Abstract
During obesity, diabetes and hypertension inevitably coexist and cause innumerable health disparities. In the obesity, diabetes, and hypertension triad (ODHT), deregulation of glucose and NaCl homeostasis, respectively, causes diabetes and hypertension. In the mammalian intestine, glucose is primarily absorbed by Na-glucose cotransport 1 (SGLT1) and coupled NaCl by the dual operation of Na-H exchange 3 (NHE3) and Cl-HCO3 [down-regulated in adenoma (DRA) or putative anion transporter 1 (PAT1)] exchange in the brush border membrane (BBM) of villus cells. The basolateral membrane (BLM) Na/K-ATPase provides the favorable transcellular Na gradient for BBM SGLT1 and NHE3. How these multiple, distinct transport processes may be affected in ODHT is unclear. Here, we show the novel and broad regulation by Na/K-ATPase of glucose and NaCl absorption in ODHT in multiple species (mice, rats, and humans). In vivo, during obesity inhibition of villus-cell BLM, Na/K-ATPase led to compensatory stimulation of BBM SGLT1 and DRA or PAT1, whereas NHE3 was unaffected. Supporting this new cellular adaptive mechanism, direct silencing of BLM Na/K-ATPase in intestinal epithelial cells resulted in selective stimulation of BBM SGLT1 and DRA or PAT1 but not NHE3. These changes will lead to an increase in glucose absorption, maintenance of traditional coupled NaCl absorption, and a de novo increase in NaCl absorption from the novel coupling of stimulated SGLT1 with DRA or PAT1. Thus, these novel observations provide the pathophysiologic basis for the deregulation of glucose and NaCl homeostasis of diabetes and hypertension, respectively, during obesity. These observations may lead to more efficacious treatment for obesity-associated diabetes and hypertension.—Palaniappan, B., Arthur, S., Sundaram, V. L., Butts, M., Sundaram, S., Mani, K., Singh, S., Nepal, N., Sundaram, U. Inhibition of intestinal villus cell Na/K-ATPase mediates altered glucose and NaCl absorption in obesity-associated diabetes and hypertension.
Keywords: SGLT1, DRA, PAT1, NHE3, BBM
The obesity epidemic in the United States affects more than one-third (34.9% or 78.6 million) of adults. In obesity, the prevalence of diabetes was 18.5% and hypertension 35.7% (1). The most recent available data from the Centers for Disease Control and Prevention [National Diabetes Statistics Report (2)] indicate that the prevalence of diabetes in this country is 30.3 million or 9.4% of the population, and this almost triples in Americans over the age of 65 (25.2%). Altered glucose homeostasis is central to the pathophysiology of diabetes. Along with glucose metabolism, insulin activity, and gluconeogenesis, intestinal assimilation of glucose is important in maintaining glucose homeostasis. Further, ∼75 million American adults or ∼1 out of every 3 adult Americans has high blood pressure (3). This prevalence more than doubles in Americans over the age of 65 (64.0% in men and 69.3% in women). Altered Na homeostasis is central to the pathophysiology of hypertension. Along with aldosterone and renal handling, intestinal absorption of Na is important for maintaining Na homeostasis. At a larger level, in the Western hemisphere 15% of the population suffers from obesity, diabetes, and hypertension with resultant health care disparities.
In obesity, deregulation of glucose homeostasis not only results in diabetes but also obesity because hyperglycemia promotes obesity by excessive insulin release and subsequent fat deposition (4). Similarly, altered NaCl homeostasis directly results in hypertension. However, how intestinal assimilation of NaCl and glucose may be affected in obesity to cause this ubiquitous triad is unclear. For example, in hypertensive rats, intestinal glucose assimilation was diminished (5). Likewise, in diabetic rats, coupled NaCl absorption was decreased, not increased (6). Finally, in obese mice, glucose absorption was unchanged (7). This study in multiple animal and human models determined how intestinal glucose and NaCl assimilation may be altered in obesity, thus providing some important insight into one important aspect of the pathogenesis of obesity-associated diabetes and hypertension.
The primary function of the mammalian small intestine is absorption of nutrients, electrolytes, and water. The daily absorption of ∼7.5 L of water is mediated by the absorption of Na and Cl. Coupled NaCl absorption is mediated by the dual operation of Na-H and Cl-HCO3 exchanges located in the brush border membrane (BBM) of absorptive villus but not secretory crypt cells. Intracellular pH mediates this functional coupling in the villus-cell BBM (8–10). The crypt cell only has a Cl-HCO3 exchange on the BBM; thus, it is incapable of coupled NaCl absorption 9. Na-H exchange in villus-cell BBM is mediated by the solute carrier (SLC)-9A3 [Na-H exchange 3 (NHE3)] of the SLC9 gene family, whereas Cl-HCO3 exchange is mediated by the down-regulated in adenoma (DRA; SLC26A3) and putative anion transporter 1 (PAT1; SLC26A6) of the SLC26 gene family in the mammalian intestine (11–15). The phenotype of the NHE3−/− mice, specifically diarrhea, low blood pressure, and mild metabolic acidosis, illustrates the significance of NHE3 for Na and resultant water absorption in the small intestine (16). A variety of agents have been shown to regulate NHE3, including but not limited to NO, Phorbol 12-myristate 13-acetate, glucocorticoids, butyrate, IFN-γ, TNF-α, and IL-1β (17–22).
The significance of DRA in NaCl-mediated water absorption is illustrated by the phenotype of DRA knockout mice, namely watery stool with high chloride concentration and metabolic alkalosis, which is similar to congenital chloride diarrhea in humans (23). In contrast, although PAT1 knockout mice showed diminished Cl-HCO3 exchange activity, it did not result in diarrhea, implying that, unlike DRA, PAT1 may not be directly coupled to water absorption (24). Coupled NaCl absorption has been shown to be regulated by norepinephrine, acetylcholine, vasoactive intestinal peptide, angiotensin II, neuropeptide Y, serotonin, substance P, somatostatin, encephalin, peptide YY, prostaglandin E2, IFN-γ, glucocorticoid, guanylin, and uroguanylin (13). Yet whether obesity may affect NHE3, DRA, or PAT and thus coupled NaCl absorption to cause hypertension is unknown.
Na-glucose cotransport 1 (SGLT1, SLC5A1) is the most abundant Na-dependent nutrient-absorptive process in the mammalian small intestine. It is also found in the BBM of absorptive villus but not crypt cells. SGLT1 is a secondary active transport process requiring a favorable transcellular Na gradient, which is provided by the Na/K-ATPase located in the basolateral membrane (BLM) of villus cells (25, 26). Thus, at the cellular level, regulation of SGLT1 may be at the BBM cotransporter level and at the Na-extruding capacity of the cell level. SGLT1 is not only important for sodium absorption but also critical for glucose absorption, which is the most abundant nutrient in the diet (27). Preserved SGLT1 is the basis for oral rehydration, which is the most important treatment for the number-1 cause of infant mortality in developing countries, diarrhea, in which coupled NaCl absorption is diminished and SGLT1 is preserved (9). Regulation of SGLT1 by cAMP, PKA and PKC, insulin, leptin, glucagon-like peptide 2, MAPKs, NF-κB, STAT3, and PI3K/Akt has been reported (28).
Apart from numerous agents that have been shown to affect NHE3 and SGLT1, there is emerging evidence that, in fact, NHE3 and SGLT1 regulate one another. When NHE3 was silenced in intestinal epithelial cell (IEC-18) monolayers with NHE3 small interfering RNA (siRNA), the cells demonstrated decreased NHE3 activity, mRNA, and protein. However, in NHE3-silenced cells, SGLT1 activity, mRNA, and protein in the BBM were significantly increased. Thus, inhibition of NHE3 expression compensatorily increased the expression and function of SGLT1 in the BBM of intestinal epithelial cells. Further, silencing SGLT1 in IEC-18 cells diminished SGLT1 activity, mRNA, and protein levels. However, in these cells, NHE3 activity, mRNA, and protein levels were increased. Therefore, the inhibition of SGLT1 expression stimulated the transcription and function of NHE3 and vice versa in the BBM of intestinal epithelial cells.
However, how these distinct transport processes may be coordinately regulated to disrupt glucose and NaCl homeostasis in obesity resulting in diabetes and hypertension is not known.
MATERIALS AND METHODS
Animal models
Zucker rats [Strain 185 (obese) and 186 (lean) (29–31); males, 18 wk] were obtained from Charles River Laboratories (Wilmington, MA, USA). TallyHo/JngJ mice (TOM) (32, 33) (males, 21 wk) and C57BL/6 mice (34–37) (males, 21 wk) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The animals were maintained in a 12-h light/dark cycle with free access to food and water. Animals were handled and euthanized according to Marshall University’s Institutional Animal Care and Use Committee’s ethics and regulation guidelines as accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Cell lines and RNA interference
IEC-18, a well authenticated model of rat ileal epithelial cells (38), was obtained from American Type Culture Collection (ATCC; CRL-1589). Cells were used only between passages 5 and 25 and grown in high-glucose DMEM supplemented with 0.2 U/ml of insulin, 0.5 mM β-hydroxybutyrate, and 10% fetal calf serum and incubated at 37°C with 10% CO2 in a humidified atmosphere. Transient transfections to inhibit Na/K-ATPase-α1 expression were performed as previously described by Manoharan et al. (39). Briefly, silencer predesigned negative control (scrambled siRNA; 4635; Thermo Fisher Scientific, Waltham, MA, USA) and Na/K-ATPase siRNAs (4390771 and s127474) were used for siRNA transfections. Seven days–posttransfected monolayers in 24-well plates were used for all the transport studies.
Human specimen
After informed consent, small-intestinal ileal biopsies were obtained from obese (body mass index > 35) individuals without other intestinal pathology. All the intestinal specimen from humans were obtained according to the Marshall University Institutional Review Board’s regulated and approved protocol (964144-1).
Na/K-ATPase measurement
Na/K-ATPase activity was measured as inorganic phosphate released from cellular homogenates using a method previously described by Forbush (40). Enzyme-specific activity was expressed as nanomoles of π liberated per milligram protein per minute.
Uptake studies in intact ileal villus cells and BBM vesicles
Ileal villus cells were isolated from the intestines of the experimental animals by a calcium chelation technique (9, 41). Ileal villus BBM vesicles (BBMVs) from animal and human intestines were prepared by Mg++ precipitation and differential centrifugation (42, 43). Na-glucose cotransport uptake studies in intact ileal villus cells (Zucker rats alone) and BBMVs (Zucker rats, TallyHo, and human ileal villus cells) were performed by the rapid- filtration technique as previously described (42, 44, 45). In brief, for intact ileal villus cell uptake, 10 μl of ileal villus cells were suspended in Na-free buffer (42, 44, 45). The ileal villus cells were then incubated in 90 μl of reaction medium that contained Na-buffer (42, 44, 45), 10 μCi of 3H-O-methyl glucose (OMG), and 100 μM OMG in the presence or absence of 1 mM phlorizin. At 2 min, uptake was arrested by mixing with ice-cold stop solution (Na-free buffer) containing 25 mM d-glucose. The mixture was filtered on 0.65-μm Millipore (HAWP) filters and washed twice with 5 ml ice-cold stop solution. Filters were dissolved in 5 ml scintillation fluid (Ecoscint; National Diagnostics, Atlanta, GA, USA), and radioactivity was determined in a Beckman 6500 Beta Scintillation Counter (Beckman Coulter, Brea, CA, USA). For BBMV uptakes, the uptakes were performed as for cells but were arrested at 90 s, and reaction mixture was filtered on 0.45-μm Millipore (HAWP) filters. To determine the kinetic parameters of BBM Na-glucose cotransport, BBMV uptakes were performed with increasing concentrations of extravesicular OMG (50 µM to 50 mM) at a constant time point of 30 s. The uptake data derived from these experiments were analyzed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA) for Michaelis-Menten kinetics using a nonlinear regression data analysis to derive kinetic parameters.
Na-H exchange uptake was measured in BBMV by rapid-filtration technique as previously described (46, 47). Briefly, 5 μl of BBMV was suspended in vesicle medium and incubated in 95 μl of reaction medium and with or without 1 mM amiloride (46, 47). At 60 s, uptake was arrested by mixing with ice-cold stop solution and processed as described for Na-glucose cotransport uptake studies.
Cl-HCO3 exchange uptake was measured in BBMV by rapid-filtration technique as previously described (46, 48). BBMV was suspended in vesicle medium and either 50 mM KHCO3 (vesicle gassed with 5% CO2 and 95% N2) or 50 mM potassium gluconate (vesicle gassed with 100% N2). The reaction was started by adding 5 μl of vesicle to 95 μl reaction mixture with or without 1 mM 4,4-diisothiocyanatostilbene-2,2-disulfonic acid disodium salt (DIDS), a potent anion exchange inhibitor (46, 48). The uptake was stopped at 60 s with ice-cold stop solution and processed as described above.
Uptake studies in IEC-18 cells
Na-glucose cotransport studies were performed using 3H-OMG as previously described (21, 45). Briefly, cells were washed and incubated with Na-free buffer, as described above for BBMV studies, for 10 min. Uptake was initiated by incubating the cells for 2 min in reaction medium in the presence or absence of 1 mM phlorizin and 10 mM d-glucose as described above for BBMV studies. The reaction was stopped, and the cells were washed twice with ice-cold Na-free buffer containing 25 mM d-glucose. The cells were then incubated with 1 N NaOH for 20 min at 70°C to digest the cells before the addition of 4 ml of scintillation fluid (Ecoscint; National Diagnostics). Radioactivity was determined in a Beckman 6500 Beta Scintillation Counter. SGLT1-specific uptake was calculated by subtracting uptake with and without phlorizin.
Na-H exchange was initiated in IEC-18 cells with Na-free buffer containing 10 μCi of 22Na and 1 mM NaCl in the presence or absence of 50 μM EIPA, as previously described (21, 49). Briefly, the cells were preincubated for 10 min in acid load and washed with wash buffer, following by addition of the reaction mixtures. 22Na uptake reaction was arrested at 2 min, and the cells were washed twice with ice-cold PBS and processed as described above.
Cl-HCO3 exchange was performed using 36Cl as the substrate. Briefly, cells were incubated for 10 min in gluconate buffer containing 115 mM Na gluconate, 5 mM K- gluconate, 25 mM NaHCO3, and 20 mM Tris-HEPES (pH 7.5) at room temperature. Uptake was initiated by the addition of the reaction medium containing 115 mM Na gluconate, 5 mM K-gluconate, 4 mM NaHCO3, 20 mM Tris-MES (pH 5.5), 5 mM NMG, and 2.5 mM HCl with 36HCl in the presence or absence of 1 mM DIDS. 36Cl uptake was arrested at 2 min, and the cells were washed with ice-cold gluconate buffer and processed as previously described.
Western blot study
All the Western blot experiments were performed with standard protocols and techniques. Solubilized BBM proteins from rat, mouse, human, and IEC-18 cells were separated (custom-made 8% polyacrylamide gel) and transferred to a BioTrace PVDF membrane. For immunoreactive protein determination, membranes were probed with protein-specific and species-reactive antibodies. For mice, anti-SGLT1 antibody raised in rabbit (ab14686; Abcam, Cambridge, MA, USA) was used. For rat and human samples, anti-SGLT1 antibody raised in chicken (custom antibody services; Thermo Fisher Scientific) was used. For NHE3, anti-NHE3 antibody raised in chicken (custom antibody services; Thermo Fisher Scientific) was used. Anti-DRA antibody raised in goat (sc-34939; Santa Cruz Biotechnology, Dallas, TX, USA) and anti-PAT1 antibody raised in goat (sc-26728; Santa Cruz Biotechnology) were also used for this study. Horseradish peroxidase–coupled specific secondary antibodies were used to detect the proteins before chemiluminescence with ECL Detection Reagent (GE Healthcare, Waukesha, WI, USA). The protein densities of the specific proteins were quantitated with a densitometric scanner FluorChemTM instrument (ProteinSimple, San Jose, CA, USA).
Immunofluorescence study
Immunofluorescence for SGLT1, NHE3, DRA, and PAT1 were performed as previously described (50). Briefly, a portion of ileum was embedded in Cryo-OCT compound (Leica Microsystems, Buffalo Grove, IL, USA) and frozen in liquid nitrogen. Tissue sections (5 µm) that were made with a cryomicrotome (CM 3050; Leica) were incubated in blocking buffer (3% bovine serum albumin, 0.1% Triton X-100 in PBS, pH 7.4) for 20 min at room temperature. The sections were then incubated for 1 h at room temperature with either one of the primary antibodies for SGLT1 (ab126853; Abcam), NHE3 (custom antibody services; Thermo Fisher Scientific), DRA (PA5-68530; Thermo Fisher Scientific) or PAT1 (sc-26728; Santa Cruz Biotechnlogy) at 1:400 dilution. Alexa Fluor [488 nm green (A11078)] for SGLT1 and DRA and Alexa Fluor [488 nm green (A11036) for PAT1, and 568 nm red (A11072) for NHE3 secondary antibody (all obtained from Thermo Fisher Scientific)] were used at 1:800 dilution for 1 h at room temperature. Sections were then washed thrice in PBS, and the nucleus was stained with DAPI mount [i.e., Fluoroshield mounting medium with DAPI (ab104139; Abcam)] and observed with the EVOS FL Cell Imaging System (Thermo Fisher Scientific). Images were quantified by using Alpha View software (ProteinSimple).
Protein assay
Proteins were quantified with the DC Protein Assay Kit (Lowry method) according to the manufacturer’s protocols (Bio-Rad, Hercules, CA, USA).
Statistics
Results presented represent means ± se of experiments performed and calculated by GraphPad Prism 7. All uptakes were done in triplicates. Student’s t tests were performed for statistical analysis.
RESULTS
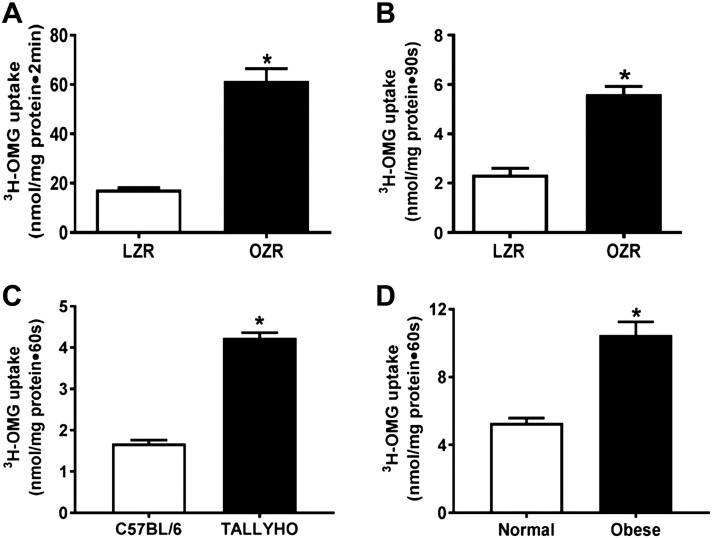
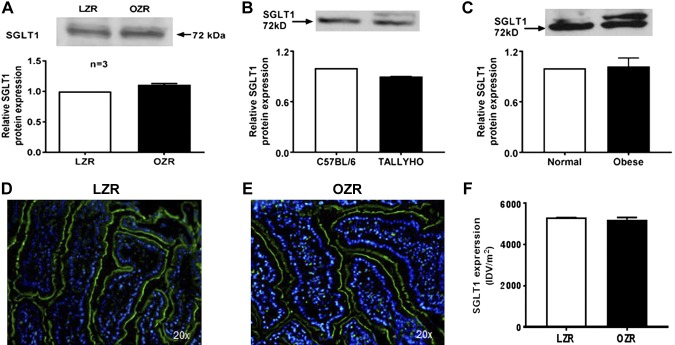
In intact villus cells isolated from obese Zucker rats (OZRs), SGLT1 activity, defined as phloridzin-sensitive Na-dependent uptake of 3-OMG, was stimulated (Fig. 1A). SGLT1 was also increased in villus-cell BBMV from OZRs (Fig. 1B). Illustrating broad applicability, SGLT1 was also increased in TOM (Fig. 1C) and obese humans (Fig. 1D). The mechanism of stimulation of SGLT1 was secondary to increased affinity (1/km) of the cotransporter for glucose [OZR: 4.8 ± 0.4 mM; lean Zucker rat (LZR): 9 ± 1; n = 4, P < 0.05)] without a change in the maximal rate of uptake of glucose (Vmax, OZR: 2.1 ± 0.1 nmol/mg pro/30 s; LZR: 1.9 ± 0.1). Consistent with this, SGLT1 protein expression was unchanged in villus cells from OZRs (Fig. 2A), TOM (Fig. 2B), or obese humans (Fig. 2C). Further, immunofluorescence studies of small intestines showed that SGLT1 immunofluorescence (green) with DAPI nuclear stain (blue) merged in LZRs (Fig. 2D) was unchanged compared with OZRs (Fig. 2E). Relative fluorescence intensity quantifications confirmed this (Fig. 2F). Collectively and broadly, these data demonstrate that, during obesity, SGLT1 was stimulated secondary to an increase in the affinity for glucose without altered SGLT1 expression.
Figure 1.
Effect of obesity on Na-dependent glucose uptake in intestinal ileal villus cells. A, B) SGLT1 was significantly stimulated in intact ileal villus cells (A) and BBMV preparations (B) from OZRs compared with LZRs. C, D) This observation was consistent in the BBMV preparations in TOM (C) and obese humans (D). For all experiments, n represents different studies performed with intestinal cells isolated from a different host each time; n = 4. *P < 0.01.
Figure 2.
Effect of obesity on SGLT1 protein. A–C) Western blot analysis showed that villus-cell SGLT1 protein levels remained unchanged in BBM between OZRs and LZRs (A), TOM and C57BL/6 (B), and obese and normal humans (C). D–F) Immunofluorescence studies of small-intestinal tissue sections showed that SGLT1 immunofluorescence (green) with DAPI nuclear stain (blue) merged in LZRs (D) is unchanged in OZRs (E) and densitometric quantification of both (F) showed no change as well. In A–C, the upper panel is a representative Western blot experiment performed at least 3 times and quantitated in the lower panels. A representative experimental pictograph is shown in D and E, and quantitation of 5 such experiments is shown in F.
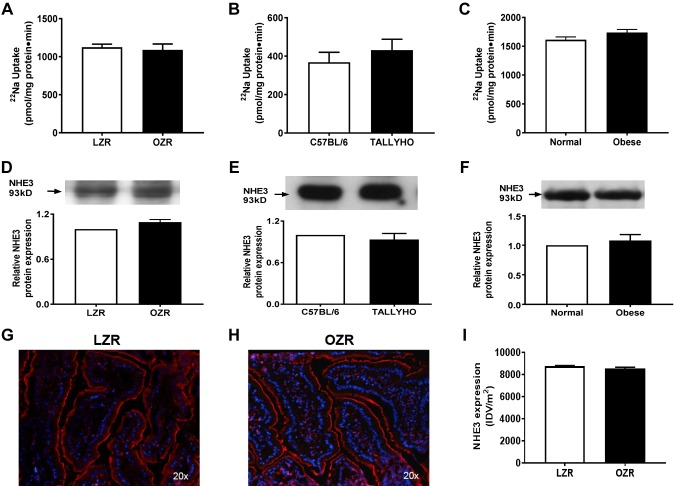
In contrast to the stimulation of SGLT1, NHE3, which with Cl-HCO3 exchange promotes coupled NaCl absorption, was unaffected in villus-cell BBMV in OZRs (Fig. 3A), TOM (Fig. 3B), and obese humans (Fig. 3C). NHE3 protein expression was also unaltered in OZRs (Fig. 3D), TOM (Fig. 3E), and obese humans (Fig. 3F). Further, immunofluorescence studies in Zucker rats confirmed that NHE3 expression (red) was unaltered between LZRs and OZRs in small-intestinal villus cells (Fig. 3G–I). In obesity, villus cell–neutral NaCl absorption is not changed because the NHE3 that mediates it (along with Cl:HCO3) is unaffected. Thus, altered NaCl homeostasis, important for hypertension, is likely not via altered neutral NaCl absorption in obesity.
Figure 3.
Na-H exchange in intestinal epithelial cells in obesity. A–C) NHE3, defined as 5-(N-ethyl-N-isopropyl)amiloride–sensitive and proton gradient–driven 22Na uptake, was unchanged in BBMVs from OZRs compared with LZRs (A). This observation was consistent in the BBMV preparations in TOM (B) and obese humans (C). D–F) Western blot analysis showed that villus-cell NHE3 protein levels remained unchanged in BBM between OZRs and LZRs (D), TOM and C57BL/6 (E), and obese and normal humans (F). G–I) Immunofluorescence studies for NHE3 expression in Zucker rats followed by densitometric analysis confirmed that NHE3 (red) was unaltered between LZRs (G) and OZRs (H) in the BBM of small-intestinal villus cells, and densitometric quantification of both (I) showed unchanged NHE3 as well. For all experiments, n represents different studies performed with intestinal cells isolated from different host each time. In D–F, the upper panel is a representative Western blot experiment performed at least 3 times and quantitated in the lower panels. A representative experimental pictograph is shown in G and H, and quantitation of 5 such experiments is shown in I; n = 4. P < 0.01 in graphs.
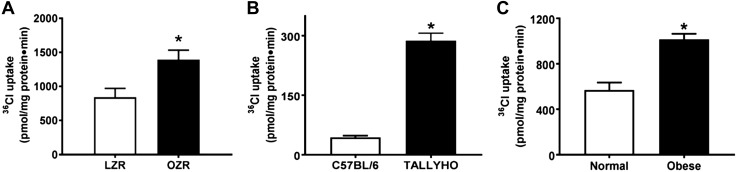
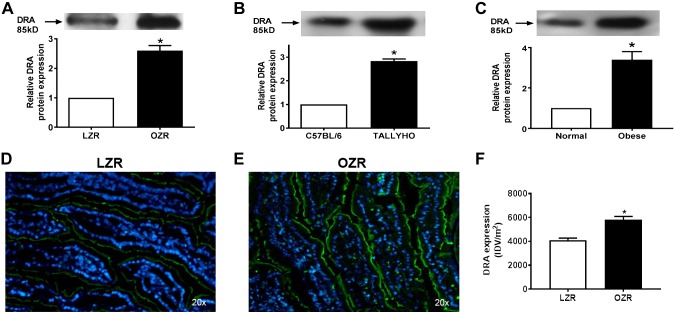
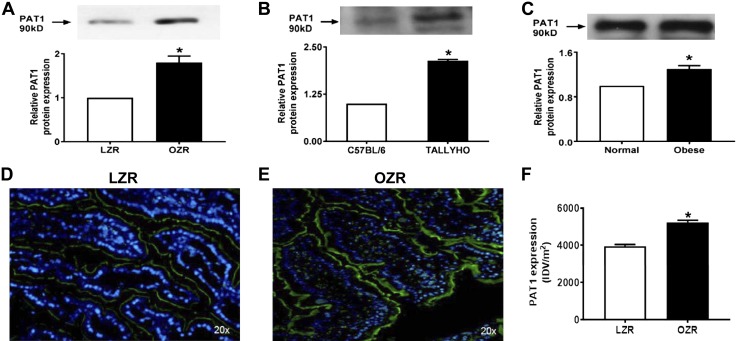
However, Cl-HCO3 exchange, mediated by DRA and PAT1, was stimulated in villus-cell BBMV from OZRs (Fig. 4A), TOM (Fig. 4B), and obese humans (Fig. 4C). The mechanism of stimulation of Cl-HCO3 exchange in OZRs was secondary to increased Vmax (OZR: 2.7 ± 0.2 nmol/mg pro/12 s; LZR: 1.7 ± 0.2; n = 3, P < 0.05) without altered Km (OZR: 6.3 ± 0.4 mM; LZR: 6.2 ± 0.5). Consistent with this, Western blot studies showed an increase in villus-cell BBM DRA protein in OZRs (Fig. 5A), TOM (Fig. 5B), and obese humans (Fig. 5C). Similarly, villus-cell BBM PAT1 was also increased in OZRs (Fig. 6A), TOM (Fig. 6B), and obese humans (Fig. 6C). Further, immunofluorescence studies showed that DRA expression (green) was increased in OZRs (Fig. 5E) compared with LZRs (Fig. 5D) in the BBM of small-intestinal villus cells during obesity (Fig. 5D–F). Similarly, PAT1 expression (green) was also increased in OZRs (Fig. 6E) compared with LZRs (Fig. 6D) in the BBM of small-intestinal villus cells in obesity (Fig. 6D–F). Thus, in obesity, BBM Cl-HCO3 exchange is stimulated secondarily to increased BBM DRA and PAT1 without altered affinity of the exchangers. Together, these data show that, in obesity, despite unaltered NHE3, Cl-HCO3 exchange is stimulated. Thus, although traditional coupled NaCl absorption is maintained, novel coupling of DRA or PAT1 with SGLT1 additionally enhances NaCl absorption in obesity in rats, mice, and humans.
Figure 4.
Effect of obesity on Cl-HCO3 exchange in intestinal epithelial cells. DIDS-sensitive and HCO3-driven 36Cl uptake was significantly increased in villus-cell BBMVs of OZRs as compared with LZRs (A), TOM vs. C57BL/6 (B), and obese human small intestine vs. normal (C). For all experiments, n represents different studies performed with intestinal cells isolated from different host each time; n = 4. *P<0.01.
Figure 5.
Effect of obesity on DRA protein. In all 3 species, small-intestinal Cl-HCO3 is mediated by DRA and PAT1. A–C) By Western blot analysis, BBM protein expression of DRA increased significantly in OZRs (A), TOM (B), and obese humans (C) compared with their respective controls. Western blot experiments were performed at least 3 times and quantitated in the lower panels. D–F) Immunofluorescence studies in Zucker rats also showed that DRA expression (green) was increased in LZRs (D) compared with OZRs (E) in the BBM of small-intestinal villus cells, and densitometric quantification of both (F) showed increased DRA during obesity as well. In D and E, a representative experimental pictograph is shown, and quantitation of 5 such experiments is shown in F. *P < 0.01.
Figure 6.
Effect of obesity on PAT1 protein. A–C) BBM protein expression of PAT1 increased significantly in OZRs (A), TOM (B), and obese humans (C) compared with their respective controls. Western blot experiments were performed at least 3 times and quantitated in the lower panels. D–F) Further, immunofluorescence studies in Zucker rats also showed that PAT1 expression (green) was increased in LZRs (D) compared with OZRs (E) in the BBM of small-intestinal villus cells, and densitometric quantification of both (F) showed increased PAT1 during obesity as well. In D and E, a representative experimental pictograph is shown and quantitation of 5 such experiments is shown in F. *P < 0.01.
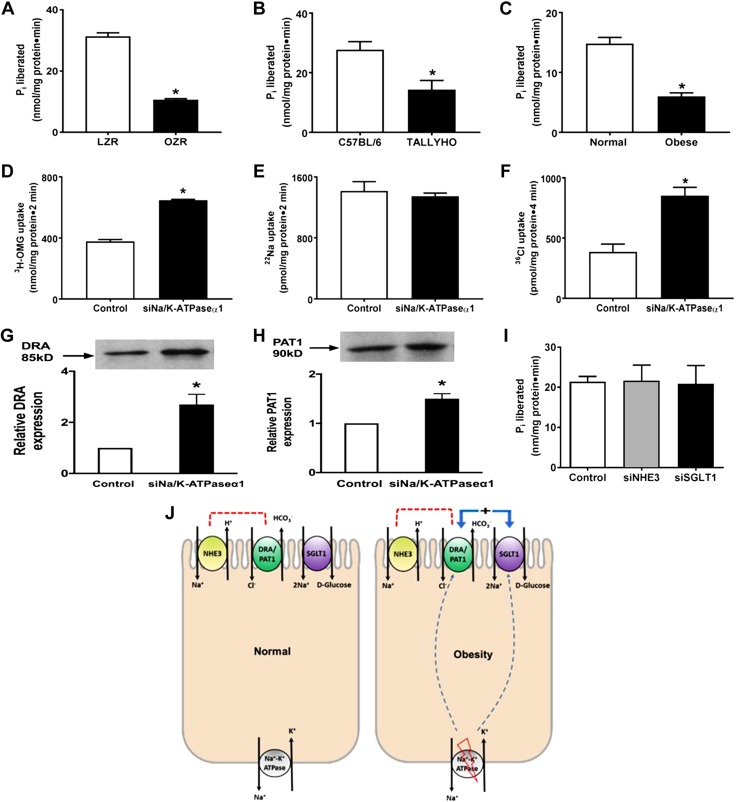
Stimulation of SGLT1 in intact villus cells (Fig. 1A) during obesity may, at least in part, be due to enhanced BLM Na/K-ATPase activity. However, Na/K-ATPase was reduced in villus cells in OZRs (Fig. 7A), TOM (Fig. 7B), and obese humans (Fig. 7C).
Figure 7.
Obesity-mediated alterations in Na/K-ATPase activity in intestinal epithelial cells. A–C) Na/K-ATPase activity was significantly reduced in villus-cell homogenates obtained from OZRs vs. LZRs (A), TOM vs. C57BL/6 (B), and obese human small intestine vs. normal (C). D–F) Transfection with siRNA for Na/K-ATPase-α1 subunit (siNa/K-ATPase-α1) in rat intestinal epithelial cells significantly stimulated Na-glucose cotransport (D), whereas Na-H exchange remained unaffected (E) as previously demonstrated and reproduced here. Further, Cl-HCO3 exchange was also stimulated (F) in siNa/K-ATPase-α1 IEC-18 cells. G, H) The BBM protein expression of the C-HCO3 exchangers, DRA (G) and PAT1 (H), was significantly increased in siNa/K-ATPase-α1 IEC-18 cells compared with controls. I) Proposed model of deregulation of glucose and Na homeostasis in obesity. J) Normal mammalian intestinal epithelial cells with traditional coupled NaCl absorption via coupling of BBM Na-H and Cl-HCO3 exchange, BBM Na-glucose cotransport and BLM Na/K-ATPase (left panel), and alterations in obesity (right panel) showing inhibition of BLM Na/K-ATPase stimulating BBM Na-glucose cotransport and C-HCO3 exchange, resulting in a novel mechanism of stimulated NaCl absorption, whereas traditional coupled NaCl absorption is maintained, thus resulting in deregulation of glucose and Na homeostasis seen in obesity. All observations are consistent with the observations from all 3 in vivo models of obesity. For all experiments, n represents different studies performed with intestinal cells isolated from different host each time. All experiments in D–H were consistently reproduced at least 3 times, each time with transfected IEC-18 cells revived from a different frozen stock. In G and H, the upper panel is a representative Western blot experiment performed at least 3 times and quantitated in the lower panels; n = 4; pi, P<0.01. *P < 0.01.
To test this hypothesis, that primary inhibition of Na/K-ATPase leads to enhanced glucose and NaCl absorption, Na/K-ATPase was directly inhibited with siRNA for its α1-subunit in IEC-18 cells. As previously demonstrated by Manoharan et al. (39) and in these experiments, SGLT1 was stimulated (Fig. 7D). The mechanism of stimulation was identical to that seen in vivo in all 3 obesity models: enhanced affinity for glucose without a change in BBM cotransporter numbers. Further, NHE3 was unaffected (Fig. 7E) in these cells. Finally, similar to in the obesity models, Cl:HCO3, mediated by DRA and PAT1, was stimulated (Fig. 7F) in these cells. The mechanism of stimulation was again increased Vmax (siRNA: 74 ± 1.2 pmol/mg pro/30 s; control: 53.4 ± 0.5; n = 3, P < 0.05) without altered Km (siRNA: 3.5 ± 0.1 mM; control:3.7 ± 0.1). Consistent with this, Western blot studies showed increased DRA (Fig. 7G) and PAT1 (Fig. 7H) expression in these cells. To determine whether inhibition of either BBM NHE3 or SGLT1 may affect BLM Na/K-ATPase, these 2 BBM transporters were silenced, and the activity of Na/K-ATPase was determined. As shown in Fig. 7I, silencing the BBM NHE3 or SGLT1 had no effect on BLM Na/K-ATPase. These data suggest that an initial direct and specific reduction in intestinal epithelial cell BLM Na/K-ATPase results in the novel stimulation and coupling of BBM SGLT1 and DRA or PAT1, promoting the enhanced glucose and NaCl absorption in obese rats, mice, and humans (Fig. 7J).
DISCUSSION
These studies indicate that in the obesity, diabetes, and hypertension triad, inhibition of intestinal villus-cell BLM Na/K-ATPase may be the initial pathophysiological alteration. Then, as compensation, BBM SGLT1 is likely stimulated. Furthermore, to maintain electro neutrality, DRA/PAT1 is also stimulated. This results in a novel additional mechanism of enhanced NaCl absorption during obesity. Further, because NHE3 is unaffected, traditional coupled NaCl absorption is also maintained. This novel coupling is twice as potent as the traditional one because SGLT1 absorbs 2 Na for each glucose and thus 2 Cl as well. Of course, stimulation of SGLT1 enhances glucose absorption as well (Fig. 7J). These observations are seen in 2 animal models of obesity and, most importantly, in human obesity as well. Further, specific RNA silencing in intestinal epithelial cells in vitro confirms the broad in vivo findings.
Therefore, these novel observations potentially provide the pathophysiologic basis for the deregulation of NaCl and glucose homeostasis of hypertension and diabetes, respectively, in obesity.
First, these studies show coupled NaCl absorption mediated by the dual operation of Na-H exchange (NHE3) and Cl-HCO3 exchange (DRA or PAT1) appears to be unaffected and preserved in hypertension of obesity (Fig. 7J) because NHE3 is unaffected in OZR (Fig. 3A), Tallyho mouse (Fig. 3B), and obese human small intestine (Fig. 3C). However, a more potent and novel pathway of NaCl absorption appears to be the cause of Na homeostasis imbalance in obesity-associated hypertension.
In contrast to NHE3, the other most prominent Na-absorptive pathway in the mammalian small intestine, namely SGLT1, was stimulated in villus-cell BBM in OZRs (Fig. 1A, B). Although the phenotype of these rats is comparable to human obesity with diabetes, hypertension, dyslipidemia, etc., it may be argued that the genotype, leptin knockout, does not have a human counterpart. Thus, a polygenic mouse model of obesity, Tallyho, was studied, and, again, here, SGLT1 was stimulated in the small intestine (Fig. 1C). SGLT1 was most tellingly stimulated in the small intestine of obese humans (Fig. 1D). The mechanism of stimulation of SGLT1 was not secondary to an increase in villus-cell BBM cotransporter numbers in Zucker rat, Tallyho mouse, or obese human intestine as demonstrated by Western blot studies (Fig. 2A–C). In agreement with these findings, immunofluorescence studies of OZRs and LZRs also did not show any change in SGLT1 expression (Fig. 2D–F). Consistent with all of these observations, kinetic studies demonstrated that, indeed, the mechanism of stimulation of SGLT1 in OZRs was secondary to an increase in the affinity of the cotransporter for glucose without a change in BBM cotransporter numbers.
Because SGLT1 stimulation was noted both at the level of the BBM as well as in intact cells from the obese intestine, Na/K-ATPase, which provides the favorable Na gradient for SGLT1, was studied. Contrary to the expectation that Na/K-ATPase may be increased to support the stimulated SGLT1, it was surprisingly diminished in Zucker rat villus cells (Fig. 7A). This unexpected observation was then confirmed in Tallyho mouse as well as in obese human small intestines (Fig. 7B, C). This led to the novel hypothesis that a primary inhibition of villus-cell BLM Na/K-ATPase in the obese intestine leads to a compensatory stimulation of BBM SGLT1 in these cells. To test this, Na/K-ATPase was directly inhibited in rat intestinal epithelial cells with siRNA for the α-subunit of Na/K-ATPase. This resulted in the inhibition of the activity of Na/K-ATPase in these cells similar to that seen in villus cells from the obese intestine of rats, mice, and humans. This lab has previously demonstrated that Na-H exchange, mediated by NHE3, was unaffected in these cells. However, SGLT1 was stimulated in these cells in a manner similar to that seen in obese animals and human. In fact, the mechanism of stimulation, secondary to an increase in the affinity of the cotransporter for glucose without an alteration in the number of cotransporters, was also identical to the mechanism of stimulation of SGLT1 in obese animals and humans. Taken together, these results support the novel hypothesis that, in obesity, a primary inhibition of BLM Na/K-ATPase in the absorptive villus cells leads to a compensatory stimulation of BBM SGLT1.
Intact tissue studies (e.g., Ussing-chamber studies) do not provide mechanistic information because they are made of villus and crypt cells, which are fundamentally different, as well as immune cells, fibroblasts, and enteric neurons, all of which can have an effect on transport. However, we agree this issue needs clarity. Thus, in this study, in all cases, ileal villus cells or BBMVs prepared from ileal enterocytes were used. Intact cells and BBMVs provide complementary mechanistic information. For example, as previously, discussed, at the level of the intact cell, BLM Na/K-ATPase has a regulatory role in the functioning of the BBM Na-dependent cotransporter. But in BBMV studies, one can decipher the effect purely at the level of the transporter without the influence of Na/K-ATPase and thus better define the mechanism of alteration. Similarly, this study concentrated on effects in the ileum. Although there is more absorption in the jejunum compared with the ileum, including via paracellular pathways and solvent drag, in the ileum, the transport is predominantly transcellular via enterocyte transporters, and that is why the studies were concentrated in this region of the gut.
Interestingly, stimulation of SGLT1 accelerates the intestinal glucose absorption that leads to excessive insulin release and fat deposition, resulting in decreased plasma glucose, which triggers repeated glucose intake and thus obesity (4). Conversely, obesity may be counteracted by inhibition of SGLT1. For example, SGLT1 knockout mice have been shown to have lower body weights, implying diminished glucose absorption leads to a lower body weight (51).
Not only does the stimulation of SGLT1 in obesity lead to enhanced glucose assimilation, it also leads to a significant increase in Na absorption because for every glucose molecule transported into the villus cells, 2 Na are also transported in the cells by SGLT1. This significant increase in positively charged Na would require a similar significant increase in Cl absorption to maintain electroneutrality. Indeed, when Cl-HCO3 exchange activity was determined in OZRs, it was increased in the BBM of villus cells. Like SGLT1, Cl-HCO3 exchange activity was also increased in Tallyho mouse intestine, and most importantly in the obese human intestine as well. Kinetic studies demonstrated that the mechanism of stimulation of Cl-HCO3 in OZRs was secondary to an increase in the number of BBM transporters without a change in the affinity of the exchanger. Cl-HCO3 exchange is mediated by DRA and PAT1 in rat, mouse, and human intestines. Consistent with kinetic studies, Western blot studies demonstrated an increase in both DRA and PAT1 in the intestines of OZRs, Tallyho mice, and, importantly, in humans as well. Taken together, stimulation of SGLT1 and DRA or PAT1 in absorptive villus cells during obesity means a new and significantly more potent means of increase in NaCl assimilation, perhaps leading to the altered NaCl homeostasis essential for hypertension seen in obesity.
Previously, it was hypothesized and confirmed with in vitro studies that a primary inhibition of BLM Na/K-ATPase activity leads to the compensatory increase in SGLT1 during obesity but not NHE3. However, the opposite, inhibition of neither BBM SGLT1 nor NHE3, affects BLM Na/K-ATPase. Thus, it was hypothesized that, in obesity, inhibition of Na/K-ATPase likely results in the stimulation of SGLT1 but not NHE3. To determine whether the de novo and more potent coupling of SGLT1 and DRA or PAT resulting in enhanced NaCl absorption is in fact present secondary to a primary inhibition of Na/K-ATPase, in vitro studies were carried out. Cl-HCO3 exchange, mediated by DRA and PAT1, was stimulated in these cells in a manner similar to that seen in obese animals and humans. In fact, the mechanism of stimulation, secondary to an increase in the number of transporters without an alteration in the affinity of the exchanger for Cl, was also identical to the mechanism of stimulation of Cl-HCO3 exchange in obese animals and humans. In these in vitro studies, scrambled controls were used, and transfection with them did not produce any nonspecific transporter effects. Further, siRNA transfection for the α1-subunit of Na/K-ATPase not only decreases mRNA expression by 60% at 4 d postconfluence but also the activity of Na/K-ATPase (39). Taken together, these results support the novel hypothesis that, in obesity, a primary inhibition of BLM Na/K-ATPase in the absorptive villus cells leads to a compensatory stimulation of BBM SGLT1 and DRA or PAT1, resulting in a de novo potent increase in NaCl assimilation during obesity.
Determination of the regulation of NHE3 and SGLT1 in obesity is made all the more interesting by the recent observation that mammalian intestinal BBM NHE3 and SGLT1 can directly regulate one another. It was shown using siRNA to silence NHE3 or SGLT1 that silencing NHE3 significantly increased the SGLT1 activity, whereas inhibiting SGLT1 stimulated NHE3 in intestinal epithelial cells (38). This would suggest that, in obesity, where SGLT1 is stimulated, NHE3 should diminish. However, because NHE3 is unaffected, this mode of regulation between SGLT1 and NHE3 appears to be lost in obesity, thus potentially contributing to the enhanced NaCl absorption necessary for hypertension associated with obesity.
In summary, the novel observations of this study in multiple species including humans and confirmed by in vitro RNA silencing have the potential to alter how we view the pathogenesis of the all-too-ubiquitous obesity, diabetes, and hypertension triad. In obesity, inhibition of BLM and Na/K-ATPase directly and selectively stimulates BBM SGLT1 with subsequent stimulation of BBM DRA or PAT1, which collectively results in stimulation of glucose and NaCl absorption. Taken together, these observations, broadly applicable in rats, mice, and humans, provide for the first time the mechanisms of enhanced intestinal glucose and NaCl absorption critical for the pathogenesis of obesity-associated diabetes and hypertension.
ACKNOWLEDGMENTS
The authors thank Dr. Usha Murughiyan (Joan C. Edwards School of Medicine, Marshall University, Huntington, WV, USA) for editorial assistance in the preparation of this manuscript. This work was supported by the American Gastroenterological Association’s Eli and Edythe Broad Student Research Fellowship to V.L.S., the U.S. National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-067420 and DK-108054, NIH National Institute of General Medical Sciences Grant P20GM121299-01A1, and the Veteran’s Administration Merit Review Grant BX003443-01 (to U.S.) B.P. and S.A. share equal first author contributions. The authors declare no conflicts of interest.
Glossary
- BBM
brush border membrane
- BBMV
BBM vesicle
- BLM
basolateral membrane
- DIDS
4,4-diisothiocyanatostilbene-2,2-disulfonic acid disodium salt
- DRA
down-regulated in adenoma
- IEC-18
intestinal epithelial cell
- LZR
lean Zucker rat
- NHE3
Na-H exchange 3
- ODHT
obesity, diabetes, and hypertension triad
- OMG
O-methyl glucose
- OZR
obese Zucker rat
- SGLT1
Na-glucose cotransport 1
- siRNA
small interfering RNA
- SLC
solute carrier
- TOM
TallyHo/JngJ mice
AUTHOR CONTRIBUTIONS
B. Palaniappan, S. Arthur, V. L. Sundaram, M. Butts, S. Sundaram, K. Mani, S. Singh, and N. Nepal performed the biological experiments, analyzed the data, and prepared the figures; and U. Sundaram originated the concept, designed the project and experiments, obtained the human biopsies, edited the manuscript, and provided overall project leadership.
REFERENCES
- 1.Saydah S., Bullard K. M., Cheng Y., Ali M. K., Gregg E. W., Geiss L., Imperatore G. (2014) Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999-2010. Obesity (Silver Spring) 22, 1888–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (2017) National Diabetes Statistics Report, US Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 3.Centers for Disease Control and Prevention (2016) High Blood Pressure Fact Sheet, High Blood Pressure in the United States. U.S. Department of Health and Human Services, Atlanta, GA, USA [Google Scholar]
- 4.Lang F., Böhmer C., Palmada M., Seebohm G., Strutz-Seebohm N., Vallon V. (2006) (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178 [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Aguayo I., Torreblanca J., Angeles de la Hermosa M., Mate A., Planas J. M., Vázquez C. M. (2001) Morphological and functional abnormalities in the ileum of rats with spontaneous hypertension: studies on SGLT1 protein. Scand. J. Gastroenterol. 36, 494–501 [DOI] [PubMed] [Google Scholar]
- 6.He P., Zhao L., Zhu L., Weinman E. J., De Giorgio R., Koval M., Srinivasan S., Yun C. C. (2015) Restoration of Na+/H+ exchanger NHE3-containing macrocomplexes ameliorates diabetes-associated fluid loss. J. Clin. Invest. 125, 3519–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung L., Kang J., Rayyan E., Bhakta A., Barrett B., Larsen D., Jelinek R., Willey J., Cochran S., Broderick T. L., Al-Nakkash L. (2014) Decreased basal chloride secretion and altered cystic fibrosis transmembrane conductance regulatory protein, Villin, GLUT5 protein expression in jejunum from leptin-deficient mice. Diabetes Metab. Syndr. Obes. 7, 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donowitz M., Cha B., Zachos N. C., Brett C. L., Sharma A., Tse C. M., Li X. (2005) NHERF family and NHE3 regulation. J. Physiol. 567, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundaram U., Knickelbein R. G., Dobbins J. W. (1991) pH regulation in ileum: Na(+)-H+ and Cl(-)-HCO3- exchange in isolated crypt and villus cells. Am. J. Physiol. 260, G440–G449 [DOI] [PubMed] [Google Scholar]
- 10.Zachos N. C., Tse M., Donowitz M. (2005) Molecular physiology of intestinal Na+/H+ exchange. Annu. Rev. Physiol. 67, 411–443 [DOI] [PubMed] [Google Scholar]
- 11.Donowitz M., Mohan S., Zhu C. X., Chen T. E., Lin R., Cha B., Zachos N. C., Murtazina R., Sarker R., Li X. (2009) NHE3 regulatory complexes. J. Exp. Biol. 212, 1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiela P. R., Xu H., Ghishan F. K. (2006) Apical NA+/H+ exchangers in the mammalian gastrointestinal tract. J. Physiol. Pharmacol. 57 (Suppl 7), 51–79 [PubMed] [Google Scholar]
- 13.Kato A., Romero M. F. (2011) Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 73, 261–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malakooti J., Saksena S., Gill R. K., Dudeja P. K. (2011) Transcriptional regulation of the intestinal luminal Na+ and Cl− transporters. Biochem. J. 435, 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mount D. B., Romero M. F. (2004) The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 447, 710–721 [DOI] [PubMed] [Google Scholar]
- 16.Schultheis P. J., Clarke L. L., Meneton P., Miller M. L., Soleimani M., Gawenis L. R., Riddle T. M., Duffy J. J., Doetschman T., Wang T., Giebisch G., Aronson P. S., Lorenz J. N., Shull G. E. (1998) Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat. Genet. 19, 282–285 [DOI] [PubMed] [Google Scholar]
- 17.Alrefai W. A., Tyagi S., Gill R., Saksena S., Hadjiagapiou C., Mansour F., Ramaswamy K., Dudeja P. K. (2004) Regulation of butyrate uptake in Caco-2 cells by phorbol 12-myristate 13-acetate. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G197–G203 [DOI] [PubMed] [Google Scholar]
- 18.Yun C. C., Chen Y., Lang F. (2002) Glucocorticoid activation of Na(+)/H(+) exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J. Biol. Chem. 277, 7676–7683 [DOI] [PubMed] [Google Scholar]
- 19.Amin M. R., Dudeja P. K., Ramaswamy K., Malakooti J. (2007) Involvement of Sp1 and Sp3 in differential regulation of human NHE3 promoter activity by sodium butyrate and IFN-gamma/TNF-alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G374–G382 [DOI] [PubMed] [Google Scholar]
- 20.Lenzen H., Lünnemann M., Bleich A., Manns M. P., Seidler U., Jörns A. (2012) Downregulation of the NHE3-binding PDZ-adaptor protein PDZK1 expression during cytokine-induced inflammation in interleukin-10-deficient mice. PLoS One 7, e40657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palaniappan B., Sundaram U. (2018) Direct and specific inhibition of constitutive nitric oxide synthase uniquely regulates brush border membrane Na-absorptive pathways in intestinal epithelial cells. Nitric Oxide 79, 8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coon S., Kekuda R., Saha P., Talukder J. R., Sundaram U. (2008) Constitutive nitric oxide differentially regulates Na-H and Na-glucose cotransport in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1369–G1375 [DOI] [PubMed] [Google Scholar]
- 23.Schweinfest C. W., Spyropoulos D. D., Henderson K. W., Kim J. H., Chapman J. M., Barone S., Worrell R. T., Wang Z., Soleimani M. (2006) slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 281, 37962–37971 [DOI] [PubMed] [Google Scholar]
- 24.Wang Z., Wang T., Petrovic S., Tuo B., Riederer B., Barone S., Lorenz J. N., Seidler U., Aronson P. S., Soleimani M. (2005) Renal and intestinal transport defects in Slc26a6-null mice. Am. J. Physiol. Cell Physiol. 288, C957–C965 [DOI] [PubMed] [Google Scholar]
- 25.Robinson J. D., Flashner M. S. (1979) The (Na+ + K+)-activated ATPase. Enzymatic and transport properties. Biochim. Biophys. Acta 549, 145–176 [DOI] [PubMed] [Google Scholar]
- 26.Wright E. M., Hirayama B. A., Loo D. F. (2007) Active sugar transport in health and disease. J. Intern. Med. 261, 32–43 [DOI] [PubMed] [Google Scholar]
- 27.Wright E., Loo D., Hirayama B., Turk E. (2006) Sugar absorption. In: Physiology of Gastrointestinal Tract, 4th Ed. (Johnson L., Barrett K. E., Ghishan F., Merchant J., Said H., Wood J., eds.), pp. 1653–1666, Elsevier/Academic Press, San Diego, CA, USA: [Google Scholar]
- 28.Poulsen S. B., Fenton R. A., Rieg T. (2015) Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 24, 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bickel C. A., Knepper M. A., Verbalis J. G., Ecelbarger C. A. (2002) Dysregulation of renal salt and water transport proteins in diabetic Zucker rats. Kidney Int. 61, 2099–2110 [DOI] [PubMed] [Google Scholar]
- 30.Kurtz T. W., Morris R. C., Pershadsingh H. A. (1989) The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension 13, 896–901 [DOI] [PubMed] [Google Scholar]
- 31.Bray G. A. (1977) The Zucker-fatty rat: a review. Fed. Proc. 36, 148–153 [PubMed] [Google Scholar]
- 32.Kim J. H., Saxton A. M. (2012) The TALLYHO mouse as a model of human type 2 diabetes. Methods Mol. Biol. 933, 75–87 [DOI] [PubMed] [Google Scholar]
- 33.Joost H. G., Schürmann A. (2014) The genetic basis of obesity-associated type 2 diabetes (diabesity) in polygenic mouse models. Mamm. Genome 25, 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao X., Dillon K. D., McEntee M. F., Saxton A. M., Kim J. H. (2014) Islet insulin secretion, β- cell mass, and energy balance in a polygenic mouse model of type 2 diabetes with obesity. J. Inborn Errors Metab. Screen. 2, 1–6 [Google Scholar]
- 35.Sung Y. Y., Lee Y. S., Jung W. H., Kim H. Y., Cheon H. G., Yang S. D., Rhee S. D. (2005) Glucose intolerance in young TallyHo mice is induced by leptin-mediated inhibition of insulin secretion. Biochem. Biophys. Res. Commun. 338, 1779–1787 [DOI] [PubMed] [Google Scholar]
- 36.Rhee S. D., Sung Y. Y., Lee Y. S., Kim J. Y., Jung W. H., Kim M. J., Lee M. S., Lee M. K., Yang S. D., Cheon H. G. (2011) Obesity of TallyHO/JngJ mouse is due to increased food intake with early development of leptin resistance. Exp. Clin. Endocrinol. Diabetes 119, 243–251 [DOI] [PubMed] [Google Scholar]
- 37.Parkman J. K., Mao X., Dillon K., Gudivada A., Moustaid-Moussa N., Saxton A. M., Kim J. H. (2016) Genotype-dependent metabolic responses to semi-purified high- sucrose high-fat diets in the TALLYHO/jng vs. C57bl/6 mouse during the development of obesity and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 124, 622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coon S., Kekuda R., Saha P., Sundaram U. (2011) Reciprocal regulation of the primary sodium absorptive pathways in rat intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 300, C496–C505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manoharan P., Gayam S., Arthur S., Palaniappan B., Singh S., Dick G. M., Sundaram U. (2015) Chronic and selective inhibition of basolateral membrane Na-K-ATPase uniquely regulates brush border membrane Na absorption in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 308, C650–C656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbush B., III (1983) Assay of Na,K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal. Biochem. 128, 159–163 [DOI] [PubMed] [Google Scholar]
- 41.Sundaram U., Coon S., Wisel S., West A. B. (1999) Corticosteroids reverse the inhibition of Na-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol. 276, G211–G218 [DOI] [PubMed] [Google Scholar]
- 42.Sundaram U., Wisel S., Rajendren V. M., West A. B. (1997) Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol. 273, G913–G919 [DOI] [PubMed] [Google Scholar]
- 43.Sundaram U., West A. B. (1997) Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am. J. Physiol. 272, G732–G741 [DOI] [PubMed] [Google Scholar]
- 44.Sundaram U., Wisel S., Fromkes J. J. (1998) Unique mechanism of inhibition of Na+-amino acid cotransport during chronic ileal inflammation. Am. J. Physiol. 275, G483–G489 [DOI] [PubMed] [Google Scholar]
- 45.Turner J. R., Black E. D. (2001) NHE3-dependent cytoplasmic alkalinization is triggered by Na(+)-glucose cotransport in intestinal epithelia. Am. J. Physiol. Cell Physiol. 281, C1533–C1541 [DOI] [PubMed] [Google Scholar]
- 46.Coon S., Sundaram U. (2003) Unique regulation of anion/HCO3- exchangers by constitutive nitric oxide in rabbit small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G1084–G1090 [DOI] [PubMed] [Google Scholar]
- 47.Manokas T., Fromkes J. J., Sundaram U. (2000) Effect of chronic inflammation on ileal short-chain fatty acid/bicarbonate exchange. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G585–G590 [DOI] [PubMed] [Google Scholar]
- 48.Manoharan P., Coon S., Baseler W., Sundaram S., Kekuda R., Sundaram U. (2013) Prostaglandins, not the leukotrienes, regulate Cl(-)/HCO(3)(-) exchange (DRA, SLC26A3) in villus cells in the chronically inflamed rabbit ileum. Biochim. Biophys. Acta 1828, 179–186 [DOI] [PubMed] [Google Scholar]
- 49.Hodges K., Gill R., Ramaswamy K., Dudeja P. K., Hecht G. (2006) Rapid activation of Na+/H+ exchange by EPEC is PKC mediated. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G959–G968 [DOI] [PubMed] [Google Scholar]
- 50.Kumar K. M., Aruldhas M. M., Banu S. L., Sadasivam B., Vengatesh G., Ganesh K. M., Navaneethabalakrishnan S., Navin A. K., Michael F. M., Venkatachalam S., Stanley J. A., Ramachandran I., Banu S. K., Akbarsha M. A. (2017) Male reproductive toxicity of CrVI: in-utero exposure to CrVI at the critical window of testis differentiation represses the expression of Sertoli cell tight junction proteins and hormone receptors in adult F1 progeny rats. Reprod. Toxicol. 69, 84–98 [DOI] [PubMed] [Google Scholar]
- 51.Gorboulev V., Schürmann A., Vallon V., Kipp H., Jaschke A., Klessen D., Friedrich A., Scherneck S., Rieg T., Cunard R., Veyhl-Wichmann M., Srinivasan A., Balen D., Breljak D., Rexhepaj R., Parker H. E., Gribble F. M., Reimann F., Lang F., Wiese S., Sabolic I., Sendtner M., Koepsell H. (2012) Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]