Abstract
The role of malformed or dilated branches of iliac vessels in causing pelvic pain is not well understood. Such vessels may entrap nerves of the lumbosacral (LS) plexus against the pelvic sidewalls, producing symptoms not typically encountered in gynecological practice, including sciatica and refractory urinary and/or anorectal dysfunction. We describe cases of sciatica in which laparoscopy revealed compression of the LS plexus by variant superior gluteal veins (SGVs). In demonstrating an improvement in patient symptoms after decompression, we identify this neurovascular conflict as a potential intrapelvic cause of sciatica. This study is a retrospective case series (Canadian Task Force Classification II-3). Nerve decompression laparoscopies were performed in São Paulo, Brazil. Thirteen female patients undergoing laparoscopy for sciatica with no clear spinal or musculoskeletal causes were included in this study. In all cases, we identified LS entrapment by aberrant SGVs, and performed decompression by vessel ligation. The average preoperative visual analog scale score of 9.62 ± 0.77 decreased significantly to 2.54 ± 2.88 post-operatively (P < 0.001). The success rate (defined as ≥ 50% improvement in visual analog scale score) was 92.3%, over a follow-up of 13.2 ± 10.6 months. Our case series demonstrates a high success rate and significant decrease in pain scores after laparoscopic intrapelvic decompression, thereby identifying pelvic nerve entrapment by aberrant SGVs as a potential yet previously unrecognized cause of sciatica. This intrapelvic neurovascular conflict—the SGV syndrome—should be considered in cases of sciatica with no identifiable spinal or musculoskeletal etiology.
INTRODUCTION
Pelvic congestion syndrome is a well-known cause of cyclic pelvic pain. Patients commonly present with pelvic pain without any evidence of inflammatory disease. The pain is worse during the premenstrual period and pregnancy, and can be exacerbated by fatigue and standing [1–4]. Dilated ovarian veins and subsequent pelvic varicosities identified in symptomatic patients are believed to be the underlying structural etiology in this multifactorial syndrome [1, 5].
However, what is far less recognized is the fact that dilated or variant branches of the internal or external iliac vessels can entrap the nerves of the lumbosacral (LS) plexus against the pelvic sidewalls. This produces symptoms largely unfamiliar to a gynecologic practice, such as sciatica and refractory urinary and/or anorectal dysfunction [3, 4].
In this case series, we describe 13 patients presenting with sciatica in the absence of any clear spinal or musculoskeletal lesions. Laparoscopy revealed compression of the LS nerve roots by aberrant superior gluteal veins (SGVs) (Fig. 1). Surgical decompression by SGV ligation resulted in symptomatic improvement, thereby identifying a previously unrecognized neurovascular conflict as a potential intrapelvic cause of sciatica—an entity we describe as the SGV syndrome.
Fig. 1.
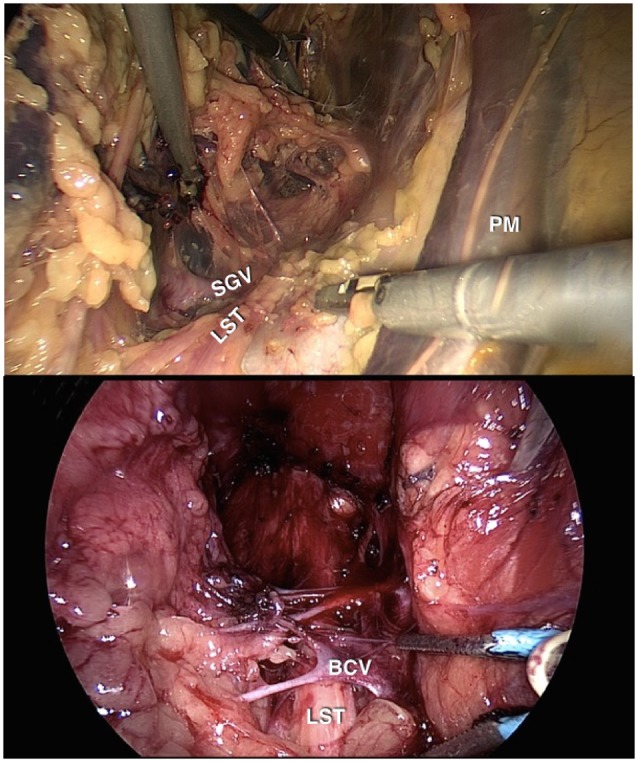
Normal SGV and variant SGV anatomy. Left: Normal SGV enters the pelvis in between the LST (LS trunk) and underlying piriformis muscle. Right: Variant SGV (BCV) enters the pelvis anterior to the LST, entrapping it against the underlying piriformis muscle.
MATERIALS AND METHODS
Retrospective observational data of 13 consecutive female patients with sciatica who underwent laparoscopic decompression of the sacral plexus due to entrapment by the SGV were collected. Patients belonged to the Pelvic Neurodysfunction Clinic or the private practice of NL in São Paulo, Brazil, and were operated between 2012 and 2016.
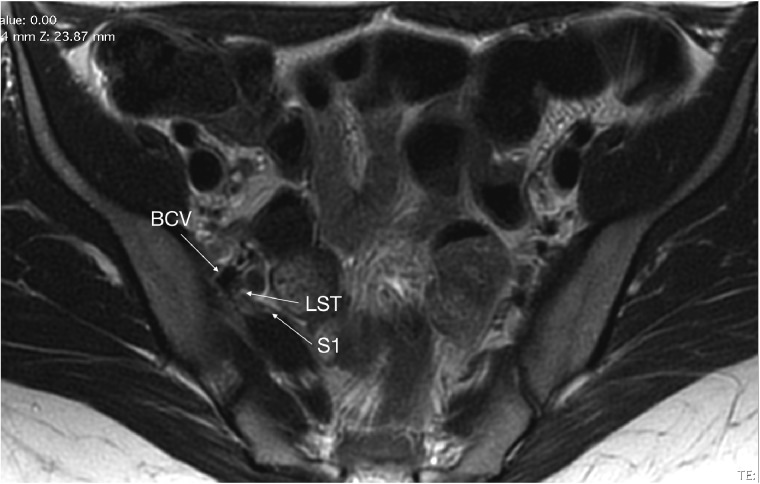
Patients were selected for laparoscopic intervention based on clinical neuropelveological and urodynamic assessment, which mapped the topography of the nerve entrapment at an intrapelvic level. The neuropelveological work up included detailed dermatome mapping with response to sharp and light touch, as well as the assessment of sacral nerve reflexes. Urodynamics was used to differentiate entrapments of the proximal portion of the nerve roots (which cause detrusor overactivity) from distal entrapments (which cause urgency due to hypersensitivity). All patients had previously failed conservative management for sciatica including pharmacotherapy and physiotherapy. Underlying spinal or musculoskeletal lesions were ruled out by orthopedic, neurosurgical and radiological evaluation. Two patients also had LS plexus entrapment by their SGVs visualized by pelvic MR neurography (Fig. 2). Pre-operative assessment included a visual analog scale (VAS) score for pain.
Fig. 2.
MRI of variant SGV. Variant SGV (BCV) compressing LST (LS trunk) and S1 nerve roots.
Written informed consent for the proposed procedure and authorization of case data and images for research and educational purposes was obtained from each patient before surgery.
All surgeries were performed by the same surgeon. Intrapelvic exploration of the iliolumbar and obturator fossae and isolation of the LS plexus was performed via laparoscopic retroperitoneal dissection. Variant SGV branches were defined as those superior to and therefore compressing LS nerve roots against the piriformis muscle and/or the pelvic brim. These variant veins were sealed using bipolar energy and transected, thereby detrapping the underlying nerves (Fig. 3).
Fig. 3.
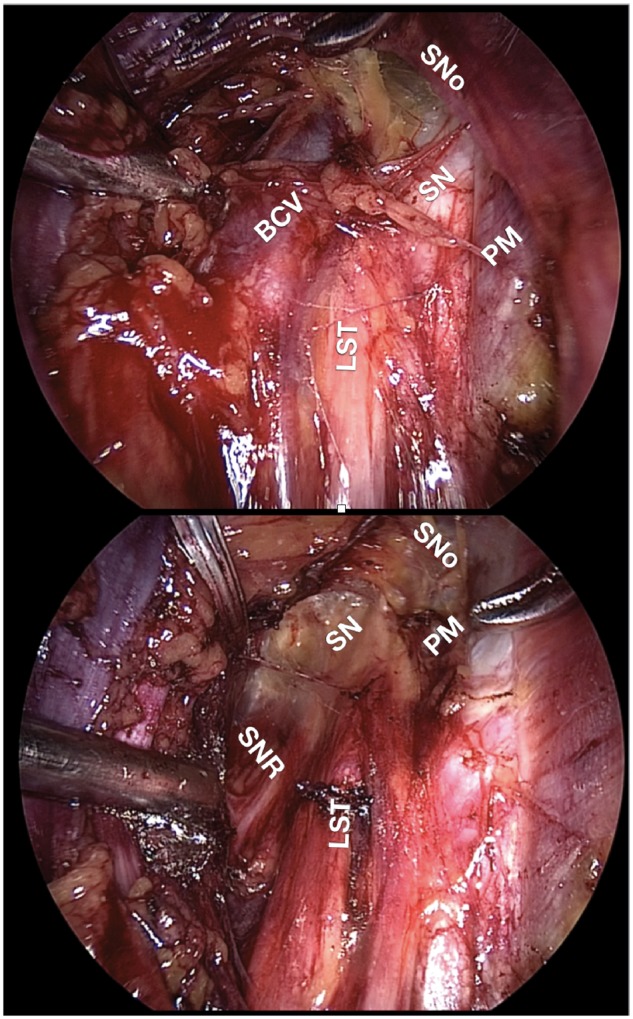
Intra-operative findings before and after decompression. Top: Variant SGV (BCV) compressing LST, LS trunk; SN, sciatic nerve. Bottom: LST, SN and SNR (sacral nerve roots) visible after variant SGV ligation. SNo, sciatic notch; PM, psoas muscle.
Patients were followed post-operatively with clinical assessment of symptoms. A post-operative VAS score was repeated at each follow-up visit and the VAS score from the final follow-up interview was used to determine improvement from pre-operative scores. Any new motor deficits and adverse symptoms after surgery were assessed qualitatively. The duration of post-operative pain flare was calculated based on changes in reported symptoms and comparison of serial VAS scores.
Descriptive statistics were used to determine central tendency (as both mean and median values) and variability of data collected. A paired t-test was used to compare pre- and post-operative VAS scores. Success after surgery was defined as a 50% or more improvement in VAS scores. A P value of <0.05 was regarded as statistically significant. All statistical analyses were performed using Microsoft Excel.
RESULTS
Among our 13 cases, the average age was 35.9 ± 7.36 years. The average time from onset of symptoms to diagnosis was 3.88 ± 3.09 years, and most patients had at least one previous surgery (Table I).
Table I.
Pre-operative patient characteristics
| Patient characteristics | Mean | Median | SD |
|---|---|---|---|
| Age | 35.93 | 35.22 | ±7.36 |
| Previous surgeries | 0.85 | 1.00 | ±0.80 |
| Interval between onset of symptoms and diagnosis (years) | 3.88 | 3.00 | ±3.09 |
| Pre-operative VAS score | 9.62 | 10.00 | ±0.77 |
All cases had a variant SGV that was ligated intraoperatively. One patient also had a variant superior gluteal artery which was also ligated. The mean operative time was 144.54 ± 55.10 min.
Average pre-operative VAS score was 9.62 ± 0.77, which decreased significantly to 2.54 ± 2.88 post-operatively (P < 0.001). A total of 12 (92.3%) patients had a 50% or more improvement in VAS scores (Table II). Patients reported post-decompression pain for a mean duration of 5.67 ± 3.51 months after surgery, as determined on review of serial post-operative VAS scores. Post-operative motor deficits were reported transiently for an average duration of 2.67 ± 0.58 months. No patients had any persistent motor deficits or new symptoms at their last follow-up visit. Patients were followed for an average of 13.2 ± 10.6 months after surgery.
Table II.
Post-operative results after laparoscopic decompression
| Post-operative results | Mean | Median | SD | P value |
|---|---|---|---|---|
| Operative time (min) | 144.54 | 124.00 | ±55.10 | |
| Pre-operative VAS score | 9.62 | 10.00 | ±0.77 | * |
| Post-operative VAS score | 2.54 | 2.00 | ±2.88 | <0.001 |
| Post-decompression pain duration (months) | 5.67 | 6.00 | ±3.51 | |
| Post-decompression motoric deficit duration (months) | 2.67 | 3.00 | ±0.58 | |
| Post-decompression motor deficit rate | 30.8% | |||
| Post-decompression pain rate | 84.6% | |||
| Success rate | 92.3% |
DISCUSSION
Extraspinal causes of sciatica and radicular pain remain challenges for diagnosis and treatment. Within this heterogeneous population, sciatic nerve entrapment presents with radicular pain of the lower back, buttock and/or hip, pain with sitting, and paresthesias of the affected leg [6]. Sciatic neuropathies can occur at any anatomical level along the path of the sciatic nerve, broadly classified as proximal (central) or distal (peripheral) to the gluteal region. Central causes include spinal and intrapelvic lesions, whereas peripheral causes often lie within the deep gluteal space [6, 7]. Pinpointing the level of entrapment is essential for directing management. This begins with a detailed physical exam including dermatomal mapping of pain and paresthesias, assessment of reflexes and passive and active muscle contraction tests. A neurology consultation and/or MRI may be necessary to exclude spinal causes, such as lumbar disc disease [6].
Outside of the pelvis, local trauma, pelvic and/hip fractures and surgery or space-occupying lesions in the deep gluteal space may alter anatomy, thereby compressing the sciatic nerve. Piriformis syndrome is described as buttock pain exacerbated by hip flexion when combined with internal or external rotation of the affected leg. However, these symptoms may also present secondary to entrapment by the hamstring, gluteal and obturator internus–gemellus complex muscles, fibrous bands, and/or aberrant vessels. Given the variation in possible anatomical lesions, the term deep gluteal syndrome has been adopted to encompass these deep gluteal etiologies [6, 7].
Sciatica, however, is not the only symptom observed in our case series of patients. The somatic nerves of the LS plexus innervate muscles of the inferior limbs, perineum and pelvic floor, whereas the autonomic nerves innervate the detrusor muscle, left and sigmoid colons, rectum and the vagina. The sensory branches supply the inferior limbs, gluteal region and perineum. Therefore, besides sciatica, symptoms suggestive of SGV syndrome can include: perineal or gluteal pain, anorectal dysfunction, rectal pain and/or lower urinary tract symptoms in the absence of pelvic organ prolapse or other identifiable causes [3, 4]. When patients present with sciatica with no obvious spinal or extrapelvic cause, it is important to inquire about these additional symptoms, which may indicate an intrapelvic source of entrapment.
Vascular entrapment is a recognized precipitant of chronic pain syndromes involving the abdomen, pelvis, and lower limbs. Compression of the left renal vein between the aorta and superior mesenteric artery causes left renal venous hypertension and symptoms collectively known as Nutcracker syndrome [8, 9]. In women, it is an important cause of pelvic congestion syndrome [1, 5, 8]. Similarly, the right common iliac artery can compress the left common iliac vein against the LS spine, resulting in iliac or iliofemoral venous thrombosis. This phenomenon, known as May-Thurner or Crockett syndrome, clinically manifests as lower extremity edema, pain and venous insufficiency [10, 11].
Neurovascular conflict has also been identified as an underlying cause of pain syndromes in the head, neck and upper limbs. Specifically, microvascular compression of the trigeminal nerve can result in trigeminal neuralgia. Commonly caused by a looping vessel entrapping the trigeminal nerve, patients experience lancinating pain in the distribution of the trigeminal nerve [12]. In thoracic outlet syndrome, vascular and/or musculoskeletal structures impinge the brachial plexus at the interscalene, costoclavicular, and retropectoralis spaces. This causes upper limb weakness, paresthesias and non-radicular pain [13, 14].
Similar to the neurovascular compression well described in the pathogenesis of trigeminal neuralgia and thoracic outlet syndrome, varicosities and other vascular formations may also confine nerves of the pelvis. The sacral plexus covers the pelvic sidewalls, and is covered itself by branches of the internal iliac vessels. Therefore, dilations of these vessels can entrap the sacral plexus against the structures forming the pelvic sidewalls and floor—such as the piriformis, the pelvic brim and within the pudendal (Alcock’s) canal [3, 4].
However, the clinical significance of intrapelvic nerve entrapment by aberrant vessels is far less understood. Consequently, intrapelvic neurovascular compression in symptomatic patients is likely underdiagnosed. We identified LS plexus entrapment by variant SGVs in thirteen cases of sciatica with no identifiable musculoskeletal or spinal cause. To our knowledge, this is the first report of this anatomical variant in symptomatic patients in the literature. Alleviation of symptoms after laparoscopic decompression, with a statistically significant decrease in VAS pain scores and 92.3% success rate, strongly supports our hypothesis that SGV variants may entrap the LS plexus, resulting in the clinical presentation of atypical sciatica. In symptomatic patients with no clear spinal or musculoskeletal lesions, this previously unrecognized neurovascular conflict—SGV syndrome—should be considered as a potential intrapelvic cause of their sciatica.
CONCLUSIONS
Our case series demonstrates a correlation between variant SGVs and sciatica with no musculoskeletal or spinal etiology. However, these results alone cannot be extrapolated to determine causality. Without a control population, we do not know the prevalence of this newly identified SGV variant in the asymptomatic population. The variation in branching pattern of the internal iliac artery within the general population is well described [15, 16]. On the other hand, the venous branching pattern of these vessels and their relationship to nearby nerves is far less understood. Therefore, to further explore the clinical relevance of SGV syndrome, future research directions include conducting a cadaver study of the general population. This will allow us to determine the prevalence of SGV variants in the general population, as a comparison to what is seen in our symptomatic patients.
Although two patients in our study had pre-operative MRI sequencing, the surgical indication was based on clinical diagnosis alone. Although MRI has been useful for surgical planning, its diagnostic accuracy in identifying this variant is still to be determined. Consequently, a negative MRI should not be used to rule out this condition, as specific protocols must still be investigated. Likewise, the mere presence of a variant SGV on MRI must be interpreted within the clinical context of the patient’s presentation. Radiological markers for this neurovascular conflict must be further developed and validated. Therefore, we plan to explore the utility of specific MRI sequencing in identifying variant SGVs in patients who meet clinical criteria for SGV syndrome. This will be used to understand the prevalence of variant SGVs in patients with sciatica in the absence of spinal and muscoskeletal causes. Moreover, in determining the diagnostic ability of MRI sequencing, we hope to gain an objective tool for selecting surgical candidates who would benefit from nerve decompression.
FUNDING
N.L. received research support from Medtronic Inc. and Laborie Inc. However, none of these grants is directly related to the current publication.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. d'Archambeau O, Maes M, De Schepper AM.. The pelvic congestion syndrome: role of the “nutcracker phenomenon” and results of endovascular treatment. JBR-BTR 2004; 87:1–8. [PubMed] [Google Scholar]
- 2. Ganeshan A, Upponi S, Hon LQ. et al. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol 2007; 30:1105–11. [DOI] [PubMed] [Google Scholar]
- 3. Lemos N, Possover M.. Laparoscopic approach to intrapelvic nerve entrapments. J Hip Preserv Surg 2015; 2:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Possover M, Schneider T, Henle KP.. Laparoscopic therapy for endometriosis and vascular entrapment of sacral plexus. Fertil Steril 2011; 95:756–8. [DOI] [PubMed] [Google Scholar]
- 5. Nasser F, Cavalcante RN, Affonso BB. et al. Safety, efficacy, and prognostic factors in endovascular treatment of pelvic congestion syndrome. Int J Gynaecol Obstet 2014; 125:65–8. [DOI] [PubMed] [Google Scholar]
- 6. Martin HD, Shears SA, Johnson JC. et al. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy 2011; 27:172–81. [DOI] [PubMed] [Google Scholar]
- 7. McCrory P, Bell S.. Nerve entrapment syndromes as a cause of pain in the hip, groin and buttock. Sports Med 1999; 27:261–74. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed K, Sampath R, Khan MS.. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg 2006; 31:410–6. [DOI] [PubMed] [Google Scholar]
- 9. Hartung O, Grisoli D, Boufi M. et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg 2005; 42:275–80. [DOI] [PubMed] [Google Scholar]
- 10. Petersen MJ, Brewster D.. Iliac Vein Compression Syndrome. Persp Vasc Surg Endovasc 1995; 8:91–5. [Google Scholar]
- 11. White JM, Comerota AJ.. Venous Compression Syndromes. Vasc Endovascular Surg 2017; 51:155–68. [DOI] [PubMed] [Google Scholar]
- 12. Alshukry A, Salburgo F, Jaloux L. et al. Trigeminal neuralgia (TN): a descriptive literature analysis on the diagnosis and management modalities. J Stomatol Oral Maxillofac Surg 2017; 118:251–4. [DOI] [PubMed] [Google Scholar]
- 13. Ferrante MA, Ferrante ND.. The thoracic outlet syndromes: part 1. Overview of the thoracic outlet syndromes and review of true neurogenic thoracic outlet syndrome. Muscle Nerve 2017; 55:782–93. [DOI] [PubMed] [Google Scholar]
- 14. Kuhn JE, Lebus VG, Bible JE.. Thoracic outlet syndrome. J Am Acad Orthop Surgeons 2015; 23:222–32. [DOI] [PubMed] [Google Scholar]
- 15. Bleich AT, Rahn DD, Wieslander CK. et al. Posterior division of the internal iliac artery: anatomic variations and clinical applications. Am J Obstet Gynecol 2007; 197:658 e1–5. [DOI] [PubMed] [Google Scholar]
- 16. Cook M. The relationship between the superior gluteal artery and lumbosacral plexus. Austin J Anat 2015; 2:1030. [Google Scholar]