Abstract
Adult periacetabular osteotomy (PAO) was originally performed through the classic Smith-Petersen approach for optimal operative visibility and acetabular fragment correction. Evolution towards an abductor-sparing technique significantly lowered the post-operative morbidity. The rectus-sparing approach represents a step further, but the innervation of the rectus femoris is theoretically more at risk. Although the topographic anatomy of the femoral nerve has been well described, it was never studied with specificity to surgical landmarks. The femoral nerve’s spatial relation with the anterior-inferior iliac spine (AIIS) and the amount of possible dissection in the rectus femoris and iliopsoas interval is uncertain. Seven formalin-preserved human cadaveric specimens without history of inguinal injury or surgery were dissected using the distal limb of an iliofemoral approach. The level of entry of motor innervation was measured and number of branches to the rectus femoris was noted. The average longitudinal distance from the AIIS to the first motor nerve to the rectus femoris was 8.6 ± 1.4 cm. The number of branches varied between 1 and 4 with the most common innervation pattern being composed of two segments. Dissection medial to the rectus femoris should not be carried out further than 7 cm distal to the AIIS and stretching of that interval during surgical exposure should be done cautiously. The clinical efficiency of the rectus-sparing approach should be studied further in order to confirm its advantage over the classic direct anterior approach. The study provides a better understanding of the localization and the anatomical variations of the structures encountered at the level of and below the AIIS. It also assesses the relative risk of denervation of the rectus femoris during PAO through the rectus-sparing approach. The authors recommend that the dissection medial to the rectus femoris should be carried out no further than 7 cm distal to the AIIS and stretching of that interval during surgical exposure should be done cautiously.
INTRODUCTION
Deep surgical dissection around the hip can be a complex endeavour. Whether in the case of pelvic tumours, acetabular fractures or periacetabular osteotomies (PAOs), the knowledge of the anatomy is quite essential. The selection of a surgical approach necessitates a compromise between good visibility and the respect of the integrity of the surrounding structures without impeding on surgical goals. Opting for minimal dissection will optimize recovery and reduce sequelae but it remains dependent on the pathology addressed and the surgical objectives [1–4].
Since the original description of the Bernese PAO by Ganz et al. [5] using a modified Smith-Petersen approach [6], many aspects of the surgical approach have evolved. The early approaches relied on the extensive dissection of the abductor muscles and the transection of both heads of the rectus femoris tendon. In an effort to preserve these muscles and reduce related morbidity, Murphy and Millis [7] proposed an abductor-sparing technique that focused on preserving the abductors. Similarly, studies were published aiming to preserve the rectus femoris tendons origin during PAO [2, 8].
Tissue preservation during the surgical approach and smaller surgical windows may lead to more traction on the surroundings structures, thereby potentially putting vital structures such as neurovascular bundles at risk [9]. While many authors have agreed on the rationale and value of PAO in properly-selected patients [5, 9–12], there is no consensus on the optimal approach to the pelvis. There are two main schools of thought, the first preaches for muscle detachment and reinsertion, while the other supports tissue stretching. The possible complications of these two lines of thought can be severe and may include muscle weakness post-operatively [13] in case of muscle detachment and reinsertion, or putting more strain on neurovascular structures and even causing permanent nerve damage in case of overly aggressive tissue stretching. For instance, some possible complications with the abductor-sparing technique include deficit in the territory of the femoral nerve [14]. Other factors to consider when choosing a surgical approach include the type of pathology addressed, the visibility required, the duration of surgery and the difficulty level of the intervention.
Although the trend is to favour minimally invasive procedures, their relative associated risk does not always outweigh those of their more invasive counterparts. In order to prevent possible post-operative complications of the anterior surgical approach of the hip, it is essential to master sufficient anatomical knowledge of the anterior pelvis. Many studies have described muscle insertions, osseous and ligamentous structures and relative positioning of neurovascular bundles [15–22]. However, none of those studies were developed with specific interest to the rectus-sparing approach for PAO. Despite the described excellent treatment options and the promising initial outcomes for this situation [23], the space between the anterior-inferior iliac spine (AIIS) and the first nerve branch to the rectus femoris with its possible anatomic variations, still needs to be defined to better guide the surgeons.
Hence, this study aims to define the distance separating the AIIS and the muscle entry point of the nerve supplying the rectus femoris. Our goal is to offer base knowledge to better define the exploitable muscular interval between psoas and rectus, and the extent to which its dissection can safely be performed in the mindset of providing sufficient exposure to the anterior hip and pelvic bone.
MATERIALS AND METHODS
Cadaver preparation
Seven cadavers were selected for the study (five men, two women) with a mean age of 79 (range 74–89). All cadavers were fresh frozen unprepared cadavers stored at −5°C. Before dissections, the cadavers were kept at room temperature for 48 h. Each hip was examined to assess the presence of surgical scars and the range of motion in flexion, internal rotation and external rotation. We excluded cadavers with any evidence of previous surgery or trauma that could potentially affect the anatomy of the motor innervation of the rectus femoris.
Surgical technique
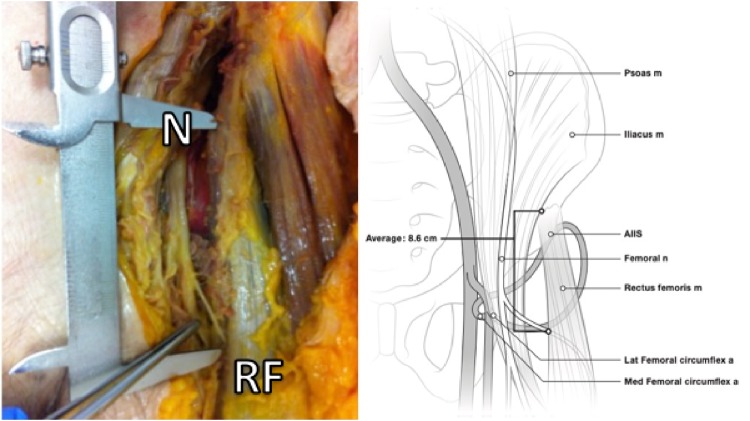
At the beginning of the surgical procedure, cadavers were placed supine on the operating room tables. The distal limb of the iliofemoral (Smith-Petersen) approach was used in an attempt to preserve all abductor muscles during the dissection. By developing the interval between the sartorius and tensor fascia lata muscles, the femoral nerve was located as it passes under the inguinal ligament (Fig. 1). Then the AIIS was exposed by reflection of the sartorius medially and the rectus femoris direct and reflected heads laterally. Finally, fine dissection of the branches of the femoral nerve was performed in order to isolate and visualize the motor branches to the rectus femoris. Both distances from the top and the centre of the AIIS to the entry of the first motor branch of the rectus femoris were measured (Fig. 2).
Fig. 1.
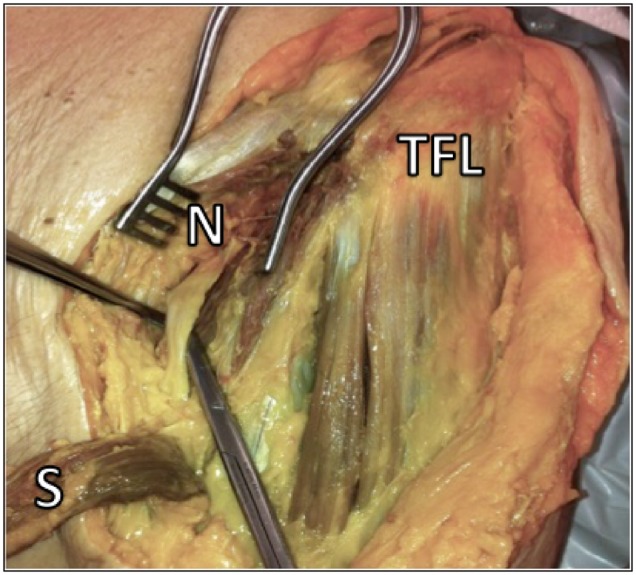
Femoral nerve (N) is identified as it passes under the inguinal ligament. After execution of a classic Smith-Petersen approach and development of the sartorius (S)–tensor fascia lata (TFL) interval (the sartorius is reflected in this photograph).
Fig. 2.
The distance from the AIIS to the first ramification of the femoral nerve (N) to the rectus femoris can be measured as seen. After reflection of the sartorius, elevation of the rectus femoris (RF) and fine dissection of the femoral nerve and its branches.
Variables collected
The exact distance between the AIIS and the first nerve ramification into the rectus femoris sheath were measured by using a millimetric calliper (Pocket vernier caliper no 184501, Helios-Preisser, Germany) and the number of motor branches that supply the muscle was determined (Fig. 3). The rectus femoris tendon and the psoas muscle were found in the 13 specimens always separated by an intervening connective tissue sheath. The thickness of the iliocapsularis muscle at the level of the labrum directly below the AIIS was measured and a note was taken if it was absent in any specimen.
Fig. 3.
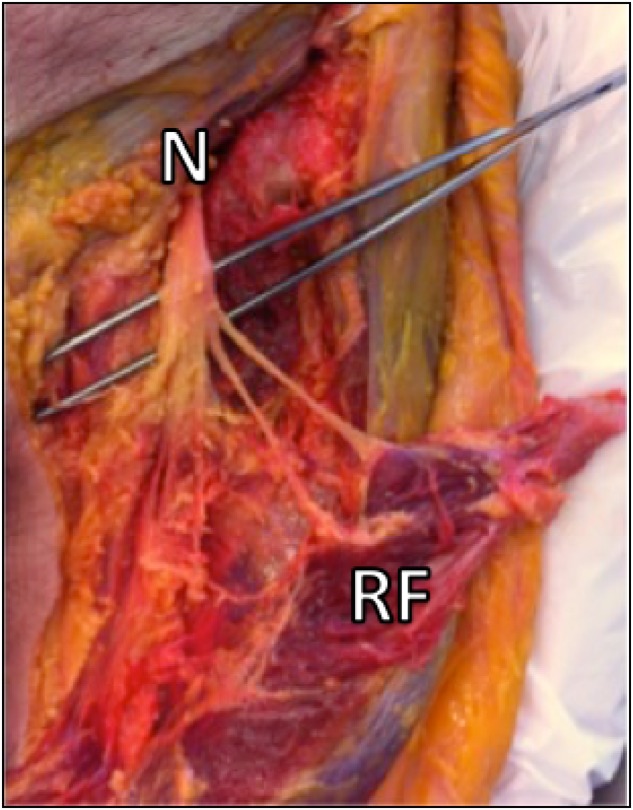
The number of nerve branches of the femoral nerve (N) innervating the rectus femoris (RF) muscle belly is identified. In this image, the rectus femoris was detached from its origin on the AIIS.
Statistical analysis
After data collection, measured lengths between the AIIS and the first ramification of the femoral nerve to the rectus femoris, the number of branches of the femoral nerve to the rectus femoris and the thickness of the iliocapsularis muscle were noted, averaged and standard deviation was calculated. The presence of a sheath surrounding the rectus femoris was also noted and probability of occurrence (%) was calculated for all appropriate data.
RESULTS
A total of 14 thighs were dissected. One hip was excluded since the rectus nerve supply had been damaged prior to dissection, most likely due to previous femoral artery punctures. In the 13 other specimens, the point of entry of the motor branch into the rectus femoris was at an average of 8.6 ± 1.4 cm from the AIIS and located in the posteromedial aspect of the muscle. The nerve divided into either 1 (1 case = 7.7%), 2 (9 cases = 69.2%), 3 (2 cases = 15.4%) or 4 (1 case = 7.7%) branches, for an average of 2.2 ± 0.7 branches. There was no significant difference between sides. The iliocapsularis muscle was identified in 71% of subjects with an average thickness of 5.5 ± 1.4 mm. The presence of a sheath surrounding the rectus was observed in 21% of the dissected specimens. All results can be seen in Table I.
Table I.
Distance of entry from the AIIS
| Specimen | Gender | Age | Side | Distance (cm) | Number of branches | Distance to artery (cm) | Rectus sheath presence | Iliocapsularis |
|
|---|---|---|---|---|---|---|---|---|---|
| Presence | Thickness (mm) | ||||||||
| 1 | M | 85 | R | 12.5 | 2 | No | Yes | 7.0 | |
| L | 9 | 2 | No | Yes | 4.0 | ||||
| 2 | M | 81 | R | 7.5 | 4 | Yes | Yes | 5.0 | |
| L | 8.6 | 3 | Yes | Yes | 6.0 | ||||
| 3 | M | 74 | R | 7.4 | 2 | No | Yes | 8.0 | |
| L | Damaged | No | Yes | 6.0 | |||||
| 4 | M | 66 | R | 8.8 | 3 | No | Yes | 6.0 | |
| L | 10 | 1 | No | Yes | 5.0 | ||||
| 5 | M | 74 | R | 7.2 | 2 | Yes | No | NA | |
| L | 9.0 | 2 | No | No | NA | ||||
| 6 | F | 84 | R | 7.8 | 2 | No | Yes | 4.0 | |
| L | 7.5 | 2 | No | Yes | 4.0 | ||||
| 7 | F | 89 | R | 7.9 | 2 | No | No | NA | |
| L | 8.2 | 2 | No | No | NA | ||||
| 8 | M | 70 | R | 5.8 | 2 | 7.7 | No | Yes | 3.0 |
| L | Damaged | 8.1 | No | Yes | 4.0 | ||||
| 9 | M | 70 | R | 7.3 | 2 | 7.6 | No | Yes | 3.0 |
| L | 5.9 | 2 | 7.0 | No | Yes | NA | |||
| 10 | M | 80 | R | 8.4 | 3 | 6.5 | No | Yes | 5.0 |
| L | 7.8 | 4 | 7.0 | No | Yes | 4.0 | |||
| 11 | M | 80 | R | 6.0 | 2 | 7.5 | No | Yes | 3.0 |
| L | 7.3 | 2 | 7.5 | No | Yes | 2.0 | |||
| 12 | M | 62 | R | 6.8 | 2 | 7.4 | No | Yes | 3.0 |
| L | 7.3 | 3 | 8.1 | No | Yes | 3.0 | |||
| 13 | M | 92 | R | 6.9 | 2 | 7.3 | No | Yes | 3.0 |
| L | Damaged | 8.0 | No | Yes | 3.0 | ||||
| 14 | M | 89 | R | 7.4 | 3 | 7.8 | No | Yes | 6.0 |
| L | 6.8 | 3 | 7.0 | No | Yes | 6.0 | |||
| 15 | F | 61 | R | 6.4 | 2 | 7.3 | No | Yes | 4.0 |
| L | 6.9 | 2 | 6.8 | No | Yes | 3.0 | |||
| 16 | M | 80 | R | 9.8 | 1 | 8.0 | No | No | NA |
| L | 8.0 | 1 | 8.0 | No | Yes | NA | |||
| Mean | 9.33 | 2.24 | 7.48 | No (91%) | Yes (88%) | 4.4 | |||
| SD | 0.47 | 0.73 | 0.47 | 1.5 | |||||
NA, Not available.
DISCUSSION
Adult PAO’s evolution towards muscle sparing approaches significantly lowered associated morbidity by decreasing the post-operative complications. However, a small incision also required more stretching of the surrounding soft tissue. Accordingly, the rectus-sparing approach is theoretically putting the neurovascular bundle of the rectus femoris at a higher risk. The current study provides a better understanding of the localization and the anatomical variations of the structures encountered at the level of and below the AIIS. It also assesses the relative risk of denervation of the rectus femoris muscle, either by an inadvertent transection of its nerve supply or by overstretching of the soft tissue during surgery. We found that the average distance between the AIIS and the first ramification of the femoral nerve to the rectus femoris was 8.6 ± 1.4 cm. The posteromedial orientation of the nerve entry into the muscle may protect it from direct trauma or stretching.
In terms of clinical application, some limitations were identified in this study. First, the relation between the nerve and the usual skin incision of the rectus-sparing approach could not be established since an extensile skin incision was executed in order to get direct and precise measures. This would have been pertinent since nerve lesions were often suggested to lead to femoral and sciatic nerve palsies complication after the abductor-sparing approach [12, 14, 24]. We can certainly presume that similar complications may be encountered through the rectus-sparing approach. Another study limitation is that all the measurements were taken manually by only one observer with relatively simple measurement devices (Pocket vernier caliper no 184501, Helios-Preisser, Germany) in order to minimize the interobserver bias. Consequently, the reliability calculations and errors in measurements could not be calculated. Another limitation is that the distance studied remain in a single plane (horizontal), which does not put into account the fact that the nerve has a three-dimensional pathway and thus the width of dissection in coronal plane and the depth of dissection in the sagittal plane need to be calculated as well. Finally, our measurements were taken on an absolute scale, unlike the relative measurements presented by Linss et al. [22] which may be more valuable. However, in a surgical setting, when draping is complete and the leg position changes during surgery, distal landmarks, on which the relative measurements are based, may not be as reliable as expected. Although it would have been interesting to consider our data in relation to the body height and the proportions of the specimen’s limb lengths, it seemed to us more practical to work with a fixed bony landmark (AIIS) and an absolute measurable distance.
The anatomy of the femoral nerve had been well described, particularly that inferior to the inguinal ligament. Measures and anatomic descriptions were most often made in relation to the antero-superior iliac spine and the inguinal ligament since these landmarks are relevant for most surgical procedures of the anterior hip [16, 17, 22, 25]. Although those studies shed light on the topography and the understanding of the femoral nerve, there were no studies describing the anatomy of motor branches to the rectus femoris during Bernese PAO in relation to relevant surgical landmarks. The exact location of the motor innervation of the rectus femoris and its distance from the AIIS or the possible anatomical variations remained unclear prior to this study. Contrary to what was presented by Linss et al. [22] who reported three cases out of 83 (3.6%) that showed five or more motor branches to the rectus femoris, in this study, we did not record a specimen with more than four innervating motor branches. We also had only one (7.7%) specimen with a single motor branch supplying the rectus femoris, compared with the 28 cases (33.7%) found in Linss’s study [22].
Major gaps in the anatomical knowledge of the anterior hip and pelvis still exist. For example, the actual function and innervation of the iliocapsularis muscle are still to be determined. Some authors have briefly described this structure and theoretical function [26, 27] and one study used it as an indicator of developmental dysplasia of the hip when hypertrophied [28], but no strategy to preserve this possibly important structure during surgical procedures have been promoted. In these studies, the iliocapsularis muscle was found to be constantly present, which is contrary to our own observations. This may be due to the still undefined anatomical variations of this muscle. It is possible that its trajectory varies sufficiently to the extent that it makes it difficult to properly identify it without completely removing the superficial structures such as the iliopsoas and rectus femoris muscles. Moreover, there is no data describing the maximum mobilization of the iliopsoas or the rectus femoris that can be safely allowed when attempting to improve the surgical exposure and reach deeper for the obturator foramen. We therefore recommend that more research be conducted in order to fully comprehend the possible implications of developing this complex surgical interval between iliopsoas and rectus femoris.
CONCLUSION
In conclusion, the distance from the AIIS to the first ramification of the femoral nerve was found to be 8.6 cm. The femoral nerve was found to have two motor branches innervating the rectus femoris in 69.2% of the observed hips. The rectus did not have any sheath separating it from the tensor fascia lata in 79% of the cases studied and the iliocapsularis muscle was found in 71% of the hips. Based on these observations, we recommend that the dissection medial to the rectus femoris be carried out no further than 7 cm distal to the AIIS. Furthermore, stretching of this area during open surgery should be done cautiously. The clinical efficiency of the rectus-sparing approach needs to be studied in further depth to confirm its advantages over the classic direct anterior approach.
Ethical approval
Each author certifies that his or her institution has approved the protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
ACKNOWLEDGEMENTS
The authors wish to thank Maged Shanin MD for manuscript preparation.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Hoppenfeld S,, deBoer P, Buckley R.. Surgical Exposures in Orthopaedics: The Anatomic Approach. Lippincott Williams & Wilkins, Google Books, 2012. [Google Scholar]
- 2. Peters CL, Erickson JA, Anderson MB. et al. Preservation of the rectus femoris origin during periacetabular osteotomy does not compromise acetabular reorientation. Clin Orthop Relat Res 2015; 473: 608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pajarinen J, Hirvensalo E.. Two-incision technique for rotational acetabular osteotomy: good outcome in 35 hips. Acta Orthop Scand 2003; 74: 133–9. [DOI] [PubMed] [Google Scholar]
- 4. Troelsen A, Elmengaard B, Søballe K.. Comparison of the minimally invasive and ilioinguinal approaches for periacetabular osteotomy: 263 single-surgeon procedures in well-defined study groups. Acta Orthop 2008; 79: 777–84. [DOI] [PubMed] [Google Scholar]
- 5. Ganz R, Klaue K, Vinh TS. et al. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res 1988; 232: 26–36. [PubMed] [Google Scholar]
- 6. Smith-Petersen MN. Approach to and exposure of the hip joint for mold arthroplasty. J Bone Joint Surg Am 1949; 31A: 40–6. [PubMed] [Google Scholar]
- 7. Murphy SB, Millis MB.. Periacetabular osteotomy without abductor dissection using direct anterior exposure. Clin Orthop Relat Res 1999; 364: 92–8. [DOI] [PubMed] [Google Scholar]
- 8. Novais EN, Kim Y-J, Carry PM. et al. The Bernese periacetabular osteotomy: is transection of the rectus femoris tendon essential? Clin Orthop Relat Res 2014; 472: 3142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hussell JG, Mast JW, Mayo KA. et al. A comparison of different surgical approaches for the periacetabular osteotomy. Clin Orthop Relat Res 1999; 363: 64–72. [PubMed] [Google Scholar]
- 10. Pogliacomi F, Stark A, Wallensten R.. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop 2005; 76: 67–74. [DOI] [PubMed] [Google Scholar]
- 11. Yasunaga Y, Yamasaki T, Ochi M.. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res 2012; 470: 3342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clohisy JC, Knaus ER, Hunt DM. et al. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res 2009; 467: 638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ezoe M, Naito M, Asayama I.. Muscle strength improves after abductor-sparing periacetabular osteotomy. Clin Orthop Relat Res 2006; 443: 161–8. [DOI] [PubMed] [Google Scholar]
- 14. Sierra RJ, Beaule P, Zaltz I. et al. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res 2012; 470: 2209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goubier J-N, Teboul F, Yeo S.. Transfer of two motor branches of the anterior obturator nerve to the motor portion of the femoral nerve: an anatomical feasibility study. Microsurgery 2012; 32: 463–5. [DOI] [PubMed] [Google Scholar]
- 16. Yang D, Morris SF.. Neurovascular anatomy of the rectus femoris muscle related to functioning muscle transfer. Plast Reconstr Surg 1999; 104: 102–6. [PubMed] [Google Scholar]
- 17. Gustafson KJ, Pinault GCJ, Neville JJ. et al. Fascicular anatomy of human femoral nerve: implications for neural prostheses using nerve cuff electrodes. J Rehabil Res Dev 2009; 46: 973.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Philippon MJ, Michalski MP, Campbell KJ. et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am 2014; 96: 1673–82. [DOI] [PubMed] [Google Scholar]
- 19. Hewitt JD, Glisson RR, Guilak F. et al. The mechanical properties of the human hip capsule ligaments. J Arthroplasty 2002; 17: 82–9. [DOI] [PubMed] [Google Scholar]
- 20. Flack N, Nicholson HD, Woodley SJ.. The anatomy of the hip abductor muscles. Clin Anat 2014; 27: 241–53. [DOI] [PubMed] [Google Scholar]
- 21. Ryan JM, Harris JD, Graham WC. et al. Origin of the direct and reflected head of the rectus femoris: an anatomic study. Arthroscopy 2014; 30: 796–802. [DOI] [PubMed] [Google Scholar]
- 22. Linss W, Fröhlich J, Schmidt M. et al. [The nerve entry into the human femoral quadriceps muscle]. Anat Anz 1990; 170: 281–7. [PubMed] [Google Scholar]
- 23. Liu L, Zheng G, Bastian JD. et al. Periacetabular osteotomy through the pararectus approach: technical feasibility and control of fragment mobility by a validated surgical navigation system in a cadaver experiment. Int Orthop 2016; 40: 1389–96. [DOI] [PubMed] [Google Scholar]
- 24. Peters CL, Erickson JA, Hines JL.. Early results of the Bernese periacetabular osteotomy: the learning curve at an academic medical center. J Bone Joint Surg Am 2006; 88: 1920–6. [DOI] [PubMed] [Google Scholar]
- 25. Sung DH, Jung J-Y, Kim H-D. et al. Motor branch of the rectus femoris: anatomic location for selective motor branch block in stiff-legged gait. Arch Phys Med Rehabil 2003; 84: 1028–31. [DOI] [PubMed] [Google Scholar]
- 26. Babst D, Steppacher SD, Ganz R. et al. The iliocapsularis muscle: an important stabilizer in the dysplastic hip. Clin Orthop Relat Res 2011; 469: 1728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ward WT, Fleisch ID, Ganz R.. Anatomy of the iliocapsularis muscle. Relevance to surgery of the hip. Clin Orthop Relat Res 2000; 374: 278–85. [DOI] [PubMed] [Google Scholar]
- 28. Haefeli PC, Steppacher SD, Babst D. et al. An increased iliocapsularis-to-rectus-femoris ratio is suggestive for instability in borderline hips. Clin Orthop Relat Res 2015; 473: 3725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]