Abstract
The paracellular gap formed by endothelial cell (EC) contraction is fundamental for endothelium permeability, but the mechanism underlying EC contraction has yet to be determined. Here, we identified the zipper-interacting protein kinase (ZIPK) as the kinase for EC contraction and myosin light chain (MLC) phosphorylation. Inhibition of ZIPK activity by pharmacological inhibitors and small interfering RNAs led to a significant decrease in the mono- and diphosphorylation of MLCs along with a contractile response to thrombin, suggesting an essential role of ZIPK in EC paracellular permeability. To assess the role of ZIPK in vivo, we established mouse lines with conditional deletion of Zipk gene. The endothelium-specific deletion of Zipk led to embryonic lethality, whereas the UBC-CreERT2–mediated deletion of Zipk by tamoxifen induction at adulthood caused no apparent phenotype. The induced deletion of Zipk significantly inhibited ischemia-reperfusion–induced blood-brain barrier dysfunction and neuronal injuries from middle cerebral artery occlusion and reperfusion, as evidenced by reduced infarct and edema volume, attenuated Evans blue dye leakage, and improved neuronal behavior. We thus concluded that ZIPK and its phosphorylation of MLC were required for EC contraction and ischemic neuronal injuries. ZIPK may be a prospective therapeutic target for stroke.—Zhang, Y., Zhang, C., Zhang, H., Zeng, W., Li, S., Chen, C., Song, X., Sun, J., Sun, Z., Cui, C., Cao, X., Zheng, L., Wang, P., Zhao, W., Zhang, Z., Xu, Y., Zhu, M., Chen, H. ZIPK mediates endothelial cell contraction through myosin light chain phosphorylation and is required for ischemic-reperfusion injury.
Keywords: MLC phosphorylation, stroke, ischemia-reperfusion injury
Endothelial cells (ECs) line the intimal layer of the blood vessel and form a semipermeable barrier. In addition to the transport and exchange of essential solutes and nutrients, EC permeability is important for multiple physiologic processes, such as inflammation, transendothelial migration of immune cells, thrombosis, angiogenesis, arteriogenesis, and others (1–4). Thus, EC is essential for physiologic homeostasis and for adaptive responses to stresses imposed by pathologic disorders. Abnormalities of endothelial permeability may give rise to diseases such as sepsis, systemic capillary leak syndrome, multiple sclerosis, stroke, and reperfusion injuries (5–9).
Endothelial permeability reflects transcellular and paracellular mechanisms, in which the latter mechanism is considered a primary and efficient mechanism for permeability (10). Paracellular permeability is primarily determined by cell-cell gaps between the contacts of ECs. Physiologically, the EC contacts are formed by 2 types of junctional complexes: adherens junctions (AJs) and tight junctions (TJs). TJs regulate the diffusion of water, ions, and small molecules, whereas AJs have more diverse functions in the regulation of edema, matrix exposure, and leukocyte transendothelial migration (10–12). AJs are primarily composed of transmembrane vascular endothelial cadherin proteins between adjacent ECs and AJs anchored to the cytoskeleton (13, 14). Upon EC contraction, the reorganized cytoskeleton breaks the contact and forms intercellular gaps. The resulting gaps allow macromolecules and inflammatory cells to cross the endothelium layer (15). During this process, EC contraction or cytoskeleton reorganization have been considered as limiting steps for permeability regulation.
Similar to smooth muscle cells, ECs contract through actomyosin interactions involving regulatory myosin light chain (MLC) phosphorylation (16, 17). Upon stimulation with cytokines, proinflammatory factors, and bioactive lipids, MLC is phosphorylated by a kinase or kinases and hence regulates the EC barrier (18). However, the identity of the kinase remains controversial. During smooth muscle contraction, MLC kinase (MLCK) has been well documented to be the exclusive kinase for MLC phosphorylation (19, 20). Several reports have indicated that the long isoform of MLCK is the kinase for MLC phosphorylation and is necessary for EC permeability (18, 21, 22). However, our previous report shows that freshly isolated endothelium has no long isoform of MLCK expression, and deletion of total MLCK in the endothelium has no apparent effect on EC permeability and MLC phosphorylation (23). Thus, the calcium-calmodulin (CaM)–dependent MLCK is not required for MLC phosphorylation and permeability of endothelium. This conclusion is further supported by the observations from other groups as well (24, 25). Interestingly, some kinases, such as integrin-like kinase, zipper-interacting protein kinase (ZIPK), and P21-activated kinase, also have kinase activity of MLC phosphorylation in muscle and nonmuscle cells (26–28). Whether these kinases contribute to EC MLC phosphorylation has yet to be determined.
ZIPK, a serine-threonine kinase, belongs to the death-associated protein kinase (DAPK) family including DAPK1, DAPK2 and DAPK3 (also named ZIPK) (29). DAPKs consist of an N-terminal kinase region, a CaM-binding region, ankyrin repeats, a cytoskeleton-binding region, a C-terminal death region, and a highly conserved catalytic core. Unlike MLCK and other DAPK members, ZIPK activity is independent of calcium-CaM signaling because it lacks a CaM-binding domain. In smooth muscle, ZIPK potentially phosphorylates MLC, C-kinase–activated protein phosphatase 1 inhibitor 17, and myosin-targeting subunit 1 of MLC phosphatase, thereby regulating smooth muscle contraction in a Ca2+-independent manner (30–32). In nonmuscle cells, ZIPK catalyzes MLC phosphorylation and thereby regulates apoptotic membrane blebbing, cell motility, and cytokinesis (27, 33–35). The underlying mechanism may involve interplaying with transcriptional factors, including activating transcription factor 4, signal transducer and activator of transcription 3 in the nucleus (36), and the actin filament that is necessary for extrusion of lamellipodia-like protrusions and remodeling of actin stress fibers (37). However, whether ZIPK is involved in MLC phosphorylation of ECs during the contraction process remains to be determined. In this report, we first examined the role of ZIPK in MLC phosphorylation of ECs and found that inhibition of ZIPK abolished EC MLC phosphorylation and contraction. This suggests an essential role of ZIPK in EC contraction-mediated paracellular permeability. Endothelium-specific deletion of ZIPK led to embryonic defect, whereas the deletion of ZIPK in adult mice showed no apparent physiologic alteration. However, the latter mice showed a significant protection against neuronal injuries and ischemia-reperfusion–induced impairment of the blood-brain barrier (BBB). These findings suggest that ZIPK-mediated EC permeability is necessary for responding to pathological stresses. Inhibition of ZIPK activity may be a promising therapeutic target for endothelial permeability-related diseases.
MATERIALS AND METHODS
Cell culture and thrombin treatment
Primary cultured HUVECs were grown within 3–10 passages in medium 199 containing 20% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), thymidine (MilliporeSigma, Burlington, MA, USA), acidic human fibroblast growth factor (PeproTech, Rocky Hill, NJ, USA), HEPES (MilliporeSigma), and penicillin-streptomycin (Thermo Fisher Scientific). The culture dishes were precoated with 0.1% rat-tail collagen (BD Biosciences, San Jose, CA, USA). HUVEC manipulations for this study were approved by the First Affiliated Hospital of Nanjing Medical University (2012-SR-153).
The murine brain microvessel ECs (BMECs) were isolated and cultured according to the method previously reported (38, 39). Briefly, the brains were dissected and the meninges containing blood vessels were peeled off and discarded. Using 15% dextran, the capillary fragments were isolated from brain tissues with the removal of cerebellum, brain stem, and white matter. After digestion with collagenases, the BMECs were harvested and cultured in DMEM containing 20% fetal bovine serum, 1% l-glutamine (Thermo Fisher Scientific), 0.25 ng/ml endothelial growth factor (R&D Systems, Minneapolis, MN, USA), 0.001 ng/ml basic fibroblast growth factor (R&D Systems), 0.1 ng/ml vitamin C, 4 ng/ml hydrocortisone, 0.68 µg/ml heparin sodium, and penicillin-streptomycin.
HUVECs and BMECs were cultured for 2 d after reaching confluence. The cells were exposed to thrombin (MilliporeSigma) in the presence or absence of ZIPK inhibitors, staurosporine (STS; Selleck Chemicals, Houston, TX, USA), and Tc-DAPK6 (Tocris Bioscience, Bristol, United Kingdom).
Animals
Male C57BL/6 mice were purchased from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). The mice were maintained at the National Resource Center for Mutant Mice (Nanjing University) and the Nanjing Normal University specific-pathogen-free grade animal facility (Nanjing, China). All animal procedures were performed in accordance with the guidelines of the Animal Care and Use Committee of Nanjing University and Nanjing Normal University.
ZIPK knockout mouse generation
The Zipk-targeting vector was constructed using a bacterial artificial chromosome retrieval method (40). In brief, the Zipk locus encoding the full-length ZIPK protein was retrieved from a C57BL/6 bacterial artificial chromosome clone (provided by the Sanger Institute, Hinxton, United Kingdom) by a retrieval vector containing 2 homologous arms. Exons 2–5 were flanked by 2 loxP sites which can be cut by Cre recombinase and an frt-Neo-frt (frt is the recombination site of flpase) cassette as a positive selection marker. Embryonic stem cells were electroporated with the linearized targeting vector, selected with neomycin, and the positive clones were expanded. Chimeric mice were generated by injecting a positive clone into C57BL/6 blastocysts followed by transfer into pseudopregnant mice.
To ablate the Zipk gene specifically in ECs, the chimeric mice were crossed with Tie2-Cre transgenic mice, which express noninducible Cre recombinase under the control of the receptor tyrosine kinase (Tie2) promoter. To delete the Zipk gene globally in adult mice, the floxed mice were crossed with UBC-CreERT2 transgenic mice containing the human ubiquitin C (UBC) promoter driving expression of Cre ERT2 recombinase, in which Cre activity can be induced by tamoxifen (MilliporeSigma) injection. To ablate the Zipk gene specifically in smooth muscle cells, the Zipk floxed mice were crossed with SMA-Cre transgenic mice containing the smooth muscle actin (SMA) promoter driving expression of the Cre recombinase, which expressed Cre in smooth muscle. For induction of Cre activation, tamoxifen was dissolved in sunflower oil at 10 mg/ml and intraperitoneally injected (0.1 ml/20–25 g body weight) for 5 consecutive days. Approximately 4–6 wk after injection, the mice were subjected to subsequent experiments.
Transfection of ZIPK small interfering RNAs
To knock down the expression of ZIPK, 90%-confluent HUVECs were transfected with ZIPK small interfering RNAs (siRNAs; the sequences are listed in Supplemental Table S2) or nontargeting siRNAs [negative control (NC)–siRNAs] (MilliporeSigma) using LipoMax transfection reagent (Sudgen, Nanjing, China) or Lipofectamine 2000 (Thermo Fisher Scientific). The cells were stimulated with thrombin after 48 h of transfection.
Real-time quantitative PCR
Total RNA from tissues was extracted with Trizol Reagent (Thermo Fisher Scientific). cDNA was synthesized by using the PrimeScript RT Reagent Kit (Takara, Kyoto, Japan) and quantified by quantitative PCR using a SYBR Green kit (Vazyme, Nanjing, China) in a StepOnePlus cycler (Thermo Fisher Scientific) according to the manufacturer’s instructions. The PCR primers were as follows: mouse Zipk forward, 5′-CATGTTGCTGGACAAGCACG-3′; reverse: 5′-ATGCTCCACATGTCAGCCTC-3′. mRNA expression was normalized by glyceraldehyde 3-phosphate dehydrogenase (Gapdh) to evaluate the relative level.
Western blotting
Lysates from tissues or cells were subjected to electrophoresis on a 10% or 12% wt/vol polyacrylamide gel and transferred onto PVDF membrane (0.22 or 0.45 µm; MilliporeSigma). The transferred membranes were blocked with 5% wt/vol skim milk and sequentially probed with specific primary antibodies and the appropriate horseradish peroxidase–conjugated secondary antibody. The signals were then visualized by incubation in Clarity Western ECL Substrate (Bio-Rad, Hercules, CA, USA) prior to scanning (Tanon 4500; Tanon Science & Technology, Shanghai, China). The primary antibodies used are specific to MLC (Cell Signaling Technology, Danvers, MA, USA), monophosphorylated MLC (pMLC) Serine 19 (Cell Signaling Technology), diphosphorylated MLC (ppMLC) Threonine 18/ Serine 19 (Cell Signaling Technology), CD31 (BD Biosciences), and ZIPK (Sudgen). The intensity of the bands was determined by ImageJ software (National Institutes of Health, Bethesda, MA, USA).
Immunofluorescence staining
Anesthetized mice were perfused with PBS followed by 4% paraformaldehyde (PFA; MilliporeSigma) wt/vol in PBS. The tissues were fixed with 4% PFA at 4°C for 4 h, dehydrated overnight with 30% sucrose and embedded in O.C.T. compound (Sakura, Torrance, CA, USA). The tissues were sectioned at a thickness of 10 μm by a freezing microtome (Leica CM1950; Leica Microsystems, Buffalo Grove, IL, USA). The tissue sections or cells grown on rat collagen-coated coverslips were rinsed with PBS with 0.1% Tween 20, permeabilized with 0.2% Triton X-100 (MilliporeSigma) in PBS, and blocked with 5% normal goat serum (Thermo Fisher Scientific).
After overnight incubation with primary antibodies at 4°C, the slides were probed with the corresponding fluorescent secondary antibodies (Thermo Fisher Scientific). The resultant slides were then incubated with DAPI (MilliporeSigma) to visualize the cell nuclei, and fluorescent phalloidin (MilliporeSigma) stained the actin filaments. The slides were then washed and mounted with ProLong Gold Antifade Mountant (Thermo Fisher Scientific) and imaged using a confocal microscope (Nikon A1; Nikon, Tokyo, Japan). Image processing was performed by using Adobe Photoshop software (Adobe, San Jose, CA, USA).
For whole-mount staining of the embryo (41), the embryo was dissected and rinsed with PBS. The sample was then fixed with 4% PFA for 2 h, rinsed with PBS for 15 min, and blocked with 10% normal goat serum for 3 h. The embryo was then incubated with anti-CD31 antibody at 4°C overnight with gentle shaking. After rinsing with PBS, the embryo was probed with the corresponding fluorescent secondary antibody and imaged as previously described.
Transendothelial electrical resistance
The endothelial barrier function was evaluated by assessing the transendothelial electrical resistance (TEER) with electrodes connected to a Millicell ERS-2 Volt-Ohm Meter (MilliporeSigma) (42). Briefly, confluent HUVECs were seeded on a Transwell insert (0.4-μm pore size, 6.5-mm diameter) in a 24-well culture plate (Costar, Cambridge, MA, USA). After culture for 3 d, the medium from both the upper and lower chamber was refreshed prior to the addition of thrombin to the upper chamber. The electrical resistance value was recorded, and the calculated TEER values were expressed by Ω × cm2.
In vivo endothelial permeability
The in vivo endothelial permeability was evaluated by leakage of Evans blue dye (EBD; MilliporeSigma) according to the method previously described in Gautam et al. (43). Briefly, C57BL/6 mice were intravenously injected with 100 μl EBD (2% in PBS). Four hours after injection, the mice were anesthetized prior to flushing blood EBD with 20 ml PBS through left cardiac ventricle perfusion. The organs were dissected and homogenized with 1 ml saline per 100 mg of tissue. The resultant homogenates were incubated with 2 volumes of formamide for 18 h at 60°C and then centrifuged at 12,000 g for 20 min. The supernatant was collected and the absorbance at 620 nm was measured on a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT, USA).
Vessel contractility assay
The second-order branches of the mesenteric artery were isolated and mounted for isometric tension measurements (610M; Danish Myo Technology, Aarhus, Denmark) as previously reported in He et al. (44). After equilibrating in preoxygenated HEPES-Tyrode buffer [137 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 10 mM HEPES (pH 7.4)] at 37°C for 30 min, the resting tension was adjusted to a value comparable to 100 mmHg pressure in vivo. The artery segments were equilibrated for 20 min before testing contractile capacity by exposure to 124 mM KCl-containing buffer [15.7 mM NaCl, 124 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 10 mM HEPES (pH 7.4)]. The contractile activity of the vessels in response to KCl, norepinephrine (NE), endothelin-1 (ET-1), phenylephrine (PE) and acetylcholine (ACh) was measured.
Blood pressure measurement
The systolic and mean blood pressures of conscious adult mice were recorded using a tail-cuff system (ALC Noninvasive Blood Pressure System; Shanghai Alcott Biotech, Shanghai, China) as previously reported in He et al. (44). Mice were kept in a warm box (32°C) and the blood pressure was measured for 20 min at the same time every day. The mice were trained for 7 d before the measurements were recorded.
Electrocardiogram recordings
Surface electrocardiogram was performed as previously described in Frasier et al. (45). Briefly, the mice were anesthetized with 1.25% vol/vol avertin. Leads were placed through the skin. After the heart rate was stabilized, 5 min of baseline recordings were collected with Powerlab 8130 ML870 (ADInstruments, Sydney, Australia).
Middle cerebral artery occlusion procedure
The male animals were blindly and randomly assigned in our study. Transient cerebral ischemia was generated in 8–10-wk-old male mice (22–25 g body weight) by middle cerebral artery occlusion (MCAO) as previously described in Chen et al. (46). Briefly, the mice were anesthetized, and the body temperature was maintained at 37 ± 0.5°C. A 6-0 monofilament nylon suture with a heat-rounded tip was inserted into the right external carotid artery until the plug reached the start site of the middle cerebral artery. A successful surgery was determined by a >70% decrease of local brain blood flow monitored by a Doppler flowmeter (Perimed, Järfälla, Sweden). After 60 min of occlusion and 24 h of reperfusion, the mice were euthanized and their brains were successively cut in a coronal direction. The section thickness was 1 mm.
Brain infarct volume and edema assessment
The infarct volume was assessed by 2, 3, 5-triphenyltetrazolium chloride (TTC; MilliporeSigma) staining according to the protocol previously described in Bederson et al. (47). Briefly, the brain sections were immersed in a 2% TTC solution at 37°C for 20 min in the dark. The resulting pale gray color (TTC negative) represents damaged areas, and the red color (TTC positive) represents normal areas. The infarct volumes were calculated as a percentage of the infarcted volume vs. the ipsilateral hemisphere. Brain edema was determined as the increased volume of the ipsilateral hemisphere compared with the noninfarcted volume of the contralateral hemisphere. The volumes were quantified by ImageJ software.
BBB disruption evaluation
The ischemia-reperfusion–induced BBB disruption was evaluated by leakage of EBD. One hundred microliters EBD (2% in PBS) was injected into mice via tail vein at 20 h postreperfusion. Four hours after reperfusion, the brain was dissected for preparing coronal sections at 2-mm thickness. The EBD in the brain was visualized by photographing whole brain and brain slices. EBD concentration in the brain was measured by brain extraction and absorbance measurement at 620 nm.
Neurological function assessment
Two investigators blinded to the mouse groups performed the tests. At 24 h after the MCAO surgery, a modified neurologic severity score (mNSS), which comprises a motor test and beam balance test, was used to assess neurologic deficits (46, 48). One point was given when a mouse failed to perform a test or lacked a tested reflex.
Statistical analyses
Statistical analyses of the data were performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). All biological replicates and group sizes used for statistical analyses are indicated in the figure legends. The results are presented as the means ± sd or sem. A 2-tailed unpaired (or paired) Student’s t test was used to determine the significance of differences between 2 groups. A value of P < 0.05 was considered significant.
RESULTS
ZIPK protein is abundantly expressed in ECs in vivo
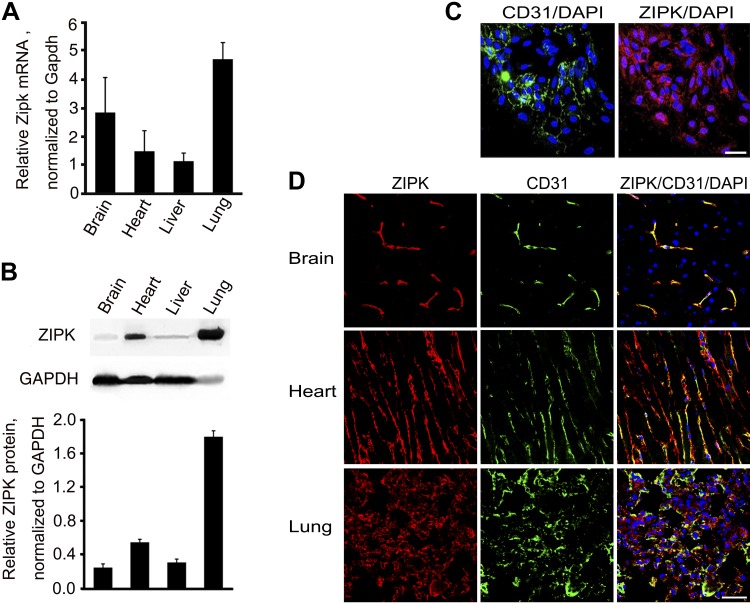
We first measured Zipk mRNA expression in mouse organs and found that the brain, heart, liver, and lung expressed abundant Zipk mRNA (Fig. 1A), which was consistent with previous reports (37). For ZIPK protein measurement, we evaluated several commercial anti-mouse ZIPK antibodies but none worked well in both Western blots and immunofluorescence assays. Thus, we prepared a hybridoma clone (Z2 clone) expressing an mAb against mouse ZIPK (Sudgen) by immunizing mice with intact mouse ZIPK protein. Western blot assay showed a positive band of about 52 Kd in the BMEC sample of C57BL/6 mice, and a significantly decreased band in tamoxifen-induced Zipk knockout (KO) BMECs (Supplemental Fig. S1). We validated this antibody by immunostaining ZIPK in aorta and found a remarkable decrease in ZIPK protein in mutant aorta (Supplemental Fig. S2). With this antibody, we measured ZIPK protein in mouse organs, including brain, liver, lung, and heart. The relative expression levels in the brain and liver were substantially lower than the levels in the lung and heart (Fig. 1B). To investigate ZIPK protein expression in ECs, we performed immunofluorescence staining with Z2 anti-mouse ZIPK and anti-CD31 antibodies. First, we stained the ZIPK protein of primary cultured BMECs and found that ZIPK protein diffusely distributed in the cytoplasm and nuclei of CD31-positive cells (Fig. 1C). In the brain tissue, ZIPK protein was predominantly expressed in CD31-positive cells, whereas no apparent signal was found in the CD31-negative region (Fig. 1D). Because the neurons were well delineated by NeuN staining within the CD31-negative region (Supplemental Fig. S3), it implied no apparent expression of ZIPK in neurons. In heart tissue, ZIPK protein was detected both in CD31-positive and -negative cells (Fig. 1D). Cardiac troponin T (cTNT)-positive cells occupied the most area of the CD31-negative region, indicating that the cardiac muscle cells also express ZIPK abundantly (Supplemental Fig. S3). Similarly, in lung tissue, ZIPK was expressed in CD31-positive and a few CD31-negative cells (Fig. 1D). This observation suggests an abundant expression of ZIPK in ECs and implies an essential role of ZIPK in the endothelium.
Figure 1.
Expression of ZIPK in ECs of different tissues. A) Expression of Zipk mRNA in the brain, heart, liver, and lung tissues of mice was measured by quantitative PCR and normalized by Gapdh mRNA; n = 3. B) ZIPK protein in mouse brain, heart, liver, and lung tissues was measured by Western blotting and normalized by GAPDH; n = 3. C) Immunostaining of ZIPK (red), CD31 (green), and nuclei (DAPI; blue) of primary cultured BMECs. Scale bar, 10 μm. D) Immunostaining of ZIPK (red), CD31 (green), and nuclei (DAPI; blue) of brain, heart, and lung tissues of mice. Scale bar, 20 μm.
ZIPK is required for contraction and MLC phosphorylation in ECs
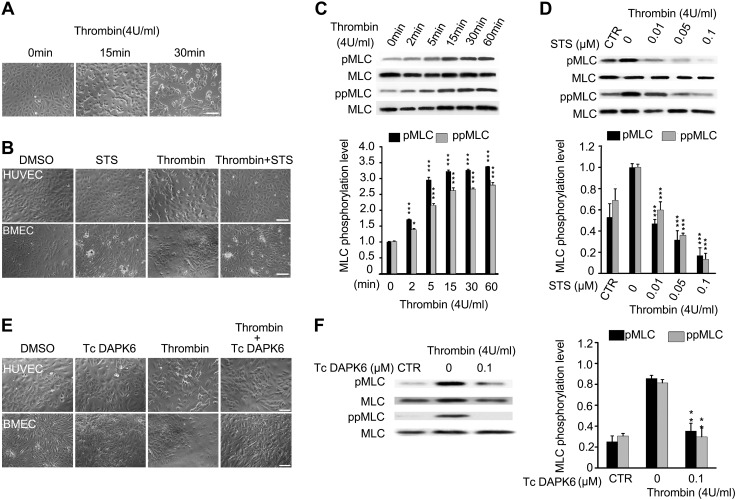
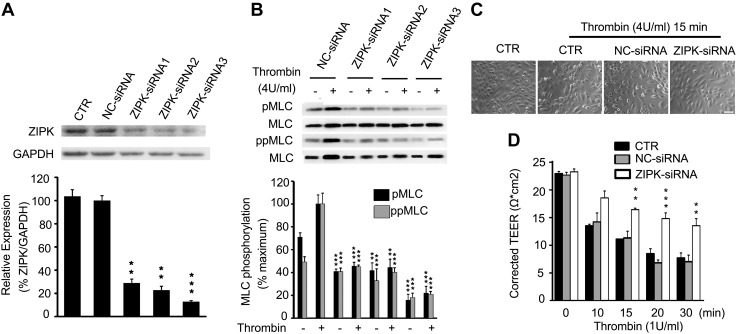
Because MLC phosphorylation is necessary for contractile responses of muscle and nonmuscle cells (20, 49, 50), we speculated that ZIPK might regulate EC contraction through phosphorylating MLC during the regulation of endothelial permeability. We first detected the contraction of HUVECs in response to thrombin. Thrombin is an agonist of the protease-activated receptor and acts as a vascular EC mediator of cell contraction (51). Upon incubation with thrombin (4 U/ml), HUVECs initiated a contraction in terms of cell rounding and cell-cell gap formation. The cells contracted slightly within 10 min, and apparently within 15 min and maintained for at least 30 min (Fig. 2A). We then measured the effects of STS, a broadly-acting protein kinase inhibitor with known effect against ZIPK (52), on thrombin-induced EC contraction. Upon incubation with STS, both the HUVECs and BMECs showed an apparent inhibition of cell contraction, as evidenced by substantially fewer cells rounding up (Fig. 2B). Consistent with the contraction behavior, both the pMLC and ppMLC levels were rapidly increased after thrombin exposure (Fig. 2C). Accordingly, the mono- and diphosphorylation of MLCs was inhibited in a dose-dependent manner (Fig. 2D). We also applied Tc-DPAK6, a more specific inhibitor of ZIPK (53), to above experimentation. As shown in Fig. 2E, F, upon exposing HUVECs to thrombin and Tc-DAPK6, both cell contraction and MLC phosphorylation were significantly suppressed as well. Because Tc-DAPK6 potentially inhibited Rho-associated, coiled-coil–containing protein kinase II (ROCKII) activity (54), we next applied 3 independent siRNAs to knock down ZIPK expression in HUVECs, which further confirmed the effects above. After transfection with these siRNAs, all sets of HUVECs showed about 70–85% decrease in ZIPK protein (Fig. 3A). Upon stimulation with thrombin, the HUVECs transfected with siRNAs showed a significant decrease in MLC phosphorylation and contractility (Fig. 3B, C). Quantification showed that the thrombin-induced MLC phosphorylation in siRNA-transfected cells was significantly inhibited (P < 0.001) to the level comparable with that in the absence of thrombin stimulation. As a control, NC-siRNA led to no alteration of MLC phosphorylation in response to thrombin (Supplemental Fig. S4).
Figure 2.
Thrombin-induced EC contraction and MLC phosphorylation are suppressed by ZIPK inhibitors. A) Contraction of HUVECs induced by thrombin (4 U/ml) for 15 and 30 min; n = 3. B) Inhibition effects of STS (0.01 μM) on HUVECs and BMECs contractile in response to thrombin (4 U/ml); n = 3. C) MLC phosphorylation in HUVECs upon thrombin (4 U/ml) treatment at different time points; n = 3. D) Inhibition effects of STS on MLC phosphorylation in HUVECs induced by thrombin (4 U/ml) for 15 min; n = 3. Control (CTR) represents the cells without thrombin and STS treatment. E) Inhibition effects of Tc-DAPK6 (0.1 μM) on HUVECs and BMECs contractile in response to thrombin (4 U/ml); n = 3. F) Inhibition effects of Tc-DAPK6 (0.1 μM) on MLC phosphorylation in HUVECs induced by thrombin (4 U/ml) treatment; n = 3. CTR represents the cells without thrombin and Tc-DAPK6 treatment. All the above data are presented as means ± sd. Scale bars, 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3.
Suppression of thrombin-induced EC contraction and MLC phosphorylation by siRNA-mediated ZIPK knockdown. A) ZIPK expression in HUVECs was knocked down by siRNAs. B) Reduction of MLC phosphorylation in HUVECs with down-regulated ZIPK. C) Inhibition effects on contractile response to thrombin in HUVECs with down-regulated ZIPK. D) TEER values in HUVECs with thrombin (1 U/ml) exposure at different time points. The data were obtained from 3 independent experiments (means ± sd). Scale bar, 100 μm. **P < 0.01, ***P < 0.001.
To assess the role of ZIPK in the EC barrier in vitro, we measured TEER of HUVECs transfected with ZIPK siRNA. Without thrombin treatment, all groups of monolayer cells showed high TEER values (∼22 Ω × cm2). After treating with thrombin (1 U/ml) for 10 min, the control cells or NC-siRNA–transfected cells displayed an apparent decrease in TEER value and lasted for up to 30 min, whereas the ZIPK siRNA-transfected cells showed a moderate decrease (Fig. 3D). Statistical analysis showed significant differences among the groups (P < 0.01). Collectively, our results showed that the inhibition of ZIPK caused an apparent decrease in contraction and MLC phosphorylation of HUVECs, suggesting an essential role of ZIPK in EC contraction and permeability.
Phenotypic characterization of ZIPK KO mice
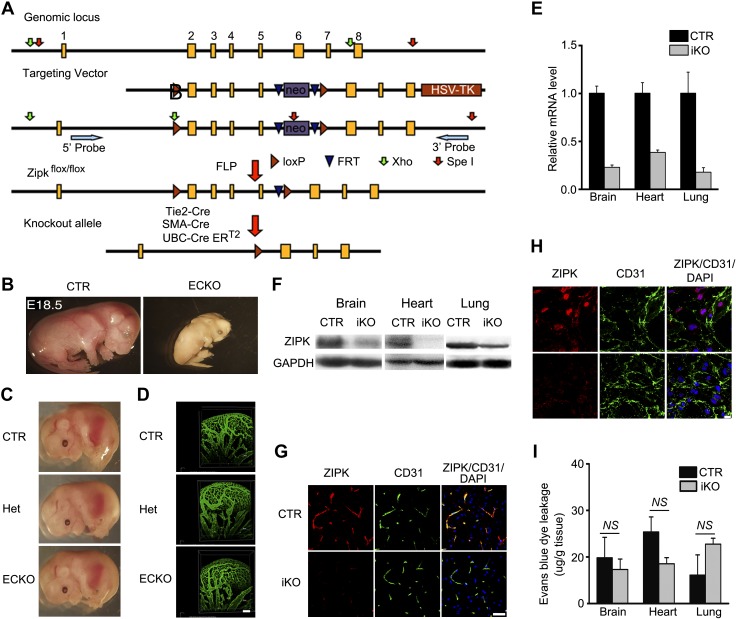
To investigate the in vivo role of ZIPK, we established a line with a C57BL/6 genetic background by inserting 2 loxP sites at the 5′ and 3′ ends of exons 2–5 of the Zipk gene. The floxed mice (Zipkflox/flox) were then crossed with Tie2-Cre transgenic mice to generate EC-specific KO (ECKO) mice (Tie2-Cre–Zipkflox/flox) (Fig. 4A). Crossing Tie2-Cre−–Zipkflox/flox mice with Tie2-Cre+–Zipkflox/+ mice gave no homozygotes (Tie2-Cre–Zipkflox/flox) (Supplemental Table S1), indicating an embryonic lethality of ECKO mice. We then examined the mice at different embryonic stages and found that the ECKO mice died from approximately embryonic d 17 (E17) to E18.5. The dead mutant embryos appeared pale in color and small in size (Fig. 4B), whereas the living E14.5 embryos showed normal color appearance and body size (Fig. 4C). The number of embryos with different genotypes suggested that the embryos obeyed the Mendelian ratio (Supplemental Table S1). Because EC is an essential component for the formation of blood vessels during development, we then examined the vascular network of the embryonic brains by whole-mount immunostaining with anti-CD31 antibody (41). Because the murine blood vessel starts to develop from E6.5 (55) and Tie2-Cre is expressed at approximately E6.5 in ECs (56), we subjected E11.5 embryos to phenotypic analysis. As shown in Fig. 4D, the homozygote brain vessels showed no apparent abnormalities, as evidenced by the normal global morphology, regular branches, and normal color, indicating that the development of the vascular network was not affected. Although the cause of the embryonic death was unclear thus far, this result showed a critical role of endothelial ZIPK in embryonic development. Previous observation from global KO of ZIPK that shows embryonic death around E8.5 further supports this conclusion (57).
Figure 4.
Characterization of ZIPK KO mice. A) Targeting strategy of Zipk floxed mice. B) Representative murine embryos (E18.5) of Zipk ECKO mice. C) Representative murine embryos of Zipk ECKO (E14.5); n = 8. D) CD31 staining of brain blood vessel system in Zipk ECKO murine embryos (E11.5); n = 3. Scale bar, 100 μm. E) Quantitative PCR detection of Zipk mRNA expression in iKO mouse tissues including brain, heart, and lung. The data were normalized as folds of the level identified in the control; n = 3. F) Western blotting analysis of ZIPK protein expression in the tissues of iKO mice. n = 3. G) Decreased ZIPK expression in iKO brain parenchyma tissue detected by immunostaining. ZIPK (red), CD31 (green) and DAPI (blue). Scale bar, 10 μm. H) Decreased ZIPK expression in primary cultured BMECs of iKO mice detected by immunostaining. ZIPK (red), CD31 (green) and DAPI (blue). Scale bar, 10 μm. I) EBD leakage in mouse brain, heart, and lung tissues under physiological conditions; n = 3. Data are presented as means ± sem. NS: P > 0.05.
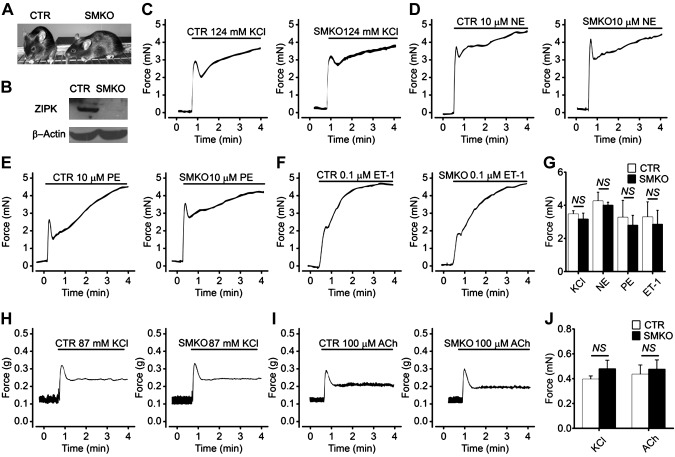
To assess the role of ZIPK in adult endothelium, we generated a line with an inducible global deletion of ZIPK by crossing the Zipkflox/flox with UBC-CreERT2–transgenic mice (Fig. 4A). Because ZIPK is reported to regulate smooth muscle contraction that potentially affects endothelium function (58), we simultaneously generated a line with a smooth muscle–specific KO (SMKO) of ZIPK. We crossed Zipkflox/flox mice with SMA-Cre transgenic mice and generated a line of SMA-Cre–Zipkflox/flox (Fig. 5A). Western blot assays showed that ∼90% ZIPK protein was deleted in gut smooth muscle (Fig. 5B). The genotypes of the floxed mice were confirmed by Southern blot (Supplemental Fig. S5) and sequencing. The Zipk SMKO mice exhibited normal size, normal body weight, and normal physiologic behaviors. Macrophenotypic analyses showed no apparent histologic alterations in the brain, liver, heart, kidney, jejunum, airway, and mesenteric artery (unpublished results). The blood pressure was not altered as well (Supplemental Fig. S6). Upon stimulation with KCl, NE, PE, and ET-1, the mutant mesenteric artery smooth muscles showed a typical initial robust followed by sustained contraction (Fig. 5C–G). The mutant jejunum smooth muscle, however, showed a typical robust followed by a rapid relaxation when stimulated with KCl or ACh (Fig. 5H–J). Quantitation showed no difference of maximal force tension of mesenteric artery or peak force of jejunum smooth muscle between the control group and Zipk SMKO group (P > 0.05). We thus concluded that ZIPK deletion did not affect smooth muscle contraction. We then crossed Zipkflox/flox mice with UBC-CreERT2–transgenic mice and obtained the global and inducible KO (iKO) mice (UBC-CreERT2+/+–Zipkflox/flox). After induction with tamoxifen, Zipk mRNA levels in the brain, heart, and lung of iKO mice were reduced significantly in contrast with the control littermates (UBC-CreERT2−/−–Zipkflox/flox or UBC-CreERT2−/−–Zipkflox/+, control) (Fig. 4E), and ZIPK protein was accordingly decreased (Fig. 4F). We further confirmed the ablation of ZIPK in brain by immunofluorescence staining (IF) (Fig. 4G). Consistently, the significantly reduced expression of ZIPK protein in mutant BMECs cultured from iKO brain was determined by IF and Western blotting (Fig. 4H and Supplemental Fig. S1). These data indicated a high KO efficiency of ZIPK in iKO mice. After tamoxifen induction, the mutant mice appeared normal in terms of appearance, physiological behavior, life span, and the weights of the body as well as the organs (brain, heart, liver, lung, and kidney) (Supplemental Fig. S7A–F). Histological examinations showed no apparent alterations of blood vessels in the brain, heart, liver, and lung tissues as well (Supplemental Fig. S7G). The blood pressure of iKO mice was comparable with that of control mice (unpublished results). In addition, the mutant mice showed anesthetized heart rate and electrocardiogram waveform properties that were comparable with controls (P > 0.05) (Supplemental Fig. S8). This macrophenotypic analysis showed that the inducible deletion of ZIPK does not apparently affect mice physiological activities. The mutant mice showed a comparable leakage of EBD under physiological conditions (Fig. 4I), indicating the physiological endothelial permeability in vivo was not affected.
Figure 5.
Contractile responses of ZIPK-deficient smooth muscle to stimulations. A) Appearance of SMKO mice. B) Ablation of ZIPK in the jejunum smooth muscle of SMKO mice. C–F) Contractile response of mesenteric artery muscle to 124 mM KCl [control (CTR): n = 8; KO: n = 8] (C), 10 μM NE (CTR: n = 3; SMKO: n = 4) (D), 10 μM PE (CTR: n = 3; SMKO: n = 4) (E), and 0.1 μM ET-1 (CTR: n = 4; SMKO: n = 4) (F). G) Quantitation for the maximal force tension of mesenteric artery smooth muscle. H, I) Contractile response of jejunum smooth muscle to 87 mM KCl (CTR: n = 4; SMKO: n = 5) (H) and 100 μM ACh (CTR: n = 4; SMKO: n = 6) (I). J) Quantitation for the peak forces of jejunum smooth muscle contraction. Data are presented as means ± sem. NS: P > 0.05.
Taken together with results from the phenotypic analysis of ZIPK-KO mice, we conclude that EC-specific expression of ZIPK is required for embryonic development and that ZIPK expression may not be required for adult mice, at least under physiological conditions.
Global deletion of ZIPK significantly attenuates ischemic-reperfusion injury in the adult brain
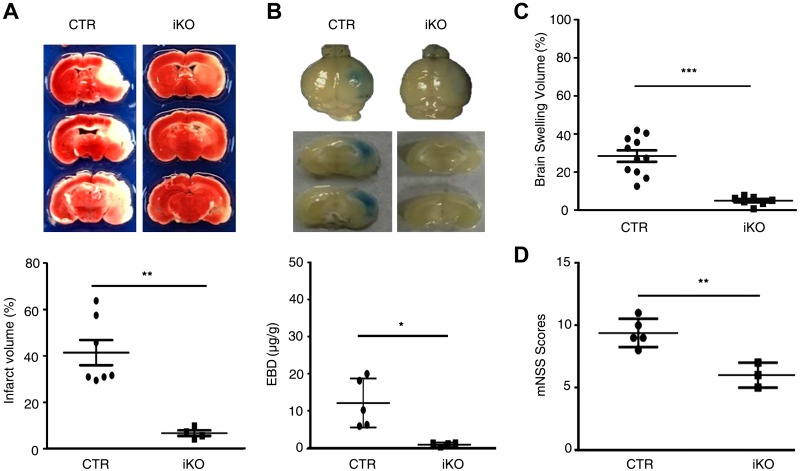
Because BBB disruption is considered essential for ischemic-reperfusion injury of the brain (9), we selected the MCAO animal model for investigating the in vivo role of ZIPK under pathological conditions. After 24 h of reperfusion immediately following 60 min of occlusion, the brains were dissected and subjected to measurements of infarct volume and permeability of EBD. The infarcted brains were visualized by staining with TTC and the relative infarct volumes were quantified using ImageJ. In the control wild-type brain (these mice received tamoxifen treatment at the same time as the iKO mice), ∼40% of the ipsilateral hemisphere volume showed TTC-negative staining. In Zipk iKO mice, however, the TTC-negative staining volume was strikingly reduced to ∼13%, which was significantly smaller than that of control (P < 0.01) (Fig. 6A). This observation indicates an apparent protective effect of ZIPK depletion on neuronal injury. To examine the BBB dysfunction of the ZIPK-deficient brain, we intravenously injected EBD into the MCAO mice and examined EBD leakage. After occlusion for 60 min and immediate reperfusion for 24 h, the control wild-type brains showed an obvious blue area, whereas the iKO mutant brains showed a substantially smaller and fainter blue area (Fig. 6B). Quantification of EBD leakage into brain tissues showed that the amount of the dye in wild-type brains was significantly higher than that in mutant brains (P < 0.05). This result suggested a protective effect of ZIPK deletion on ischemia-reperfusion–induced BBB disruption, which was further supported by the evidence from brain edema assays. Brain edema is primarily caused by BBB disruption and features an increase in tissue volume that results from infiltration of macromolecules, such as albumin, into the parenchymal tissue followed by water movement from the blood. We assessed the severity of brain edema by measuring the increased volume of the damaged brain, which was determined by the volume of the contralateral hemisphere volume minus the ipsilateral hemisphere volume. As shown in Fig. 6C, the edema volumes of iKO mice were significantly smaller than those of wild-type controls (P < 0.01). We next investigated the neuronal protective effects of ZIPK depletion by an mNSS evaluation. As shown in Fig. 6D, the control wild-type littermates displayed typical neuronal behavior deficits, revealed by the average score ranging from 8 to 11 at 24 h after reperfusion (12 total scores). For Zipk iKO mice, the behavior deficits were significantly attenuated and the average mNSS score ranged from 5 to 7. This result shows a significant effect of ZIPK deletion on protection from ischemia-reperfusion injury.
Figure 6.
ZIPK tamoxifen iKO in adults reduces brain infarct volume, leakage of EBD, and neurological scores. A) TTC staining revealing brain damage of control (CTR) (n = 7) and iKO mice (n = 4). B) Leakage of EBD in CTR (n = 4) and iKO (n = 5) brain tissues. C) Increased volumes of the damaged compared with the noninfarcted hemisphere-revealing edema of CTR (n = 11) and iKO (n = 7) brains. D) Analysis of mNSS score of CTR (n = 5) and iKO (n = 3) animals 24 h after reperfusion. Data are presented as means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001.
ZIPK-deficient BMECs displayed a reduced contractility and MLC phosphorylation in response to thrombin
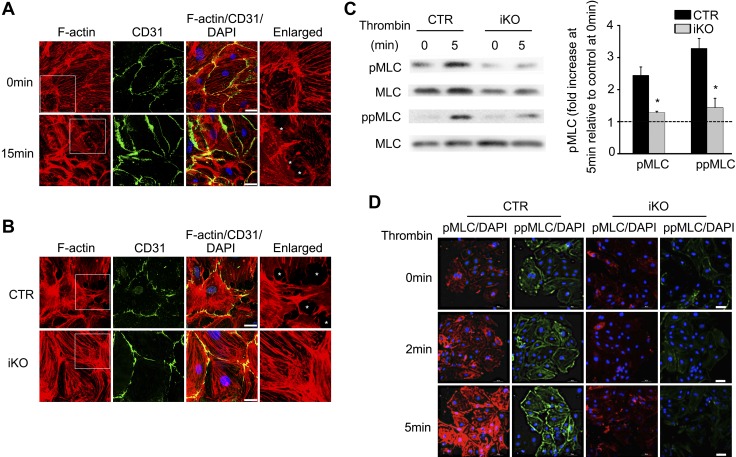
To determine whether the effect of ZIPK deletion on ischemic-reperfusion injury was caused by ZIPK-mediated EC contraction, we isolated BMECs from the mutant brains and subjected them to measurements of contractility and MLC phosphorylation. ZIPK ablation in BMECs was confirmed by immunostaining and Western blotting (Fig. 4H and Supplemental Fig. S1). We first evaluated the contractile response of BMECs to thrombin. Upon stimulation with 4 U/ml thrombin, the BMECs from control mice showed apparent contraction and enlarged cell-cell gaps within 15 min (Fig. 7A). We then compared the gap formation of the mutant BMECs with that of control BMECs at 15 min after thrombin stimulation. Several gaps were formed among the wild-type control cells, whereas no obvious cell-cell gap was observed among the mutant cells (Fig. 7B). In above experiments, we also examined the F-actin cytoskeleton of the cells. In control cells, the F-actin filaments became short and dense after thrombin stimulation, showing a typical cytoskeleton reorganization of a contracting cell. The F-actin filaments of iKO BMECs did not apparently change when treated with thrombin. This result indicated that ZIPK was required for BMEC contraction and F-actin cytoskeleton reorganization.
Figure 7.
ZIPK depletion inhibits BMEC contraction and MLC phosphorylation upon thrombin treatment. A) CD31 (green) and F-actin (red) staining of BMECs showed the opening of intercellular gap after 15 min of thrombin (4 U/ml) exposure. Asterisks (*) indicate the gaps. Scale bars, 10 μm. B) CD31 (green) and F-actin (red) staining of control (CTR) wild-type vs. ZIPK mutant BMECs showed the decreased intercellular gap. Asterisks (*) indicate the gaps. Scale bars, 10 μm. C) Western blot analysis of mono- and diphosphorylation of MLC in ZIPK-deficient and CTR BMECs. CTR: n = 4; iKO: n = 4. Data are presented as means ± sem. *P < 0.05. Dashed line indicates the basal level of phosphorylated MLC at 0 min. D) Mono- (red) and diphosphorylation (green) of MLC staining in BMEC deficiency of ZIPK (iKO) (n = 3) vs. CTR wild type (n = 3). Scale bars, 20 μm.
We then measured the MLC phosphorylation of BMECs in response to thrombin. As expected, both pMLC and ppMLC were elevated in control BMECs (Fig. 7C), whereas the phosphorylation level in the mutant BMECs did not increase obviously. Quantification showed that at 5 min after stimulation, both pMLC and ppMLC levels in the mutant BMECs were significantly lower than those of wild-type control BMECs (P < 0.05). The immunofluorescence assay showed a similar result (Fig. 7D). These data showed that, in BMECs, ZIPK was required for both mono- and diphosphorylation of MLC in response to thrombin stimulation. Moreover, we observed that the distribution of pMLC and ppMLC was different in the BMECs after 2 and 5 min of thrombin exposure. Although most of the pMLC localized in the perinuclear cytoplasmic region, the ppMLC localized in the cell periphery. This result was consistent with previous studies (54, 59, 60). The distribution pattern might reflect distinct roles of pMLC and ppMLC during EC contraction. Because ROCKII activation in EC leads to a similar distribution pattern (59), we suggest ZIPK might serve as a downstream effector of ROCKII during contraction.
DISCUSSION
Several physiological and pathological stimuli, such as cytokines, inflammatory mediators, thrombin, and LPS, potently regulate endothelial permeability through receptors, and EC contraction as well as cytoskeleton reorganization have been emphasized in this regulatory process (10, 60). However, the linkage between stimuli and contraction initiation was incompletely clear. In this report, we found that ZIPK was abundantly expressed in ECs and that inhibition of ZIPK significantly inhibited MLC phosphorylation and contraction of ECs along with a disruptive regulation of endothelial permeability. Our findings suggest that ZIPK is required for EC contract initiation, whereby catalyzing MLC phosphorylation. Because several signaling molecules, such as signal transducer and activator of transcription 3, IL-6 family cytokines, and DAPK (32, 61, 62), are capable of regulating ZIPK activation or phosphorylation, ZIPK thus serves as a key signal linker between the agonist receptors and EC contraction. According to this linkage, additional signaling scenarios associated with EC permeability will be identified conveniently. Based on our current knowledge, we might briefly propose a signal-transduction pathway that converges on MLC phosphorylation during the regulation of paracellular permeability. The agonist receptors [e.g., G protein-coupled receptors (GPCRs)] activate signal modules followed by activation or phosphorylation of ZIPK, and the activated ZIPK catalyzes MLC phosphorylation, resulting in EC contraction and paracellular gap formation. This pathway includes the following features: 1) ZIPK is the primary (if not exclusive) kinase for mono- and di phosphorylation of MLC, 2) Activation of ZIPK may be regulated by Rho and ROCK through GPCR activation (31, 59, 63), and 3) ZIPK-regulated EC contraction is necessary for gap formation. Because the ZIPK iKO mice showed a significant inhibition of microvascular permeability in response to ischemia-reperfusion but showed no obvious disruptive permeability under physiological conditions, we do not emphasize the importance of ZIPK-mediated EC permeability under resting conditions. Notably, in addition to the regulation of endothelial permeability, ZIPK in ECs is also required for embryonic development. This effect may be attributable to other functions of ZIPK, such as the regulation of apoptosis and cell migration (27, 36).
BMECs are the main component of the BBB and are located between the blood and neurons, thereby controlling the exchange of substances and cells. The BBB is maintained by multiple functional components, including the TJ-sealed capillary ECs, astrocyte endfeet, pericytes, and extracellular matrix (64, 65). The integrity of the BBB is important for the maintenance of brain physiological homeostasis and responses to pathological stresses. In ischemia stroke, the pathology of the injured brain features neuronal necrosis and a disrupted or dysfunctional BBB. However, the contribution of BBB dysfunction in ischemia-reperfusion injury is not completely understood. Here, we found that the deletion of ZIPK led to a significant inhibition of neuronal injury along with BBB leakage. The ZIPK-mediated paracellular permeability appears to be essential for BBB integrity and neuron damage. Although the ZIPK iKO mice drive an inducible and global deletion of Zipk gene, the protective effect of ZIPK deletion on stroke might be unlikely contributed by other cells, such as neuron and smooth muscle cells, because the neurons either did not express or express lower ZIPK, and ZIPK deletion did not affect the function of smooth muscle cells. Stroke is a leading cause of death, and more than 80% of strokes are ischemic, usually because of a blood clot (66). During thrombolysis treatment of stroke, how to protect the neuronal tissue from ischemia-reperfusion injury is a critical challenge. Current therapeutic strategies are mainly based on antioxidants and free radical scavengers (67, 68). Our findings suggest that ZIPK may be an alternative target for preventing this injury. Indeed, some compounds (e.g., STS) with inhibitory activity on ZIPK have more or less neuronal protective effects in stroke injury, although these compounds are usually used as inhibitors of other kinases, such as PKC (69). Our data provide strong evidence that ZIPK-mediated EC MLC phosphorylation is an essential mechanism of stroke injury. Because global KO of ZIPK at adulthood did not result in any phenotypic alteration in ZIPK iKO mice, we predicted no critical toxic effect of ZIPK inhibition during stroke therapy.
In addition, there are reports showing that ZIPK may regulate smooth muscle contractility in a calcium-independent manner (52, 58). However, we found here that ZIPK-deficient smooth muscle showed a comparable contractile response to KCl depolarization and GPCR agonists. Our loss-of-function evidence suggests that ZIPK is not required for smooth muscle contraction at least under physiological conditions.
In summary, our data suggest that ZIPK is the kinase responsible for MLC phosphorylation of EC and is necessary for the regulation of endothelial permeability. We demonstrate that BBB dysfunction induced by ischemia-reperfusion is significantly attenuated in ZIPK-depleted mice. This observation suggests that ZIPK-regulated EC permeability may be a prospective target for the protection of ischemic-reperfusion injury.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Qianqian Zhang, Wen Yuan, and Tengxiang Ma (all from Nanjing Normal University, College of Life Sciences, Nanjing, China) for assistance in preparation of the revised version of this manuscript. The authors thank Dr. Jincai Luo from Peking University (Beijing, China) for providing primary HUVECs and Dr. Yin Lu from the Nanjing University of Chinese Medicine (Nanjing, China) for the transendothelial electrical resistance measurement of the endothelial cell monolayer. The study was supported by the National Natural Science Foundation of China (31371356, 30971540, and 31330034; Beijing, China), the Jiangsu Major Natural Science Foundation of Higher Education (13KJA180004), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors declare no conflicts of interest.
Glossary
- ACh
acetylcholine
- AJ
adherens junction
- BBB
blood-brain barrier
- BMEC
brain microvessel EC
- CaM
calmodulin
- DAPK
death-associated protein kinase
- EBD
Evans blue dye
- EC
endothelial cell
- ECKO
EC-specific KO
- ET-1
endothelin-1
- Gapdh
glyceraldehyde 3-phosphate dehydrogenase
- iKO
inducible KO
- KO
knockout
- MCAO
middle cerebral artery occlusion
- MLC
myosin light chain
- MLCK
MLC kinase
- mNSS
modified neurologic severity score
- NE
norepinephrine
- PE
phenylephrine
- PFA
paraformaldehyde
- pMLC
monophosphorylated MLC
- ppMLC
diphosphorylated MLC
- ROCKII
Rho-associated, coiled-coil–containing protein kinase II
- siRNA
small interfering RNA
- SMKO
smooth muscle–specific KO
- STS
staurosporine
- TEER
transendothelial electrical resistance
- TJ
tight junction
- TTC
2, 3, 5-triphenyltetrazolium chloride
- ZIPK
zipper-interacting protein kinase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Zhang, C. Zhang, H. Zhang, W. Zeng, S. Li, C. Chen, X. Song, J. Sun, Z. Sun, C. Cui, X. Cao, L. Zheng, P. Wang, and W. Zhao performed research; Y. Zhang, C. Zhang, W. Zeng, S. Li, J. Sun, and Z. Zhang analyzed data; and Y. Xu, M. Zhu, and H. Chen designed the research and wrote the manuscript.
REFERENCES
- 1.Nourshargh S., Hordijk P. L., Sixt M. (2010) Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378 [DOI] [PubMed] [Google Scholar]
- 2.Korn C., Augustin H. G. (2015) Mechanisms of vessel pruning and regression. Dev. Cell 34, 5–17 [DOI] [PubMed] [Google Scholar]
- 3.Schaefer A., Hordijk P. L. (2015) Cell-stiffness-induced mechanosignaling - a key driver of leukocyte transendothelial migration. J. Cell Sci. 128, 2221–2230 [DOI] [PubMed] [Google Scholar]
- 4.Van Hinsbergh V. W., Tasev D. (2015) Platelets and thromboxane receptors: pivotal players in arteriogenesis. Cardiovasc. Res. 107, 400–402 [DOI] [PubMed] [Google Scholar]
- 5.Opal S. M., van der Poll T. (2015) Endothelial barrier dysfunction in septic shock. J. Intern. Med. 277, 277–293 [DOI] [PubMed] [Google Scholar]
- 6.Xie Z., Ghosh C. C., Patel R., Iwaki S., Gaskins D., Nelson C., Jones N., Greipp P. R., Parikh S. M., Druey K. M. (2012) Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood 119, 4321–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlokovic B. V. (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg G. A., Estrada E. Y., Dencoff J. E. (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 29, 2189–2195 [DOI] [PubMed] [Google Scholar]
- 9.Shi Y., Zhang L., Pu H., Mao L., Hu X., Jiang X., Xu N., Stetler R. A., Zhang F., Liu X., Leak R. K., Keep R. F., Ji X., Chen J. (2016) Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 7, 10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta D., Malik A. B. (2006) Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86, 279–367 [DOI] [PubMed] [Google Scholar]
- 11.Bazzoni G., Dejana E. (2004) Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 84, 869–901 [DOI] [PubMed] [Google Scholar]
- 12.Dejana E., Orsenigo F., Lampugnani M. G. (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121, 2115–2122 [DOI] [PubMed] [Google Scholar]
- 13.Lampugnani M. G., Corada M., Caveda L., Breviario F., Ayalon O., Geiger B., Dejana E. (1995) The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J. Cell Biol. 129, 203–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannotta M., Trani M., Dejana E. (2013) VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 [DOI] [PubMed] [Google Scholar]
- 15.Komarova Y., Malik A. B. (2010) Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 72, 463–493 [DOI] [PubMed] [Google Scholar]
- 16.Schnittler H. J., Wilke A., Gress T., Suttorp N., Drenckhahn D. (1990) Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J. Physiol. 431, 379–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia J. G., Davis H. W., Patterson C. E. (1995) Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J. Cell. Physiol. 163, 510–522 [DOI] [PubMed] [Google Scholar]
- 18.Vandenbroucke E., Mehta D., Minshall R., Malik A. B. (2008) Regulation of endothelial junctional permeability. Ann. N. Y. Acad. Sci. 1123, 134–145 [DOI] [PubMed] [Google Scholar]
- 19.He W. Q., Peng Y. J., Zhang W. C., Lv N., Tang J., Chen C., Zhang C. H., Gao S., Chen H. Q., Zhi G., Feil R., Kamm K. E., Stull J. T., Gao X., Zhu M. S. (2008) Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology 135, 610–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somlyo A. P., Somlyo A. V. (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 [DOI] [PubMed] [Google Scholar]
- 21.Yuan S. Y., Wu M. H., Ustinova E. E., Guo M., Tinsley J. H., De Lanerolle P., Xu W. (2002) Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ. Res. 90, 1214–1221 [DOI] [PubMed] [Google Scholar]
- 22.Tinsley J. H., De Lanerolle P., Wilson E., Ma W., Yuan S. Y. (2000) Myosin light chain kinase transference induces myosin light chain activation and endothelial hyperpermeability. Am. J. Physiol. Cell Physiol. 279, C1285–C1289 [DOI] [PubMed] [Google Scholar]
- 23.Yu Y., Lv N., Lu Z., Zheng Y. Y., Zhang W. C., Chen C., Peng Y. J., He W. Q., Meng F. Q., Zhu M. S., Chen H. Q. (2012) Deletion of myosin light chain kinase in endothelial cells has a minor effect on the lipopolysaccharide-induced increase in microvascular endothelium permeability in mice. FEBS J. 279, 1485–1494 [DOI] [PubMed] [Google Scholar]
- 24.Shinde A. V., Motiani R. K., Zhang X., Abdullaev I. F., Adam A. P., González-Cobos J. C., Zhang W., Matrougui K., Vincent P. A., Trebak M. (2013) STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci. Signal. 6, ra18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolwijk J. A., Zhang X., Gueguinou M., Zhang W., Matrougui K., Renken C., Trebak M. (2016) Calcium signaling is dispensable for receptor regulation of endothelial barrier function. J. Biol. Chem. 291, 22894–22912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J., Mahavadi S., Sriwai W., Hu W., Murthy K. S. (2006) Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem. J. 396, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu S., Ikebe M. (2004) ZIP kinase is responsible for the phosphorylation of myosin II and necessary for cell motility in mammalian fibroblasts. J. Cell Biol. 165, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockton R. A., Schaefer E., Schwartz M. A. (2004) p21-activated kinase regulates endothelial permeability through modulation of contractility. J. Biol. Chem. 279, 46621–46630 [DOI] [PubMed] [Google Scholar]
- 29.Bialik S., Kimchi A. (2006) The death-associated protein kinases: structure, function, and beyond. Annu. Rev. Biochem. 75, 189–210 [DOI] [PubMed] [Google Scholar]
- 30.Niiro N., Ikebe M. (2001) Zipper-interacting protein kinase induces Ca(2+)-free smooth muscle contraction via myosin light chain phosphorylation. J. Biol. Chem. 276, 29567–29574 [DOI] [PubMed] [Google Scholar]
- 31.Hagerty L., Weitzel D. H., Chambers J., Fortner C. N., Brush M. H., Loiselle D., Hosoya H., Haystead T. A. (2007) ROCK1 phosphorylates and activates zipper-interacting protein kinase. J. Biol. Chem. 282, 4884–4893 [DOI] [PubMed] [Google Scholar]
- 32.MacDonald J. A., Eto M., Borman M. A., Brautigan D. L., Haystead T. A. (2001) Dual Ser and Thr phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett. 493, 91–94 [DOI] [PubMed] [Google Scholar]
- 33.Sato N., Kamada N., Muromoto R., Kawai T., Sugiyama K., Watanabe T., Imoto S., Sekine Y., Ohbayashi N., Ishida M., Akira S., Matsuda T. (2006) Phosphorylation of threonine-265 in Zipper-interacting protein kinase plays an important role in its activity and is induced by IL-6 family cytokines. Immunol. Lett. 103, 127–134 [DOI] [PubMed] [Google Scholar]
- 34.Mills J. C., Stone N. L., Erhardt J., Pittman R. N. (1998) Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 140, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosoba K., Komatsu S., Ikebe M., Kotani M., Wenqin X., Tachibana T., Hosoya H., Hamao K. (2015) Phosphorylation of myosin II regulatory light chain by ZIP kinase is responsible for cleavage furrow ingression during cell division in mammalian cultured cells. Biochem. Biophys. Res. Commun. 459, 686–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai T., Matsumoto M., Takeda K., Sanjo H., Akira S. (1998) ZIP kinase, a novel serine/threonine kinase which mediates apoptosis. Mol. Cell. Biol. 18, 1642–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murata-Hori M., Fukuta Y., Ueda K., Iwasaki T., Hosoya H. (2001) HeLa ZIP kinase induces diphosphorylation of myosin II regulatory light chain and reorganization of actin filaments in nonmuscle cells. Oncogene 20, 8175–8183 [DOI] [PubMed] [Google Scholar]
- 38.Navone S. E., Marfia G., Invernici G., Cristini S., Nava S., Balbi S., Sangiorgi S., Ciusani E., Bosutti A., Alessandri G., Slevin M., Parati E. A. (2013) Isolation and expansion of human and mouse brain microvascular endothelial cells. Nat. Protoc. 8, 1680–1693 [DOI] [PubMed] [Google Scholar]
- 39.Borovski T., Verhoeff J. J., ten Cate R., Cameron K., de Vries N. A., van Tellingen O., Richel D. J., van Furth W. R., Medema J. P., Sprick M. R. (2009) Tumor microvasculature supports proliferation and expansion of glioma-propagating cells. Int. J. Cancer 125, 1222–1230 [DOI] [PubMed] [Google Scholar]
- 40.Liu P., Jenkins N. A., Copeland N. G. (2003) A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fantin A., Vieira J. M., Plein A., Maden C. H., Ruhrberg C. (2013) The embryonic mouse hindbrain as a qualitative and quantitative model for studying the molecular and cellular mechanisms of angiogenesis. Nat. Protoc. 8, 418–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monaghan-Benson E., Wittchen E. S. (2011) In vitro analyses of endothelial cell permeability. Methods Mol. Biol. 763, 281–290 [DOI] [PubMed] [Google Scholar]
- 43.Gautam N., Olofsson A. M., Herwald H., Iversen L. F., Lundgren-Åkerlund E., Hedqvist P., Arfors K. E., Flodgaard H., Lindbom L. (2001) Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat. Med. 7, 1123–1127 [DOI] [PubMed] [Google Scholar]
- 44.He W. Q., Qiao Y. N., Zhang C. H., Peng Y. J., Chen C., Wang P., Gao Y. Q., Chen C., Chen X., Tao T., Su X. H., Li C. J., Kamm K. E., Stull J. T., Zhu M. S. (2011) Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 301, H584–H591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frasier C. R., Wagnon J. L., Bao Y. O., McVeigh L. G., Lopez-Santiago L. F., Meisler M. H., Isom L. L. (2016) Cardiac arrhythmia in a mouse model of sodium channel SCN8A epileptic encephalopathy. Proc. Natl. Acad. Sci. USA 113, 12838–12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J., Sanberg P. R., Li Y., Wang L., Lu M., Willing A. E., Sanchez-Ramos J., Chopp M. (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688 [DOI] [PubMed] [Google Scholar]
- 47.Bederson J. B., Pitts L. H., Germano S. M., Nishimura M. C., Davis R. L., Bartkowski H. M. (1986) Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17, 1304–1308 [DOI] [PubMed] [Google Scholar]
- 48.Li Y., Chopp M., Chen J., Wang L., Gautam S. C., Xu Y. X., Zhang Z. (2000) Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J. Cereb. Blood Flow Metab. 20, 1311–1319 [DOI] [PubMed] [Google Scholar]
- 49.Kamm K. E., Stull J. T. (2001) Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 276, 4527–4530 [DOI] [PubMed] [Google Scholar]
- 50.Bresnick A. R. (1999) Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26–33 [DOI] [PubMed] [Google Scholar]
- 51.Coughlin S. R. (2005) Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3, 1800–1814 [DOI] [PubMed] [Google Scholar]
- 52.Borman M. A., MacDonald J. A., Haystead T. A. (2007) Staurosporine inhibition of zipper-interacting protein kinase contractile effects in gastrointestinal smooth muscle. Biochem. Cell Biol. 85, 111–120 [DOI] [PubMed] [Google Scholar]
- 53.Fujiwara N., Usui T., Ohama T., Sato K. (2016) Regulation of beclin 1 protein phosphorylation and autophagy by protein phosphatase 2A (PP2A) and death-associated protein kinase 3 (DAPK3). J. Biol. Chem. 291, 10858–10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Ghabkari A., Deng J. T., McDonald P. C., Dedhar S., Alshehri M., Walsh M. P., MacDonald J. A. (2016) A novel inhibitory effect of oxazol-5-one compounds on ROCKII signaling in human coronary artery vascular smooth muscle cells. Sci. Rep. 6, 32118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drake C. J., Fleming P. A. (2000) Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95, 1671–1679 [PubMed] [Google Scholar]
- 56.Schlaeger T. M., Bartunkova S., Lawitts J. A., Teichmann G., Risau W., Deutsch U., Sato T. N. (1997) Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. USA 94, 3058–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kocher B. A., White L. S., Piwnica-Worms D. (2015) DAPK3 suppresses acini morphogenesis and is required for mouse development. Mol. Cancer Res. 13, 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ihara E., Moffat L., Borman M. A., Amon J. E., Walsh M. P., MacDonald J. A. (2009) Ca2+-independent contraction of longitudinal ileal smooth muscle is potentiated by a zipper-interacting protein kinase pseudosubstrate peptide. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G361–G370 [DOI] [PubMed] [Google Scholar]
- 59.Hirano M., Hirano K. (2016) Myosin di-phosphorylation and peripheral actin bundle formation as initial events during endothelial barrier disruption. Sci. Rep. 6, 20989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sukriti S., Tauseef M., Yazbeck P., Mehta D. (2014) Mechanisms regulating endothelial permeability. Pulm. Circ. 4, 535–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato N., Kawai T., Sugiyama K., Muromoto R., Imoto S., Sekine Y., Ishida M., Akira S., Matsuda T. (2005) Physical and functional interactions between STAT3 and ZIP kinase. Int. Immunol. 17, 1543–1552 [DOI] [PubMed] [Google Scholar]
- 62.Mukhopadhyay R., Ray P. S., Arif A., Brady A. K., Kinter M., Fox P. L. (2008) DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol. Cell 32, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu J., Miller C. T., Kislitsyna K., Laine G. A., Stewart R. H., Cox C. S., Uray K. S. (2012) Decreased myosin phosphatase target subunit 1(MYPT1) phosphorylation via attenuated rho kinase and zipper-interacting kinase activities in edematous intestinal smooth muscle. Neurogastroenterol. Motil. 24, 257–266, e109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neuwelt E. A., Bauer B., Fahlke C., Fricker G., Iadecola C., Janigro D., Leybaert L., Molnár Z., O’Donnell M. E., Povlishock J. T., Saunders N. R., Sharp F., Stanimirovic D., Watts R. J., Drewes L. R. (2011) Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 12, 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Obermeier B., Daneman R., Ransohoff R. M. (2013) Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., Das S. R., de Ferranti S., Després J. P., Fullerton H. J., Howard V. J., Huffman M. D., Isasi C. R., Jiménez M. C., Judd S. E., Kissela B. M., Lichtman J. H., Lisabeth L. D., Liu S., Mackey R. H., Magid D. J., McGuire D. K., Mohler E. R., III, Moy C. S., Muntner P., Mussolino M. E., Nasir K., Neumar R. W., Nichol G., Palaniappan L., Pandey D. K., Reeves M. J., Rodriguez C. J., Rosamond W., Sorlie P. D., Stein J., Towfighi A., Turan T. N., Virani S. S., Woo D., Yeh R. W., Turner M. B.; Writing Group Members ; American Heart Association Statistics Committee ; Stroke Statistics Subcommittee (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–e360 [DOI] [PubMed] [Google Scholar]
- 67.Slemmer J. E., Shacka J. J., Sweeney M. I., Weber J. T. (2008) Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr. Med. Chem. 15, 404–414 [DOI] [PubMed] [Google Scholar]
- 68.Prabhakaran S., Ruff I., Bernstein R. A. (2015) Acute stroke intervention: a systematic review. JAMA 313, 1451–1462 [DOI] [PubMed] [Google Scholar]
- 69.Hara H., Onodera H., Yoshidomi M., Matsuda Y., Kogure K. (1990) Staurosporine, a novel protein kinase C inhibitor, prevents postischemic neuronal damage in the gerbil and rat. J. Cereb. Blood Flow Metab. 10, 646–653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.