Abstract
Lung cancer is the most common cause of cancer mortality in male and female patients in the US. The etiology of non-small cell lung cancer (NSCLC) is not fully defined, but new data suggest that estrogens and growth factors promote tumor progression. In this work, we confirm that estrogen receptors (ER), both ERα and ERβ, occur in significant proportions of archival NSCLC specimens from the clinic, with receptor expression in tumor cell nuclei and in extranuclear sites. Further, ERα in tumor nuclei was present in activated forms as assessed by detection of ER phosphorylation at serines-118 and −167, residues commonly modulated by growth factor receptor as well as steroid signaling. In experiments using small interfering RNA (siRNA) constructs, we find that suppressing expression of either ERα or ERβ elicits a significant reduction in NSCLC cell proliferation in vitro. Estrogen signaling in NSCLC cells may also include steroid receptor coactivators (SRC), as SRC-3 and MNAR/PELP1 are both expressed in several lung cell lines, and both EGF and estradiol elicit serine phosphorylation of SRC-3 in vitro. EGFR and ER also cooperate in promoting early activation of p42/p44 MAP kinase in NSCLC cells. To assess new strategies to block NSCLC growth, we used Faslodex alone and with erlotinib, an EGFR kinase inhibitor. The drug tandem elicited enhanced blockade of the growth of NSCLC xenografts in vivo, and antitumor activity exceeded that of either agent given alone. The potential for use of antiestrogens alone and with growth factor receptor antagonists is now being pursued further in clinical trials.
Keywords: estradiol, estrogen receptor-alpha, estrogen receptor-beta, steroids, non-small cell lung cancer, epidermal growth factor receptor, steroid receptor coactivator
Introduction
Lung cancer is the most common cause of cancer mortality in both male and female patients in the US. It is estimated that more than 180,000 new cases of non-small cell lung cancer (NSCLC) will be diagnosed this year in the US, and about 165,000 patients will succumb to the disease. Survival rates from NSCLC are unacceptably low [1, 2], and new therapeutic options are urgently needed. The etiology of most NSCLC is not fully defined, but several studies suggest a role of estrogens in progression [2–5]. Clearly, cigarette smoking remains the primary risk factor for lung cancer with 85–90% of all lung cancer patients having smoked cigarettes at some time. Remaining lung cancer cases occur in nonsmokers, mainly women. In the past, men had higher lung cancer incidence due to higher smoking rates. However, rates of smoking in women have increased, with a 600% increase in the death rate from lung cancer leading to a “full blown epidemic” as noted by the US Surgeon General [2]. Mortality from lung cancer in women now exceeds that from breast cancer.
Estrogen status appears to be a significant factor in lung cancer in women, with evidence that exogenous and endogenous estrogen may play a role in development of lung cancer, especially adenocarcinoma [2, 5, 6]. Women have naturally higher circulating estrogen levels than men that may increase their susceptibility to lung cancer. In addition, estrogen biosynthesis due to activity of aromatase is reported in lung, suggesting that estrogens are produced locally in women and men and could affect tumor development [4, 7]. Despite earlier conflicting reports on the presence of ER in lung [8], new work clearly shows that both ERα and ERβ mRNA and protein are expressed in malignant lung epithelial cells [4, 9–11]. Moreover, these receptors may play important biologic roles in lung and respond to antiestrogens. In lung tumor cells in the laboratory, estrogens stimulate cell proliferation and enhance tumor progression in vivo. Blockade of this pathway by competition for estrogen binding to ER is the basis of the therapeutic tamoxifen, a partial agonist that limits proliferative effects of estrogen in breast cancer. However, in the uterine endometrium, tamoxifen has more prominent agonist effects and promotes growth, and it appears to have similar effects in lung [9, 11]. In contrast, we and others expect that new agents, such as Faslodex, an antiestrogen that downregulates ER [12], and aromatase inhibitors [13], downregulators of local estrogen production in tissues, may have previously-unsuspected use to suppress lung cancer [4, 13].
Extra-nuclear and nuclear ER were postulated in early concepts of steroid interaction with target cells, such as breast and ovary [14], with the transcriptional activity of estrogen mediated by high-affinity ER in cell nuclei [15]. On estrogen binding in target cells, ER is phosphorylated and undergoes a conformational change that allows receptor dimerization and association of estrogen-ER complexes with specific estrogen response elements (ERE) in DNA, leading to transcription. Nuclear actions of estrogen are dependent, in part, on the subtype (ERα, ERß) of ER, the gene promoter, and the steroid receptor co-activator and co-repressor proteins that modulate transcription [15, 16]. In addition, ER also regulates gene expression without direct binding to DNA. This occurs by protein-protein interaction with other transcription factors, such as AP-1, and with extranuclear signaling complexes that, in turn, modulate downstream gene transcription. Extranuclear signaling, such as MAPK activation, has a rapid onset and is mediated by ER in or tethered to membrane. In lung, as in breast, extra-nuclear ER appear to derive from the same transcript as nuclear ER [4, 17–19]. Nuclear and extranuclear ER act in concert with growth factor signaling pathways, such as EGFR [20, 21], to promote growth and survival [4, 11, 22]. The EGFR family of receptors, including EGFR (HER-1) and HER-2, are also implicated in lung cancer pathogenesis [23, 24]. This receptor axis is associated with progression of malignancy, inhibition of apoptosis and angiogenesis. EGFR receptor antibodies or EGFR tyrosine kinase inhibitors elicit growth inhibition of lung tumors expressing these receptors. Moreover, EGFR/HER receptors regulate ligand-independent ER activation. Molecular details of interaction between ER and EGFR/HER are emerging, and ER is an important locus for signal convergence [4, 21, 22, 25]. In gene knockout mice lacking ERα, both estrogen- and EGF-stimulated growth in target tissue is blocked, while, in knockout mice lacking ERβ, disruption of normal lung development occurs [26]. Thus, ER may mediate transcription by integrating signals from growth factor pathways as well as from estrogen binding [27–30]. Although some reports suggest that extranuclear ER is an alternative protein [31, 32], most studies indicate that extranuclear and nuclear ER derive from the same transcript [17, 33–35]. Post-translational ER modification can elicit membrane targeting [36–38], and association of ER with other adaptor or signaling proteins (e.g. shc, c-src or MNAR) may occur [39–41].
2. Experimental
2.1. Cell culture
Human non-small cell lung cancer cells (NCI-H23 [H23], A549) and breast cancer cells (MCF-7 and SKBR3) were from ATCC. MCF-7 tumor cells with HER-2 overexpression (MCF-7/HER-2) were prepared as before [28]. Cell lines were routinely maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS, Invitrogen/Life Technologies, Carlsbad, CA), 2 mM L-glutamine and 1% Antibiotic-Antimycotic solution 100X, (Mediatech, Herndon, VA). For estrogen-free conditions, media were changed 48 h before experiments to phenol-red free RPMI 1640 with 0.1% dextran-coated, charcoal-treated (DCC) FBS [28].
2.2. Assay of ERα and ERβ by immunohistochemistry in human lung tumors
Patient specimens and information were collected under Institutional Review Board-approved and Health Insurance Portability and Accountability Act-compliant protocols at the University of California at Los Angeles Medical Center.
Formalin-fixed, paraffin-embedded tissue specimens were cut in 4-μm sections and placed on slides. Standard immunohistochemical (IHC) procedures were then followed for staining [4, 11, 42, 43]. In brief, sections were deparaffinazed in xylenes and hydrated in graded alcohols. Antigen recovery was done by incubating slides in 10 mM sodium citrate buffer (pH 6.0) in a heated water bath at 90°C for 30–45 min. To reduce non-specific background staining, slides were blocked with “Background SNIPER” (Biocare Medical, Concord, CA) for 10 min. Primary antibodies used included: ERα rabbit polyclonal antibody HC-20 (Santa Cruz Biotechnology, Santa Cruz, CA) and ER-β polyclonal antibody (Panvera/Invitrogen). Primary antibody detection was accomplished using the MACH 3 rabbit horseradish peroxidase (HRP) polymer kit (Biocare Medical). In order to visualize antibody-antigen complex, the DAB500 chromogen system from Biocare Medical was used.
2.3. Assay of phosphorylated ERα by immunohistochemistry
Standard IHC methods were followed as described above. Specific antibodies that detect only serine phosphorylated ERα were used [44]. Primary antibodies included anti-phosphoserine-118-ERα (S118) mouse monoclonal antibody 16J4 (Cell Signaling technology, cat#: 2511) [45] and anti-phosphoserine-167 ERα (S167) rabbit polyclonal antibody 31478 (Abcam, Cambridge, UK) [41]. Antigen recovery and antibody dilutions were standardized for both antibodies.
2.4. Suppression of ER expression by use of small inhibitory RNA (siRNA) and cell proliferation assays
NCI-H23 cells were transfected with vehicle alone (control), non-specific random siRNAs (Dharmacon, Lafayette, CO) or ESR1 and ESR2 siRNA smartpool (Dharmacon), directed to nuclear ERα and ERβ, respectively, using Lipofectamine RNAiMAX (Invitrogen). At 24 hrs after transfection, RT/PCR was performed to determine knockdown of both estrogen receptors. For proliferation assays, cells were transfected with lipofectamine alone, non-specific siRNA, ERα siRNA or ERβ siRNA. After 24 hours, cells were cultivated in vitro as before [19], with proliferation at 72 hrs after treatment determined by cell counts and compared with that of paired cells exposed only to vehicle control.
2.5. Total RNA isolation and RT-PCR
Total RNA was extracted from selected cells using TRIzol® Reagent (Invitrogen). Reverse transcription and RT-PCR was done with the SuperScript One-Step RT-PCR with Platinum Taq kit (Invitrogen). Briefly, 1 μg of total RNA was reverse-transcribed to first strand cDNA for 30 min at 55°C with SuperScript reverse transcriptase. PCR amplification of cDNA was performed using the following primers described by Mollerup et al. [3]: Primers for ERα were ERA1 (forward): 5’-AATTCAGATAATCGACGCCAG-3’ and ERA2 (reverse): 5’-GTGTTTCAACATTCTCCCTCCTC-3’ resulting in a PCR product of 345-bp, for ERβ, primers ERB1 (forward): 5’-TAGTGGTCCATCGCCAGTTAT-3’ and ERB2 (reverse): 5’-GGGAGCCACACTTCACCAT-3’ with an expected PCR product of 393 bp. For control of RNA integrity and relative abundance normalization, 36B4 protein [46]was amplified using primers 36B4-P3 (forward): 5’-TGTTTCATTGTGGGAGCAGA-3’ and 36B4-P4 (reverse): 5’-AAGGAGAAGGGGGAGATGTT-3’, with an expected product of 478-bp. PCR was performed with Platinum Taq polymerase (Invitrogen). Enzyme activation was done at 94°C for 2 min. Amplification was for 40 cycles with denaturation at 94 for 15 sec, annealing at 55 for 30 sec and extension at 68°C for 1 min. Followed by a final extension at 72°C for 10 min. Amplicons resolved on agarose gels were visualized by ethidium bromide staining.
2.6. Immunoprecipitation and Western Blots
Before each experiment, NCI-H23 cells were maintained in estrogen-free conditions for 24–48 h [19, 21]. After serum deprivation, cells were treated 6 hrs with 2 nM estradiol-17β, 2 nM Faslodex (fulvestrant; ICI 182,780; AstraZeneca), 1 nM epidermal growth factor (EGF) with or without 7.5 μM erlotinib (Tarceva; Genentech). Total lysates (1 mg) were incubated overnight at 4°C with 5 μl of anti-phosphoserine antibody (clone PSR-45, Sigma). The following day, immunoprecipitates were washed 5 times in mild lysis. Protein lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. Immunodetection was done with anti-human SRC-3/AIB1 antibody (Affinity Bioregents). Visualization was done with enhanced chemiluminescent agents from Amersham Biosciences.
Western Blots of NSCLC cells were done by running 50 μl of cell lysate as described above. Immnunodetection was done with anti-SRC-3 antibody (Affinity Bioregents) and MNAR/PELP-1 antibody (Bethyl Laboratories, Inc.).
2.7. Activation of p42/p44 MAP kinase in NSCLC cells in vitro
Phosphoryaltion of p42/p44 mitogen-activated protein kinase (MAPK) in NCI-H23 cells was determined as before [19, 28]. In brief, cells were grown in media with 0.1% DCC-FBS for 72 hrs followed by selected treatments. Thereafter, cell extracts were prepared and MAPK was immunoprecipitated with anti-p42/p44-MAPK antibody, with MAPK activity in immunoprecipitates assessed by ELISA [19, 28].
2.8. Growth of human NSCLC xenografts in nude mice in vivo
Ovariectomized nude mice at 6 weeks of age were obtained from Harlan Sparague-Dawley. (Indianapolis, IN). Mice were primed with extended-release pellets of estradiol-17β (Innovative Research of America, Sarasota, Florida). To determine antitumor effects of Faslodex (fulvestrant; AstraZeneca) and erlotinib (Tarceva; Genentech) alone and in combination, NCI-H23 cells were implanted as subcutaneous xenografts in the ovariectomized nude mice with estrogen supplement [19, 28]. When tumors grew to 50–75 cubic mm, mice were randomized to different treatment groups, including control, Faslodex (5 mg subcutaneous, weekly for 21 days), erlotinib (25 mg/kg by oral gavage, daily for 21 days) or a combination of Faslodex and erlotinib.
Tumor volumes for mice in experimental and control groups were measured every 3 to 4 days, with tumor volume calculated by (l × w × h), where l is tumor length, w is tumor with and h is tumor height in mm. Data are presented as the mean ± SEM for tumor volumes measured in cubic mm. Data were analyzed by use of student’s t-test and ANOVA statistical approaches as reported before [28].
3. Results
3.1. ERα and ERβ are both expressed in archival human NSCLC specimens from the clinic
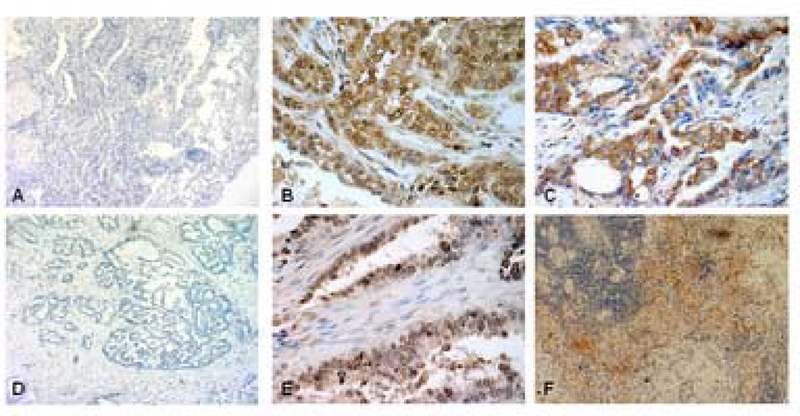
The prevalence of ERα and ERβ in human NSCLC specimens was assessed by standard IHC methods using archival formalin-fixed, paraffin-embedded human tumor specimens. Figure 1 presents representative examples of IHC staining patterns for ERα and ERβ. Appropriate tissue and reagent controls were done to confirm specificity [43]. For example, in the absence of antibodies for ERα or ERβ, no specific staining was observed (see Fig. 1A and 1D). Staining in tumor cell nuclei was observed for both ERα (Fig.1B) and ERβ (Fig.1E). Furthermore, NSCLC tumors also exhibited specific extranuclear staining for both ERα (Fig.1C) and ERβ (Fig1F).
Figure 1.
Immunohistochemical detection of ERα and ERβ proteins in archival human lung adenocarcinomas. Formalin-fixed paraffin-embedded tumors were processed for IHC using ERα Ab HC-20 (Santa Cruz) and Panvera/Invitrogen ERβ Ab. Panels A, B and C show control specimens with no addition of primary antibody for ERα, specific nuclear staining of ERα, and specific extranuclear staining of ERβ, respectively. Panels D,E and F display control specimens with no primary antibody for ERβ, specific nuclear staining of ERβ, and specific extranuclear staining of ERβ, respectively.
In total, we have examined 65 archival NSCLC specimens. As noted above, specific ER immunoreactivity commonly occurs in nuclear and extranuclear sites (see cellular distribution in Table 1). Among the NSCLC specimens assayed, we find that more than 45% and 52% had specific nuclear staining for ERα and ERβ, respectively. In addition, about 75% and 69% demonstrated specific extranuclear staining for ERα and ERβ, respectively (see Table 1).
Table 1.
Estrogen receptor distribution in archival non-small cell lung tumors*
| ER-alpha | ER-beta | |
|---|---|---|
| NUCLEAR | 45% | 52% |
| Female | 49% (22/45) | 51% (23/45) |
| Male | 35% (07/20) | 55% (11/20) |
| EXTRANUCLEAR | 75% | 69% |
| Female | 78% (35/45) | 64% (29/45) |
| Male | 70% (14/20) | 80% (16/20) |
Sixty five non-small cell lung tumors were studied as indicated in the Table. IHC staining as done for ERα and ERβ. Samples were scored for nuclear (cut off score ≥ 10% cell nuclei stain positive) and extra-nuclear (deposit of ≥ 10% extra-nuclear cell staining positive) deposits of specific staining.
3.2. Specific serine residues in ERα are phoshorylated in archival NSCLC specimens
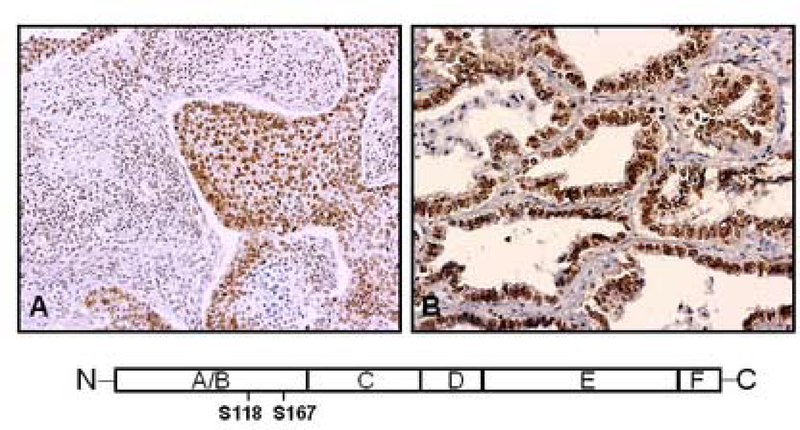
Phosphorylation of ERα at serine-118 and serine-167 residues may be mediated by enhanced tumor cell activity of MAPK and growth factors, such as EGF [4, 47]. Such events may prove important in understanding cross-communication among these signaling pathways. In IHC studies of archival NSCLC specimens that express ERα, we find significant phosphorylation of serine-118 (Fig. 2A) and serine-167 (Fig. 2B) residues in ERα, with such staining localized predominantly to tumor cell nuclei. We find phosphorylation of serine-167 in ERα in 14 of 16 (87.5%) ER-positive archival lung adenocarcinoma specimens studied to date. It is possible that the presence of phosphoserine-ER may correlate with activated growth factor signaling pathways and responses to treatment.
Figure 2.
Phosphorylated ERα is present in archival human NSCLC specimens. Formalin-fixed, paraffin-embedded NSCLC specimens with expression of ERα were prepared for IHC. Anti-phosphoserine-118 ER (A) and anti-phosphoserine-167 ER (B). Representative results are shown for two NSCLC specimens, with prominent staining observed in cell nuclei. The diagram below indicates the relative positions of serine-118 and serine-167 in the A/B domain near the N-terminal portion of ERα protein.
3.3. Steroid hormone receptor coactivators (SRC) are expressed and active in NSCLC cells
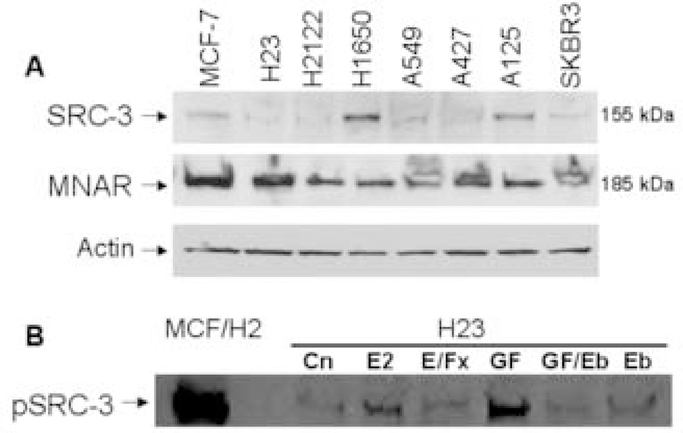
Nuclear receptor coactivators play an important role in modulating ER transcriptional activity [22, 41, 48], and human NSCLC cells are reported to express some of these molecules [5, 9, 11, 25]. In Fig. 3A, we show findings from a screen of 6 different human NSCLC cell lines and compare the expression of SRC-3 (AIB1) and MNAR/PELP1 with that found in 2 human breast cancer cell lines (MCF-7 and SKBR3). Expression of SRC-3 is variable among the several NSCLC cells, and significant levels of MNAR are present in all NSCLC cells evaluated. Since phosphorylation of SRC-3 by growth factor receptor-mediated signaling in breast tumors is associated with activation of transcription [48], we assessed the effects of estradiol and EGF on serine phosphorylation of SRC-3 in NCI-H23 NSCLC cells. As shown in Fig. 3B, both estrogen and EGF promote SRC-3 phosphoryaltion. Moreover, the antiestrogen, Faslodex, and the EGFR kinase inhibitor, erlotinib, counteract these actions. Thus, selected NSCLC cells appear to be equipped not only with ER but also with SRC proteins to amplify and modulate the biologic response to estradiol and possibly to growth factor receptor signaling.
Figure 3.
Steroid receptor coactivator proteins in archival human NSCLC specimens. A) Steroid receptor coactivators are expressed and active in NSCLC cells. We assessed the presence of SRC-3 (AIB1) and MNAR/PELP1 with specific antibodies by Western Blot. Levels of staining in lung cells are compared with those in MCF-7 and SKBR3 breast cells. Actin loading controls show a relatively equal distribution of protein among the samples. B) Estrogen and EGF induce rapid serine phosphorylation of nuclear receptor coactivator SRC-3/AIB1. NCI-H23 NSCLC cells were serum-depleted and then treated with estradiol-17β (E2) alone or with Faslodex (E/Fx); or with epidermal growth factor (GF), erlotinib (Eb) or both agents (GF/Eb). Lysates were immunoprecipitated with anti-phosphoserine antibody as described in methods. Immunoblotting of the resulting immunoprecipitates was performed with anti-SRC-3 (AIB1) antibody. MCF-7 breast cancer cells with overexpression of HER-2 receptors (MCF/H2) are known to express phosphorylated SRC-3 and represent positive controls.
3.4. Suppression of ERα and ERβ protein expression reduces proliferation in NSCLC cells
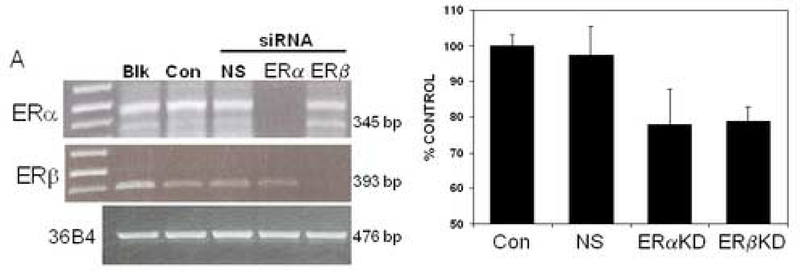
To examine the biologic activity of ERα and ERβ in NSCLC cells, we used short interfering RNA (siRNA) expression vectors to down-regulate expression of these proteins (Fig. 4A). Treatment of NSCLC cells with siRNA directed to ERα and ERβ effectively reduced mRNA expression of ERα and ERβ, respectively, whereas nonspecific siRNA treatment had no effect on the amounts of ERα or ERβ detected (see Fig.4A). Of note, expression levels of the control protein, 36B4, did not change with these different treatments (Fig.4A).
Figure 4.
Specific siRNAs suppress ERα and ERβ expression and also elicit reduced proliferation of NCI-H23 NSCLC cells. NCI-H23 cells were transfected with vehicle alone (Blk), lipofectamine alone (Con) or non-specific (NS) small inhibitory RNAs (siRNA). For ERα and ERβ silencing, smartpool ESR1 siRNA (ERα) and ESR2 (ERβ) siRNA were used, respectively. A) In the left panel, after 24 hrs, total RNA was isolated and RT/PCR was performed. The expected ER mRNA transcripts of 345-bp (ERα) and 393 bp (ERβ) were present in controls and NS-transfected cells. ERα and ERβ transcripts were significantly reduced in those cells transfected with active ER-siRNA. Corresponding amplification of control 36B4 mRNA was used for semiquantitative analysis of RNA. B) The right panel shows cell proliferation after knockdowns of estrogen receptors. Cells were transfetected with lipofectamine control only (CON), non-specific siRNA (NS), ERα siRNA (ERα KD) or ERβ si RNA (ERβ KD). Cells were then counted 96 hours after trasnsfection as before [4]. Results are presented as percent of vehicle-treated control cell proliferation. Treatment of cells with specific siRNAs for ERα and ERβ elicit a significant reduction in cell growth as compared to controls, with P<0.05.
Cell proliferation in vitro was then assessed after transfection of NSCLC cells with lipofectamine control alone, control siRNA or specific siRNA for knockdowns of ERα or ERβ. In addition, paired cells exposed only to vehicle were cultivated in parallel as an additional control. After transfection, lipofectamine and non-specific siRNA control cells exhibited significant levels of cell proliferation equal to that of vehicle controls (Fig. 4B). However, as shown in Fig. 4B, NSCLC cell proliferation was significantly reduced by suppressing expression of either ERα or ERβ, as compared with that of control-transfected NSCLC cells. These findings suggest that ERα and ERβ are both important to sustain NSCLC proliferation in vitro.
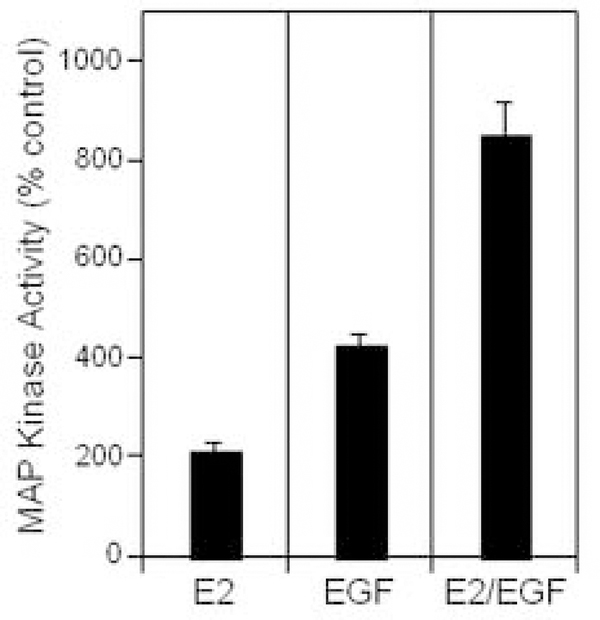
3.5. Estradiol and EGF induce early activation of MAPK in human NSCLC cells
The p42/p44 MAPK cascade serves an important role in regulating proliferation in lung cells. This signaling pathway is initiated at the membrane and terminates in the nucleus, resulting in phosphorylation of nuclear transcription factors. We tested acute effects and interactions of estrogen and EGF on MAPK phosphorylation (Fig. 5). The data show that both estrogen and EGF rapidly activate MAPK, with combination therapy eliciting supra-additive increases in MAPK stimulation. The mechanism of the dual effect may involve transactivation of EGFR by ER [35] or EGFR-independent mechanisms. Our data confirm other reports of ER-EGFR cross-talk occurring rapidly after either estrogen or growth factor stimulation of cells [4, 9, 11, 21]. These experiments offer evidence that ER and EGFR signaling may cooperate in modulating important cell functions, such as regulation of MAPK. EGF-mediated effects on ER-dependent, but E2-independent, transcription are also reported in other target tissues [20, 49, 50].
Figure 5.
Rapid activation of p42/p44 MAP kinase by estradiol (E2) and EGF in NCI-H23 lung tumor cells. Cells were grown in media with 0.1% DCC-treated serum for 72 h followed by treatment with control, EGF (10 ng/ml), estradiol (10 nM) or both agents for 10 min. Cell extracts were prepared, and MAPK was immunoprecipitated using anti-p42/p44 MAPK antibody, with MAP activities in immunoprecipitates assayed by ELISA [19, 28]. Results are expressed as % control (n=3).
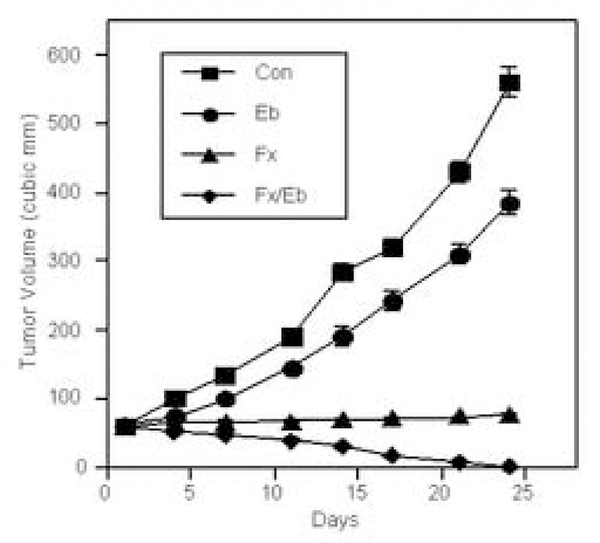
3.6. Combination therapy with Faslodex and erlotinib elicits marked blockade of the growth of human NSCLC xenografts in vivo
In view of the cooperative interactions between ER and EGFR noted in studies above, we used specific inhibitors of ER and EGFR signaling to block these pathways simultaneously in order to determine if this intervention (e.g. tandem drug therapy) would improve antitumor efficacy. Thus, Faslodex, a pure antiestrogen, and erlotinib, an EGFR tyrosine kinase inhibitor, were tested as single and dual therapies. Thus, studies with another well-characterized NSCLC cell line, A549, were conducted. Human A549 tumor xenografts were grown in vivo with or without Faslodex, erlotinib or both agents (see Fig. 6). The results indicate that combined therapy with Faslodex and erlotinib is superior to treatment with either drug administered alone (P<0.001). These in vivo studies provide a rationale for further evaluation of this novel strategy for NSCLC using an ER downregulator with an EGFR tyrosine kinase inhibitor.
Figure 6.
Faslodex and erlotinib block growth of lung tumor xenografts in vivo. A549 lung tumor cells were implanted as subcutaneous xenografts in ovariectomized nude mice with estrogen supplement [19, 28]. When tumors grew to 50–75 cubic mm, mice were randomized to different treatment groups, including control, Faslodex (a pure antiestrogen) (5 mg SQ weekly to 21 days), erlotinib (Tarceva, an EGFR TKI) (25 mg/kg by oral gavage daily for 21 days) or a combination of Faslodex and erlotinib. Data are presented as the mean ± SEM for tumor volumes measured in cubic mm. Tumor volumes of mice in the combination arm were significantly different from control, Faslodex and erlotinib (all at P<0.001, ANOVA). In preliminary studies, mice with NSCLC xenografts were treated with Faslodex at two different doses, 1 or 5 mg SQ weekly; by day 21, tumors showed modest regression after low-dose Faslodex (1 mg), but more profound inhibition occurred with high-dose Faslodex therapy (5 mg), the dose used in experiments above.
4.0. Discussion
Data from the present study confirm earlier work showing that estrogen as well as growth factors promote the progression of human NSCLC [4, 9–11]. Although previously not recognized, it is now known that estradiol promotes the growth of NSCLC [4, 11]. NSCLC cells harbor a complete estrogen signaling system, including estrogen receptors, estrogen-responsive elements in DNA and steroid hormone receptor coactivators. Using controlled homogenization and quantitative subcellular fractionation, a method to promote purity and low contamination of cell fractions [4, 19, 51–53], we reported previously that specific estrogen binding is enriched predominantly in lung tumor cell nuclei [14]. However, significant binding also occurs in plasma membrane fractions, with specific estrogen binding in membranes enriched to 25-times whole homogenate levels, accounting for about 20% of total cell estrogen binding [35]. Estrogen binding in lung tumor cell membranes is saturable, ligand-specific and high-affinity, indicating characteristics of a true estrogen receptor [4, 19]. These findings are consistent with earlier work showing high-affinity estrogen binding in rat lung [54], as well as a new report that confirms expression of a high-affinity, limited capacity ER in human NSCLC cells [55]. Further, both ERα and ERβ, as well as aromatase (a key enzyme for biosynthesis of estrogen), occur in a large percentage of clinical NSCLC specimens [56, 57]. Both ER forms occur in tumor cell nuclei and also in extranuclear sites, where they concentrate in caveolae or lipid rafts, regions highly enriched with critical signaling molecules including EGFR and HER-2 [4]. Further, we find significant cross-communication between growth factor and estrogen signaling pathways, so that the interaction between these pathways may offer a potent therapeutic target in NSCLC. Data from the clinic also show that combined overexpression of EGFR and ERα correlate with poor outcome in patients with lung cancer [42], a finding consistent with this hypothesis.
There is convincing evidence that extranuclear and nuclear ER in target cells derive from the same transcript [17, 34, 46], but how can nuclear receptors localize to extra-nuclear sites? Post-translational modification of ER may provide an answer [4, 36–38]. ERα has 2 cysteines, Cys-447 and −530, in the ligand-binding domain (LBD) accessible for S-acylation, allowing linkage of palmitate to the S-atom of cysteine. This type of modification accounts for membrane association of other signaling molecules (e.g. the amino acid sequence surrounding Cys447 in ERα has homology with that of S-palmitoylated Cys133 of caveolin-1 [36, 37]. ERα is palmitoylated in vivo [4, 37, 38], and point mutation of Cys447 in ERα blocks palmitoylation [4, 37]. Although ER mutated at Cys447 retains estradiol binding affinity similar to that of wild-type ERα, the mutant ER does not localize to plasma membrane and is markedly deficient in promoting estrogeninduced activation of MAPK and transcription [4, 38, 58]. Further, recent experiments suggest that palmitoylation is associated with promotion of proliferation in NSCLC cells. Treatment with the palmitoyltransferase inhibitor, 2-bromohexadecanoic acid [37], before estrogen elicits significant suppression of lung tumor cell growth as compared with controls [4]. Other data also indicate that ER-Cys447 modification is important for ER action [4, 37, 38]. Of note, the ERβ LBD has amino acid sequence with conserved cysteine residues homologous to that in ERα, and this may be a common feature allowing membrane localizatioñ.
If biologic interactions between ER and EGFR/HER promote lung cancer growth, this signaling axis could offer a new target to treat NSCLC [4, 5, 11, 25, 37, 38]. A link between EGFR and steroid receptors has also been suggested before. Activation of EGFR was first associated with tumorigenesis with discovery that EGFR gene is a retroviral oncogene of the erythroblastosis virus (v-erb B) [59]. Further, the v-erb A gene, derived from a nuclear hormone receptor gene, is contained in the same virus. Erb B is the dominant transforming activity, and erb A provides a block in differentiation [59]. Co-selection of these genes, erb A and erb B, by the transforming retrovirus raises the possibility that this combination is important for cell transformation, suggesting functional links between the nuclear receptor family (erb A) and transforming activity of the erb B family (EGFR, HER-2). In view of it’s known oncogenic role in human breast cancer, ER, a member of the erb A gene family, is a prime suspect for a nuclear receptor cooperating with EGFR activation in NSCLC [60].

Another way to alter estrogen signaling in a target tissue is via estrogen biosynthesis. Aromatase is a cytochrome P-450 enzyme complex found in many tissues (e.g. breast and ovary) [13]. It mediates the final, rate-limiting step in estrogen synthesis, catalyzing conversion of androstenedione and testosterone to estrone and estradiol, respectively. Estrogen levels are reportedly elevated in female lung cancer patients as compared to women of similar age without lung cancer [5, 61]. Aromatase is also reported in lung, suggesting that estrogens produced locally in women and men may affect lung tumor development. If this is true, aromatase inhibitors may also have utility to block estrogen synthesis and consequent estrogen-dependent activation of ER in lung cancer (see Weinberg et al) [57]. The potential for using these agents to treat lung tumors remains to be determined.
In recent clinical trials of endocrine treatment, hormone replacement therapy was reported to associate with reduced survival in women with lung cancer [5, 62]. In another pertinent trial, patients with primary breast cancer were randomized after 2–3 years of adjuvant tamoxifen to sequential treatment with aromatase inhibitor or continuation of tamoxifen. After several years, those assigned to aromatase inhibitor showed a trend to reduced incidence of lung cancer as compared to those on continued tamoxifen [63], suggesting that lung is responsive to selected endocrine agents. In earlier work, it was found that tamoxifen was not an effective antitumor drug in lung cancer, since it promotes lung cell growth as in the uterus [5, 9, 11, 25]. Further, preclinical data showing enhanced antitumor effects by combined therapy with antagonists of ER and EGFR in NSCLC led to a Phase I study with 22 patients to assess the safety and tolerability of gefitinib, an EGFR kinase inhibitor, with Faslodex in postmenopausal women. Trial data show the drug tandem is well-tolerated and active in advanced lung cancer, with final data pending [64]. Results of the present studies with combination drug therapies that simultaneously block estrogen and EGFR signaling may help to advance more clinical-translational initiatives in patients afflicted with NSCLC.
Acknowledgments
We would like to thank Dr. Hermes J. Garbán for his support in the preparation of this manuscript. Research supported by funding from NCI Lung Cancer SPORE Program at UCLA (P50 CA90388), Hamburger Fund of the Jonsson Cancer Center and Stiles Program in Integrative Oncology (in vitro work).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bunn PA Jr., Early-stage non-small-cell lung cancer: current perspectives in combined-modality therapy. Clin Lung Cancer, 2004. 6(2): p. 85–98. [DOI] [PubMed] [Google Scholar]
- 2.Patel JD, Bach PB, and Kris MG, Lung cancer in US women: a contemporary epidemic. Jama, 2004. 291(14): p. 1763–8. [DOI] [PubMed] [Google Scholar]
- 3.Mollerup S, et al. , Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer, 2002. 37(2): p. 153–9. [DOI] [PubMed] [Google Scholar]
- 4.Pietras RJ, et al. , Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids, 2005. 70(5–7): p. 372–81. [DOI] [PubMed] [Google Scholar]
- 5.Stabile LP and Siegfried JM, Estrogen receptor pathways in lung cancer. Curr Oncol Rep, 2004. 6(4): p. 259–67. [DOI] [PubMed] [Google Scholar]
- 6.Taioli E and Wynder EL, Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst, 1994. 86(11): p. 869–70. [DOI] [PubMed] [Google Scholar]
- 7.Pezzi V, et al. , Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol, 2003. 87(2–3): p. 181–9. [DOI] [PubMed] [Google Scholar]
- 8.Beattie CW, Hansen NW, and Thomas PA, Steroid receptors in human lung cancer. Cancer Res, 1985. 45(9): p. 4206–14. [PubMed] [Google Scholar]
- 9.Hershberger PA, et al. , Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res, 2005. 65(4): p. 1598–605. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch EA, et al. , Estrogen acutely stimulates endothelial nitric oxide synthase in H441 human airway epithelial cells. Am J Respir Cell Mol Biol, 1999. 20(4): p. 658–66. [DOI] [PubMed] [Google Scholar]
- 11.Stabile LP, et al. , Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res, 2002. 62(7): p. 2141–50. [PubMed] [Google Scholar]
- 12.Howell A, et al. , ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer, 2000. 89(4): p. 817–25. [DOI] [PubMed] [Google Scholar]
- 13.Brodie A, Aromatase inhibitor development and hormone therapy: a perspective. Semin Oncol, 2003. 30(4 Suppl 14): p. 12–22. [DOI] [PubMed] [Google Scholar]
- 14.Jensen EV and DeSombre ER, Estrogen-receptor interaction. Science, 1973. 182(108): p. 126–34. [DOI] [PubMed] [Google Scholar]
- 15.Evans RM, The steroid and thyroid hormone receptor superfamily. Science, 1988. 240(4854): p. 889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzenellenbogen BS and Frasor J, Therapeutic targeting in the estrogen receptor hormonal pathway. Semin Oncol, 2004. 31(1 Suppl 3): p. 28–38. [DOI] [PubMed] [Google Scholar]
- 17.Razandi M, et al. , Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol, 1999. 13(2): p. 307–19. [DOI] [PubMed] [Google Scholar]
- 18.Norfleet AM, et al. , Antibodies to the estrogen receptor-alpha modulate rapid prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. Faseb J, 2000. 14(1): p. 157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marquez DC and Pietras RJ, Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene, 2001. 20(39): p. 5420–30. [DOI] [PubMed] [Google Scholar]
- 20.Curtis SW, et al. , Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci U S A, 1996. 93(22): p. 12626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez DC, et al. , Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine, 2001. 16(2): p. 73–81. [DOI] [PubMed] [Google Scholar]
- 22.Gruber CJ, et al. , Production and actions of estrogens. N Engl J Med, 2002. 346(5): p. 340–52. [DOI] [PubMed] [Google Scholar]
- 23.Lynch TJ, et al. , Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med, 2004. 350(21): p. 2129–39. [DOI] [PubMed] [Google Scholar]
- 24.Paez JG, et al. , EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science, 2004. 304(5676): p. 1497–500. [DOI] [PubMed] [Google Scholar]
- 25.Stabile LP, et al. , Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res, 2005. 65(4): p. 1459–70. [DOI] [PubMed] [Google Scholar]
- 26.Patrone C, et al. , Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol, 2003. 23(23): p. 8542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietras RJ, Chen H-W, Tsai E. Therapeutic targeting of growth factor and estrogen receptor signaling pathways in human non-small cell lung cancer. in Proc Lung Cancer SPORE Investigator’s Meeting. 2004. [Google Scholar]
- 28.Pietras RJ, et al. , HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene, 1995. 10(12): p. 2435–46. [PubMed] [Google Scholar]
- 29.Pietras RJ, Biologic basis of sequential and combination therapies for hormone-responsive breast cancer. Oncologist, 2006. 11(7): p. 704–17. [DOI] [PubMed] [Google Scholar]
- 30.Pietras RJ, Interactions between estrogen and growth factor receptors in human breast cancers and the tumor-associated vasculature. Breast J, 2003. 9(5): p. 361–73. [DOI] [PubMed] [Google Scholar]
- 31.Filardo EJ, et al. , Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol, 2002. 16(1): p. 70–84. [DOI] [PubMed] [Google Scholar]
- 32.Thomas P, et al. , Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology, 2005. 146(2): p. 624–32. [DOI] [PubMed] [Google Scholar]
- 33.Pietras RJ, et al. , Improved antitumor therapy with Herceptin and Faslodex for dual targeting of HER-2 and estrogen receptor signaling pathways in human breast cancers with overexpression of HER-2/neu gene. Breast Cancer Res treatment, 2003. 82(Suppl 1): p. 12–13. [Google Scholar]
- 34.Watson CS and Gametchu B, Membrane-initiated steroid actions and the proteins that mediate them. Proc Soc Exp Biol Med, 1999. 220(1): p. 9–19. [DOI] [PubMed] [Google Scholar]
- 35.Levin ER, Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol, 2005. 19(8): p. 1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acconcia F, et al. , S-palmitoylation modulates human estrogen receptor-alpha functions. Biochem Biophys Res Commun, 2004. 316(3): p. 878–83. [DOI] [PubMed] [Google Scholar]
- 37.Acconcia F, et al. , Does palmitoylation target estrogen receptors to plasma membrane caveolae? IUBMB Life, 2003. 55(1): p. 33–5. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Haynes MP, and Bender JR, Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A, 2003. 100(8): p. 4807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song RX, et al. , The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A, 2004. 101(7): p. 2076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietras RJ, Nemere I, and Szego CM, Steroid hormone receptors in target cell membranes. Endocrine, 2001. 14(3): p. 417–27. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita H, et al. , Phosphorylation of estrogen receptor alpha serine 167 is predictive of response to endocrine therapy and increases postrelapse survival in metastatic breast cancer. Breast Cancer Res, 2005. 7(5): p. R753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai H, et al. , Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res, 2005. 11(14): p. 5084–9. [DOI] [PubMed] [Google Scholar]
- 43.Press M, et al. , Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids, 2002. 67(9): p. 799–813. [DOI] [PubMed] [Google Scholar]
- 44.Al-Dhaheri MH and Rowan BG, Application of phosphorylation site-specific antibodies to measure nuclear receptor signaling: characterization of novel phosphoantibodies for estrogen receptor alpha. Nucl Recept Signal, 2006. 4: p. e007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell RA, et al. , Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem, 2001. 276(13): p. 9817–24. [DOI] [PubMed] [Google Scholar]
- 46.Marquez DC, et al. , Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol, 2006. 246(1–2): p. 91–100. [DOI] [PubMed] [Google Scholar]
- 47.Kato S, et al. , Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science, 1995. 270(5241): p. 1491–4. [DOI] [PubMed] [Google Scholar]
- 48.Font de Mora J and Brown M, AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol, 2000. 20(14): p. 5041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Improta-Brears T, et al. , Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc Natl Acad Sci U S A, 1999. 96(8): p. 4686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ignar-Trowbridge DM, et al. , Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A, 1992. 89(10): p. 4658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pietras RJ and Szego CM, Estrogen receptors in uterine plasma membrane. J Steroid Biochem, 1979. 11(4): p. 1471–83. [DOI] [PubMed] [Google Scholar]
- 52.Pietras RJ and Szego CM, Partial purification and characterization of oestrogen receptors in subfractions of hepatocyte plasma membranes. Biochem J, 1980. 191(3): p. 743–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pietras RJ and Szego CM, Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature, 1977. 265(5589): p. 69–72. [DOI] [PubMed] [Google Scholar]
- 54.Morishige WK and Uetake CA, Receptors for androgen and estrogen in the rat lung. Endocrinology, 1978. 102(6): p. 1827–37. [DOI] [PubMed] [Google Scholar]
- 55.Dougherty SM, et al. , Gender difference in the activity but not expression of estrogen receptors alpha and beta in human lung adenocarcinoma cells. Endocr Relat Cancer, 2006. 13(1): p. 113–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubey S, Siegfried JM, and Traynor AM, Non-small-cell lung cancer and breast carcinoma: chemotherapy and beyond. Lancet Oncol, 2006. 7(5): p. 416–24. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg OK, et al. , Aromatase inhibitors in human lung cancer therapy. Cancer Res, 2005. 65(24): p. 11287–91. [DOI] [PubMed] [Google Scholar]
- 58.Reese JC and Katzenellenbogen BS, Mutagenesis of cysteines in the hormone binding domain of the human estrogen receptor. Alterations in binding and transcriptional activation by covalently and reversibly attaching ligands. J Biol Chem, 1991. 266(17): p. 10880–7. [PubMed] [Google Scholar]
- 59.Beug H and Graf T, Co-operation between viral oncogenes in avian erythroid and myeloid leukaemia. Eur J Clin Invest, 1989. 19(6): p. 491–502. [DOI] [PubMed] [Google Scholar]
- 60.Sellers WR and Meyerson M, EGFR gene mutations: a call for global x global views of cancer. J Natl Cancer Inst, 2005. 97(5): p. 326–8. [DOI] [PubMed] [Google Scholar]
- 61.Tiuriunova AM, C.E., Mironenko TV, et al. , Hormonal balance in women with lung cancer and its changes after combined treatment. Vopr Onkol, 1986. 32: p. 2–30. [PubMed] [Google Scholar]
- 62.Ganti AK, et al. , Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol, 2006. 24(1): p. 59–63. [DOI] [PubMed] [Google Scholar]
- 63.Coombes RC, et al. , A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med, 2004. 350(11): p. 1081–92. [DOI] [PubMed] [Google Scholar]
- 64.Traynor AM, Schiller J, Stabile l, Kolesar J, Belani C, Hoang T, Dubey S, Eickhoff J, Marcotte S and Siegfried J, Combination therapy with gefitinib and fulvestrant (G/F) for women with non-small cell lung cancer (NSCLC). Proc. ASCO, 2005. 23: p. 676s. [Google Scholar]