Abstract
Na+/H+ exchanger regulatory factor 1 (NHERF1; also known as ezrin-radixin-moesin–binding phosphoprotein 50) is a PSD-95, disc large, zona occludens-1 adapter that acts as a scaffold for signaling complexes and cytoskeletal-plasma membrane interactions. NHERF1 is crucial to β-2-adrenoceptor (β2AR)-mediated activation of cystic fibrosis transmembrane conductance regulator (CFTR) in epithelial cells, and NHERF1 has been proposed to mediate the recycling of internalized β2AR back to the cell membrane. In the current study, we assessed the role of NHERF1 in regulating cAMP-mediated signaling and immunomodulatory functions in airway smooth muscle (ASM). NHERF1 knockdown attenuated the induction of (protein kinase A) phospho–vasodilator-stimulated phosphoprotein (p-VASP) by isoproterenol (ISO), prostaglandin E2 (PGE2), or forskolin (FSK) as well as the induction of p–heat shock protein 20 after 4 h of stimulation with ISO and FSK. NHERF1 knockdown fully abrogated the ISO-, PGE2-, and FSK-induced IL-6 gene expression and cytokine production without affecting cAMP-mediated phosphodiesterase 4D (PDE4D) gene expression, phospho–cAMP response element–binding protein (p-CREB), and cAMP response element (CRE)–Luc, or PDGF-induced cyclin D1 expression. Interestingly, NHERF1 knockdown prevented ISO-induced chromatin-binding of the transcription factor CCAAT-enhancer–binding protein-β (c/EBPβ). c/EBPβ knockdown almost completely abrogated the cAMP-mediated IL-6 but not PDE4D gene expression. The differential regulation of cAMP-induced signaling and gene expression in our study indicates a role for NHERF1 in the compartmentalization of cAMP signaling in ASM.—Pera, T., Tompkins, E., Katz, M., Wang, B., Deshpande, D. A., Weinman, E. J., Penn, R. B. Specificity of NHERF1 regulation of GPCR signaling and function in human airway smooth muscle.
Keywords: EBP50, compartmentalized signaling, PKA, c/EBP, asthma
GPCR signaling is a complex cascade of events subject to regulation at multiple loci inside the cell. Following ligand binding, a conformational change occurs in the 3-dimensional structure of the GPCR, initiating a series of events resulting in the generation of second messenger molecules and activation of downstream signaling effectors that regulate cellular functions. For many GPCRs, ligand binding will also initiate the desensitization and down-regulation of receptors, which may limit their signaling abilities. During the process of receptor down-regulation GPCRs are internalized via clathrin-coated pits and are subsequently recycled back to the cell membrane, or they can be sorted into endosomes, which destines them for lysosomal degradation.
Na+/H+ exchanger (NHE) regulatory factor 1 [NHERF1; also known as ezrin-radixin-moesin (ERM)-binding phosphoprotein 50] contains postsynaptic density protein 95 (PSD-95), disc large, zona occludens-1 (PDZ) domains, which enable protein-protein interactions with molecules containing PDZ-binding motifs. In addition, its ERM domain renders it capable of binding to the actin cytoskeleton. NHERF1 was initially identified as a cofactor required for the cAMP-dependent protein kinase (PKA)-mediated inhibition of the NHE in kidney brush border membranes (1).
Hall et al. (2) was the first to demonstrate a direct interaction of NHERF1 with GPCRs, in which NHERF1 was shown to interact with the PDZ-binding motif (D-S/T-x-L) in the C terminus of the β-2-adrenoceptor (β2AR). These initial studies described the potential of NHERF1 to function as a signaling molecule that transduces β2AR signaling independently of PKA to regulate NHE. Subsequent work by the von Zastrow lab also revealed that NHERF1 is required for the efficient recycling of internalized β2AR (3). Impaired NHERF1 binding to β2AR, imposed either by truncation of NHERF1 PDZ domains or mutations in the β2AR C terminus (PDZ-binding motifs), leads to diminished recycling of internalized β2AR back to the cell membrane, instead diverting receptors to lysosomes for degradation. The ERM domain of NHERF1, which allows interaction of NHERF1 with the actin cytoskeleton, was similarly crucial for efficient recycling of β2AR. Since these initial studies, multiple GPCRs, including parathyroid hormone receptor, κ opioid receptor, P2Y purinoceptor 1, C-C chemokine receptor 5, calcitonin receptor–like receptor, and thromboxane A2 receptor, have been shown to bind NHERF1 to modulate their down-regulation and recycling dynamics (4). In addition to its role in receptor trafficking, NHERF1 has been shown to form complexes to either promote C-X-C motif chemokine receptor 2 (CXCR2) – phospholipase C-3β (PLC3β) (5) or inhibit platelet-derived growth factor receptor (PDGFR) - phosphatase and tensin homolog (PTEN), frizzled class receptor 4 (Fzd4) – disheveled (Dvl) (6, 7) signaling.
Moreover, NHERF1 has been shown to bind the A-kinase anchoring protein ezrin to form a signaling complex with PKA to promote immunomodulatory actions of cAMP in T cells (8, 9) or to promote the stability and cAMP-mediated activation of cystic fibrosis transmembrane conductance regulator (CFTR) in epithelial cells (10–13). The ability of NHERF1 to regulate GPCR desensitization or recycling, to direct GPCR signaling, and to engage in formation of signaling complexes makes it very well positioned to affect signaling and functional outcomes in cells. Although numerous studies by our group and others have examined the regulation and functional significance of cAMP/PKA signaling in airway smooth muscle (ASM) cells (14–25), no studies to date have examined the role of NHERF1 in ASM. Herein, we delineate the regulatory role of NHERF1 in Gs-coupled GPCR signaling in human ASM cells.
MATERIALS AND METHODS
Human ASM cell isolation and cell culture
Human ASM cultures were established as previously described (26) from human airways obtained from lung transplant donors under procedures approved by the University of Maryland, and the Thomas Jefferson University Institutional Review Board. Characterization of these cells regarding immunofluorescence of smooth muscle actin and agonist-induced changes in cytosolic calcium has been previously reported (27). Third to sixth passage cells were plated at a density of 104 cells/cm2 and maintained in Ham’s F-12 medium supplemented with 10% fetal bovine serum. Cells were growth arrested 24 h prior to stimulation by washing once in PBS and refeeding with serum-free Ham’s F-12 medium.
Small interfering RNA–mediated knockdown of NHERF1 in ASM
Small interfering RNA (siRNA) On-Targetplus Smartpool oligos (Dharmacon, Lafayette, CO, USA) directed against NHERF1 or CCAAT-enhancer–binding protein-β (c/EBPβ) or scrambled (SCR; control) siRNA oligos were annealed at 37°C for 1 h; 5 μg of the annealed oligo mixture was used to transfect human ASM cells using Dharmafect 1 (Dharmacon) per the manufacturer’s instruction. Twenty-four hours after transfection, the cells were replated for subsequent assessment of protein expression, cAMP accumulation, or gene expression.
cAMP accumulation in cultured ASM cells
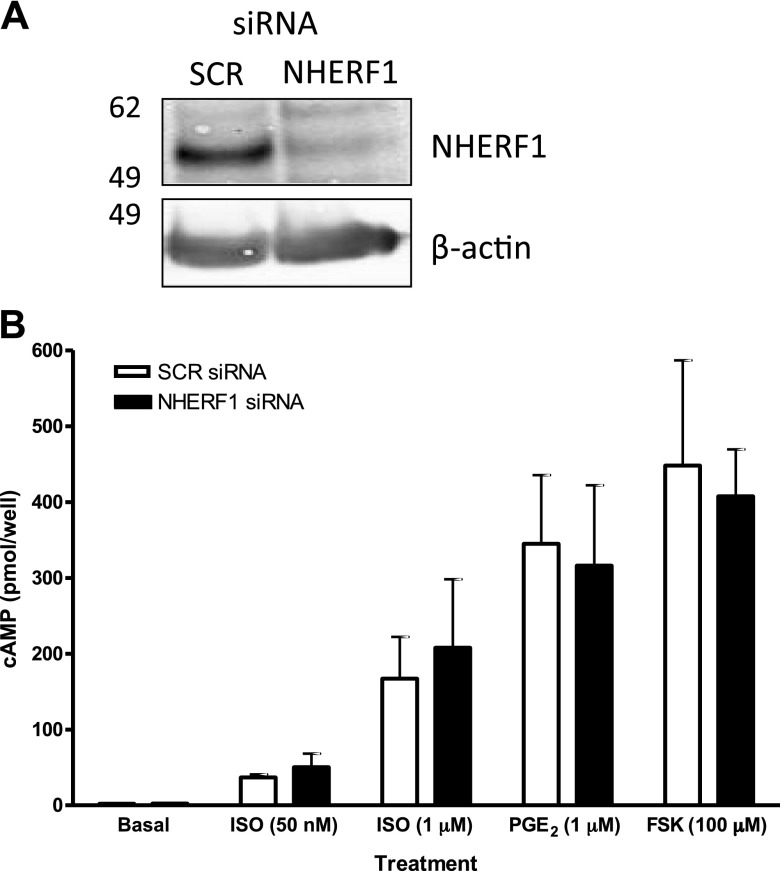
Cells transfected with SCR or NHERF1 siRNA were plated into 24-well plates and analyzed for agonist-induced cAMP accumulation as previously described (27). Briefly, cells were growth arrested, washed, and stimulated with PBS containing 300 µM ascorbic acid, 1 mM 3-isobutyl-1-methylxanthine, and either vehicle, isoproterenol (ISO; 50 nM or 1 µM), prostaglandin E2 (PGE2; 1 µM), or forskolin (FSK; 100 µM) for 10 min at 37°C. Reactions were quenched by buffer aspiration and addition of 400 µl cold 100% ethanol. Isolated cAMP was subsequently quantified by radioimmunoassay (27). Data are expressed as picomoles cAMP/well.
Immunoblot analysis
Cells were growth arrested for 24 h, refed with serum-free medium, and stimulated for the indicated times. Cells were then washed with ice-cold PBS and solubilized in RIPA buffer (25 mM Tris HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with protease and phosphatase inhibitor cocktail (Biotool, Houston, TX, USA). For cell fractionation, a subcellular protein fractionation kit for cultured cells (Thermo Fisher Scientific, Waltham, MA, USA) was used according to the manufacturer’s instructions. Lysates were buffered with Laemmli solution and loaded and separated on 10% or 4–20% gradient Tris-glycine polyacrylamide gels (Criterion; Bio-Rad, Hercules, CA, USA). Blots were probed with specific antibodies directed against NHERF1 and phosphorylated (p)–heat shock protein 20 (HSP20; Abcam, Cambridge, MA, USA), vasodilator-stimulated phosphoprotein (VASP; BD Biosciences, San Jose, CA, USA), p–cAMP response element–binding protein (CREB), c/EBPβ (Cell Signaling Technology, Danvers, MA, USA), and β-actin (MilliporeSigma, Burlington, MA, USA).
Quantitative PCR
Total RNA was isolated by standard procedures using Trizol (Thermo Fisher Scientific) and Direct-zol RNA Miniprep Kit (Zymo Research, Irvine, CA, USA) converted to cDNA, and mRNA abundance was assessed by quantitative PCR as previously described (28). Primers used are GAPDH forward: 5′-CCCTTCATTGACCTCAACTACATGGT-3′, and reverse: 5′-TGATGACAAGCTTCCCGTTCTCAG-3′; IL-6 forward: 5′-AAGTCCTGATCCAGTTCCTG-3′, and reverse: 5′-GCGCAGAATGAGATGAGTTG-3′; and phosphodiesterase 4D (PDE4D) forward: 5′-ATCCATGCTGCAGATGTTGTCC-3′, and reverse: 5′-AAAGCCCACAGCCAAATGATGG-3′. All primers were designed to span an intron.
Luciferase reporter assay
For luciferase assays, stable expression of a reporter construct for cAMP response element (CRE)-Luc was established in human telomerase reverse transcriptase ASM cells using lentivirus (Cignal Lenti luciferase reporter viral particles; SA Biosciences, Frederick, MD, USA) as per the manufacturer’s recommendations. Stable lines were selected and maintained in complete medium containing puromycin. Cells were plated into 24-well plates post–NHERF1 knockdown, allowed to attach overnight, and then serum–deprived for 24 h. Cells were subsequently stimulated for 6 h and then harvested in passive lysis buffer according to the manufacturer’s instructions and stored at −20°C. After thawing, 20 µl of cell lysate was loaded per well of a white 96-well plate, and firefly luciferase substrate reagent (100 µl; Luciferase Assay System; Promega, Madison, WI, USA) was added to each well. Luminescence was measured using a microplate luminometer (Synergy 2; BioTek, Winooski, VT, USA).
Statistical analysis
Data analysis was performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA), and data are expressed as means ± sem. Group comparisons were performed using 2-way or 1-way ANOVA followed by Bonferroni’s multiple comparison test. A value of P < 0.05 was considered sufficient to reject the null hypothesis.
RESULTS
NHERF1 regulates cAMP signaling in human ASM cells
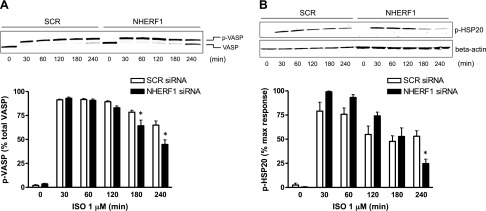
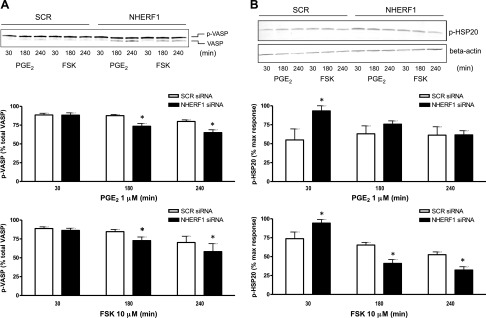
In primary human ASM cells, expression of NHERF1 was confirmed by Western blotting and quantitative PCR (unpublished results). Transfection with siRNA directed against NHERF1 consistently resulted in >80% knockdown of NHERF1 protein levels (Fig. 1A) as determined by Western blotting. Knockdown of NHEFR1 did not affect acute (10 min stimulation) ISO-, PGE2-, or FSK-induced cAMP production in human ASM cells (Fig. 1B). However, a time-course of ISO-stimulated phosphorylation of the PKA substrate VASP revealed that knockdown of NHERF1 resulted in decreased VASP phosphorylation after 180 and 240 min of stimulation (Fig. 2A). In addition, ISO-induced HSP20 phosphorylation after 4 h of stimulation was lower under NHERF1 knockdown conditions compared with SCR controls (Fig. 2B). Similarly, PGE2- and FSK-induced VASP phosphorylation was reduced after 180 and 240 min of stimulation (Fig. 3). Interestingly, at 30 min of stimulation, PGE2- and FSK-induced HSP20 phosphorylation was increased under NHERF1 knockdown condition, whereas at 180 and 240 min poststimulation, FSK-induced p-HSP20 was lower under NHERF1 knockdown vs. SCR controls (Fig. 3). PGE2-induced pHSP20 at 180 and 240 min was not different between NHERF1 and SCR conditions.
Figure 1.
NHERF1 knockdown does not affect cAMP accumulation in human ASM cells. A) NHERF1 knockdown was confirmed by immunoblotting; a representative blot is shown. B) After SCR or NHERF1 siRNA transfection, cells were stimulated with cAMP-elevating agents for 10 min in the presence of IBMX. Data are means ± sem; n = 3.
Figure 2.
NHERF1 knockdown decreases ISO-induced VASP (A) and HSP20 (B) phosphorylation in human ASM cells. Cells were stimulated with ISO (0–240 min) post–siRNA transfection and cell lysates collected. Representative blot is shown. Data are means ± sem; n = 5. Two-way ANOVA followed by Bonferroni multiple comparison test. *P < 0.05 vs. own SCR control.
Figure 3.
Role of NHERF1 in PGE2- and FSK-induced VASP and HSP20 phosphorylation in human ASM cells. Cells were stimulated with PGE2 (A) or FSK (B) (30–240 min) post–siRNA transfection and cell lysates collected. Representative blots are shown. Data are means ± sem; n = 4–7 (PGE2) or n = 3–5 (FSK). Two-way ANOVA followed by Bonferroni multiple comparison test. *P < 0.05 vs. own SCR control.
NHERF1 differentially regulates cAMP-driven gene expression and cyclin D1 expression in human ASM cells
Among the important functions of ASM are its immunodulatory and synthetic functions, including the production of cytokines that cooperate in the regulation of allergic airway inflammation (29, 30). Stimulation of human ASM cells with ISO, PGE2, or FSK increased IL-6 gene expression by ∼6-fold as determined by quantitative PCR (Fig. 4A). NHERF1 knockdown resulted in a 90–100% inhibition of IL-6 gene expression for all 3 cAMP-elevating agents. NHERF1 knockdown did not have an effect on TNF-α–induced IL-6 gene expression (SCR: 26.7 ± 2.8; NHERF1: 22.8 ± 8.3 fold basal). Similar results were obtained for IL-6 cytokine protein measured by ELISA (Fig. 4B). Conversely, PDE4D mRNA expression induced by ISO and PGE2 was not affected by NHERF1 knockdown; FSK-induced PDE4D gene expression was modestly affected but not statistically different (Fig. 4C). Combined average Ct values from all experiments for the SCR vehicle condition were GAPDH, 16.7 ± 0.2; IL-6, 25.7 ± 0.6; and PDE4D, 24.5 ± 0.6.
Figure 4.
NHERF1 differentially regulates cAMP-driven gene expression and IL-6 production in human ASM cells. Cells were stimulated with ISO (1 µM), PGE2 (1 µM), or FSK (10 µM) post–siRNA transfection for either 4 h (mRNA) or 24 h (ELISA). A, B) IL-6 mRNA (A) was quantified by quantitative PCR and normalized to SCR basal; IL-6 concentration (B) in cell supernatants was determined by ELISA. C) PDE4D mRNA was quantified by quantitative PCR and normalized to SCR basal. Data are means ± sem; n = 5 (mRNA) or n = 7 (ELISA); 2-way ANOVA followed by Bonferroni multiple comparison test. *P < 0.05 vs. SCR control.
Immunoblot analysis of ISO-induced (1 µM; 30–240 min) CREB phosphorylation suggested that NHERF1 knockdown did not affect ISO-induced p-CREB (Fig. 5A). Furthermore, ISO-, PGE2-, and FSK-induced CRE-Luc activity was not affected by NHERF1 knockdown (Fig. 5B). Similarly, the ability of cAMP-elevating agents to inhibit PDGF-induced cyclin D1 expression was not limited by NHERF1 knockdown (Fig. 5C).
Figure 5.
NHERF1 knockdown does not limit cAMP-driven CREB signaling in human ASM cells. A) Cells were stimulated with ISO (1 µM; 30–240 min), and p-CREB was determined in whole cell lysates. B) Human telomerase reverse transcriptase–expressing human ASM cells stably transfected with CRE-Luc reporter construct were stimulated with ISO (1 µM), PGE2 (1 µM), or FSK (10 µM) for 6 h, lysates were collected, and luciferase activity was determined. C) For cyclin D1 expression analysis, cells were stimulated with PDGF (10 ng/ml) with or without ISO (1 µM), PGE2 (1 µM), or FSK (10 µM) post–siRNA transfection and cell lysates collected. Representative blots are shown. Data are means ± sem; n = 3. Two-way ANOVA followed by Bonferroni multiple comparison test. *P < 0.05 vs. own SCR control.
NHERF1 mediates cAMP-driven gene expression via c/EBPβ
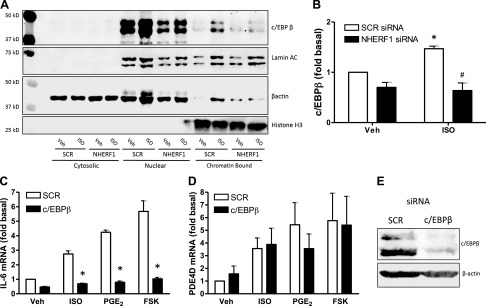
In order to evaluate the role of NHERF1 in ISO-mediated activation of the transcription factor c/EBPβ, we performed cell fractionation experiments and evaluated the expression of c/EBPβ in cytosolic, nuclear, and nuclear chromatin–bound fractions. c/EBPβ was detected in the nuclear but not the cytosolic fractions. Stimulation with ISO (1 µM; 30 min) increased the c/EBPβ chromatin-bound nuclear fraction, indicating increased c/EBPβ binding to DNA. This ISO-induced increase in c/EBPβ binding to DNA was fully abrogated by NHERF1 knockdown (Fig. 6A, B). cAMP-driven IL-6, but not PDE4D, gene expression (Fig. 6C, D) was eliminated by c/EBPβ knockdown (60 ± 5%; Fig. 6E). Collectively, these data demonstrate an important role for NHERF1 in directing cAMP signaling toward c/EBPβ, driving IL-6 production, but not toward CREB pathways.
Figure 6.
c/EBPβ knockdown eliminates cAMP-mediated IL-6 gene expression in human ASM cells. A) Human ASM cells were stimulated with ISO (1 µM) for 30 min post–siRNA transfection, and cell fractionation was performed. c/EBPβ, lamin A/C, β-actin, and histone [3H] expression in the cytosolic, nuclear, and chromatin-bound nuclear fraction was determined by immunoblotting. Representative blots are shown. B) Quantification of c/EBPβ expression in the chromatin-bound nuclear fraction; histone [3H] was used as a loading control. C, D) IL-6 (C) and PDE4D (D) mRNA was quantified by quantitative PCR and normalized to SCR basal. Cells were stimulated with ISO (1 µM), PGE2 (1 µM), or FSK (10 µM) post–siRNA transfection for 4 h. E) c/EBP knockdown was confirmed by immunoblotting; a representative blot is shown. Data are means ± sem; n = 3. Two-way ANOVA (gene expression) or 1-way ANOVA (c/EBPβ chromatin binding), followed by Bonferroni multiple comparison test. *P < 0.05 vs. own SCR control.
DISCUSSION
In the current study, we demonstrate that NHERF1 regulates various aspects of cAMP signaling and cAMP-mediated functions in ASM cells. Regulation of signaling at the receptor locus (i.e., receptor desensitization, internalization, and recycling) has been studied extensively using heterologous and overexpression systems. However, the impact of these regulatory mechanisms on cell functions in physiologic model systems still needs to be determined for many processes.
Compartmentalized signaling involves signaling inputs being transduced via multiple independent signaling cascades to engage different effectors and modulate distinct cell functions; it appears to be dependent, in large part, on the ability of scaffolding proteins to form signaling complexes that route signaling. In recent years, there has been a growing appreciation of how signaling is compartmentalized in various pathways. cAMP pathways are compartmentalized by formation of various signaling complexes facilitated by A-kinase anchoring proteins (AKAP) of which now 30 have been identified (reviewed in ref. 31). In addition, cAMP signaling may be directed by the coupling of Gs subunits to distinct adenylyl cyclase isoforms (32) and by the involvement of distinct PDE isoforms (33, 34). In its ability to form signaling complexes with PKA and to bind ezrin, NHERF1 resembles AKAPs.
Although initially discovered as a regulator of NHE transporter in kidney epithelial cells, NHERF1 has since emerged as a regulator of a surprisingly wide range of signaling pathways affecting multiple cell functions. NHERF1 interactions with GPCRs have been characterized best for the parathyroid hormone (PTH) receptor. The Friedman Lab has demonstrated an important role for NHERF1 in PTH-receptor endocytosis and recycling dynamics (35–38). NHERF1 has also been identified as a binding partner for CFTR, promoting cAMP-mediated activation of CFTR as well as CFTR shuttling from the Golgi to the plasma membrane (10, 12, 39), which may be highly relevant to cystic fibrosis given that both activity and transport of mutant CFTR to the plasma membrane are impaired in this disease. Regulation of Gs-coupled receptors in ASM, including the β2AR, is highly relevant to obstructive lung diseases. Of note, with respect to Gq signaling, we determined that NHERF1 knockdown does not affect muscarinic M3 acetylcholine receptor–mediated calcium mobilization in M3-expressing ASM cells (unpublished results).
In T cells, NHERF1 forms a macromolecular complex with ezrin and PKA, which allows cAMP-mediated inhibition of T-cell receptor signaling (40). In this particular setting, NHERF1 appears to act in the capacity of a scaffold required to form the signaling complex as well as to enable the localizing of the complex to lipid rafts (41). This indicates that the two broadly defined roles of NHERF1, complex formation and molecular shuttling and localization, are not mutually exclusive and may even act concomitantly to regulate signaling.
The phosphorylation status of NHERF1 is subject to regulation by multiple kinases (including GRK6A, Akt, PKC, cdc2, and RSK1) and phosphatases (PP1 and PP2A). At least 11 serine and threonine phosphorylation sites on NHERF1 have been identified thus far (reviewed in ref. 31). In addition, NHERF1 protein expression may also be subject to regulation (42, 43). Increased expression of NHERF1 has been observed in hepatic (44), biliary (45), and colorectal (46) cancer cells.
In the current study of ASM cells, NHERF1 knockdown did not affect acute cAMP production and early cAMP signaling (30 min–2 h), but it did impair signaling (VASP and HSP20 phosphorylation) at 3–4 h of stimulation. The delayed nature of this effect suggests that deficient receptor recycling is the underlying mechanism. This is consistent with observations from the von Zastrow group (3), showing the absence of NHERF1 results in increased lysosomal degradation of β2AR after 4 h of stimulation. However, given that we observed a trend toward reduced p-VASP and p-HSP20 induction with FSK stimulation, receptor internalization may not be the only mechanism contributing to the decrease in cAMP signaling with NHERF1 knockdown.
Our data show no discernable role for NHERF1 in regulating CREB pathways. NHERF1 knockdown did not affect cAMP-driven CREB phosphorylation, CRE-Luc activity, or PDE4D mRNA expression. CRE binding sites in the PDE4D promotor region have been identified as crucial for cAMP-induced up-regulation of PDE4D mRNA expression (47). Another study has shown that in murine NHERF1-null mesenchymal stem cells, the phosphorylation status of CREB is not changed compared with wild-type cells (48).
c/EBPβ is a member of the Basic Leucine Zipper (bZIP) family of transcriptional activators that was initially named NF-IL6 when it was identified as a c/EBP homolog that binds to CCAAT consensus sequences in the IL-6 gene (49). c/EBPβ promoter binding is often supportive of, but not required for, NF-κB– and AP1-mediated transcription, and c/EBPβ promotes transcription of a wide range of proinflammatory cytokines (reviewed in ref. 50). c/EBPβ was shown to promote increased IL-8 release from cultured asthmatic human ASM cells (51). Of note, this study also suggested that nuclear translocation of c/EBPβ is not required for transcriptional activity in ASM, and that despite similar levels of nuclear c/EBPβ expression between cells from healthy and asthmatic tissues, c/EBPβ DNA binding is increased in asthmatic ASM cells. This is consistent with observations by us and others (52), which indicate that, at least in some cell types, c/EBPβ is localized in the nucleus, and changes in DNA binding, rather than nuclear translocation, govern transcriptional activity. cAMP-mediated regulation of c/EBP-mediated transcription has long been established (53, 54), but only one previous study linked NHERF1 to transcriptional activation at the CCAAT promoter sequences, showing a requirement for NHERF1 expression for the type IIa sodium phosphate cotransporter gene transcription (55). Though additional regulation of cAMP production at later time points has not been excluded, our current study identifies NHERF1 as a critical adapter in cAMP-driven c/EBPβ DNA binding, leading to IL-6 gene expression and cytokine production.
Collectively, these data suggest a role for NHERF1 in maintaining cAMP and PKA signaling as well as in compartmentalizing cAMP signaling, with a crucial role for NHERF1 in promoting c/EBPβ-dependent IL-6 production but not in CREB- or CRE-driven processes.
ACKNOWLEDGMENTS
This study was funded by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants HL58506, HL136209 (to R.B.P.), and HL140064 (to T.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- ASM
airway smooth muscle
- β2AR
β-2-adrenoceptor
- c/EBPβ
CCAAT-enhancer–binding protein β
- CFTR
cystic fibrosis transmembrane conductance regulator
- CRE
cAMP response element
- CREB
cAMP response element–binding protein
- EBP50
ezrin-radixin-moesin–binding phosphoprotein 50
- ERM
ezrin radixin moesin
- FSK
forskolin
- HSP20
heat shock protein 20
- ISO
isoproterenol
- NHE
Na+/H+ exchanger
- NHERF1
NHE regulatory factor
- PDE4D
phosphodiesterase 4D
- PDZ
PSD-95, disc large, zona occludens-1
- PGE2
prostaglandin E2
- PKA
cAMP-dependent protein kinase
- SCR
scrambled
- siRNA
small interfering RNA
- VASP
vasodilator-stimulated phosphoprotein
AUTHOR CONTRIBUTIONS
T. Pera designed and performed research, analyzed data, and wrote the manuscript; E. Tompkins, M. Katz, B. Wang, and D. A. Deshpande performed experiments and analyzed data; E. J. Weinman designed research; and R. B. Penn designed the research and wrote the manuscript.
REFERENCES
- 1.Weinman E. J., Steplock D., Wang Y., Shenolikar S. (1995) Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J. Clin. Invest. 95, 2143–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall R. A., Premont R. T., Chow C. W., Blitzer J. T., Pitcher J. A., Claing A., Stoffel R. H., Barak L. S., Shenolikar S., Weinman E. J., Grinstein S., Lefkowitz R. J. (1998) The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 392, 626–630 [DOI] [PubMed] [Google Scholar]
- 3.Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401, 286–290 [DOI] [PubMed] [Google Scholar]
- 4.Ardura J. A., Friedman P. A. (2011) Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol. Rev. 63, 882–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Y., Wang S., Holcomb J., Trescott L., Guan X., Hou Y., Brunzelle J., Sirinupong N., Li C., Yang Z. (2014) Crystallographic analysis of NHERF1-PLCβ3 interaction provides structural basis for CXCR2 signaling in pancreatic cancer. Biochem. Biophys. Res. Commun. 446, 638–643 [DOI] [PubMed] [Google Scholar]
- 6.Pan Y., Weinman E. J., Dai J. L. (2008) Na+/H+ exchanger regulatory factor 1 inhibits platelet-derived growth factor signaling in breast cancer cells. Breast Cancer Res. 10, R5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler D. S., Barrick S. R., Grubisha M. J., Brufsky A. M., Friedman P. A., Romero G. (2011) Direct interaction between NHERF1 and Frizzled regulates β-catenin signaling. Oncogene 30, 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornez I., Taskén K. (2010) Spatiotemporal control of cyclic AMP immunomodulation through the PKA-Csk inhibitory pathway is achieved by anchoring to an Ezrin-EBP50-PAG scaffold in effector T cells. FEBS Lett. 584, 2681–2688 [DOI] [PubMed] [Google Scholar]
- 9.Stokka A. J., Mosenden R., Ruppelt A., Lygren B., Taskén K. (2009) The adaptor protein EBP50 is important for localization of the protein kinase A-Ezrin complex in T-cells and the immunomodulating effect of cAMP. Biochem. J. 425, 381–388 [DOI] [PubMed] [Google Scholar]
- 10.Li C., Krishnamurthy P. C., Penmatsa H., Marrs K. L., Wang X. Q., Zaccolo M., Jalink K., Li M., Nelson D. J., Schuetz J. D., Naren A. P. (2007) Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131, 940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo M. J., Amaral M. D., Zaccolo M., Farinha C. M. (2016) EPAC1 activation by cAMP stabilizes CFTR at the membrane by promoting its interaction with NHERF1. J. Cell Sci. 129, 2599–2612 [DOI] [PubMed] [Google Scholar]
- 12.Monterisi S., Favia M., Guerra L., Cardone R. A., Marzulli D., Reshkin S. J., Casavola V., Zaccolo M. (2012) CFTR regulation in human airway epithelial cells requires integrity of the actin cytoskeleton and compartmentalized cAMP and PKA activity. J. Cell Sci. 125, 1106–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamprecht G., Seidler U. (2006) The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G766–G777 [DOI] [PubMed] [Google Scholar]
- 14.Ammit A. J., Lazaar A. L., Irani C., O’Neill G. M., Gordon N. D., Amrani Y., Penn R. B., Panettieri R. A., Jr (2002) Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am. J. Respir. Cell Mol. Biol. 26, 465–474 [DOI] [PubMed] [Google Scholar]
- 15.Billington C. K., Hall I. P., Mundell S. J., Parent J.-L., Panettieri R. A., Jr., Benovic J. L., Penn R. B. (1999) Inflammatory and contractile agents sensitize specific adenylyl cyclase isoforms in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 21, 597–606 [DOI] [PubMed] [Google Scholar]
- 16.Billington C. K., Pascual R. M., Hawkins M. L., Penn R. B., Hall I. P. (2001) Interleukin-1beta and rhinovirus sensitize adenylyl cyclase in human airway smooth-muscle cells. Am. J. Respir. Cell Mol. Biol. 24, 633–639 [DOI] [PubMed] [Google Scholar]
- 17.Deshpande D. A., Theriot B. S., Penn R. B., Walker J. K. (2008) Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 22, 2134–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncharova E. A., Billington C. K., Irani C., Vorotnikov A. V., Tkachuk V. A., Penn R. B., Krymskaya V. P., Panettieri R. A., Jr (2003) Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am. J. Respir. Cell Mol. Biol. 29, 19–27 [DOI] [PubMed] [Google Scholar]
- 19.Guo M., Pascual R. M., Wang S., Fontana M. F., Valancius C. A., Panettieri R. A., Jr., Tilley S. L., Penn R. B. (2005) Cytokines regulate beta-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry 44, 13771–13782 [DOI] [PubMed] [Google Scholar]
- 20.Horvat S. J., Deshpande D. A., Yan H., Panettieri R. A., Codina J., DuBose T. D., Jr., Xin W., Rich T. C., Penn R. B. (2012) A-kinase anchoring proteins regulate compartmentalized cAMP signaling in airway smooth muscle. FASEB J. 26, 3670–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong K. C., Gandhi U., Martin T. J., Anz C. B., Yan H., Misior A. M., Pascual R. M., Deshpande D. A., Penn R. B. (2008) Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry 47, 9279–9288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misior A. M., Deshpande D. A., Loza M. J., Pascual R. M., Hipp J. D., Penn R. B. (2009) Glucocorticoid- and protein kinase A-dependent transcriptome regulation in airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 41, 24–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misior A. M., Yan H., Pascual R. M., Deshpande D. A., Panettieri R. A., Jr., Penn R. B. (2008) Mitogenic effects of cytokines on smooth muscle are critically dependent on protein kinase A and are unmasked by steroids and cyclooxygenase inhibitors. Mol. Pharmacol. 73, 566–574 [DOI] [PubMed] [Google Scholar]
- 24.Morgan S. J., Deshpande D. A., Tiegs B. C., Misior A. M., Yan H., Hershfeld A. V., Rich T. C., Panettieri R. A., An S. S., Penn R. B. (2014) β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J. Biol. Chem. 289, 23065–23074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mundell S. J., Olah M. E., Panettieri R. A., Benovic J. L., Penn R. B. (2001) Regulation of G protein-coupled receptor-adenylyl cyclase responsiveness in human airway smooth muscle by exogenous and autocrine adenosine. Am. J. Respir. Cell Mol. Biol. 24, 155–163 [DOI] [PubMed] [Google Scholar]
- 26.Panettieri R. A., Murray R. K., DePalo L. R., Yadvish P. A., Kotlikoff M. I. (1989) A human airway smooth muscle cell line that retains physiological responsiveness. Am. J. Physiol. 256, C329–C335 [DOI] [PubMed] [Google Scholar]
- 27.Murray R. K., Fleischmann B. K., Kotlikoff M. I. (1993) Receptor-activated Ca influx in human airway smooth muscle: use of Ca imaging and perforated patch-clamp techniques. Am. J. Physiol. 264, C485–C490 [DOI] [PubMed] [Google Scholar]
- 28.Saxena H., Deshpande D. A., Tiegs B. C., Yan H., Battafarano R. J., Burrows W. M., Damera G., Panettieri R. A., Dubose T. D., Jr., An S. S., Penn R. B. (2012) The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br. J. Pharmacol. 166, 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damera G., Tliba O., Panettieri R. A., Jr (2009) Airway smooth muscle as an immunomodulatory cell. Pulm. Pharmacol. Ther. 22, 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koziol-White C. J., Panettieri R. A., Jr (2011) Airway smooth muscle and immunomodulation in acute exacerbations of airway disease. Immunol. Rev. 242, 178–185 [DOI] [PubMed] [Google Scholar]
- 31.Vaquero J., Nguyen Ho-Bouldoires T. H., Clapéron A., Fouassier L. (2017) Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: from signaling regulation to clinical relevance. Oncogene 36, 3067–3079 [DOI] [PubMed] [Google Scholar]
- 32.Johnstone T. B., Agarwal S. R., Harvey R. D., Ostrom R. S. (2018) cAMP signaling compartmentation: adenylyl cyclases as anchors of dynamic signaling complexes. Mol. Pharmacol. 93, 270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baillie G. S. (2009) Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 276, 1790–1799 [DOI] [PubMed] [Google Scholar]
- 34.Johnstone T. B., Smith K. H., Koziol-White C. J., Li F., Kazarian A. G., Corpuz M. L., Shumyatcher M., Ehlert F. J., Himes B. E., Panettieri R. A., Jr., Ostrom R. S. (2018) PDE8 is expressed in human airway smooth muscle and selectively regulates cAMP signaling by β2-Adrenergic receptors and adenylyl cyclase 6. Am. J. Respir. Cell Mol. Biol. 58, 530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B., Bisello A., Yang Y., Romero G. G., Friedman P. A. (2007) NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J. Biol. Chem. 282, 36214–36222 [DOI] [PubMed] [Google Scholar]
- 36.Wang B., Yang Y., Abou-Samra A. B., Friedman P. A. (2009) NHERF1 regulates parathyroid hormone receptor desensitization: interference with beta-arrestin binding. Mol. Pharmacol. 75, 1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sneddon W. B., Syme C. A., Bisello A., Magyar C. E., Rochdi M. D., Parent J. L., Weinman E. J., Abou-Samra A. B., Friedman P. A. (2003) Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50). J. Biol. Chem. 278, 43787–43796 [DOI] [PubMed] [Google Scholar]
- 38.Wheeler D., Sneddon W. B., Wang B., Friedman P. A., Romero G. (2007) NHERF-1 and the cytoskeleton regulate the traffic and membrane dynamics of G protein-coupled receptors. J. Biol. Chem. 282, 25076–25087 [DOI] [PubMed] [Google Scholar]
- 39.Hall R. A., Ostedgaard L. S., Premont R. T., Blitzer J. T., Rahman N., Welsh M. J., Lefkowitz R. J. (1998) A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc. Natl. Acad. Sci. USA 95, 8496–8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh K., Sakakibara M., Yamasaki S., Takeuchi A., Arase H., Miyazaki M., Nakajima N., Okada M., Saito T. (2002) Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol. 168, 541–544 [DOI] [PubMed] [Google Scholar]
- 41.Brdicková N., Brdicka T., Andera L., Spicka J., Angelisová P., Milgram S. L., Horejsí V. (2001) Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Lett. 507, 133–136 [DOI] [PubMed] [Google Scholar]
- 42.Helms C., Cao L., Krueger J. G., Wijsman E. M., Chamian F., Gordon D., Heffernan M., Daw J. A., Robarge J., Ott J., Kwok P. Y., Menter A., Bowcock A. M. (2003) A putative RUNX1 binding site variant between SLC9A3R1 and NAT9 is associated with susceptibility to psoriasis. Nat. Genet. 35, 349–356 [DOI] [PubMed] [Google Scholar]
- 43.Ediger T. R., Kraus W. L., Weinman E. J., Katzenellenbogen B. S. (1999) Estrogen receptor regulation of the Na+/H+ exchange regulatory factor. Endocrinology 140, 2976–2982 [DOI] [PubMed] [Google Scholar]
- 44.Shibata T., Chuma M., Kokubu A., Sakamoto M., Hirohashi S. (2003) EBP50, a beta-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 38, 178–186 [DOI] [PubMed] [Google Scholar]
- 45.Clapéron A., Guedj N., Mergey M., Vignjevic D., Desbois-Mouthon C., Boissan M., Saubaméa B., Paradis V., Housset C., Fouassier L. (2012) Loss of EBP50 stimulates EGFR activity to induce EMT phenotypic features in biliary cancer cells. Oncogene 31, 1376–1388 [DOI] [PubMed] [Google Scholar]
- 46.Lin Y. Y., Hsu Y. H., Huang H. Y., Shann Y. J., Huang C. Y., Wei S. C., Chen C. L., Jou T. S. (2012) Aberrant nuclear localization of EBP50 promotes colorectal carcinogenesis in xenotransplanted mice by modulating TCF-1 and β-catenin interactions. J. Clin. Invest. 122, 1881–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Jeune I. R., Shepherd M., Van Heeke G., Houslay M. D., Hall I. P. (2002) Cyclic AMP-dependent transcriptional up-regulation of phosphodiesterase 4D5 in human airway smooth muscle cells. Identification and characterization of a novel PDE4D5 promoter. J. Biol. Chem. 277, 35980–35989 [DOI] [PubMed] [Google Scholar]
- 48.Liu L., Alonso V., Guo L., Tourkova I., Henderson S. E., Almarza A. J., Friedman P. A., Blair H. C. (2012) Na+/H+ exchanger regulatory factor 1 (NHERF1) directly regulates osteogenesis. J. Biol. Chem. 287, 43312–43321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. (1990) A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 9, 1897–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poli V. (1998) The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 273, 29279–29282 [DOI] [PubMed] [Google Scholar]
- 51.John A. E., Zhu Y. M., Brightling C. E., Pang L., Knox A. J. (2009) Human airway smooth muscle cells from asthmatic individuals have CXCL8 hypersecretion due to increased NF-kappa B p65, C/EBP beta, and RNA polymerase II binding to the CXCL8 promoter. J. Immunol. 183, 4682–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundfeldt K., Ivarsson K., Carlsson M., Enerbäck S., Janson P. O., Brännström M., Hedin L. (1999) The expression of CCAAT/enhancer binding protein (C/EBP) in the human ovary in vivo: specific increase in C/EBPbeta during epithelial tumour progression. Br. J. Cancer 79, 1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alberini C. M., Ghirardi M., Metz R., Kandel E. R. (1994) C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell 76, 1099–1114 [DOI] [PubMed] [Google Scholar]
- 54.Tae H. J., Zhang S., Kim K. H. (1995) cAMP activation of CAAT enhancer-binding protein-beta gene expression and promoter I of acetyl-CoA carboxylase. J. Biol. Chem. 270, 21487–21494 [DOI] [PubMed] [Google Scholar]
- 55.Clark B. J., Murray R. D., Salyer S. A., Tyagi S. C., Arumugam C., Khundmiri S. J., Lederer E. D. (2016) Protein-DNA interactions at the opossum Npt2a promoter are dependent upon NHERF-1. Cell Physiol. Biochem. 39, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]