Abstract
The endoplasmic reticulum (ER) plays a central role in cellular stress responses via mobilization of ER stress coping responses, such as the unfolded protein response (UPR). The inositol-requiring 1α (IRE1α) is an ER stress sensor and component of the UPR. Muscle cells also have a well-developed and highly subspecialized membrane network of smooth ER called the sarcoplasmic reticulum (SR) surrounding myofibrils and specialized for Ca2+ storage, release, and uptake to control muscle excitation-contraction coupling. Here, we describe 2 distinct pools of IRE1α in cardiac and skeletal muscle cells, one localized at the perinuclear ER and the other at the junctional SR. We discovered that, at the junctional SR, calsequestrin binds to the ER luminal domain of IRE1α, inhibiting its dimerization. This novel interaction of IRE1α with calsequestrin, one of the highly abundant Ca2+ handling proteins at the junctional SR, provides new insights into the regulation of stress coping responses in muscle cells.—Wang, Q., Groenendyk, J., Paskevicius, T., Qin, W., Kor, K. C., Liu, Y., Hiess, F., Knollmann, B. C., Chen, S. R. W., Tang, J., Chen, X.-Z., Agellon, L. B., Michalak, M. Two pools of IRE1α in cardiac and skeletal muscle cells.
Keywords: calcium, calsequestrin, ER stress, sarcoplasmic reticulum
Stress responses are central to cellular physiology and pathology, and failure to adapt to stress leads to cell death. To mitigate cellular stress and re-establish homeostasis, cells must activate stress coping response mechanisms (1–3). In cells, including muscle cells, the endoplasmic reticulum (ER) plays a central role in cellular stress responses via mobilization of one of the stress coping responses, such as the unfolded protein response (UPR). The UPR involves 3 unique ER transmembrane signaling proteins: the inositol-requiring enzyme 1 (IRE1), dsRNA-activated protein kinase-like ER kinase, and activating transcription factor 6 (1, 4, 5). Activation of ER stress–induced UPR signaling pathways results in translational attenuation, transcriptional activation of genes encoding proteins involved in protein folding, and transcriptional activation of genes for components of the ER-associated degradation pathway (1, 4, 5). Under optimal conditions, IRE1, dsRNA-activated protein kinase-like ER kinase, and activating transcription factor 6 are maintained in a nominal state by binding to the binding immunoglobulin protein (BiP), an ER chaperone. Upon stress, BiP dissociates from these proteins, resulting in the activation of UPR signaling pathways (1, 5). IRE1α is the most evolutionarily conserved ER stress sensor and component of the UPR. The protein has endoribonuclease activity that splices the mRNA encoding the transcription factor X-box binding protein 1 to produce X-box binding protein 1 mRNA, encoding the stable form of the transcription factor that induces the expression of genes involved in many aspects of the protein secretory pathway, including protein folding, ER-associated degradation, and protein quality control (6).
In muscle cells, the ER is responsible for cellular housekeeping functions, among which are the synthesis, folding, post-translational modification, and transport of proteins; the synthesis of lipids and steroids; the assembly and trafficking of membranes; stress signaling; and signaling to the nucleus, cytoplasm, mitochondria, and plasma membrane (7–9). Muscle cells also have a well-developed and highly specialized membrane network of smooth ER called the sarcoplasmic reticulum (SR) surrounding myofibrils (10, 11). The SR is specialized for Ca2+ storage, release, and uptake to control muscle excitation-contraction coupling (12). The SR luminal Ca2+ binding proteins calsequestrin, histidine-rich calcium-binding protein, junctate, and sarcalumenin are responsible for Ca2+ storage, whereas the ryanodine receptor (RyR) Ca2+ release channel is responsible for Ca2+ release to trigger muscle contraction. SR or ER Ca2+ ATPase pumps Ca2+ back to the lumen of the SR, driving muscle relaxation. Additionally, the SR forms 2 distinct regions in the muscle: the longitudinal SR, which is enriched with the sarcoplasmic or ER Ca2+ ATPase pump, and the junctional SR, where the RyR and calsequestrin are localized (13, 14). Calsequestrin is involved in binding and storing Ca2+, and it comprises ∼27% by mass of all junctional SR proteins (15). Two isoforms of calsequestrin exist and are encoded by 2 different genes: cardiac muscle calsequestrin (Casq2) and skeletal muscle calsequestrin (Casq1) (16, 17). The crystal structures of Casq2 and Casq1 indicate that the proteins contain 3 thioredoxin-like domains reminiscent of ER luminal oxiodoreductases (18).
Disruption of ER functions triggers ER stress and activates IRE1α (1, 19). In skeletal muscle, the IRE1α is activated during exercise (19), starvation (20), and a high-fat diet (21). Activation of IRE1α and other branches of the UPR pathway has been implicated in many cardiovascular diseases, including hypoxia, ischemia and reperfusion, hypertrophy, pressure overload, and drug-induced insults (1, 22). Previous studies have shown that inhibition of IRE1α signaling protects the heart from cardiac fibrosis (23) and atherosclerosis (24). How IRE1α signaling is regulated in the muscle by the SR luminal environment is not known. Understanding the molecular organization of IRE1α and events controlling its activation in skeletal and cardiac muscle is necessary to assess the connection between the muscle stress coping response and cellular pathophysiology (1). Here, we report that there are 2 pools of IRE1α in cardiac and skeletal muscle cells: one localized to perinuclear ER and the other at the junctional SR, a site of Ca2+ release for myofilament activation. We also discovered that calsequestrin binds to the ER luminal domain of IRE1α to prevent its dimerization, and this may serve to squelch the activation of IRE1α at the junctional SR.
MATERIALS AND METHODS
Plasmids and site-specific mutagenesis
The mammalian expression vector encoding the human IRE1 luminal domain (IRE1-NLD) cDNA (25) was a generous gift from Dr. Randal Kaufman (Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA, USA). The triple cysteine mutant of the IRE1-NLD (C109, 148, 332A) was previously described by Groenendyk et al. (26). Full-length cDNA-encoding canine Casq2 lacking the signal sequence was cloned into pET22b vector to generate pET-Casq2 for bacterial expression of the protein. The following expression vectors were used in this study: pcDNA3.1 expression vector containing cDNA-encoding full-length Casq2, C terminus truncation of Casq2 (Δ350–390), and C terminus plus partial thioredoxin domain III truncation of Casq2 (Δ316–390) (27). The cDNA-encoding Casq2 truncations (Δ350–390 and Δ316–390) were also cloned into pET22b vector for bacterial expression and purification of recombinant proteins.
Adenovirus construct
Mammalian expression vector encoding the cDNA of red fluorescence protein (RFP) fused to full-length mouse IRE1α was cloned from ER RFP (a generous gift from Dr. Erik Snapp, Albert Einstein College of Medicine, Bronx, New York, NY, USA) and pcDNA3.1(+) mouse full-length IRE1α plasmid (a generous gift from Dr. Ko Miyoshi, Osaka University, Osaka, Japan). EcoRI and NotI restriction enzyme sites were introduced by PCR. The cDNA of full-length mouse IRE1α with the signal sequence omitted was cloned into ER RFP expression vector with the pEGFP-C1 green fluorescent protein (GFP) backbone at the C terminus of RFP. Short and flexible linker sequences encoding the amino acid sequence GGSGEFGGSG were added between the RFP and IRE1α coding sequences in the cDNA. The cDNA of RFP IRE1α was cloned and packed into adenovirus by Vector Biolabs (Malvern, PA, USA).
Protein purification
IRE1-NLD cysteine triple mutant (C109, 148, 332A) was expressed in COS-1 cells (https://web.expasy.org/cellosaurus/CVCL_0223) and purified by Ni-NTA agarose chromatography (Qiagen, Toronto, Ontario) (25, 26). COS-1 cells were transfected with IRE1-NLD or IRE1-NLD cysteine triple mutant expression vector, harvested, and lysed in a buffer containing 25 mM Tris Cl (pH 8.0), 150 mM NaCl, and 1% Nonidet P-40. Cell lysates were centrifuged at 16,000 g for 30 min at 4°C, and supernatant was used for protein purification. Ni-NTA agarose purification was performed by following the manufacturer’s protocol (30230; Qiagen, Germantown, MD, USA) under native conditions in a binding buffer containing 50 mM NaH2PO4, 500 mM NaCl, and 10 mM imidazole (pH 8.0) (26). The IRE1-NLD or IRE1-NLD cysteine triple mutant proteins were eluted with 250 mM imidazole (26).
Native Casq2 and Casq1 proteins were purified from pig heart and rabbit skeletal muscle, respectively (28, 29). In brief, 200–250 g of muscle was homogenized in a buffer containing 0.1 M KH2PO4 (pH 7.1), 1 mM EDTA, and 2.66 M ammonium sulfate (65% saturation) followed by ammonium sulfate (85% saturation) precipitation, diethylaminoethyl chromatography, and phenyl Sepharose CL-4B chromatography (GE Healthcare Life Sciences, Mississauga, Ontario) (28, 29). Calsequestrin-containing fractions were eluted from phenyl Sepharose CL-4B with a buffer containing 10 mM CaCl2 (28, 29). Fractions containing calsequestrin were pooled and stored at −80°C in a buffer containing 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.4), 150 mM KCl, 500 μM CaCl2, and 250 μM EGTA. All procedures were carried out at 4°C, and all buffers contained a cocktail of protease inhibitors (30). Full-length recombinant Casq2 and truncated Casq2 were expressed in E. coli BL21(DE3) cells (Thermo Fisher Scientific, Waltham, MA, USA) and purified with Ni-NTA affinity column chromatography following the manufacturer’s protocol (30230; Qiagen) under native conditions.
Microscale thermophoresis
Microscale thermophoresis analyses were carried out using a Monolith NT.115 instrument (NanoTemper Technologies, London, United Kingdom) or Monolith NT.LabelFree instrument (NanoTemper Technologies). Proteins were labeled using the Monolith NT Protein Labeling Kit RED-NHS (MO-C030; NanoTemper Technologies) following the manufacturer’s protocol. All experiments were carried out at room temperature in standard capillaries (for labeled IRE1-NLD or IRE1-NLD cysteine triple mutant) or in hydrophobic capillaries (for labeled calsequestrin) with 20% light-emitting diode power (fluorescence lamp intensity) and 40% microscale thermophoresis power (infrared laser intensity). The assay buffer contained 50 mM HEPES (pH 7.4), 150 mM KCl, 500 μM CaCl2, 250 μM EGTA, 0.05% Tween 20, and 2.5% glycerol. CaCl2 and EGTA concentrations were adjusted to obtain the desired free Ca2+ concentration: 80 μM (350 μM CaCl2 and 850 μM EGTA), 125 μM (175 μM CaCl2 and 50 μM EGTA), and 1000 μM (1100 μM CaCl2 and 100 μM EGTA). Free Ca2+ concentration was calculated using the Ca-EGTA Calculator TS v1.3 web tool (31).
Ca2+ binding to full-length Casq2 or truncated Casq2 were carried out using a Monolith NT.LabelFree instrument in standard capillaries with 20% light-emitting diode power and 40% microscale thermophoresis power. The proteins were incubated for 10 min in a buffer containing 50 mM HEPES (pH 7.4), 150 mM KCl, 0.1% pluronic F-127, and 50 μM EGTA. An increasing concentration of CaCl2 [0.01–20 mM in 50 mM HEPES (pH 7.4) and 150 mM KCl] was used. All microscale thermophoresis data were analyzed by Monolith Affinity Analysis v.2.2.6 software.
Surface plasmon resonance analysis
Surface plasmon resonance (SPR) was performed to monitor the interaction between IRE1-NLD and calsequestrin (GE Healthcare Life Sciences, Waukesha, WI, USA). The Biacore sensor chip CM5 was activated using a 1:1 dilution of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide:N-hydroxysuccinimide as previously described by Groenendyk et al. (26). Purified IRE1-NLD protein was diluted in 10 mM sodium acetate (pH 5) injected over the activated CM5 chip and captured at a flow rate of 5 μl/min to a total of ∼2000 response units (RUs). Uncoupled amine reactive sites on the carboxymethyl dextran surface were then blocked by an injection of 1 M ethanolamine (pH 9.0). An uncoupled reference lane was generated to subtract background binding. The running buffer was composed of 10 mM HEPES (pH 7.2), 150 mM KCl, 1 mM EDTA, and 0.005% P20. Purified calsequestrin protein was diluted (10,000–39 nM), and the dilution series was performed in triplicate. Purified IRE1-NLD triple cysteine mutant protein (26) was coupled to a CM5 chip to a total of ∼1500 RUs followed by addition of an increasing concentration of calsequestrin (10,000–39 nM). For each measurement, the signal was corrected against the control surface response to eliminate any refractive index changes due to buffer change. The data were collected at 25°C at a flow rate of 30 µl/min to minimize mass transfer effects. Kinetic analysis was performed using the BiaEvaluation software (GE Healthcare Life Sciences) with a 1:1 Langmuir binding model. Association and dissociation rates and affinity were calculated for each experiment and averaged. The binding response signal in RUs was continuously recorded and presented graphically as a function of time. All experiments and analyses were conducted on a BIACore T200 instrument (GE Healthcare).
Immunoprecipitation
COS-1 cells were cotransfected with pED-IRE1-NLD-6His expression vector and pcDNA3.1 expression vector containing cDNA-encoding full-length Casq2 or truncated Casq2 using TurboFect transfection reagent (R0531; Thermo Fisher Scientific). At 48 h after transfection, cells were washed and harvested with 600 μl lysis buffer containing 50 mM HEPES (pH 7.4), 200 mM NaCl, 2% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, and a mixture of protease inhibitors. The lysate was incubated on ice for 30 min and centrifuged at 13,000 g for 15 min at 4°C. Two microliters of antibodies [control IgG, mouse anti-6xHis (MA1-21315; Thermo Fisher Scientific) or rabbit anti-calsequestrin (ab3516; Abcam, Cambridge, MA, USA)] was added to the supernatant and the mixture was incubated overnight at 4°C with rotation. A 10% slurry of protein A/G Sepharose CL-4B beads (100 μl) was added, and the mixture was incubated for an additional 4 h with rotation at 4°C. Beads were pelleted and washed 3 times with buffer containing 50 mM HEPES (pH 7.4), 200 mM NaCl, and 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate and then once more with buffer containing 50 mM HEPES (pH 7.4) and 200 mM NaCl. Pellets were resuspended in 30 μl of sample buffer and loaded on an SDS-PAGE gel followed by immunoblot analysis with mouse anti-6xHis or rabbit anti-calsequestrin antibodies and then with goat anti-mouse (AP200P; MilliporeSigma, Burlington, MA, USA) or mouse anti-rabbit light-chain specific horseradish peroxidase–conjugated antibodies.
IRE1α imaging in skeletal muscle and cardiomyocytes
For histologic analysis, paraffin sections of rabbit hind leg muscle were prepared and processed by the Alberta Diabetes Institute HistoCore Facility at the University of Alberta. Heat-induced epitope retrieval was used to break potential protein cross-linking during fixation. Tissue sections were heated in 10 mM sodium citrate (pH 6.0) at 90–95°C for 20 min. Sections were permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature, then blocked with a solution containing 5% bovine serum albumin and 2% goat serum in PBS. Sections were incubated with primary antibodies (diluted in blocking buffer) for 18 h, washed with PBS, and incubated with Alexa Fluor 488–conjugated goat anti-rabbit IgG (A11034, 1:100; Thermo Fisher Scientific) or Alexa Fluor 546–conjugated goat anti-mouse IgG antibodies (A11003, 1:100; Thermo Fisher Scientific). Sections were washed with PBS and mounted with Prolong Diamond Antifade Mountant (P36961; Thermo Fisher Scientific) and visualized using a Leica TCS SP5 confocal microscope with Leica inverted DMI 6000 B microscope base (Leica Microsystems, Buffalo Grove, IL, USA). Images were acquired with oil immersion objectives ×40 and numerical aperture (NA) 1.25 or ×100 and NA 1.44 at 22.5°C. For Alexa Fluor 488 visualization, the argon laser was used with excitation at 488 nm and emission peak at 525 nm. For Alexa Fluor 488 visualization (detects rabbit anti-RyR, -IRE1α, and -obscurin), the argon laser was used with excitation at 488 nm and emission peak at 525 nm. For Alexa Fluor 546 (detects mouse anti-RyR, -α-actinin, and -Casq1), the HeNe laser was used with excitation at 543 nm and emission peak at 573 nm. The following primary antibodies were used: rabbit anti-ryanodine receptor (32) at a 1:500 dilution, mouse anti-ryanodine receptor 1 (33) at a 1:200 dilution, rabbit anti-IRE1α (ab37073; Abcam) at a 1:200 dilution, rabbit anti-obscurin (ab121652; Abcam) at a 1:2500 dilution, mouse anti-α-actinin (A7811; MilliporeSigma) at a 1:800 dilution, and mouse anti-Casq1 VIIID1-2C at a 1:40 dilution (34) (generous gift from Dr. K. P. Campbell, University of Iowa, Iowa City, Iowa). Skeletal muscle sections were also stained with DAPI (62248; Thermo Fisher Scientific) and FITC-conjugated concanavalin A (1:50). Images were acquired with Leica Application Suite Advanced Fluorescence (Leica LAS-AF; Leica Microsystems) microscopy software, exported as a Leica Image File format, and processed using ImageJ software (https://imagej.net/Fiji/Downloads) with 8-bit image type.
Overlap of IRE1α with calsequestrin or ryanodine receptor signals was analyzed using ImageJ software. A straight line was drawn along the triad (junctional SR + T-tubule) at the longitudinal axis of muscle fiber and identified as a region of interest. The fluorescence signal intensity of each channel (green for Alexa Fluor 488, red for Alexa Fluor 546) for each immunostained section was obtained using the corresponding region of interest, and the values were plotted along the X axis (distance in micrometers) to identify regions of overlap.
Ventricular myocytes from GFP-RyR2 knock-in mice (35) were isolated using retrograde aortic perfusion as previously described by Hunt et al. (36). Freshly isolated cells were collected by centrifugation; reintroduced with Ca2+ (0.5 mM); resuspended in minimum essential medium (Thermo Fisher Scientific) supplemented with 0.2% fetal bovine serum, insulin (1 μg/ml), transferrin (0.55 μg/ml), selenium (0.5 ng/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), 2 mM glutamine, 4 mM NaHCO3, 10 mM HEPES (pH 7.4), and 10 μM blebbistatin; plated on glass coverslips precoated with laminin (50 µg/ml); and cultured in 5% CO2 at 37°C in 6-well dishes. After 4–6 h, unattached myocytes were gently removed by PBS wash, and fresh culture medium were added to the wells. The IRE1α adenovirus was added to the culture medium at a multiplicity of infection of 1000. Culture media were changed every day. After 5 d in culture, the coverslips were gently washed with PBS and mounted on an inverted Nikon A1R scanning confocal microscope system equipped with a Nikon ×60 and NA 1.2 Plan-Apochromat water immersion objective and selective excitation and emission filters. Excitation light was provided by argon (488 nm, Sapphire; Coherent, Santa Clara, CA, USA) and yellow diode (561 nm, Sapphire; Coherent) lasers to detect GFP (ExcitationMax 488 nm/EmissionMax 510 nm) and IRE1α abundance (ExcitationMax 581 nm/EmissionMax 644 nm) in cardiomyocytes. Basic image processing and spectral fluorescence unmixing for codetection and analysis of GFP and IRE1α fluorescence signals were performed using the NIS Elements AR v.4.13 software (Nikon, Tokyo, Japan).
Immunostaining of mouse embryonic fibroblasts
Ern1−/− mouse embryonic fibroblasts (lacking IRE1α) and wild-type mouse embryonic fibroblasts (a generous gift from Dr. Randal Kaufman) were fixed with 3.7% paraformaldehyde (15710; Electron Microscopy Sciences, Hatfield, PA, USA) and 0.1% glutaraldehyde for 12 min at 37°C. Cells were permeabilized with 0.05% Saponin diluted in PBS for 10 min, washed with PBS, and blocked with 5% goat normal serum in PBS with 0.05% saponin for 1 h followed by incubation with anti–IRE1α (ab37073; Abcam) at 1:200 dilution and Alexa Fluor 488–conjugated goat anti-rabbit IgG (A11034, 1:100; Thermo Fisher Scientific). Slides were mounted with Prolong Diamond Antifade Mountant and visualized with a Leica TCS SP5 confocal microscope.
IRE1α cross-linking
The homobiofunctional protein cross-linker disuccinimidyl suberate (21555; Thermo Fisher Scientific) was dissolved in DMSO at a final concentration of 10 mM. His-IRE1-NLD was diluted to a final concentration of 3.6 µM in a reaction buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 250 μM EGTA, 500 μM CaCl2, 0.05% Tween 20, and 5% glycerol. Casq2 was added to a final concentration of 18 μM (1–5 M ratio, IRE1-NLD to Casq2). Mixtures were incubated with 20-fold molar excess of disuccinimidyl suberate for 1 h at 22.5°C. The reaction was then quenched for 15 min with 100 mM Tris (pH 7.4) followed by SDS-PAGE (12% acrylamide). Proteins were transferred to a nitrocellulose membrane followed by immunoblotting with mouse anti-6xHis antibodies (MA1-21315; Thermo Fisher Scientific).
Subcellular fractionation
SR membrane fractions were isolated from rabbit skeletal muscle as previously described by Saito et al. (37). The hind leg muscle was collected from New Zealand white rabbits (1–3 kg weight), and the muscle was homogenized in buffer containing 250 ml of 300 mM sucrose and 5 mM imidazole-HCl (pH 7.4). The homogenate was centrifuged at 7700 g for 10 min at 4°C. The supernatant was saved, and the pellets were rehomogenized with the same buffer. The supernatants from both homogenates were combined and centrifuged. The microsomal pellet (containing longitudinal and terminal cisternae of SR vesicles) was obtained by centrifugation of the low-speed supernatant for 90 min at 110,000 g at 4°C. The microsomal pellet was resuspended in homogenization buffer and layered onto a sucrose gradient consisting of 45% (wt/wt) sucrose (1.6 M), 38% sucrose (1.3 M), 32% sucrose (1.1 M), and 27% sucrose (0.8 M) in 5 mM imidazole-HCl (pH 7.4). The gradient was centrifuged overnight at 70,000 g for 90 min at 4°C. The membrane fractions at the interfaces between the gradients were collected and diluted with 5 mM imidazole-HCl (pH 7.4) followed with centrifugation at 125,000 g for 2 h at 4°C. The pellets were resuspended in homogenization buffer and stored at −80°C until use.
Two hundred microliters of each fraction obtained from the sucrose gradient was further fractionated with the OptiPrep system (MilliporeSigma). OptiPrep (60% iodoxanol in water) was diluted to 25, 22, 19, 16, 10, 7, and 5% iodoxanol in a homogenization buffer containing 10 mM HEPES (pH 7.4), 250 mM sucrose, 1 mM EDTA, and 1 mM EGTA. The OptiPrep gradient was centrifuged at 184,501 g in a Beckman SW55 Ti swinging bucket rotor for 6 h. Twelve fractions (300 μl per fraction) were collected from the top of the gradient followed by incubation overnight at −20°C. A total of 1.2 ml of 90% acetone was used to precipitate proteins. Precipitated proteins were centrifuged at 16,100 g for 10 min at 4°C. The pellets were washed with 200 μl of 100% ethanol and recentrifuged at 16,100 g for 10 min at 4°C. The final pellets were dissolved in 100 μl of SDS-PAGE sample buffer followed by SDS-PAGE and immunoblot analyses.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v.7.0 (GraphPad Software, La Jolla, CA, USA). The Student’s t test was used to compare the means of 2 independent groups, and 1-way ANOVA was used to compare the means of 3 or more independent groups, with a P value determined to be significant if <0.05.
RESULTS
IRE1α is localized to junctional SR and perinuclear space in skeletal muscle and cardiomyocytes
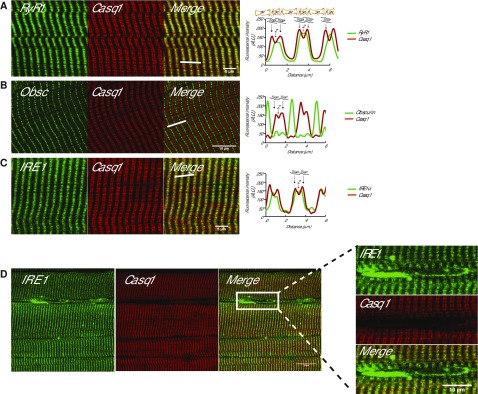
We examined the intracellular distribution of IRE1α, an ER stress coping response sensor and signaling molecule (1, 19–21), to address its molecular organization and regulation in muscle cells. Anti-RyR1 and anti-calsequestrin antibodies were used as markers of the junctional SR membrane (38), and anti-obscurin antibodies, a sarcomeric protein localized to the M-line (39), was used as a marker for the region occupied by the longitudinal SR. As expected, RyR1 and calsequestrin were colocalized to the junctional SR (Fig. 1A) (40) but not to the longitudinal SR stained with anti-obscurin antibodies (Fig. 1B). Next, we used anti-IRE1α antibodies (Supplemental Fig. S1) to localize IRE1α in muscle cells. Surprisingly, we discovered IRE1α–positive staining in the junctional SR that overlapped with anti-calsequestrin staining (Fig. 1C). This was in addition to the anticipated IRE1α–positive staining in the nuclear envelope and the perinuclear region containing the ER membrane network (Fig. 1D), a cellular region lacking any detectable staining for both calsequestrin and RyR1 (Fig. 1D). The perinuclear region ER can also be identified using concanavalin A staining (Supplemental Fig. S2).
Figure 1.
Localization of IRE1α in skeletal muscle. A) Immunostaining longitudinal sections of skeletal muscle with antibodies for RyR1 or Casq1. Right panel, graphic representation of overlap between RyR1 and Casq1. The white bars indicate the scanned area represented in the graphs. Location of the triad (T) (junctional SR + T-tubule membrane) and the Z line are indicated in the graphs. B) Immunostaining of skeletal muscle sections with anti-obscurin antibodies (Obsc) indicating the location of the M-band or with anti-calsequestrin antibodies (Casq1). The white bars indicate the scanned area represented in the graphs. C) Immunostaining of skeletal muscle with antibodies for IRE1α or Casq1. Right panel, graphic representation of overlap between IRE1α and Casq1. The white bars indicate the scanned area represented in the graphs. D) Immunostaining of IRE1α and Casq1 in the perinuclear region of skeletal muscle. Right panel shows a large magnification of the perinuclear space section indicated by the rectangle.
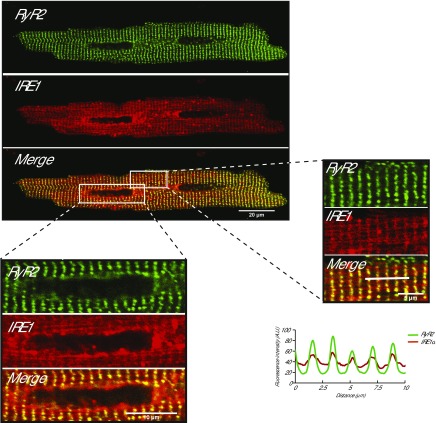
Next, we examined IRE1α localization in isolated cardiomyocytes that express GFP-tagged RyR2 (35). Similar to the skeletal muscle (Fig. 1), IRE1α colocalized with RyR2 in the cardiac junctional SR (Fig. 2) and was detected in the nuclear envelope and perinuclear ER membrane region, which did not show detectable RyR2 signal (Fig. 2). Thus, we concluded that both skeletal and cardiac muscle cells contained 2 pools of IRE1α, one localized at the perinuclear region and the other at the junctional SR containing calsequestrin and RyR1 (or RyR2).
Figure 2.
IRE1α in isolated cardiomyocytes. Isolated rat cardiomyocytes from GFP-tagged RyR2 knock-in transgenic mice (35) were transduced with adenovirus packed with the RFP-tagged IRE1α (IRE1α). Large magnification of the sarcomere and perinuclear areas is shown as indicated by the boxes. Graphic representation of overlap between RyR2 and IRE1α is shown. The white bars indicate the scanned area represented in the graphs.
The luminal domain of IRE1α interacts with calsequestrin, an SR junctional protein
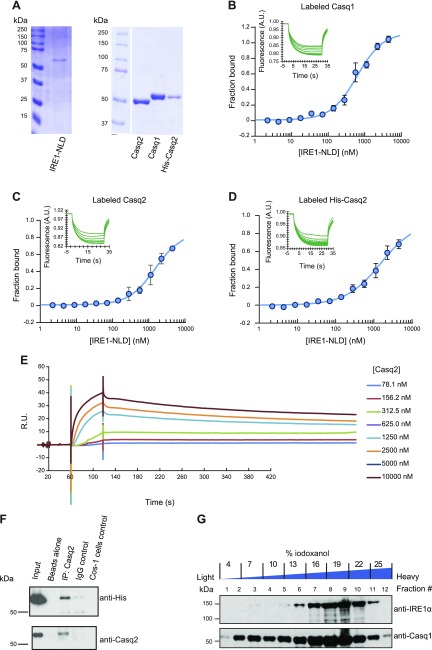
Previously, the ER resident oxidoreductase protein disulfide isomerase A6 (PDIA6) was identified as an IRE1α binding protein that modulates IRE1α activity (26, 41). Calsequestrin contains 3 thioredoxin domains (18) typical for ER resident oxidoreductases (42). Therefore, we asked whether, in muscle cells, calsequestrin can also form complexes with IRE1α at the junctional SR. To evaluate this, tissue-purified Casq1 and Casq2 and recombinant His-tagged Casq2 (Fig. 3A) were tested for direct binding to IRE1-NLD. Using microscale thermophoresis, we discovered that native skeletal muscle Casq1 bound to IRE1-NLD with a Kd of 698 nM (Fig. 3B). Both native Casq2 and recombinant His-tagged Casq2 also bound to IRE1-NLD with a Kd of 2 μM (Fig. 3C, D). Calreticulin was used as a negative control and showed no binding to IRE1-NLD as expected (Supplemental Fig. S3).
Figure 3.
Calsequestrin (Casq1 and Casq2) binds to the ER luminal domain of IRE1α. A) SDS-PAGE of proteins used for microscale thermophoresis analysis. B) Casq1 protein was covalently labeled with a red fluorescent tag and incubated with increasing amounts of the purified IRE1-NLD protein followed by microscale thermophoresis. Each data point is the mean of 3 independent microscale thermophoresis measurements. C) Fluorescently labeled native Casq2 protein was incubated with increasing amounts of purified IRE1-NLD protein followed by microscale thermophoresis. Each data point is the mean of 3 independent microscale thermophoresis measurements; error bars represent the se. D) Recombinant Casq2 protein was covalently labeled with a red fluorescent tag and incubated with increasing amounts of purified IRE1-NLD protein followed by microscale thermophoresis. Each data point is the mean of 6 independent microscale thermophoresis measurements. Normalized microscale thermophoresis time traces are shown in graphs B–D. E) IRE1-NLD was immobilized on a CM5 chip followed by flow of increasing concentrations of Casq2 as indicated in the figure and analyzed by SPR. F) His-tagged ER luminal domain of IRE1α (IRE1-NLD) and Casq2 were expressed in COS-1 cells followed by immunoprecipitation with anti-His antibodies or IgG control. Immunoblot analysis was carried out with anti-His or anti-Casq2 antibodies. Immunoprecipitation experiments were performed in triplicate with representative blot shown. G) OptiPrep gradient fractionation of heavy SR vesicles (junctional SR) followed by immunoblot analysis with anti-IRE1α and anti-calsequestrin (Casq1). A.U., arbitrary units; R.U., relative units.
We used SPR (BIACore) (Fig. 3E) and immunoprecipitation techniques (Fig. 3F) to further examine Casq2-IRE1α interactions. BIACore analysis revealed that Casq2 interacted with the luminal domain of IRE1α in a concentration-dependent manner (Fig. 3E). Next, His-tagged IRE1-NLD and Casq2 were expressed in COS-1 cells followed by immunoprecipitation with anti-Casq2 antibodies (Fig. 3F). Full-length Casq2 coimmunoprecipitated with His-tagged IRE1-NLD (Fig. 3F). Finally, OptiPrep gradient fractionation of heavy SR vesicles (enriched in junctional SR) showed that calsequestrin and IRE1α were enriched in the fractions containing heavy SR vesicles (Fig. 3G, fractions #6-11). These findings demonstrated that IRE1α colocalized with Casq1 and Casq2 at the junctional SR and that calsequestrin formed complexes with the ER luminal domain of IRE1α.
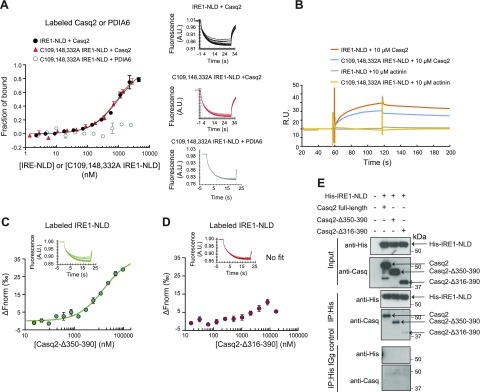
There are 3 cysteine residues in IRE1-NLD [i.e., Cys109, Cys148, and Cys332 (43)] that are essential for binding of the oxidoreductase PDIA6 to IRE1α (26). Because calsequestrin also contains 3 thioredoxin domains (18), which is typical for ER resident oxidoreductases (42), we asked whether the cysteine residues in IRE1α were involved in the binding of calsequestrin. Mutation of 3 cysteines in IRE1-NLD did not have any effect on calsequestrin binding to IRE1-NLD as measured by microscale thermophoresis (Fig. 4A) or by BIACore (Fig. 4B) techniques, indicating that calsequestrin binding to the IRE1α luminal domain did not involve the IRE1α cysteine residues.
Figure 4.
Casq2 binding to IRE1-NLD. A) Casq2 protein was covalently labeled with a red fluorescent tag and incubated with increasing amounts of IRE1-NLD or IRE1-NLD triple cysteine mutant (C109, 148, 332A IRE1-NLD) protein followed by microscale thermophoresis. Covalently labeled PDIA6 binding to the IRE1-NLD triple cysteine mutant was used as a control (26). Normalized microscale thermophoresis time traces are shown to the right of the graph. Each data point is the mean of 3 independent microscale thermophoresis measurements. B) Casq2 was injected over immobilized IRE1-NLD or immobilized C109, 148, 332A IRE1-NLD. α-Actinin was used as a negative control. C) Labeled N-terminus IRE1-NLD (IRE1-NLD) protein was incubated with increasing concentrations of truncated calsequestrin (Casq2Δ350–390) followed by microscale thermophoresis analysis. Normalized time traces are shown on the top of the graph. D) Recombinant IRE1-NLD protein was covalently labeled with a red fluorescent tag and incubated with increasing amounts of residues 316–390–truncated Casq2 (Casq2 Δ316–390) followed by microscale thermophoresis analysis. Normalized time traces are shown on the top of the graph. E) His-tagged ER IRE1-NLD (His-IRE1-NLD) and full-length or truncated (Casq2Δ350–390 or Casq2Δ316–390) Casq2 were expressed in COS-1 cells followed by immunoprecipitation with anti-His antibodies or IgG control. Immunoblot analysis was carried out with anti-His or anti-Casq2 antibodies. Immunoprecipitation experiments were performed in triplicate with a representative blot shown. The location of full-length, Δ316–390 and Δ350–390 calsequestrin is indicated by the arrows. A.U., arbitrary units; δFnorm, normalized fluorescence unit, 1000 × [Fnorm(bound) – Fnorm(unbound)]; R.U., relative units.
Finally, we asked whether binding of calsequestrin to the IRE1α luminal domain was sensitive to changes in Ca2+ concentration. Microscale thermophoresis analysis indicated that complex formation between native Casq2 or recombinant Casq2 and the luminal domain of IRE1α was independent of Ca2+ at concentrations ranging from 80 to 1000 µM (Supplemental Fig. S4).
Mapping of calsequestrin binding to IRE1α
Structurally, in addition to the 3 thioredoxin domains, Casq2 contains an acidic C-terminal domain (18), a site of high-capacity Ca2+ binding (44, 45). To map the region of Casq2 protein involved in binding to IRE1α, we expressed in E. coli and purified 2 Casq2 truncated proteins (Supplemental Fig. S5A), then analyzed their ability to bind Ca2+ and IRE1-NLD. As expected, full-length calsequestrin bound Ca2+ with a Kd value of 1 mM (Supplemental Fig. S5B). The Casq2 Δ350–390 protein, missing the 41 C-terminal acidic aa residues (Supplemental Fig. S5C), exhibited Ca2+ binding with a Kd value similar to that seen for a full-length protein (Supplemental Fig. S5B). In contrast, Casq2 Δ316–390 protein, containing only 11 acidic aa residues of the third thioredoxin domain, showed no measurable Ca2+ binding (Supplemental Fig. S5D).
We used microscale thermophoresis analysis to test whether truncated Casq2 could bind to IRE1-NLD. Calsequestrin truncated at the C-terminal acidic region (Casq2-Δ350–390) bound to the IRE1-NLD (Fig. 4C). Deletion of an additional 34 aa residues (Casq2-Δ316–390) resulted in loss of binding to IRE1-NLD (Fig. 4D). This was supported by coimmunoprecipitation experiments showing that both full-length Casq2 and Casq2 Δ350–390 expressed in COS-1 cells were efficiently pulled down with His IRE1-NLD, whereas Casq2-Δ316–390 was not (Fig. 4E). Similar to the full-length Casq (Supplemental Fig. S4), Casq2-Δ350–390 binding to IRE1-NLD was independent of Ca2+. Thus, we concluded that the last 34 amino acid residues in the third thioredoxin domain of calsequestrin that forms 2 short α-helices and 2 short β-sheets of calsequestrin (Supplemental Fig. S6) were important for the binding of Casq2 to IRE1-NLD.
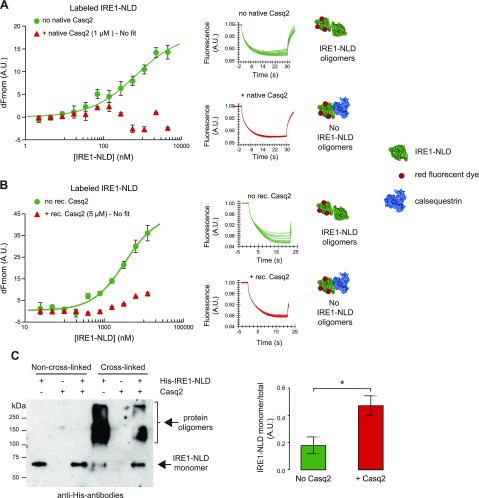
Calsequestrin prevents dimerization of IRE1α via interaction with the IRE1α luminal domain
The dimerization and oligomerization of the IRE1-NLD brings the cytosolic domains of IRE1α into close proximity (46–48). The process reconstitutes IRE1α endoribonuclease activity, which is a key step in the activation of the IRE1α branch of the UPR pathway (46–48). Based on our findings, we hypothesized that the binding of calsequestrin to IRE1α interferes with IRE1α dimerization. To test this hypothesis, we developed an IRE1α dimerization assay using microscale thermophoresis (Supplemental Fig. S7) and carried out an IRE1α dimerization or cross-linking assay. In the absence of calsequestrin, IRE1-NLD underwent the expected dimerization with increasing concentrations of IRE1-NLD (Fig. 5A, B, green traces). Strikingly, in the presence of either native (Fig. 5A) or recombinant (Fig. 5B) Casq2, the dimerization of IRE1α cytosolic domains was not detected. To further examine how calsequestrin inhibited the oligomerization of IRE1-NLD, we carried out the dimerization or cross-linking analysis of IRE1α in the absence or presence of calsequestrin. Upon addition of cross-linker, there was a substantial decrease in IRE1α monomers (Fig. 5C). In agreement with the microscale thermophoresis analysis, there was a significant proportion of IRE1α protein remaining in monomeric form in the presence of Casq2, consistent with the reduced formation of IRE1α multimers (Fig. 5C). Taken together, these findings demonstrate that the binding of calsequestrin to IRE1-NLD impeded oligomerization of IRE1α.
Figure 5.
Calsequestrin prevents IRE1α dimerization. A) Fluorescent-labeled IRE1-NLD was incubated with increasing concentrations of unlabeled IRE1-NLD in the absence (no Casq2) or presence (+ Casq2) of native Casq2 followed by microscale thermophoresis analysis. Normalized microscale thermophoresis time traces are shown to the right of the graph. Each data point is the mean of 3 independent microscale thermophoresis measurements. B) Recombinant IRE1-NLD protein was covalently labeled with a red fluorescent tag and incubated with increasing concentration of unlabeled IRE1-NLD in the absence (no rec. Casq2) or presence (+ rec. Casq2) of recombinant Casq2 followed by microscale thermophoresis. Normalized time traces are shown to the right of the graph. Each data point is the mean of 3 independent microscale thermophoresis measurements. C) Cross-linking of IRE1-NLD in the absence and presence of Casq2 was carried out as described in Materials and Methods. The abundance of IRE1-NLD monomer relative to the total IRE1-NLD is shown in the graph; n = 3. *P = 0.0307.
DISCUSSION
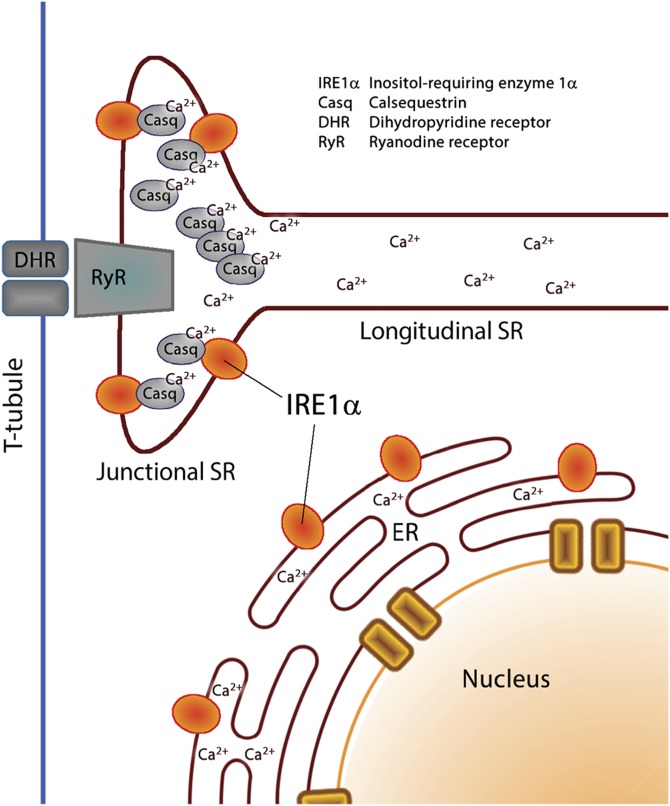
In eukaryotic cells, including muscle cells, the ER is responsible for many basic cellular processes, such as stress responses, protein synthesis and folding, synthesis of lipids and sterols, storage and release of intracellular Ca2+, and signaling to the nucleus, mitochondria, and plasma membrane (1, 8, 49). In cardiomyocytes and skeletal muscle cells, many of the ER housekeeping functions are the responsibility of the perinuclear rough and smooth ER (50). The ER of muscle is further structurally and functionally subspecialized into longitudinal and junctional SR, which are instrumental in the regulation of excitation-contraction coupling to facilitate muscle mechanical functions (51, 52) but less involved with respect to cellular processes traditionally associated with the ER (50). In this study, we discovered that there are 2 pools of IRE1α, one in the perinuclear area corresponding to the ER-like network of intracellular membrane and the second one at the junctional SR (Fig. 6). This specialized region of the SR membrane network is enriched in the RyR and Ca2+ release channel and Ca2+ binding and buffering protein calsequestrin (Fig. 6) and the site of Ca2+ release for myofilament activation (12–14). Importantly, we discovered that, at the junctional SR, IRE1-NLD interacts with calsequestrin, preventing IRE1α oligomerization. The binding of IRE1α to calsequestrin at the junctional SR may represent a unique strategy for squelching IRE1α signaling under physiologic conditions when junctional SR experiences repeated fluctuations of SR Ca2+ concentration. This strategy might serve to insulate IRE1α signaling and function in SR, leaving IRE1α in the perinuclear ER to remain responsive to cellular stress and to activate UPR independent of the constant fluctuations in Ca2+ concentration that occur in the SR.
Figure 6.
A schematic representation of 2 pools of IRE1α in muscle SR or ER. In muscle ER, the membrane network is organized into a perinuclear ER and a highly specialized smooth ER called SR (50). IRE1α is found in the perinuclear area corresponding to the ER-like network of intracellular membrane and at the junctional SR that is enriched in the RyR and Ca2+ release channel and Ca2+ binding protein calsequestrin and is the site of Ca2+ release for myofilament activation.
Several proteins have been identified as components of junctional SR to support Ca2+ release to trigger muscle contraction (e.g., RyR Ca2+ release channel) and the Ca2+ storage proteins calsequestrin, junctin, triadin, junctate, junctophilins, and mitsugumin 56 (13). Calsequestrin interacts with RyR, triadin, and junctin in the junctional SR and regulates RyR activity (17, 53–58). Binding of calsequestrin to stromal interaction molecule 1 (STIM1) has also been observed in nonmuscle cells with forced overexpression of calsequestrin, and this interaction suppresses store-operated Ca2+ entry (59). Calsequestrin binding to triadin, junctin, and STIM1 involves the C-terminal acidic high-capacity Ca2+ binding domain of the protein. This site is independent from binding of calsequestrin to IRE1α because the deletion of the calsequestrin C-terminal high-capacity Ca2+ binding domain (Δ350–390) had no effect on the interaction between the truncated calsequestrin and IRE1-NLD.
It is apparent that IRE1α signaling involves interaction with different proteins (including phosphatases, kinases, apoptosis-related proteins, and the cytoskeleton) that modulate its activity through binding to its cytoplasmic domain (1, 60, 61). It is well documented that, in nonexcitable cells, BiP binds IRE1α, directly inhibiting its activity under nonstress conditions, and dissociates from IRE1α to trigger its activity when ER stress is induced (47, 62–64). However, deletion of the BiP binding domain of IRE1α does not cause constitutive IRE1α kinase activity (65, 66), indicating there is additional complexity in BiP-dependent regulation of IRE1α. PDIA6, an oxidoreductase and ER luminal resident protein, has also been identified as an IRE1α binding partner (26, 41) and modulator of IRE1α activity (26), and, more recently, Hsp47 (67) and COX-2 (68) were identified as regulators of IRE1α. Here, we show that calsequestrin is a novel IRE1α interaction partner that constitutes the complex regulatory network controlled by IRE1α, one that is specialized at the junctional SR in the muscle.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Monika Dabrowska (University of Alberta, Edmonton, AB, Canada) for technical help. The pRL-IXFL X-box binding protein 1 splicing reporter and pED-IRE1-NLD-His6-KDEL expression vectors were a generous gift from Dr. R. Kaufman (Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA, USA). Mouse anti–skeletal muscle calsequestrin VIIID1-2C was a generous gift from Dr. K. P. Campbell (University of Iowa, Iowa City, IA, USA). This work was supported by the Canadian Institutes of Health Research Grants MOP-15291, MOP-15415, MOP-53050, and PS-153325 to M.M.; a grant from the SynAD Program (University of Alberta) to M.M.; a generous donation from the Kenneth and Sheelagh McCourt family; Canadian Institutes of Health Research Grant MOP-15291 and PS-153325 to L.B.A.; U.S. National Institutes of Health, National Heart, Lung and Blood Institute Grant R35-HL144980 to B.C.K.; National Natural Science Foundation of China, Grant 31871420 and 81602448 to J.F.T.; and by research grants from the Heart and Stroke Foundation of Alberta, Northwest Territories and Nunavut, the Natural Sciences Engineering Research Council of Canada, and the Heart and Stroke Foundation Chair in Cardiovascular Research to S.R.W.C. The authors declare no conflicts of interest.
Glossary
- BiP
binding immunoglobulin protein
- Casq1
skeletal muscle calsequestrin
- Casq2
cardiac muscle calsequestrin
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IRE1
inositol-requiring enzyme 1
- IRE1-NLD
IRE1 luminal domain
- PDIA6
protein disulfide isomerase A6
- RFP
red fluorescence protein
- RU
response unit
- RyR
ryanodine receptor
- SPR
surface plasmon resonance
- SR
sarcoplasmic reticulum
- UPR
unfolded protein response
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Q. Wang designed experiments, analyzed data, performed biochemical, biophysical, and cell biological experiments, and wrote the manuscript; J. Groenendyk designed experiments, carried out siRNA screening experiments, performed biochemical experiments, and wrote the manuscript; T. Paskevicius designed experiments and carried out protein expression and purification experiments; W. Qin designed experiments and carried out biochemical analyses; K. C. Kor and B. C. Knollmann designed experiments and carried out experiments with cardiomyocytes; Y. Liu, F. Hiess, and S. R. W. Chen designed and carried out experiments with adenovirus transduction; and J. Tang, X.-Z. Chen, L. B. Agellon, and M. Michalak designed experiments, analyzed data, and wrote the manuscript.
REFERENCES
- 1.Groenendyk J., Agellon L. B., Michalak M. (2013) Coping with endoplasmic reticulum stress in the cardiovascular system. Annu. Rev. Physiol. 75, 49–67 10.1146/annurev-physiol-030212-183707 [DOI] [PubMed] [Google Scholar]
- 2.Cao S. S., Kaufman R. J. (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 21, 396–413 10.1089/ars.2014.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dicks N., Gutierrez K., Michalak M., Bordignon V., Agellon L. B. (2015) Endoplasmic reticulum stress, genome damage, and cancer. Front. Oncol. 5, 11 10.3389/fonc.2015.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M., Kaufman R. J. (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335 10.1038/nature17041 [DOI] [PubMed] [Google Scholar]
- 5.Hetz C., Papa F. R. (2018) The unfolded protein response and cell fate control. Mol. Cell 69, 169–181 10.1016/j.molcel.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 6.Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N. H., Arias C., Lennon C. J., Kluger Y., Dynlacht B. D. (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 27, 53–66 10.1016/j.molcel.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 7.Schwarz D. S., Blower M. D. (2016) The endoplasmic reticulum: structure, function and response to cellular signaling. Cell. Mol. Life Sci. 73, 79–94 10.1007/s00018-015-2052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krebs J., Agellon L. B., Michalak M. (2015) Ca2+ homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 460, 114–121 10.1016/j.bbrc.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Braakman I., Hebert D. N. (2013) Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 5, a013201 10.1101/cshperspect.a013201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi A. E., Dirksen R. T. (2006) Sarcoplasmic reticulum: the dynamic calcium governor of muscle. Muscle Nerve 33, 715–731 10.1002/mus.20512 [DOI] [PubMed] [Google Scholar]
- 11.Reddish F. N., Miller C. L., Gorkhali R., Yang J. J. (2017) Calcium dynamics mediated by the endoplasmic/sarcoplasmic reticulum and related diseases. Int. J. Mol. Sci. 18, E1024 10.3390/ijms18051024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisner D. A., Caldwell J. L., Kistamás K., Trafford A. W. (2017) Calcium and excitation-contraction coupling in the heart. Circ. Res. 121, 181–195 10.1161/CIRCRESAHA.117.310230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone V., Randazzo D., Del Re V., Sorrentino V., Rossi D. (2015) Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers. J. Muscle Res. Cell Motil. 36, 501–515 10.1007/s10974-015-9421-5 [DOI] [PubMed] [Google Scholar]
- 14.Chopra N., Knollmann B. C. (2013) Triadin regulates cardiac muscle couplon structure and microdomain Ca2+ signalling: a path towards ventricular arrhythmias. Cardiovasc. Res. 98, 187–191 10.1093/cvr/cvt023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costello B., Chadwick C., Saito A., Chu A., Maurer A., Fleischer S. (1986) Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J. Cell Biol. 103, 741–753 10.1083/jcb.103.3.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Györke S., Stevens S. C., Terentyev D. (2009) Cardiac calsequestrin: quest inside the SR. J. Physiol. 587, 3091–3094 10.1113/jphysiol.2009.172049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knollmann B. C. (2009) New roles of calsequestrin and triadin in cardiac muscle. J. Physiol. 587, 3081–3087 10.1113/jphysiol.2009.172098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S., Trumble W. R., Liao H., Wesson C. R., Dunker A. K., Kang C. H. (1998) Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 5, 476–483 10.1038/nsb0698-476 [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Ruas J. L., Estall J. L., Rasbach K. A., Choi J. H., Ye L., Boström P., Tyra H. M., Crawford R. W., Campbell K. P., Rutkowski D. T., Kaufman R. J., Spiegelman B. M. (2011) The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 13, 160–169 10.1016/j.cmet.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul P. K., Bhatnagar S., Mishra V., Srivastava S., Darnay B. G., Choi Y., Kumar A. (2012) The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol. Cell. Biol. 32, 1248–1259 10.1128/MCB.06351-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierre N., Deldicque L., Barbé C., Naslain D., Cani P. D., Francaux M. (2013) Toll-like receptor 4 knockout mice are protected against endoplasmic reticulum stress induced by a high-fat diet. PLoS One 8, e65061 10.1371/journal.pone.0065061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marambio P., Toro B., Sanhueza C., Troncoso R., Parra V., Verdejo H., García L., Quiroga C., Munafo D., Díaz-Elizondo J., Bravo R., González M. J., Diaz-Araya G., Pedrozo Z., Chiong M., Colombo M. I., Lavandero S. (2010) Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim. Biophys. Acta 1802, 509–518 10.1016/j.bbadis.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 23.Groenendyk J., Lee D., Jung J., Dyck J. R., Lopaschuk G. D., Agellon L. B., Michalak M. (2016) Inhibition of the unfolded protein response mechanism prevents cardiac fibrosis. PLoS One 11, e0159682 10.1371/journal.pone.0159682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tufanli O., Telkoparan Akillilar P., Acosta-Alvear D., Kocaturk B., Onat U. I., Hamid S. M., Çimen I., Walter P., Weber C., Erbay E. (2017) Targeting IRE1 with small molecules counteracts progression of atherosclerosis. Proc. Natl. Acad. Sci. USA 114, E1395–E1404 10.1073/pnas.1621188114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C. Y., Wong H. N., Schauerte J. A., Kaufman R. J. (2002) The protein kinase/endoribonuclease IRE1alpha that signals the unfolded protein response has a luminal N-terminal ligand-independent dimerization domain. J. Biol. Chem. 277, 18346–18356 10.1074/jbc.M112454200 [DOI] [PubMed] [Google Scholar]
- 26.Groenendyk J., Peng Z., Dudek E., Fan X., Mizianty M. J., Dufey E., Urra H., Sepulveda D., Rojas-Rivera D., Lim Y., Kim D. H., Baretta K., Srikanth S., Gwack Y., Ahnn J., Kaufman R. J., Lee S. K., Hetz C., Kurgan L., Michalak M. (2014) Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci. Signal. 7, ra54 10.1126/scisignal.2004983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatti G., Trifari S., Mesaeli M., Parker J. M., Michalak M., Meldolesi J. (2001) Head-to-tail oligomerization of calsequestrin: a novel mechanism for heterogeneous distribution of endoplasmic reticulum luminal proteins. J. Cell Biol. 154, 525–534 10.1083/jcb.200103002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLennan D. H., Wong P. T. (1971) Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 68, 1231–1235 10.1073/pnas.68.6.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cala S. E., Jones L. R. (1983) Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J. Biol. Chem. 258, 11932–11936 [PubMed] [Google Scholar]
- 30.Milner R. E., Baksh S., Shemanko C., Carpenter M. R., Smillie L., Vance J. E., Opas M., Michalak M. (1991) Calreticulin, and not calsequestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum. J. Biol. Chem. 266, 7155–7165 [PubMed] [Google Scholar]
- 31.Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. (1992) CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques 12, 870–874, 876–879 [PubMed] [Google Scholar]
- 32.McPherson P. S., Kim Y. K., Valdivia H., Knudson C. M., Takekura H., Franzini-Armstrong C., Coronado R., Campbell K. P. (1991) The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron 7, 17–25 10.1016/0896-6273(91)90070-G [DOI] [PubMed] [Google Scholar]
- 33.Campbell K. P., Knudson C. M., Imagawa T., Leung A. T., Sutko J. L., Kahl S. D., Raab C. R., Madson L. (1987) Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J. Biol. Chem. 262, 6460–6463 [PubMed] [Google Scholar]
- 34.Knudson C. M., Chaudhari N., Sharp A. H., Powell J. A., Beam K. G., Campbell K. P. (1989) Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J. Biol. Chem. 264, 1345–1348 [PubMed] [Google Scholar]
- 35.Hiess F., Vallmitjana A., Wang R., Cheng H., ter Keurs H. E., Chen J., Hove-Madsen L., Benitez R., Chen S. R. (2015) Distribution and function of cardiac ryanodine receptor clusters in live ventricular myocytes. J. Biol. Chem. 290, 20477–20487 10.1074/jbc.M115.650531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt D. J., Jones P. P., Wang R., Chen W., Bolstad J., Chen K., Shimoni Y., Chen S. R. (2007) K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem. J. 404, 431–438 10.1042/BJ20070135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito A., Seiler S., Chu A., Fleischer S. (1984) Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J. Cell Biol. 99, 875–885 10.1083/jcb.99.3.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanner J. T., Georgiou D. K., Joshi A. D., Hamilton S. L. (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2, a003996 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young P., Ehler E., Gautel M. (2001) Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J. Cell Biol. 154, 123–136 10.1083/jcb.200102110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorgensen A. O., Shen A. C., Campbell K. P. (1985) Ultrastructural localization of calsequestrin in adult rat atrial and ventricular muscle cells. J. Cell Biol. 101, 257–268 10.1083/jcb.101.1.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eletto D., Eletto D., Dersh D., Gidalevitz T., Argon Y. (2014) Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol. Cell 53, 562–576 10.1016/j.molcel.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozlov G., Määttänen P., Thomas D. Y., Gehring K. (2010) A structural overview of the PDI family of proteins. FEBS J. 277, 3924–3936 10.1111/j.1742-4658.2010.07793.x [DOI] [PubMed] [Google Scholar]
- 43.Liu C. Y., Xu Z., Kaufman R. J. (2003) Structure and intermolecular interactions of the luminal dimerization domain of human IRE1alpha. J. Biol. Chem. 278, 17680–17687 10.1074/jbc.M300418200 [DOI] [PubMed] [Google Scholar]
- 44.Park H., Park I. Y., Kim E., Youn B., Fields K., Dunker A. K., Kang C. (2004) Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J. Biol. Chem. 279, 18026–18033 10.1074/jbc.M311553200 [DOI] [PubMed] [Google Scholar]
- 45.Beard N. A., Dulhunty A. F. (2015) C-terminal residues of skeletal muscle calsequestrin are essential for calcium binding and for skeletal ryanodine receptor inhibition. Skelet. Muscle 5, 6 10.1186/s13395-015-0029-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J., Liu C. Y., Back S. H., Clark R. L., Peisach D., Xu Z., Kaufman R. J. (2006) The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl. Acad. Sci. USA 103, 14343–14348 10.1073/pnas.0606480103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C. Y., Schröder M., Kaufman R. J. (2000) Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 275, 24881–24885 10.1074/jbc.M004454200 [DOI] [PubMed] [Google Scholar]
- 48.Li H., Korennykh A. V., Behrman S. L., Walter P. (2010) Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. USA 107, 16113–16118 10.1073/pnas.1010580107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumann O., Walz B. (2001) Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 205, 149–214 10.1016/S0074-7696(01)05004-5 [DOI] [PubMed] [Google Scholar]
- 50.Michalak M., Opas M. (2009) Endoplasmic and sarcoplasmic reticulum in the heart. Trends Cell Biol. 19, 253–259 10.1016/j.tcb.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 51.Wray S., Burdyga T. (2010) Sarcoplasmic reticulum function in smooth muscle. Physiol. Rev. 90, 113–178 10.1152/physrev.00018.2008 [DOI] [PubMed] [Google Scholar]
- 52.Bers D. M. (2014) Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu. Rev. Physiol. 76, 107–127 10.1146/annurev-physiol-020911-153308 [DOI] [PubMed] [Google Scholar]
- 53.Beard N. A., Wei L., Dulhunty A. F. (2009) Ca2+ signaling in striated muscle: the elusive roles of triadin, junctin, and calsequestrin. Eur. Biophys. J. 39, 27–36 10.1007/s00249-009-0449-6 [DOI] [PubMed] [Google Scholar]
- 54.Lee J. M., Rho S. H., Shin D. W., Cho C., Park W. J., Eom S. H., Ma J., Kim D. H. (2004) Negatively charged amino acids within the intraluminal loop of ryanodine receptor are involved in the interaction with triadin. J. Biol. Chem. 279, 6994–7000 10.1074/jbc.M312446200 [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi Y. M., Alseikhan B. A., Jones L. R. (2000) Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J. Biol. Chem. 275, 17639–17646 10.1074/jbc.M002091200 [DOI] [PubMed] [Google Scholar]
- 56.Györke I., Hester N., Jones L. R., Györke S. (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86, 2121–2128 10.1016/S0006-3495(04)74271-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L., Kelley J., Schmeisser G., Kobayashi Y. M., Jones L. R. (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272, 23389–23397 10.1074/jbc.272.37.23389 [DOI] [PubMed] [Google Scholar]
- 58.Gaburjakova M., Bal N. C., Gaburjakova J., Periasamy M. (2013) Functional interaction between calsequestrin and ryanodine receptor in the heart. Cell. Mol. Life Sci. 70, 2935–2945 10.1007/s00018-012-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L., Zhang L., Li S., Zheng Y., Yan X., Chen M., Wang H., Putney J. W., Luo D. (2015) Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci. Rep. 5, 11349 10.1038/srep11349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraskiewicz H., FitzGerald U. (2012) InterfERing with endoplasmic reticulum stress. Trends Pharmacol. Sci. 33, 53–63 10.1016/j.tips.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 61.Hetz C. (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 10.1038/nrm3270 [DOI] [PubMed] [Google Scholar]
- 62.Bertolotti A., Zhang Y., Hendershot L. M., Harding H. P., Ron D. (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 10.1038/35014014 [DOI] [PubMed] [Google Scholar]
- 63.Oikawa D., Kimata Y., Kohno K., Iwawaki T. (2009) Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp. Cell Res. 315, 2496–2504 10.1016/j.yexcr.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 64.Ma K., Vattem K. M., Wek R. C. (2002) Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J. Biol. Chem. 277, 18728–18735 10.1074/jbc.M200903200 [DOI] [PubMed] [Google Scholar]
- 65.Oikawa D., Kimata Y., Kohno K. (2007) Self-association and BiP dissociation are not sufficient for activation of the ER stress sensor Ire1. J. Cell Sci. 120, 1681–1688 10.1242/jcs.002808 [DOI] [PubMed] [Google Scholar]
- 66.Kimata Y., Oikawa D., Shimizu Y., Ishiwata-Kimata Y., Kohno K. (2004) A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol. 167, 445–456 10.1083/jcb.200405153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sepulveda D., Rojas-Rivera D., Rodríguez D. A., Groenendyk J., Köhler A., Lebeaupin C., Ito S., Urra H., Carreras-Sureda A., Hazari Y., Vasseur-Cognet M., Ali M. M. U., Chevet E., Campos G., Godoy P., Vaisar T., Bailly-Maitre B., Nagata K., Michalak M., Sierralta J., Hetz C. (2018) Interactome screening identifies the ER luminal chaperone Hsp47 as a regulator of the unfolded protein response transducer IRE1α. Mol. Cell 69, 238–252.e7 10.1016/j.molcel.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 68.Groenendyk J., Paskevicius T., Urra H., Viricel C., Wang K., Barakat K., Hetz C., Kurgan L., Agellon L. B., Michalak M. (2018) Cyclosporine A binding to COX-2 reveals a novel signaling pathway that activates the IRE1α unfolded protein response sensor. Sci. Rep. 8, 16678 10.1038/s41598-018-34891-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.