symptomatic improvement with leukotriene antagonists but no effect on esophageal eosinophilia.27 The authors do not note this improvement, however, in clinical practice where patients on montelukast (for their asthma management) have active EoE. In addition, cysteinyl leukotriene levels in esophageal mucosal biopsies of children with EoE were similar to those of controls.28 A small series of children with EoE had no symptomatic or histologic response to cromolyn sodium.13
Fig. 2.
Proposed algorithm for treatment of EoE.
RISKS
As expected, 40% of children treated with prednisone for EoE exhibit systemic side effects, such as hyperphagia and weight gain.5 Up to 15% of patients receiving FP may develop esophageal candidiasis, although this is usually found incidentally on follow-up endoscopy, is not associated with esophageal inflammation, and may not be of clinical significance.4-6 To minimize this risk, the authors instruct patients to not eat or drink for 30 minutes after drug administration and then drink a small amount of liquid to wash the esophageal mucosa (see Table 2 for FP and OVB dosing regimens and instructions). The incidence of esophageal candidiasis is decreasing with careful attention to drug administration.
There has been no definitive evidence of adrenal suppression with topical steroids. In the placebo-controlled trial with OVB there were no signs of adrenal suppression; serum cortisol levels were similar between pretreatment, post-treatment, and placebo-groups.9 Long-term data regarding bone disease and/or growth rates are not yet available in patients with EoE. Asthma studies indicate that children receiving inhaled steroids grow 1 to 2 cm less than their counterparts; this height deficit does not accumulate but does persist into adulthood.29,30 Prospective long-term studies using large EoE patient databases are needed to evaluate this.31,32
SUMMARY AND UNMET NEEDS
Swallowed FP and OVB are effective first-line pharmacologic therapies for EoE and an alternative to dietary restrictions. Side effects are minimal without evidence of Cushing syndrome, as seen in treatment with systemic CSs. Recent preliminary studies suggest that higher dosing and/or improved delivery may be needed to improve efficacy of these medications.19 New studies on alternative delivery systems and different CSs (eg, ciclesonide) are encouraging. As knowledge of EoE expands, newer questions arise. Several of these are listed, recognizing that some have partial answers and others are without any answers at present. The authors hope this list will stimulate interests in the study of EoE:
Do the various formulations of topical CSs differ in efficacy and/or side-effect profile?
What are the optimal delivery mechanisms, dose strength, and dosing frequencies for topical CSs for induction and maintenance of remission?
What is the best length of treatment to induce remission?
What are long-term side effects of prolonged topical CS therapy (eg, linear growth, bone health, and adrenal suppression)? Are these adverse effects reversible or irreversible?
To what degree are adverse effects modified by simultaneous use of topical CSs for other conditions (eg, asthma and allergic rhinitis)?
Is there a benefit to cooling down the inflamed esophageal strictures with topical CSs or diet elimination prior to dilation?
Are CSs useful in the burnt-out esophagus without active eosinophilic inflammation but poor motility/compliance due to their effects on the fibrotic pathway?
Could PPI, mast cell stabilizers, or leukotriene antagonists be additive or synergistic with CS?
Should topical CSs be used in combination with diet and/or dilation therapy?
KEY POINTS.
Topical corticosteroids (CSs) (eg, swallowed fluticasone propionate [FP] and oral viscous budesonide [OVB]) are effective first-line therapies for pediatric eosinophilic esophagitis.
Topical CSs have minimal known side effects when used for treatment of eosinophilic esophagitis.
Systemic CSs have significant adverse effects and are now reserved for urgent situations where topical CSs are not effective or in patients who require rapid improvement in symptoms.
ACKNOWLEDGMENTS
We wish to extend our sincere appreciation to Ms Sharon McPheeters for her secretarial assistance and expert attention to this work.
Footnotes
Disclosures: E.M. Contreras has no disclosures; S.K. Gupta is a consultant for Meritage Pharmacia, QOL Medical, and Receptos Inc. He is in the speaker’s bureau for both Abbott Nutrition and Nestle.
REFERENCES
- 1.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–92 [quiz: 693]. [DOI] [PubMed] [Google Scholar]
- 2.Faubion WA Jr, Perrault J, Burgart LJ, et al. Treatment of eosinophilic esophagitis with inhaled corticosteroids. J Pediatr Gastroenterol Nutr 1998;27:90–3. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology 2002;122:1216–25. [DOI] [PubMed] [Google Scholar]
- 4.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006;131:1381–91. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol 2008;6:165–73. [DOI] [PubMed] [Google Scholar]
- 6.Boldorini R, Mercalli F, Oderda G. Eosinophilic oesophagitis in children: responders and non-responders to swallowed fluticasone. J Clin Pathol 2013;66: 399–402. [DOI] [PubMed] [Google Scholar]
- 7.Ammoury RF, Rosenman MB, Roettcher D, et al. Incidental gastric eosinophils in patients with eosinophilic esophagitis: do they matter? J Pediatr Gastroenterol Nutr 2010;51:723–6. [DOI] [PubMed] [Google Scholar]
- 8.Rothenberg ME, Children’s Hospital Medical Center C. A double blinded, randomized trial of swallowed 1760 mcg fluticasone propionate versus placebo in the treatment of eosinophilic esophagitis. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 Jan 10]. In: ClinicalTrials.gov [Internet]. Available at: http://clinicaltrials.gov/ct2/show/NCT00426283. NLM Identifier: NCT00426283. [Google Scholar]
- 9.Dohil R, Newbury R, Fox L, et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 2010;139:418–29. [DOI] [PubMed] [Google Scholar]
- 10.Lee JJ, Fried AJ, Hait E, et al. Topical inhaled ciclesonide for treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2012;130:1011 [author reply: 1011–2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder S, Fleischer DM, Masterson JC, et al. Successful treatment of eosinophilic esophagitis with ciclesonide. J Allergy Clin Immunol 2012;129:1419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer MJ, Halpin DM, Stein K. Inhaled ciclesonide versus inhaled budesonide or inhaled beclomethasone or inhaled fluticasone for chronic asthma in adults: a systematic review. BMC Fam Pract 2006;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005;3:1198–206. [DOI] [PubMed] [Google Scholar]
- 14.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2011;9:400–9.e1. [DOI] [PubMed] [Google Scholar]
- 15.Aptalis Pharma. Six month safety follow-up study for PR-021 [multicenter, randomized, double-blind, placebo-controlled, safety and tolerability phase 1/2a study of two dosing regimens of EUR-1100 for oral use, in eosinophilic esophagitis subjects]. Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 Jan 10]. In: ClinicalTrials.gov [Internet]. Available at: http://clinicaltrials.gov/ct2/show/NCT01498497. NLM Identifierr: NCT 01498497. [Google Scholar]
- 16.Aceves SS, Bastian JF, Newbury RO, et al. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol 2007; 102:2271–9 [quiz: 2280]. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman SS, Rother KI. Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J Toxicol Environ Health B Crit Rev 2013;16:399–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hait E, Lee J, Fried A, et al. Neocate® Nutra is as effective as sucralose as a de-livery vehicle for oral viscous budesonide to treat eosinophilic esophagitis in children [abstract]. J Pediatr Gastroenterol Nutr 2013;57:e4. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Collins M, Lewis J, et al. Efficacy and safety of oral budesonide suspension (OBS) in pediatric subjects with eosinophilic esophagitis (EoE): results from the double-blind, placebo-controlled PEER study. Gastroenterol Clin North Am 2011;140:S179. [Google Scholar]
- 20.Liacouras CA, Wenner WJ, Brown K, et al. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr 1998;26:380–5. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell JM, Blanchard C, Collins MH, et al. Glucocorticoid-regulated genes in eosinophilic esophagitis: a role for FKBP51. J Allergy Clin Immunol 2010;125: 879–88.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy 2010;65:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellon ES, Sheikh A, Speck O, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012;143:321–4.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlag C, Pfefferkorn S, Brockow K, et al. Serum eosinophil cationic protein is superior to mast cell tryptase as marker for response to topical corticosteroid therapy in eosinophilic esophagitis. J Clin Gastroenterol 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Subbarao G, Rosenman MB, Ohnuki L, et al. Exploring potential noninvasive biomarkers in eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr 2011; 53:651–8. [DOI] [PubMed] [Google Scholar]
- 26.Franciosi JP, Hommel KA, Bendo CB, et al. PedsQL eosinophilic esophagitis module: feasibility, reliability, and validity. J Pediatr Gastroenterol Nutr 2013;57: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attwood SE, Lewis CJ, Bronder CS, et al. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut 2003;52:181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta SK, Peters-Golden M, Fitzgerald JF, et al. Cysteinyl leukotriene levels in esophageal mucosal biopsies of children with eosinophilic inflammation: are they all the same? Am J Gastroenterol 2006;101:1125–8. [DOI] [PubMed] [Google Scholar]
- 29.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006;354:1985–97. [DOI] [PubMed] [Google Scholar]
- 30.Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med 2012;367:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Straumann A, Hruz P. What’s new in the diagnosis and therapy of eosinophilic esophagitis? Curr Opin Gastroenterol 2009;25:366–71. [DOI] [PubMed] [Google Scholar]
- 32.Dellon ES, Erichsen R, Pedersen L, et al. Development and validation of a registry-based definition of eosinophilic esophagitis in Denmark. World J Gastroenterol 2013;19:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]