Abstract
Femoroacetabular impingement syndrome (FAIS) surgery can produce improvements in function and patient satisfaction; however, data on muscle assessment and kinematics of high mobility tasks of post-operative patients is limited. The purpose of this study was to evaluate kinematics and muscle activity during a deep squat task, as well as muscle strength in a 2-year follow-up FAIS corrective surgery. Eleven cam morphology patients underwent motion and electromyography capture while performing a squat task prior and 2-years after osteochondroplasty and were BMI-, age- and sex-matched to 11 healthy control (CTRL) participants. Isometric muscle strength, flexibility and patient-reported outcome measures (PROMs) were also evaluated. Post-operative FAIS was significantly weaker during hip flexion (23%) and hip flexion-with-abduction (25%) movements when compared with CTRL, no improvements in squat depth were observed. However, post-operative FAIS increased the pelvic range of motion during the squat descent (P = 0.016) and ascent (P = 0.047). They had greater peak activity for the semitendinosus and total muscle activity for the gluteus medius, but decreased peak activity for the glutei and rectus femoris during squat descent; greater total muscle activity for the tensor fascia latae was observed during squat ascent (P = 0.005). Although not improving squat depth, post-operative patients increased pelvic ROM and showed positive PROMs. The muscle weakness associated with hip flexion and flexion-with-abduction observed at the follow-up can be associated with the alterations in the muscle activity and neuromuscular patterns. Rehabilitation programs should focus on increasing pelvis and hip muscles flexibility and strength.
INTRODUCTION
Cam-type morphology is an aspherical bony extension at the anterolateral and anterosuperior femoral head, characterized by elevated alpha angles (i.e. axial and radial alpha angles >50.5° or 60°, respectively) [1–4], and a leading factor to femoroacetabular impingement syndrome (FAIS) and early hip osteoarthritis (OA) [5–7]. The presence of the cam morphology along with a lower femoral neck-shaft angle [4, 8] can contribute to the onset of symptoms including groin or hip pain during activity or during sustained periods of hip flexion, such as sitting [6, 9]. The cam-FAIS can be confirmed through imaging (e.g. computed tomography—CT) and patients typically test positive during a flexion, adduction and internal rotation physical examination [10, 11].
FAIS symptoms are first treated with conservative methods [12–15]; however, once conservative methods are exhausted, surgical correction, an osteochondroplasty of the femoral head–neck junction [16], is often required [12, 17, 18]. The surgery for FAIS is done through either open [19–21] or arthroscopic [22–26] procedures. Patient-reported outcome measures (PROMs) have indicated that both surgical methods are effective at reducing pain and improving quality of life [27–30].
Although PROMs have given insight into the success of surgical correction on patients with FAIS, only a limited number of studies have objectively compared patients before and after surgery using biomechanical outcomes [31–37] mainly during gait. This task does not place patients in a near impinged position. Only three studies have compared FAIS patients pre- and post-operatively during tasks with extreme hip flexion [32, 37, 38]. For the surgical treatment of femoroacetabular impingement, it is unknown how this affects the muscle strength at the hip during isometric contraction or joint biomechanics and muscle activity of the hip muscles during activities with a large range of motion. Therefore, comparing the strength, kinematics and muscle activity during a deep squat task in FAIS patients before and at 2-year following surgery can provide insight into optimizing function for those suffering from FAIS.
The purpose of this study was to examine if post-operative FAIS patients have improved the squat depth, pelvic and hip range of motion, hip muscle strength, or differ their hip muscle activity pattern compared with their pre-operative condition.
MATERIALS AND METHODS
This study had a prospective, matched case–control design (level III evidence). Eleven male patients with unilateral symptomatic cam-FAIS were compared with eleven male body mass index (BMI)-, age-matched-healthy controls (CTRL)—Table I. FAIS patients had a positive impingement test and presented a cam morphology >50.5° and 60° in the oblique axial and radial planes, respectively [1–4, 39, 40]. For the purpose of this study, CTRL participants were also submitted to CT scan before their participation to discard the presence of the cam morphology. Participants were excluded if they had any musculoskeletal or neurological disorders, degenerative diseases, previous major lower limb injuries or a BMI >30 kg m−2. FAIS participants went for motion analysis testing before receiving surgery and at minimum 2 years post-operatively (25.05 ± 1.13 months), whereas CTRL participants performed the testing protocol once. The study was approved by the hospital and university research ethics boards, and all participants signed and provided informed consent before their participation in the study.
Table I.
Group demographics and patient-reported outcome measures for HOOS questionnaire
| Groups | FAIS |
Control | ||
|---|---|---|---|---|
| Pre-operative | Post-operative | |||
| Group size (n) | 11 | 11 | ||
| Age (years) | 34.1 ± 7.4 | 36.2 ± 7.4 | 33.1 ± 7.2 | |
| Height (m) | 1.77 ± 0.06 | 1.78 ± 0.07 | 1.74 ± 0.10 | |
| Weight (kg) | 80.0 ± 10.3 | 81.0 ± 10.4 | 77.3 ± 13.9 | |
| BMI (kg m−2) | 25.4 ± 2.7 | 25.6 ± 3.6 | 25.4 ± 3.2 | |
| Sit-and-reach test | 29.8 ± 8.4 | 25.8 ± 9.4 | 24.2 ± 8.3 | |
| α-Angle (°) | 3:00 position | 54.0 ± 7.2a,b | 45.6 ± 6.7 | 43.3 ± 4.7 |
| 1:30 position | 66.3 ± 5.4a,b | 52.5 ± 9.1 | 53.0 ± 4.9 | |
| HOOS symptoms | 70.0 ± 10.7a,b | 81.4 ± 10.0b | 99.1 ± 2.0 | |
| HOOS pain | 70.0 ± 16.1a,b | 90.0 ± 8.3 | 98.9 ± 3.8 | |
| HOOS activities daily living | 81.7 ± 15.0a,b | 95.4 ± 6.6 | 99.6 ± 1.3 | |
| HOOS sport/recreation | 56.8 ± 25.1a,b | 83.0 ± 13.7 | 98.3 ± 5.7 | |
| HOOS quality of life | 39.2 ± 21.8a,b | 65.9 ± 21.5b | 97.2 ± 9.4 | |
aSignificant difference (P < 0.05) compared with FAIS post-op.
bSignificant difference (P < 0.05) compared with CTRL.
Four of the FAIS patients underwent corrective surgery, an osteochondroplasty of the femoral head–neck junction, with an open approach with surgical dislocation and seven had surgery with an arthroscopic approach, all performed by the same surgeon. Surgeries were followed by a standard 6-week physiotherapy program.
After completing the CT scan examination, all participants were transferred to the motion capture laboratory at the local university where they completed the Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire and performed two trials of sit-and-reach flexibility test with the feet level at 20 cm [41]. Wireless electromyography (EMG) probes (BTS FreeEMG 300, Padova, Italy) were placed on the ‘rectus femoris’ (RF), ‘biceps femoris’ long head (BF), ‘semitendinosus’ (ST), ‘gluteus medius’ (GMed), ‘gluteus maximus’ (GMax) and ‘tensor fasciae latae’ (TFL) muscles of both limbs according to SENIAM guidelines to record muscle activity [42]. Two maximal voluntary isometric contractions (MVICs) were captured using a hand-held dynamometer (model 01163, Lafayette Instrument, Lafayette, LA, USA) for each task and were separated by a 30 s resting interval (Table II). A comparison between flexion/extension strength ratio was also performed in order to determine leg strength imbalance.
Table II.
Hip muscle strength produced during MVIC and hand-held dynamometer (HHD) placement
| Movement | Muscles | Illustration | Normalized torque (Nm kg−1) |
||
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Pre-op | Post-op | CTRL | |||
| Hip flexiona | ‘Rectus femoris’ |
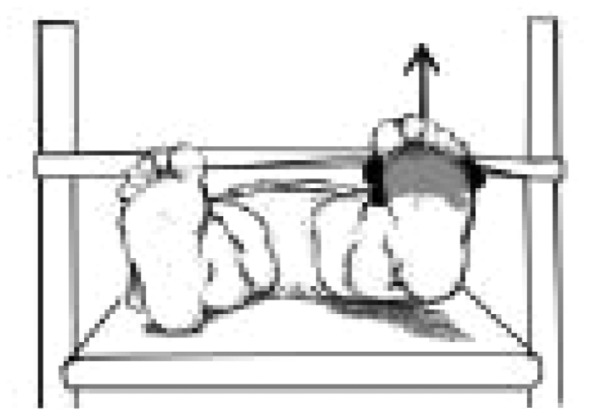
|
1.78 ± 0.51 | 1.70 ± 0.68 | 2.16 ± 0.60 |
| Hip extension | ‘Gluteus maximus’ |
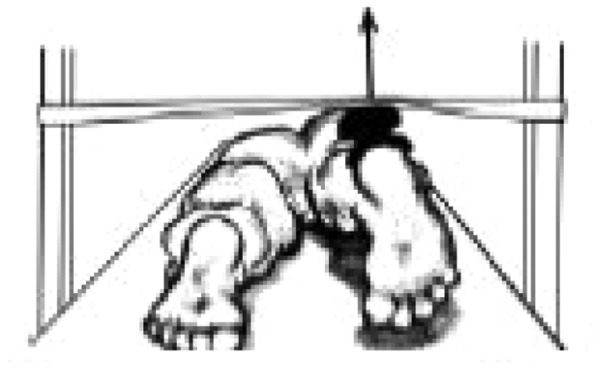
|
1.84 ± 0.56 | 1.70 ± 0.71 | 1.47 ± 0.46 |
| ‘Biceps femoris’ | |||||
| ‘Semitendinosus’ | |||||
| Hip abduction | ‘Gluteus medius’ |
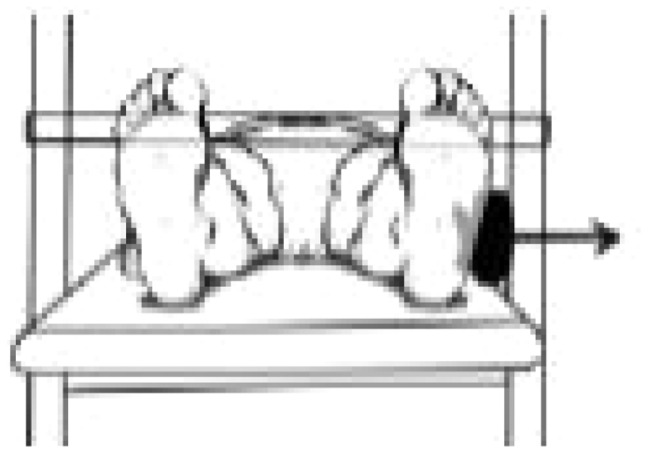
|
1.54 ± 0.31 | 1.47 ± 0.41 | 1.59 ± 0.47 |
| Hip flexion with hip abductiona | ‘Tensor fasciae latæ’ |
|
1.49 ± 0.40 | 1.27 ± 0.53 | 1.61 ± 0.48 |
Source: Illustrations in Table II have been partially presented in a publication (Catelli DS et al. Asymptomatic participants with a femoroacetabular deformity demonstrate stronger hip extensors and greater pelvis mobility during the deep squat task. Orthop J Sports Med 2018; 6(7):1–10. Copyright © 2018 SAGE Publishing. doi:10.1177/2325967118782484); The arrow represents the location of the HHD and the direction of the force vector.
aSignificant difference (P < 0.05) between FAIS post–op and CTRL.
Three-dimension motion analysis was collected using 10 infrared cameras (MX-13, Vicon, Oxford, UK) and 45 retroreflective skin markers placed on anatomical landmarks as the UOMAM marker set [43]. Participants completed five deep squats to their maximal depth at a controlled and self-selected pace. They were instructed to place their feet hip-width apart, directed anteriorly, with toes and heels in full contact with the ground during the entire squat cycle. The squat trials were separated into descending and ascending phases, and squat depth was normalized with respect to their leg length; the distance between the anterior superior iliac-spine to the medial malleolus. EMG and motion data were exported into a custom built Matlab script (MathWorks, Natick, MA, USA) for extraction and processing. Motion trajectories were filtered using a Woltring filter (MSE = 15 mm2). Pelvic and hip sagittal ROM, along with peak hip flexion, peak hip abduction and peak knee flexion were extracted and averaged between the five trials. EMG data were filtered using a bandpass filter (20–450 Hz) and rectified. From the rectified signal, peak linear envelope (PeakLE) and total muscle activity (iEMG—integral of the linear envelope signal) were determined for each muscle during each phase of the squat and normalized by their MVIC. The data were then averaged between the five trials and with respect to each group in order to be analysed. All data were explored for normality. Comparisons between pre- and post-operative were made using either a paired t-test or its non-parametric equivalent Wilcoxon signed rank test. To compare differences between the FAIS conditions and the CTRL group, a one-way ANOVA was used with a Bonferroni post-hoc comparison, to determine where significant differences occurred (CI = 95%). The effect size was calculated with Cohen’s d and was considered as either small (d = 0.2), medium (d = 0.5) or large (d = 0.8).
RESULTS
The FAIS patients reported significantly improved HOOS on all measures on their follow-up compared with their pre-operative values (Table I). No significant differences amongst the groups were found in the sit-and-reach flexibility test.
Squat depth for the three groups is illustrated in Fig. 1. Between the pre-operative and post-operative conditions for the FAIS group, there was no change in squat depth as they achieved as average a squat depth of 33.1% leg length and 34.2% leg length (0% representing the lowest squat depth), respectively. The CTRL group was able to squat significantly lower (27.0%) than both the pre- and post-operative FAIS conditions (P < 0.01; d > 0.65), suggesting a moderate to high effect size.
Fig. 1.
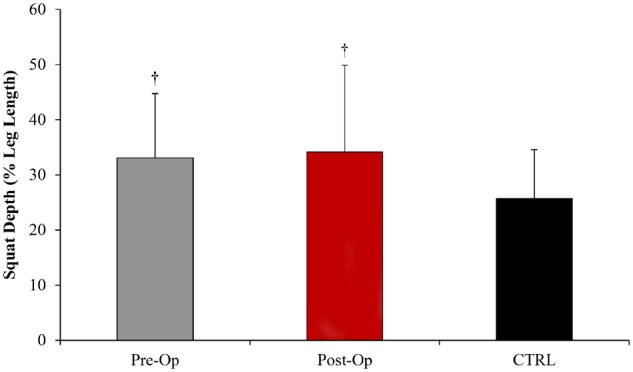
Squat depth normalized to the percentage of leg length for the FAIS group before and after surgery, compared to the healthy CTRL. †Significant difference (P < 0.01) compared with CTRL.
At 2-year follow-up, FAIS patients showed decreased muscle strength compared with their pre-operative values for all hip MVIC tasks, but the results were not statistically significant. When comparing strength measures to the CTRL group, while there were no differences with the pre-operative condition, post-surgery the FAIS patients were significantly weaker during hip flexion (P = 0.032, d = 0.83) and hip abduction with flexion (P = 0.027, d = 0.87) tasks (Table II). A comparison between flexion/extension strength ratio (FAIS pre-op 1.08 ± 0.46; FAIS post-op 1.28 ± 1.00 and CTRL 1.68 ± 0.94) showed a significant difference when comparing the pre-operative patients with the CTRL (P = 0.034, d = 0.83).
Pelvic sagittal ROM was significantly greater post-operatively compared with the pre-operative condition for both descent (P = 0.016, d = 0.87) and ascent (P = 0.047, d = 0.68) phases of the squat in both cases suggesting a high to moderate effect size (Table III). Although the FAIS group was able to achieve greater peak hip flexion following surgery, the difference was not significant (P = 0.054, d = 0.66) between pre- and post-operative conditions, nor for hip sagittal ROM. No significant differences were observed in hip abduction, and knee flexion (Table III), as well as when compared with the CTRL group.
Table III.
Hip and pelvis kinematics during the descent and ascent phases of the squat
| FAIS pre-op | FAIS post-op | CTRL | |
|---|---|---|---|
| Pelvic ROM (°)—squat descenta | 9.0 ± 4.5 | 16.0 ± 6.2 | 11.7 ± 7.8 |
| Pelvic ROM (°)—squat ascenta | 8.9 ± 3.4 | 14.7 ± 7.3 | 10.4 ± 7.3 |
| Hip ROM (°) | 91.4 ± 24.2 | 101.3 ± 7.2 | 101.1 ± 7.3 |
| Peak hip flexion (°) | 95.4 ± 19.5 | 104.1 ± 8.8 | 103. ± 8.6 |
| Peak hip abduction (°) | 13.3 ± 6.2 | 11.6 ± 4.8 | 13.5 ± 3.3 |
| Peak knee flexion (°) | 123.0 ± 15.1 | 121.3 ± 20.3 | 118.2 ± 7.3 |
aSignificant difference (P < 0.05) between FAIS pre- and post-op.
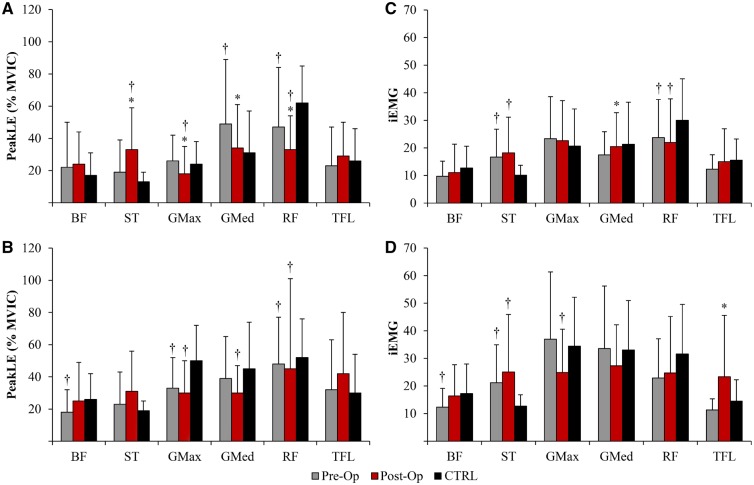
During the squat descent phase, the post-operative patients had decreased PeakLE for the glutei (GMax and GMed) and RF muscles, but increased for the ST muscle, when compared with their pre-operative values (Fig. 2A). No differences in PeakLE existed between pre-operative and post-operative conditions during the squat ascent phase (Fig. 2B). The post-operative patients had increased iEMG for the GMed and the TFL muscles during the squat descent (Fig. 2C) and ascent (Fig. 2D) phases, respectively when compared with their pre-operative condition. Both hamstring muscles (BF and ST) had an increase in iEMG for both phases of the squat; however, it did not reach significance.
Fig. 2.
EMG peak linear envelope (PeakLE) and total activity (iEMG) for the biceps femoris (BF), semitendinosus (ST), gluteus maximus (GMax), gluteus medius (GMed), rectus femoris (RF) and tensor fasciae latea (TFL) muscles during descent (A and C) and ascent (B and D) phases of the squat task. *Significant difference (P < 0.05) compared with FAIS pre-op. †Significant difference (P < 0.05) compared with CTRL.
DISCUSSION
The osteochondroplasty of the femoral head–neck junction has been chosen as an option to relieve pain for cam-FAIS patients; however, with only a few studies objectively measuring the biomechanical outcomes before and after surgery [32–34, 36, 44], the evidence is primarily limited to PROMs. Of the few biomechanical studies, most have made comparisons during gait that does not require an extreme ROM of the hip, such as a deep squatting task. Using the squat task to compare the biomechanics and muscle activity in patients before and after surgery can empirically evaluate if the patients have improved their function and mobility at near impingement. In our study, we found that the post-operative participants have increased pelvic ROM, but this did not translate to improved squat depth or an increase in hip ROM. At the 2-year follow-up, post-operative patients were weaker when compared with their matched-healthy CTRLs when tested isometrically for hip flexion and hip flexion combined with abduction. Also, the maximum muscle activity and total muscle activity changed during squat performance.
Several studies examined the biomechanics of a squat movement or have reported squat data in pre-operative patients with FAIS [38, 45–48]. Four of the previous studies examined maximal squat depth [8, 38, 48, 49], whereas the other study had patients perform a squat to 25% of the total body height at a controlled speed [45]; thus making it difficult to compare our results to the latter. Only two studies had compared squat depth between pre- and post-operative conditions [32, 37]. Our study showed an average squat depth of 33.1% (pre-op) and 34.2% (post-op) of the leg length, which are similar values measured by previous studies [32, 37]. Only one of these studies found a significant difference between pre- and post-operative conditions [32] whereas the most recent one did not show any statistical significance between the conditions [37] like the present work. For both studies [32, 37] their cohort had similar mixed surgical approaches as the current study. We cannot conclusively determine if squat depth has changed following corrective surgery. In our study, the maximum squat depth on the post-operative patients did not improve from the pre-operative condition, and it remained still higher than on the controls.
Normally when standing, the pelvis remains in neutral position, and while squatting, it tilts anteriorly and returns to its neutral position at the deepest part of the squat. When standing up, the pelvis tilts anteriorly once more and returns to its neutral position when upright. At the 2-year follow-up, the FAIS patients have shown increased pelvic mobility during the squat task, although no differences in peak hip flexion or hip ROM were observed. Previous studies also did not find any significant differences in hip kinematics during a squat task [32]. An improving in the pelvic ROM, but not at the hip joint, could help to justify why the ultimate measurement, the squat depth, was not improved, although being performed with different kinematics. Perhaps less pain had a positive effect on the pelvic ROM that was not transferred to the hip, limiting the squat depth performance. The hypothesis raised is that after many years of dealing with pain at end ranges of motion while waiting for corrective surgery may have caused soft tissue stiffening and contraction imbalance of the muscles surrounding the hip joint. This was verified by the flexion/extension hip strength ratio analysis, where the pre-operative patients showed an anteroposterior muscle force imbalance when compared with the healthy CTRLs. A recent systematic review has suggested that in patients with hip stiffness, a capsular release may be appropriate [50] during an FAIS corrective surgery. Soft tissue stiffness would affect movement, especially during closed chain tasks such as the squat. Perhaps the laxity of stiff soft tissue structures may be a strategy to allow also hip ROM improvements. We measured sit-and-reach flexibility and found no difference between the pre- and post-operative values. As this test limits the flexibility measured of the back and hamstrings only, future research should compare the active and passive ROM of the hip in patients before and after the corrective surgery. Also, aftercare rehabilitation should aim at improving the flexibility of the soft tissues and muscles surrounding the pelvis and hip, which may improve joint mobility during open and closed chain tasks.
Muscle strength is another important factor that will need to be addressed following surgery in FAIS patients. The post-operative FAIS patients were weaker during pure hip flexion and hip flexion-with-abduction compared with the CTRL participants during the isometric test. Therefore, aftercare rehabilitation should focus on improving the muscle strength during these movements, as the strength gain in combination with flexibility will improve hip mobility. Previous studies on hip muscle strength in pre-operative FAIS patients during isometric tasks showed that hip flexors and TFL muscles were also significantly weaker compared with a control group [51]. Although the differences in this study arose only at the 2-year follow-up patients, the conclusions remain the same, as patients with FAIS present muscle weakness for the hip flexors muscle groups; which also led to a muscle imbalance regarding the ratio between hip flexion and extension strength, when compared with the CTRL participants. As muscle weakness in OA individuals can be an indicator of progression [51, 52], the assumption that FAIS could potentially lead to hip OA [6, 7, 53] is asserted. Therefore, the findings of this study support the assessment of hip muscle strength in routine clinical examinations to help diagnose FAIS [51, 54].
Post-operative FAIS patients used differently the muscle activity synergies in the hip compared with pre-operative conditions. PeakLE EMG has shown a higher peak activation of the ST, while both glutei muscle and the RF lowered during the descent phase of the squat. The total muscle activation also demonstrated an increase for the GMed and the TFL during squat descending and ascending, respectively. Excessive ST activation in symptomatic cam-FAIS during the squat has already been reported [49]. During the descent phase of the squat, the ST acts eccentrically to control the movement, in the FAIS the ST over activates to compensate the hip flexor weakness and allow the task to be performed; as a biarticular muscle, it can be associated with the limited pre-operative pelvic tilt and highlights the muscle unbalance. GMax total muscle activation was also significantly lower at the 2-year follow-up when compared with the CTRL group. Perhaps the muscle peak activation reduction observed in the glutei, and RF muscles have been caused by microarchitectural changes, such as surgery trauma, leading to a rearrangement of the muscular tissue [55, 56]. Although positive improvements were reached post-operatively in the ‘function, sports and recreational activities’ section of the HOOS questionnaire, it is uncertain if the FAIS in our study maintained their same pre-operative activity levels following surgery, as this might have influenced the decrease in strength observed in our findings. Future research should examine muscle fiber composition and architecture in FAIS patients prior to and following corrective surgery [56].
This study had certain limitations. First, our cohort consisted of only male participants; however, as the cam morphology only is statistically more prevalent in males [57–59], this study concentrated only on this population. Still, future surgical studies on FAIS should include females for a sex comparison. Second, this research did not focus on comparing surgical approaches since our cohort was not large enough for achieving a meaningful power. However non-parametric analysis showed no differences in the analysed variables amongst the approaches. It has been suggested that the arthroscopic approach offers better muscle preservation, which could provide a better joint function [28, 30, 60]. As a minimum of 2 years was used for the patients’ reassessment, we believe that no short-term benefits between the two approaches would have arisen after 2 years, also in a mixed approach cohort, a 2-year follow-up have showed that FAIS surgical correction was associated with decreased T1ρ and bone mass density, improving the overall health of the hip joint [37]. Additionally, one systematic review has shown that one approach is not significantly superior to the other [30]. Third, we did not control for the speed of the squat between participants. Participants were instructed to squat at a controlled and self-selected pace. The speed of movement could affect the joint moments and EMG variables, however, we believe that speed would have minimal effect on kinematics variables.
Although at the two-year follow-up surgical correction analysis the cam-FAIS patients did not improve the squat depth, they have shown increased pelvic ROM and positive PROMs. The weakness of muscles associated with hip flexion and flexion-with-abduction were also observed at the follow-up, which may be associated with the alterations in the muscle activity and neuromuscular patterns. The use of squat test pre- and post-surgical correction of cam morphology can provide valuable information for the clinical practice while identifying pelvic mobility in a dynamic task. The rehabilitation program should focus on increasing the flexibility and strength of muscles around the pelvis and hip, with particular attention on strengthening the hip flexors and TFL muscles. Increasing flexibility of other lower extremity muscles should not be overlooked as it will improve mobility during closed kinetic/kinematic chain tasks. Implementation of hip muscular strength measurement before and after surgery may provide additional insights into the rehabilitation program, as muscle weakness may have caused a change in the muscular contraction strategy, and also as a tool to evaluate muscle strength balance.
ACKNOWLEDGEMENTS
The authors wish to thank Mr Kevin Dwyer MSc, Mrs Giulia Mantovani PhD and Mr K.C. Geoffrey Ng PhD, from the Human Movement Biomechanics Laboratory, University of Ottawa, for their help with data collection; and the research staff of the Division of Orthopedic Surgery at The Ottawa Hospital for help in patient recruitment and data collection, as well as the Hans K. Uhthoff Graduate Fellowship Award.
FUNDING
This work was supported by the Science without Borders Scholarship (Brazil) (1098/13-06); Canadian Institutes of Health Research (97778A) and National Sciences and Engineering Research Council of Canada (106769-2013).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Beaulé PE, Hynes K, Parker G. et al. Can the alpha angle assessment of cam impingement predict acetabular cartilage delamination? Clin Orthop Relat Res 2012; 470: 3361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nötzli H, Wyss T, Stoecklin C. et al. The contour of the femoral head–neck junction as a predictor for the risk of anterior impingement: commentary. J Bone Joint Surg 2002; 84: 556–60. [DOI] [PubMed] [Google Scholar]
- 3. Rakhra KS, Sheikh AM, Allen D. et al. Comparison of MRI alpha angle measurement planes in femoroacetabular impingement. Clin Orthop Relat Res 2009; 467: 660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ng KG, Lamontagne M, Beaulé PE.. Differences in anatomical parameters between the affected and unaffected hip in patients with bilateral cam-type deformities. Clin Biomech 2016; 33: 13–9. [DOI] [PubMed] [Google Scholar]
- 5. Ganz R, Leunig M, Leunig-Ganz K. et al. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res 2008; 466: 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganz R, Parvizi J, Beck M. et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003; 417: 112–20. [DOI] [PubMed] [Google Scholar]
- 7. Leunig M, Ganz R.. Femoroacetabular impingement. A common cause of hip complaints leading to arthrosis. Unfallchirurg 2005; 108: 9–10, 12–7. [DOI] [PubMed] [Google Scholar]
- 8. Ng KG, Lamontagne M, Adamczyk AP. et al. Patient-specific anatomical and functional parameters provide new insights into the pathomechanism of cam FAI. Clin Orthop Relat Res 2015; 473: 1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clohisy JC, Knaus ER, Hunt DM. et al. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res 2009; 467: 638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byrd J. Femoroacetabular impingement in athletes: current concepts. Am J Sports Med 2014; 42: 737–51. [DOI] [PubMed] [Google Scholar]
- 11. Reiman MP, Goode AP, Cook CE. et al. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med 2015; 49: 811.. [DOI] [PubMed] [Google Scholar]
- 12. Loudon JK, Reiman MP.. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport 2014; 15: 82–90. [DOI] [PubMed] [Google Scholar]
- 13. Griffin DR, Dickenson EJ, O'Donnell J. et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med 2016; 50: 1169–76. [DOI] [PubMed] [Google Scholar]
- 14. Wall PDH, Fernandez M, Griffin DR. et al. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R 2013; 5: 418–26. [DOI] [PubMed] [Google Scholar]
- 15. Casartelli NC, Bizzini M, Kemp J. et al. What treatment options exist for patients with femoroacetabular impingement syndrome but without surgical indication? Br J Sports Med 2018; 52: 552–553. [DOI] [PubMed] [Google Scholar]
- 16. Beaulé PE, Le Duff MJ, Zaragoza E.. Quality of life following femoral head–neck osteochondroplasty for femoroacetabular impingement. J Bone Joint Surg Am 2007; 89: 773–9. [DOI] [PubMed] [Google Scholar]
- 17. Emara K, Samir W, Motasem EH. et al. Conservative treatment for mild femoroacetabular impingement. J Orthop Surg (Hong Kong) 2011; 19: 41–5. [DOI] [PubMed] [Google Scholar]
- 18. Hunt D, Prather H, Hayes MH. et al. Clinical outcomes analysis of conservative and surgical treatment of patients with clinical indications of prearthritic, intra-articular hip disorders. PM R 2012; 4: 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ribas M, Marín-Peña OR, Regenbrecht B. et al. Hip osteoplasty by an anterior minimally invasive approach for active patients with femoroacetabular impingement. Hip Int 2007; 17: 91–8. [DOI] [PubMed] [Google Scholar]
- 20. Lincoln M, Johnston K, Muldoon M. et al. Combined arthroscopic and modified open approach for cam femoroacetabular impingement: a preliminary experience. Arthroscopy 2009; 25: 392–9. [DOI] [PubMed] [Google Scholar]
- 21. Laude F, Sariali E, Nogier A.. Femoroacetabular impingement treatment using arthroscopy and anterior approach. Clin Orthop Relat Res 2009; 467: 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiland DE, Philippon MJ.. Arthroscopic technique of femoroacetabular impingement. Oper Tech Orthop 2005; 15: 256–60. [Google Scholar]
- 23. Philippon MJ, Stubbs AJ, Schenker ML. et al. Arthroscopic management of femoroacetabular impingement: osteoplasty technique and literature review. Am J Sports Med 2007; 35: 1571–80. [DOI] [PubMed] [Google Scholar]
- 24. Byrd JWT, Jones KS.. Arthroscopic femoroplasty in the management of cam-type femoroacetabular impingement. Clin Orthop Relat Res 2009; 467: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Philippon MJ, Schenker ML.. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med 2006; 25: 299–308. [DOI] [PubMed] [Google Scholar]
- 26. Horisberger M, Brunner A, Herzog RF.. Arthroscopic treatment of femoroacetabular impingement of the hip: a new technique to access the joint. Clin Orthop Relat Res 2010; 468: 182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joseph R, Pan X, Cenkus K. et al. Sex differences in self-reported hip function up to 2 years after arthroscopic surgery for femoroacetabular impingement. Am J Sports Med 2016; 44: 54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Philippon M, Briggs K, Yen Y-M. et al. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br 2009; 91: 16–23. [DOI] [PubMed] [Google Scholar]
- 29. Larson CM, Giveans MR.. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy 2008; 24: 540–6. [DOI] [PubMed] [Google Scholar]
- 30. Botser IB, Smith TW, Nasser R. et al. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy 2011; 27: 270–8. [DOI] [PubMed] [Google Scholar]
- 31. Malagelada F, Del Carmen VA, Barke SJ. et al. The anterior mini-open approach for femoroacetabular impingement: gait and functional assessment at one year post-surgery. Ann Phys Rehabil Med 2015; 58: 60–5. [DOI] [PubMed] [Google Scholar]
- 32. Lamontagne M, Brisson N, Kennedy MJ. et al. Preoperative and postoperative lower-extremity joint and pelvic kinematics during maximal squatting of patients with cam femoroacetabular impingement. J Bone Joint Surg 2011; 93: 40–5. [DOI] [PubMed] [Google Scholar]
- 33. Brisson N, Lamontagne M, Kennedy MJ. et al. The effects of cam femoroacetabular impingement corrective surgery on lower-extremity gait biomechanics. Gait Posture 2013; 37: 258–63. [DOI] [PubMed] [Google Scholar]
- 34. Rylander JH, Shu B, Andriacchi TP. et al. Preoperative and postoperative sagittal plane hip kinematics in patients with femoroacetabular impingement during level walking. Am J Sports Med 2011; 39: 36S–42S. [DOI] [PubMed] [Google Scholar]
- 35. Rylander J, Shu B, Favre J. et al. Functional testing provides unique insights into the pathomechanics of femoroacetabular impingement and an objective basis for evaluating treatment outcome. J Orthop Res 2013; 31: 1461–8. [DOI] [PubMed] [Google Scholar]
- 36. Bedi A, Dolan M, Hetsroni I. et al. Surgical treatment of femoroacetabular impingement improves hip kinematics. Am J Sports Med 2011; 39: 43S–49S. [DOI] [PubMed] [Google Scholar]
- 37. Beaulé PE, Speirs AD, Anwander H. et al. Surgical correction of cam deformity in association with femoroacetabular impingement and its impact on the degenerative process within the hip joint. J Bone Joint Surg 2017; 99: 1373–81. [DOI] [PubMed] [Google Scholar]
- 38. Diamond LE, Bennell KL, Wrigley TV. et al. Squatting biomechanics in individuals with symptomatic femoroacetabular impingement. Med Sci Sports Exerc 2017; 49: 1520–9. [DOI] [PubMed] [Google Scholar]
- 39. Nouh MR, Schweitzer ME, Rybak L. et al. Femoroacetabular impingement: can the alpha angle be estimated? Am J Roentgenol 2008; 190: 1260–2. [DOI] [PubMed] [Google Scholar]
- 40. Hack K, Di Primio G, Rakhra K. et al. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am 2010; 92: 2436–44. [DOI] [PubMed] [Google Scholar]
- 41. Wells KF, Dillon EK.. The sit and reach—a test of back and leg flexibility. Res Q Am Assoc Health Phys Educ Recreat 1952; 23: 115–8. [Google Scholar]
- 42. Hermens HJ, Freriks B, Merletti R. et al. SENIAM 8: European Recommendations for Surface Electromyography. Enschede, The Netherlands: Roessingh Research and Development, 1999. [Google Scholar]
- 43. Mantovani G, Lamontagne M.. How different marker sets affect joint angles in inverse kinematics framework. J Biomech Eng 2017; 139: 044503.. [DOI] [PubMed] [Google Scholar]
- 44. Kennedy MJ, Lamontagne M, Beaulé PE.. Femoroacetabular impingement alters hip and pelvic biomechanics during gait: walking biomechanics of FAI. Gait Posture 2009; 30: 41–4. [DOI] [PubMed] [Google Scholar]
- 45. Kumar D, Dillon A, Nardo L. et al. Differences in the association of hip cartilage lesions and cam-type femoroacetabular impingement with movement patterns: a preliminary study. PM R 2014; 6: 681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mantovani G, Ng KG, Lamontagne M.. Regression models to predict hip joint centers in pathological hip population. Gait Posture 2016; 44: 48–54. [DOI] [PubMed] [Google Scholar]
- 47. Kowalski E, Catelli DS, Lamontagne M.. Side does not matter in healthy young and older individuals—examining the importance of how we match limbs during gait studies. Gait Posture 2019; 67: 133–136. [DOI] [PubMed] [Google Scholar]
- 48. Lamontagne M, Kennedy MJ, Beaulé PE.. The effect of cam FAI on hip and pelvic motion during maximum squat. Clin Orthop Relat Res 2009; 467: 645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Catelli DS, Kowalski E, Beaulé PE. et al. Asymptomatic participants with a femoroacetabular deformity demonstrate stronger hip extensors and greater pelvis mobility during the deep squat task. Orthop J Sports Med 2018; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ortiz-Declet V, Mu B, Chen AW. et al. Should the capsule be repaired or plicated after hip arthroscopy for labral tears associated with femoroacetabular impingement or instability? A systematic review. Arthroscopy 2018; 34: 303–18. [DOI] [PubMed] [Google Scholar]
- 51. Casartelli NC, Maffiuletti NA, Item-Glatthorn JF. et al. Hip muscle weakness in patients with symptomatic femoroacetabular impingement. Osteoarthritis Cartilage 2011; 19: 816–21. [DOI] [PubMed] [Google Scholar]
- 52. Brandt KD. Is a strong quadriceps muscle bad for a patient with knee osteoarthritis? Ann Intern Med 2003; 138: 678–9. [DOI] [PubMed] [Google Scholar]
- 53. Leunig M, Beaulé PE, Ganz R.. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res 2009; 467: 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kelly BT, Williams RJ, Philippon MJ.. Hip arthroscopy: current indications, treatment options, and management issues. Am J Sports Med 2003; 31: 1020–37. [DOI] [PubMed] [Google Scholar]
- 55. Addison O, Marcus RL, Lastayo PC. et al. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014; 2014: 309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meyer DC, Hoppeler H, von Rechenberg B. et al. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res 2004; 22: 1004–7. [DOI] [PubMed] [Google Scholar]
- 57. Kassarjian A, Brisson M, Palmer WE.. Femoroacetabular impingement. Eur J Radiol 2007; 63: 29–35. [DOI] [PubMed] [Google Scholar]
- 58. Allen D, Beaule PE, Ramadan O. et al. Prevalence of associated deformities and hip pain in patients with cam-type femoroacetabular impingement. J Bone Joint Surg Br 2009; 91–B:589–94. [DOI] [PubMed] [Google Scholar]
- 59. Beck M, Kalhor M, Leuning M. et al. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005; 87–B: 1012–8. [DOI] [PubMed] [Google Scholar]
- 60. Papalia R, Del Buono A, Franceschi F. et al. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop 2012; 36: 903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]