Abstract
Influenza virus causes infected cells to generate large numbers of lipid droplets. Because the virus envelope contains substantial cholesterol, we applied atorvastatin (ATV) to Madin-Darby Canine Kidney cells before infecting them. Five micromolars ATV, within physiologic range, strongly (>95%) inhibits reproduction of influenza A as measured by PCR of viral RNA, plaque assay for viable virus, and production of virus nucleoprotein (NP). Inhibition of any of the following can suppress formation of lipid droplets (>−50%) but does not interfere with the production of NP: endoplasmic reticulum stress, autophagy, or production of reactive oxygen substances (ROS). We conclude that, regardless of whether this widely used statin, which is generally considered to be safe, can prevent infection or minimize its severity, inhibition of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase pathway to protect against infection by influenza virus or to mitigate its severity warrants further exploration.—Episcopio, D., Aminov, S., Benjamin, S., Germain, G., Datan, E., Landazuri, J., Lockshin, R. A., Zakeri, Z. Atorvastatin restricts the ability of influenza virus to generate lipid droplets and severely suppresses the replication of the virus.
Keywords: autophagy, ER stress, cholesterol, statin, viral infection
Mortality from influenza A infection is estimated at 250,000–500,000/yr (1) with the highest viral loads correlating with the most severe cytotoxicity (2–4). Viral replication depends on metabolism of lipids and cholesterol. Cholesterol accounts for 44% of the total lipid content of viruses or 11–12% of their total mass (5). Because of the importance of cholesterol to viruses, interest in statins as a cheap, readily available, and easily tolerated means of reducing influenza-caused morbidity and mortality has increased (5–8). Atorvastatin (ATV; Lipitor) selectively blocks the conversion of 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) to sterol precursor mevalonate by HMG-CoA reductase (9). ATV blocks entry of positive-strand RNA viruses like Hepatitis C virus, Dengue viruses, Japanese encephalitis virus, and West Nile virus into host cells (10). Accordingly, some correlations indicate that individuals on statin regimens are less susceptible to influenza-related death (11), though others argue that statins can interfere with immune responses to infection or that there is no effect (12). The equivocal effect of statin on patients with influenza has also been proposed to reflect nonrandom sampling. Nevertheless, generic statins are globally available, cost-effective, and clinically safe for human use, making them appealing candidates for mitigating the next influenza virus outbreak (13).
Influenza and other viruses cause their host cells to generate large numbers of lipid droplets, potentially as precursors to the lipid components of the virus. Lipid droplets are organelles with a phospholipid monolayer surrounding lipids including triacylglycerides, cholesterol esters, and sterol esters (14) that are produced under several circumstances and are required for reproduction of positive-strand RNA viruses. Early researchers proposed that influenza virus does not specifically direct lipid synthesis in infected cells, unlike its effect on nucleic acid and protein synthesis (15). However, influenza virus affects lipid profiles in the host cell such as in lipid-associated and modulating proteins like virus inhibitory protein, endoplasmic reticulum (ER)-associated, IFN-inducible (viperin), 12/15 lipooxygenase, and metabolite ω-3 polyunsaturated fatty acid-derived lipid mediator protectin D1 (PD1). Both viperin and PD1 are down-regulated by influenza virus, allowing infection to be established (16, 17). Viperin colocalizes with cytosolic lipid droplets and is a versatile antiviral agent against hepatitis C virus, dengue virus, human cytomegalovirus, West Nile virus, and influenza virus (18), acting through its manipulation of lipid rafts (glycosphingolipid and cholesterol-rich regions of the plasma membrane that influenza virus uses to bud). Viperin inactivates the enzyme farnesyl pyrophosphate synthase (FPPS), which facilitates the creation of lipid rafts. Without rafts, de novo influenza virions cannot be released, thereby preventing the infection of new host cells (19). Suppression of PD1 allows activation of 12/15 lipooxygenase (20), central to the lethality and pathology of infection (16). Nevertheless, potentially promising antivirals such as 12/15 lipooxygenase inhibitors or PD1 have not entered clinical trials (21), and no viperin small molecule modulator has been described.
Here, we explore the role of metabolic changes caused by influenza ER stress—increased reactive oxygen species (ROS) and autophagy—on the increase of lipid droplets. RNA viruses induce ER stress signaling for replication (22). Eukaryotic translation initiation factor-2α/protein kinase R-like ER kinase (PERK), one of the most important branches of ER stress/unfolded protein response signaling, is activated in response to an accumulation of proteins with polyglutamine repeats and functions in the lipidation of microtubule-associated light chain 3 (LC3) and formation of autophagosomes (23). Inhibition of ER stress pathways by tauroursodeoxycholic acid, Inositol-requiring enzyme 1 pathway inhibitor, or decreasing ER protein 57-kD reduces replication of influenza virus (24, 25). The relationship of ER stress signaling to the synthesis and assembly of lipid droplets was initially considered to derive from alterations in the lipid composition of ER membranes (26, 27). However, Inositol-requiring enzyme 1 also affects lipid metabolism through X-box binding protein 1-mediated transcription of genes regulating phospholipid synthesis (28). Through this sequence, the induction of ER stress increases lipid droplets, whereas inhibition of ER function leads to accumulation of cytotoxic lipids in the cytosol (29). Although influenza infection triggers an increase in superoxide and targeting NADPH oxidase 2 reduces both superoxide and virus replication (30), it is unclear whether increased ROS directly or through ER stress also lead to the accumulation of lipid droplets in infected cells as shown in glial cells (31).
Lipid droplets within cells are also affected by increased autophagy. Autophagy is up-regulated in influenza-infected cells (4, 32). The induction of autophagy can occur through class 1 PI3K and mammalian target of rapamycin complex (mTORC)-2 or through mTORC1. Activation of class 1 PI3K/mTORC2 signaling results in the phosphorylation of protein kinase B in response to extracellular ligands (32). Activated protein kinase B then phosphorylates mTOR at Serine 2448, which activates mTORC1 (33). mTORC1 then inhibits autophagy by targeting autophagy-related protein (ATG) 13 (34). The increase in autophagy in infected cells, engulfment of lipid droplets in autophagosomes, and enrichment of lipid droplets within autophagolysosomes (35) suggests that autophagy in infected cells contributes to the increase of lipid droplets through lipophagy (36). Lipophagy during infection has been proposed to increase ATP via β-oxidation through a still uncharacterized regulatory mechanism (36).
As we report below, exposure of Madin-Darby canine kidney (MDCK) cells to ATV (Lipitor) at physiologically relevant concentrations reduced viral replication an astonishing 90–95% compared with partial and modest suppression by ER stress, ROS, or autophagy inhibitors. Our exploration of mechanisms led to the hypothesis that several pathways converged to activate HMG-CoA reductase, which converts HMG-CoA to mevalonate. Although many steps and potential limitations exist between this in vitro result and clinical practice, it appears obvious that the potential of statins to prevent or reduce the severity of infection deserves considerably more attention than it has received.
MATERIALS AND METHODS
Cell culture and treatment
MDCK cells (a gift of Dr. Anastasia Gregoriades, Queens College, Flushing, NY, USA) were maintained in DMEM with 10% fetal bovine serum (FBS), 50 U/ml penicillin, and 50 mg/ml streptomycin at 37°C under a 5% CO2 atmosphere. Prior to all infections, cells were seeded and allowed to attach overnight in maintenance medium. Cells were washed with 1-time PBS before infecting at multiplicity of infection (MOI) = 5. Sufficient virus was added by diluting virus stock with ice-cold virus-diluting medium (PBS with 0.2% bovine serum albumin, 1 mM MgCl2, 0.9 mM CaCl2, 50 U/ml penicillin, and 50 mg/ml streptomycin) and adding to cells for 1 h at room temperature. Cells were then washed once with 1-time PBS, covered with DMEM with 5% FBS, and incubated at 37°C, 5% CO2 until data collection.
Influenza A virus (IAV; A/WSN/33) was generously provided by Dr. Garcia-Sastre (Mount Sinai Medical School, NY, USA). For expansion of influenza stocks, 10-d-old specific- pathogen-free embryonated chicken eggs (SPAFAS; Charles River Laboratories, Wilmington, MA, USA) were infected with virus and incubated at 37°C for 2 d. Allantoic fluid from infected eggs was spun at 3000 rpm for 5 min, then stored at 80°C. Viral titers of influenza stock solutions were determined by plaque assay as previously described (37). Briefly, monolayers of MDCK cells were incubated overnight in DMEM containing 10% FBS and 1% pen-strep. This was followed by infection with 10-fold serial dilutions of virus suspension for 1 h at room temperature. Cells were then covered with warmed Eagle’s minimum essential medium (12-668E; BioWhittaker, Walkersville, MD, USA) containing 0.1% DEAE–dextran (D9885; MilliporeSigma, Burlington, MA, USA) and 2% purified agar (LP0028; Oxoid, Nepean, ON, Canada). This agar medium was allowed to solidify at room temperature, and the cells were incubated for 2 d at 37°C for plaque development. Prior to analysis, the solidified agar was removed, and the cells were fixed and stained with a methanol-crystal violet solution. Plaques were counted, and the virus titer was expressed as plaque-forming units per milliliter.
When appropriate, cells were treated with class III PI3K inhibitor 3-methyladenine (3MA) (M9281; MilliporeSigma) at 5 mM (38) (3, 39), salubrinal (sc-202332; Santa Cruz Biotechnology, Dallas, TX, USA) at 5 μM (40), N-acetyl cysteine (NAC) at 5 mM (ENZ-51010; Enzo Life Sciences, Farmingdale, NY, USA) (41, 42, 43). In these cases, cells were pretreated with the appropriate chemical inhibitor 1 h prior to infection and incubated at 37°C. Medium containing the inhibitory chemical were then removed from each culture plate and stored at room temperature for the duration of the infection. Cells were washed twice with 1-time PBS and infected with IAV at an MOI of 5 for 1 h at 37°C. After infection, unincorporated virus was removed, and the plates were replenished with the same medium containing the chemical inhibitors that were used during the pretreatment. Toxicity of inhibitors and appropriate doses were previously determined (22, 39). To measure toxicity of ATV (MilliporeSigma), it was dissolved in DMSO for initial stock of 16.5 mM, and this was subsequently diluted to 500 μM in DMSO, which was added to cultures to achieve final concentrations of 0.1, 1, 5, and 10 μM; no significant toxicity was encountered below 10 μM, and we therefore used 5 μM as a standard concentration.
Detection of ROS
To detect ROS in MDCK cells, the Enzo Total ROS/Superoxide detection kit (ENZ-51010; Enzo Life Sciences) was used according to the manufacturer’s instruction. Briefly, 1 × 104 cells were seeded per well in a 96-well, black wall with clear bottom plate (00913021; Corning, Corning, NY, USA) and allowed to grow overnight. The oxidative stress detection (green) reagent was used to measure ROS. MDCK cells were treated with a volume of 100 μl for 24 h or at a specified time with the ROS detection mix along with other treatments or compounds. The ROS/Superoxide Detection Mix was prepared by adding 4 ml of the oxidative stress detection reagent in 10 ml of cultured medium. Plates were read (bottom reading) after every treatment without removing the detection/treatment mix, using a BioTek Synergy HTT Microplate reader (BioTek Instruments, Winooski, VT, USA). Standard fluorescein (excitation = 490 nm, emission = 528 nm) filter sets were used.
Quantitative RT-PCR
MDCK cells were infected and treated as previously described. Total mRNA was isolated with the RNeasy Mini Kit (GE25-0500-71; MilliporeSigma) according to the manufacturer’s protocol. The RNA was amplified by using the Power SYBR Green RNA-to-Ct 1-Step Kit (4391178; Thermo Fisher Scientific, Waltham, MA, USA). The expression of IAV hemagglutinin was analyzed with the forward primer 5′-TACACCCAGTCACAATAGGAGAGTG-3′ and reverse primer 5′-CCATGCATTCATTGTCACACTTGTGG-3′, and tubulin was analyzed with the forward primer 5′-AGGATTCGCAAGCTGGCTG-3′ and the reverse primer 5′-TAATCCACAGAGAGCCGCTCC-3′. Relative viral RNA was compared with mock-infected cells and with cells treated with ATV. PCRs for each sample were done in triplicate for the target gene and tubulin. Samples of known viral titers were also analyzed to develop a standard curve of IAV particles per milliliter, to which experimental samples were normalized. Relative IAV particle release in each cell type was compared with that of mock-infected cells and presented as a ratio of infected/mock-infected cells.
Western blot, immunocytochemistry, and cytochemistry
Cells were infected and treated as previously described. Western blot analysis was performed as described by Lin et al. (44). Briefly, treated cells were scraped and washed with ice-cold 1-time PBS before whole-cell lysate proteins were collected in RIPA buffer and quantified using the BioRad protein assay and an Ultrospec III spectrophotometer (Pharmacia LKB, Uppsala, Sweden). Western blot was performed by SDS-PAGE, using primary antibodies: anti-LC3 (L7543; MilliporeSigma), anticalreticulin (12238; Cell Signaling Technology, Danvers, MA, USA), and anti-β-tubulin (sc9104, loading control; Santa Cruz Biotechnology). Positive signals were detected using ECL (RPN2132; GE Healthcare, Waukesha, WI, USA) and visualized using an Amersham Hyperfilm ECL photoradiographic film (28906835; GE Healthcare) or a Typhoon 9410 Variable Mode Imager (GE Healthcare).
Immunocytochemical analysis was performed as described by Lin et al. (44). Cells were seeded on flame-sterilized glass cover slips, allowed to attach overnight, and infected and treated as above. After treatment 24 h postinfection (HPI), cells were washed with 1-time PBS and fixed with fresh, ice-cold 4% paraformaldehyde for 10 min, washed once, permeabilized with 0.1 M Triton X-100, and stored with 1-time PBS with serum in dark at 4°C. Cells were treated for 1 h with anti-influenza α-nucleoprotein (NP) (graciously given by Dr. Adolfo Garcia-Sastre, Mount Sinai, NY, USA). Cells were then rinsed 3 times in 1-time PBS, followed by incubation with anti-mouse IgG-Alexa Fluor 488 secondary antibody (A11008; Thermo Fisher Scientific) solution for 1 h. Cells were then rinsed thrice with 1-time PBS before staining nuclei with DAPI (D8417; MilliporeSigma). Stained cells were then mounted using GelMount (M-01; Biomeda, Foster City, CA, USA) and visualized using a confocal microscope (Leica Microsystems, Wetzlar, Germany).
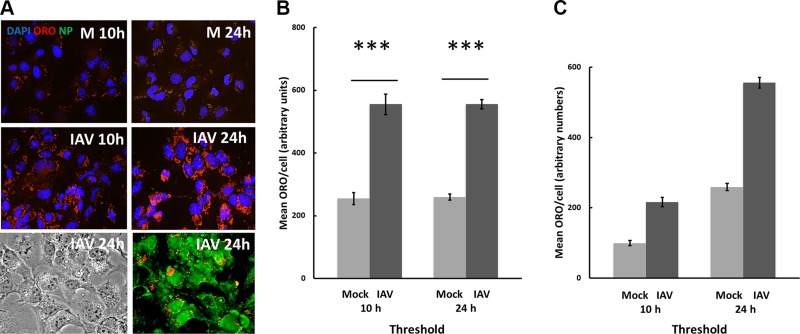
Cytochemical analysis of lipid droplets using Oil Red O (ORO) was performed as described by the manufacturer, MilliporeSigma. Briefly, after treatment and fixation, cells on coverslips were washed with 60% isopropyl alcohol and then dried for a few hours or overnight. Lipid droplets in samples were then stained with 60% ORO solution for 10 min, then rinsed 4 times with distilled water. Samples were then mounted and visualized as in immunocytochemical analysis. Each individual cell present in the field was encircled and analyzed for the area of the cell, and the intensity of the red channel was evaluated for each cell. The product of these values gave a quantity referred to as Total ORO/Cell for each cell, and the averages of the experimental conditions were compared. For comparison of uninfected vs. infected cells, 12 experiments were run. However, the quantification should be considered an approximation, both because the highest intensities of ORO are undoubtedly optically saturated and therefore nonlinear and because a nonparticulate red fluorescence, potentially ORO not yet incorporated into particles, permeates the 10-h cells (Fig. 1A). When this is subtracted by thresholding, a difference in particulate ORO becomes more apparent, as is shown in Fig. 1B, C. Each experiment is different, owing either to differences in cells or infectivity of virus, and we achieve moderately different levels of infection compared with mock-infected cells. We therefore compared each experiment to its mock-infected control, and we repeated each experiment 5 times.
Figure 1.
Influenza virus stimulates increase of lipid droplets. A) Lipid droplets are stained red (ORO) particles in both mock-infected (M) and IAV cells after 10 and 24 h, but only IAV cells display influenza NP (green); virtually all cells are infected and produce virus. Complete field shown. Original magnification, ×400. B) Although cells in culture accumulate some lipid droplets even if they are not infected (M), those that are infected (IAV) accumulate far more droplets, and tend to accumulate near the nuclei. Measuring lipid droplets as total red fluorescence per cell, lipid accumulation is found to more than double in IAV cells, which is a highly significant increase (P < 0.001). For all figures, blue = DAPI stain of nucleus; red = ORO lipid droplets, and green = immunofluorescence of antiviral NP. Except where noted, images are taken at 24 h after infection. Here, the NP image is taken using a Zeiss Apotome (optical sectioning) microscope that was not available for the other illustrations. Fields were captured for replicate slides, and the most representative fields for each condition were imported into Fiji software for quantification as described in Materials and Methods. C) The difference is more apparent when the background is subtracted. Each experiment is compared with its mock control and was repeated 5 times. *** P < 0.001 between Mock and IAV infected.
RESULTS
IAV increases lipid droplets in infected cells
The important role of lipids in viral reproduction is demonstrated by the amount of lipid in viruses, estimated to account for ∼30% of total mass (5). To understand how IAV affects lipids in infected cells, we used the influenza virus permissive MDCK cell line to evaluate the amount of lipid droplets in infected cells. We first confirmed that the cells were infected by MOI = 5 and then measured virus growth using an anti-influenza NP antibody for immunohistochemistry. Essentially all the cells were severely infected at MOI = 5 (Fig. 1A; IAV 24 h). Lipid droplets, detected by the fluorescence of ORO, are few in number and scattered in the cytoplasm at 10 through 24 h after mock infection (Fig. 1B; 10 and 24 h). Infection with IAV leads to an increase in lipid droplets and their relocalization primarily to the perinuclear region after 10 and, more pronounced, 24 HPI (Fig. 1B, C; IAV 10 h and IAV 24 h). We quantified this change by measuring total red fluorescence per cell using confocal microscopy and Fiji software (https://fiji.sc). It was not possible using our equipment to measure the relocalization of droplets. Because of background fluorescence and superimposition of droplets, it is likely that the measured change is an underestimate. Nevertheless, the measured total fluorescence more than doubled in infected cells, a highly significant (P < 0.001) increase.
IAV induces ER stress, triggering autophagy and lipid droplet formation
Stressed cells are known to accumulate lipids (45), and virus infection stresses cells because they use host machinery to synthesize viral components. We therefore evaluated the extent of ER stress in infected MDCK cells. To evaluate the timing of ER stress in influenza-infected cells, we examined ER stress marker calreticulin, a chaperone protein that is expressed more during ER stress (22). Calreticulin rose rapidly, peaking at 6 HPI, then waning afterward (Fig. 2A).
Figure 2.
Infection activates the ER stress response, followed by autophagy and production of lipid droplets. Salubrinal (SAL) lowers production of lipid droplets but not production of viral NP. A) Infection by virus causes MDCK cells to increase expression of calreticulin, an indicator of ER stress, within 3 HPI and peaking by 6 HPI. Increase in LC3-I (18 kD) and its conversion to LC3-II (16 kD) occurs later, becoming detectable by 6 HPI and peaking by 10 HPI. β-Tubulin is a loading control. B, C) SAL (100 μM), an inhibitor of the PERK pathway of ER stress, suppresses the lipid response in a dose-dependent manner. SAL, likewise, partially though not completely suppresses the formation of lipid droplets [P < 0.01 between infected (I) and infected, SAL-treated (I+SAL) cells]. D) Nevertheless, SAL does not prevent the formation of NP. Note: In this and other figures, the intensity of NP may be compared with that shown in Figs. 4 and 5, as the entire NP series illustrated is one of 3 separate series run. In all micrographs, the original magnification is ×400 and the full field is shown.
Autophagy is a stress response that occurs downstream of ER stress signaling in infected cells. We monitored autophagy through the autophagy marker LC3-II, the lipidated form of LC3-I, which localizes to the membranes of autophagosomes (46, 47). Although LC3-I (18 kD) began to rise at 6 h and peak by 10 h, LC3-I gradually converted to greater amounts of the processed LC3-II (16 kD) from 8 h, peaking at 10 h and then waning (Fig. 2A). Thus, in infected cells, ER stress peaks prior to increased up-regulation of autophagy. Therefore, IAV induces an ER stress response, followed by the activation of autophagy.
To determine whether ER stress mediates the induction of autophagy in infected cells, we used salubrinal, an effective inhibitor of ER stress through the PERK pathway (40). Salubrinal prevents the dephosphorylation of the eukaryotic initiation factor α, thereby preventing downstream signaling of the PERK pathway. Salubrinal inhibits influenza virus-induced autophagy (see Fig. 4D) and also reduces lipid droplet formation ∼33% in infected cells (Fig. 2B, C). Therefore, ER stress triggered by infection leads to autophagy, contributing to the increased formation of lipid droplets in infected cells. However, treatment with salubrinal fails to suppress the production of virus, as measured through influenza NP expression (Fig. 2D).
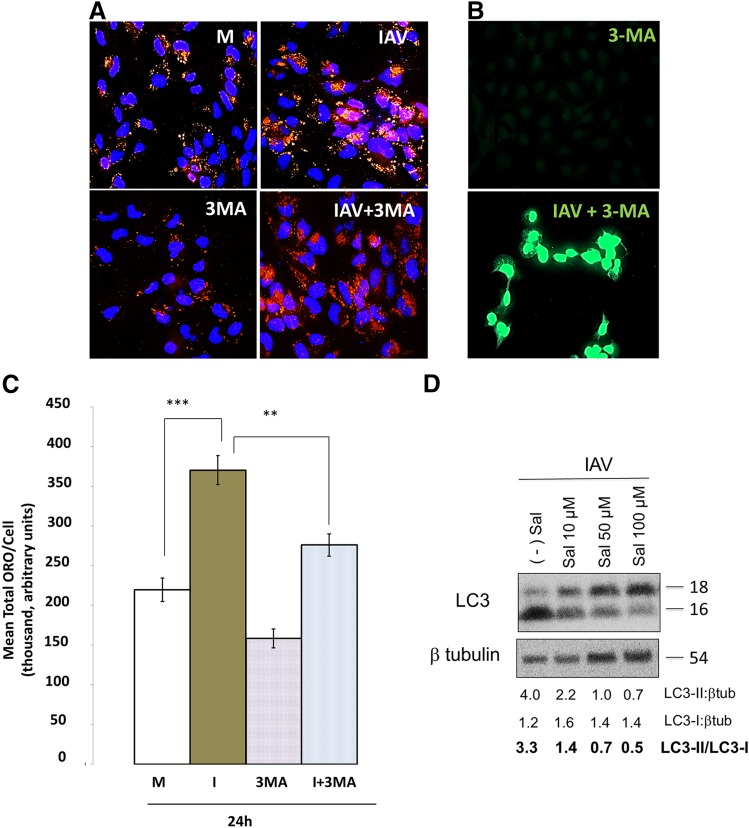
Figure 4.
Autophagy increases lipid droplets and is regulated by ER stress during infection. A) Autophagy inhibitor 3MA pretreatment at 5 mM, applied 1 h before infection, partially blocks the induction of lipid droplets by IAV (IAV+3MA vs. IAV). B) This reduction during autophagy inhibition is significant (P < 0.05). Complete field shown. Original magnification in both A and B, ×400. C) Viral replication persists in the presence of 3MA. D) Influenza-induced autophagy is inhibited by ER stress inhibitor salubrinal (Sal), suggesting ER stress regulation of influenza induced autophagy. At least 3 experiments were run for each condition. Blue = DAPI; red = ORO; green = viral NP. Triple fluorescence experiments are not shown because of technical limitations, though correspondence of lipid droplets with infection is illustrated in Fig. 5. βtub, β-tubulin. ** P<0.01, *** P<0.001.
These results coupled with our previous finding of the effect of ROS on up-regulation of influenza-induced autophagy indicate that autophagy triggered by influenza virus is regulated by ER stress and ROS in infected cells.
ROS induced by ER stress increase formation of lipid droplets
Because of ER stress, ROS affect lipid formation in Drosophila (31). To evaluate the relevance of ROS to changes in amounts of lipid droplets triggered by IAV infection, we first measured ROS production in infected MDCK cells. ROS accumulate by 24 h after the cells are infected, increasing more than 30% (Fig. 3A). Inhibition of ER stress by salubrinal decreases ROS production in uninfected cells as well as in infected cells. Salubrinal decreases ROS in infected cells by 47% compared with mock cells, whereas the antioxidant NAC likewise reduces ROS in infected cells by 40% (Fig. 3A). The ROS scavenger NAC reduces accumulation of virus-induced lipid droplets by 25% (Fig. 3B, C), indicating that ROS accumulation also contributes to the buildup of lipid droplets in infected cells. However, virus, as measured by influenza NP, nevertheless accumulates in infected cells exposed also to NAC (Fig. 3D), indicating that the inhibition of ROS accumulation does not suppress replication of virus.
Figure 3.
ER stress increases production of ROS. Inhibition of ROS production by NAC partially reduces formation of lipid droplets but does not prevent synthesis of NP. ROS were measured by fluorescein emission as described in Materials and Methods. A) Both salubrinal (Sal; 100 μM) and the reductant NAC (5 mM) individually reduce ROS both in uninfected cells (MOCK) and infected (I) cells. B, C) NAC partially (25%) suppresses the production of lipid droplets stimulated by infection (P < 0.001 for NAC vs. I+NAC and P < 0.05 for IAV+NAC vs. NAC). D) However, NAC does not suppress production of viral NP. At least 3 separate experiments were run for each condition. Measurements were at 24 HPI. In B and D, original magnifcation is ×400 and full field is shown. SEM by Student’s t-test are shown. The values indicated are arbitrary fluorescence values. *P < 0.05, ***P < 0.001.
Lysosomes can be suppressed without preventing synthesis of virus protein
We suppressed formation of autophagosomes using 3MA as described in Materials and Methods. The 3MA suppressed formation of lipid droplets, both in the presence and absence of virus, by ∼25% (Fig. 4A, B), but it did not suppress synthesis of viral NP (Fig. 4C). We achieved similar results using the less-specific class I/II PI3K inhibitor wortmannin (results not shown). By inhibiting the manifestation of stress with salubrinal, we likewise suppressed autophagy, indicating that the autophagy may be triggered by ER stress (Fig. 4D).
ATV, which blocks HMG-CoA reductase, blocks the formation of new influenza virus
Overall, our results indicate that lipid droplets can be generated through several pathways. Partial inhibition of formation of lipid droplets minimally affects the replication of virus as determined by influenza NP. ATV inhibits cholesterol biosynthesis, thus affecting the amount of cholesterol within lipid droplets. We first determined if treatment with ATV affected influenza-induced formation of lipid droplets (Fig. 5A). ATV reduced lipid droplets in uninfected cells by at least 50% (Fig. 5A, B; compare ATV with M). This is perhaps an underestimate, owing to background fluorescence. Unlike the other inhibitors we used, ATV is remarkably able to reduce lipid droplets in infected cells to mock-control levels (Fig. 5A, B; compare IAV+ATV with I).
Figure 5.
ATV reduces virus-induced lipid droplet formation and inhibits production of influenza virus. ATV at 5 μM was applied 1 h before infection, and results were determined at 24 HPI. A, B) ATV brings lipid droplets in infected (I) cells to the level of mock-infected (M) cells (A): I + ATV vs. I, and this difference is highly significant [(B): IAV vs. I+ATV, P < 0.001]. C–F) ATV treatment completely blocks reproduction of IAV by 3 criteria: expression of viral NP detected by immunofluorescence (C, F); production of virus measured by plaque assay (D); and production of viral RNA as detected by PCR (E). Because NP fluorescence was essentially undetectable for IAV+ATV, the specific ratio indicated in (F), which depends on our best assumption for background, could range from 0 to 4%. Likewise, regions of green for IAV registered as saturated, and these values could be higher, potentially lowering the IAV/(IAV+ATV) ratio. The ratios are summarized. Arb, arbitrary; PFU, plaque-forming units. These experiments were repeated 7 times. In both A and C, complete fields are shown, and original magnification is ×400. Statistics shown are sems by Student’s t-test. ***P < 0.001.
We then explored the impact on virus replication by measuring NP and live virus. In this experiment, we pretreated MDCK cells with 5 μM ATV and subsequently infected them with IAV at 5 viral particles per cell (MOI = 5). ATV reduced viral NP to nearly undetectable levels (Fig. 5C, F; compare IAV+ATV and IAV). To verify these results, we used a plaque assay to measure virus titer 24 h after infection. ATV suppressed production of live virus by 92% (Fig. 5D). Finally, we sought to measure viral RNA after 24 h by PCR. Treatment with ATV suppressed viral RNA production by 97% compared with infection alone (Fig. 5E). Thus, based on virus protein level, infectious particle, and viral RNA, the inhibition of cholesterol synthesis by ATV can seriously impede production of virus (Fig. 5G).
DISCUSSION
Given the specificity of ATV, the nearly complete suppression of viral replication in MDCK cells by ATV is presumptively closely connected to the ability of this statin to inhibit HMG-CoA reductase. This enzyme may be activated by IAV but operates under the influence of several pathways, as we hypothesize in Fig. 6.
Figure 6.
A model for the site of action for the ability of ATV to suppress replication of influenza virus. Infection stimulates ER stress, which through increased production of ROS and other mechanisms up-regulates autophagy. Increased autophagy and other presumptively metabolic changes induced by infection ultimately activate HMG-CoA reductase, which is required for the production of lipid droplets and is blocked by ATV. In the absence of ATV, production of ER stress signals and ROS and up-regulation of autophagy can be individually inhibited but still allow alternative pathways to the synthesis of lipids in infected cells. Individual blockage of any one of these metabolic processes fails to prevent synthesis of viral protein and presumptive production of virus. When HMG-CoA reductase is inhibited by ATV, production of lipid droplets and replication of virus are blocked. Under the conditions we used, the combination of 3MA and salubrinal (SAL) is too toxic to evaluate for reliable results.
Virus induces ER stress, which induces production of ROS, leading to autophagy. Autophagy and ER stress can induce formation of lipid droplets when HMG-CoA reductase is present, but whether activity of this enzyme is changed as a direct consequence of infection is not known. None of these factors by themselves seriously suppress the production of lipid droplets, which probably depends on several pathways. However, the production of lipid droplets is a necessary component of replication of virus because completely blocking increased production with ATV greatly reduces replication, demonstrated by anti-NP immunocytochemistry, plaque assay, and PCR. Because ATV is widely used to lower cholesterol, has few side effects, and is inexpensive, it might prove valuable in reducing the morbidity, mortality, and infectivity of flu.
We still have much to learn about the biology of this interaction or the relationship of HMG-CoA reductase to the replication of the virus. The effect is important because morbidity correlates highly with viral load (3, 4). Effective exploitation of the inhibition we report here will require considerably greater knowledge of the pharmacology and kinetics of the interactions on both the virus and the infected cells.
The medical goal is to translate this finding into potential therapeutics. Evidence that statins protect individuals in any way against influenza is mixed and, in general, not impressive (11, 12). Surveys of large populations have indicated that statins interfere with the effectiveness of influenza vaccinations or that they have modest, if any, influence on the frequency of infection or the severity of the disease (48, 49). Some authors argue that alleged benefits reflect nonrandom selection of patients. Nevertheless, the phenomenon reported here is real and dramatic. Statins are readily available, inexpensive, and their relatively low toxicity has been well studied for both acute and chronic use.
Chemical inhibition of cholesterol biosynthesis is a desirable strategy for studying viral-host interactions and the importance of lipids to IAV infection. Methyl-β-cyclodextrin (MβCD) was the cholesterol inhibitor of choice for many previous studies because it is pharmacologically activated early in the infectious cycle (6). MβCD is a faster acting analog of the statins. Treatment of infected cells with MβCD led to lipid raft disruption, inefficient viral fusion, reduced rapidly accelerated fibrosarcoma (RAF)/MEK/ERK activation (protein kinases involved in the MAPK-signaling pathway that stimulates cell division, defined in table of abbreviations), diminished viral RNP nuclear export, and a significant dose-dependent reduction in influenza virus infectivity (4–7). These authors hypothesized many possibilities for this reduction in infectivity: a loss of viral membrane integrity and the leakage of viral proteins such as influenza hemagglutinin (HA), influenza neuraminidase (NA), NP, and influenza matrix protein (M1) from mature virions, holes in the viral envelope, or alterations in viral structure triggered by MβCD-mediated cholesterol depletion. The requirement of cholesterol for influenza virus infectivity was affirmed when exogenous cholesterol was introduced to MβCD-treated cells, upon which the infectivity of the released viral particles was partially restored (5, 7).
It is well worth studying the mechanism by which ATV inhibits replication of virus, with the goal of eventually finding a means to attack virus replication through the pathway of cholesterol synthesis. Even if ATV proves to be pharmacologically unsuited for prophylaxis or for reduction in morbidity, its effectiveness in vitro provides us with a tool with which we can identify, with far greater precision, its mechanism of action in preventing reproduction of virus. This knowledge will be crucial in developing a more precisely targeted means of attacking the virus prior to or at an early stage of infection if statins can be used to develop new antiviral therapies.
ACKNOWLEDGMENTS
The authors thank the Queens College Core Facility for Imaging, Molecular and Cellular Biology, for access to their facilities. This work was supported in part by funding from the U.S. National Institutes of Health National Institute of General Medical Sciences (NIGMS) (MARC-USTAR) Grant T 34 GM070387 to Z.Z. The authors declare no conflicts of interest.
Glossary
- 3MA
3-methyladenine
- ATG
autophagy-related protein
- ATV
atorvastatin
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- FPPS
farnesyl pyrophosphate synthase
- HA
influenza hemagglutinin
- HMG-CoA
3-hydroxy-3-methylglutaryl–coenzyme A
- HPI
hours postinfection
- IAV
influenza A virus
- LC3
microtubule associated light chain 3
M1, influenza matrix protein 1
- MβCD
methyl-β-cyclodextrin
- MDCK
Madin-Darby canine kidney
- MOI
multiplicity of infection
- mTORC
mammalian target of rapamycin complex
- NA
influenza neuroamidase
- NAC
N-acetyl cysteine
- NP
nucleoprotein
- ORO
Oil Red O
- PD1
protectin D1
- PERK
protein kinase R-like ER kinase
- PFU
plaque-forming units
- Raf
rapidly accelerated fibrosarcoma
- ROS
reactive oxygen species
- SAL
salubrinal
- viperin
virus inhibitory protein, ER-associated, IFN-inducible
AUTHOR CONTRIBUTIONS
D. Episcopio was a major contributor of this article, performed lipid droplet and related experiments, and article revisions; S. Aminov conducted initial experiments, developed a system to detect lipid droplets, and conducted first Oil Red experiments; S. Benjamin conducted ER stress experiments; G. Germain was responsible for the production of the ROS experiments; E. Datan conducted ER stress experiments and was a major contributor to writing and revisions; J. Landazuri was responsible for viral titers and PCR and contributed to revisions; R. A. Lockshin aided in the design and evaluation of experiments, calculations, editing and design of manuscript, and revisions; and Z. Zakeri is the head of the laboratory, conceived of the study, assigned roles and supervised, and has primary responsibility for the manuscript and revisions.
REFERENCES
- 1.Rumschlag-Booms E., Rong L. (2013) Influenza virus entry: implications in virulence and future therapeutics. Adv. Virol 2013, 121924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Sayed I., Bassiouny K., Nokaly A., Abdelghani A.S., Roshdy W. (2016) Influenza A virus and influenza B virus can induce apoptosis via intrinsic or extrinsic pathways and also via NF-κB in a time and dose dependent manner. Biochem. Research Internat. 2016, 1738237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J. J., Fang D. Y., Fu J., Tian J., Zhou J. M., Yan H. J., Liang Y., Jiang L. F. (2009) Infection and replication of avian influenza H5N1 virus in an infected human. Virus Genes 39, 76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datan E., Shirazian A., Benjamin S., Matassov D., Tinari A., Malorni W., Lockshin R. A., Garcia-Sastre A., Zakeri Z. (2014) mTOR/p70S6K signaling distinguishes routine, maintenance-level autophagy from autophagic cell death during influenza A infection. Virology 452–453, 175–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X., Whittaker G. R. (2003) Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 77, 12543–12551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barman S., Nayak D. P. (2007) Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J. Virol. 81, 12169–12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marjuki H., Alam M. I., Ehrhardt C., Wagner R., Planz O., Klenk H. D., Ludwig S., Pleschka S. (2006) Membrane accumulation of influenza A virus hemagglutinin triggers nuclear export of the viral genome via protein kinase Calpha-mediated activation of ERK signaling. J. Biol. Chem. 281, 16707–16715 [DOI] [PubMed] [Google Scholar]
- 8.Mehrbod P., Hair-Bejo M., Tengku Ibrahim T. A., Omar A. R., El Zowalaty M., Ajdari Z., Ideris A. (2014) Simvastatin modulates cellular components in influenza A virus-infected cells. Int. J. Mol. Med. 34, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B., Xu L., Wang H., Yan X., Xue J., Liu F., Hu J. F. (2011) Atorvastatin exerts its anti-atherosclerotic effects by targeting the receptor for advanced glycation end products. Biochim. Biophys. Acta 1812, 1130–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villareal V. A., Rodgers M. A., Costello D. A., Yang P. L. (2015) Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antiviral Res. 124, 110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost F. J., Petersen H., Tollestrup K., Skipper B. (2007) Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 131, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 12.Havers F. P., Chung J. R., Belongia E. A., McLean H. Q., Gaglani M., Murthy K., Zimmerman R. K., Nowalk M. P., Jackson M. L., Jackson L. A., Monto A. S., Petrie J. G., Fry A. M., Flannery B. (2018) Influenza vaccine effectiveness and statin use among adults in the United States, 2011-2017. [E-pub ahead of print] Clin. Infect. Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedson D. S. (2006) Pandemic influenza: a potential role for statins in treatment and prophylaxis. Clin. Infect. Dis. 43, 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knævelsrud H., Simonsen A. (2012) Lipids in autophagy: constituents, signaling molecules and cargo with relevance to disease. Biochim. Biophys. Acta 1821, 1133–1145 [DOI] [PubMed] [Google Scholar]
- 15.Kates M., Allison A. C., Tyrell D. A., James A. T. (1962) Origin of lipids in influenza virus. Cold Spring Harb. Symp. Quant. Biol. 27, 293–301 [DOI] [PubMed] [Google Scholar]
- 16.Morita M., Kuba K., Ichikawa A., Nakayama M., Katahira J., Iwamoto R., Watanebe T., Sakabe S., Daidoji T., Nakamura S., Kadowaki A., Ohto T., Nakanishi H., Taguchi R., Nakaya T., Murakami M., Yoneda Y., Arai H., Kawaoka Y., Penninger J. M., Arita M., Imai Y. (2013) The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 153, 112–125 [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Hinson E. R., Cresswell P. (2007) The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2, 96–105 [DOI] [PubMed] [Google Scholar]
- 18.Herker E., Ott M. (2012) Emerging role of lipid droplets in host/pathogen interactions. J. Biol. Chem. 287, 2280–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munger J., Bennett B. D., Parikh A., Feng X. J., McArdle J., Rabitz H. A., Shenk T., Rabinowitz J. D. (2008) Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26, 1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam V. C., Quehenberger O., Oshansky C. M., Suen R., Armando A. M., Treuting P. M., Thomas P. G., Dennis E. A., Aderem A. (2013) Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 154, 213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orafaie A., Matin M. M., Sadeghian H. (2018) The importance of 15-lipoxygenase inhibitors in cancer treatment. Cancer Metastasis Rev. 37, 397–408 [DOI] [PubMed] [Google Scholar]
- 22.Datan E., Roy S. G., Germain G., Zali N., McLean J. E., Golshan G., Harbajan S., Lockshin R. A., Zakeri Z. (2016) Dengue-induced autophagy, virus replication and protection from cell death require ER stress (PERK) pathway activation. Cell Death Dis. 7, e2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouroku Y., Fujita E., Tanida I., Ueno T., Isoai A., Kumagai H., Ogawa S., Kaufman R. J., Kominami E., Momoi T. (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 14, 230–239 [DOI] [PubMed] [Google Scholar]
- 24.Hassan I. H., Zhang M. S., Powers L. S., Shao J. Q., Baltrusaitis J., Rutkowski D. T., Legge K., Monick M. M. (2012) Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 287, 4679–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberson E. C., Tully J. E., Guala A. S., Reiss J. N., Godburn K. E., Pociask D. A., Alcorn J. F., Riches D. W., Dienz O., Janssen-Heininger Y. M., Anathy V. (2012) Influenza induces endoplasmic reticulum stress, caspase-12-dependent apoptosis, and c-Jun N-terminal kinase-mediated transforming growth factor-β release in lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 46, 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volmer R., van der Ploeg K., Ron D. (2013) Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. USA 110, 4628–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Promlek T., Ishiwata-Kimata Y., Shido M., Sakuramoto M., Kohno K., Kimata Y. (2011) Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol. Biol. Cell 22, 3520–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sriburi R., Jackowski S., Mori K., Brewer J. W. (2004) XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Zhang K. (2012) Endoplasmic reticulum stress-associated lipid droplet formation and type II diabetes. Biochem. Res. Int. 2012, 247275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlahos R., Stambas J., Selemidis S. (2012) Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol. Sci. 33, 3–8 [DOI] [PubMed] [Google Scholar]
- 31.Liu L., Zhang K., Sandoval H., Yamamoto S., Jaiswal M., Sanz E., Li Z., Hui J., Graham B. H., Quintana A., Bellen H. J. (2015) Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z., Jiang X., Liu D., Fan Z., Hu X., Yan J., Wang M., Gao G. F. (2009) Autophagy is involved in influenza A virus replication. Autophagy 5, 321–328 [DOI] [PubMed] [Google Scholar]
- 33.Sekulić A., Hudson C. C., Homme J. L., Yin P., Otterness D. M., Karnitz L. M., Abraham R. T. (2000) A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 60, 3504–3513 [PubMed] [Google Scholar]
- 34.Corradetti M. N., Guan K. L. (2006) Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25, 6347–6360 [DOI] [PubMed] [Google Scholar]
- 35.Dall’Armi C., Devereaux K. A., Di Paolo G. (2013) The role of lipids in the control of autophagy. Curr. Biol. 23, R33–R45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiramel A. I., Brady N. R., Bartenschlager R. (2013) Divergent roles of autophagy in virus infection. Cells 2, 83–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J. W., Pearson R. B., Dennis P. B., Thomas G. (1995) Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J. Biol. Chem. 270, 21396–21403 [DOI] [PubMed] [Google Scholar]
- 38.Levine B., Kroemer G. (2008) SnapShot: macroautophagy. Cell 132, 162.e1–162.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean J. E., Wudzinska A., Datan E., Quaglino D., Zakeri Z. (2011) Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J. Biol. Chem. 286, 22147–22159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., Yuan J. (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 41.Ravikumar B., Sarkar S., Davies J. E., Futter M., Garcia-Arencibia M., Green-Thompson Z. W., Jimenez-Sanchez M., Korolchuk V. I., Lichtenberg M., Luo S., Massey D. C., Menzies F. M., Moreau K., Narayanan U., Renna M., Siddiqi F. H., Underwood B. R., Winslow A. R., Rubinsztein D. C. (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 90, 1383–1435 [DOI] [PubMed] [Google Scholar]
- 42.Liu Q., Chang J. W., Wang J., Kang S. A., Thoreen C. C., Markhard A., Hur W., Zhang J., Sim T., Sabatini D. M., Gray N. S. (2010)Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J. Med. Chem. 53, 7146–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun L., Gu L., Wang S., Yuan J., Yang H., Zhu J., Zhang H. (2012) N-acetylcysteine protects against apoptosis through modulation of group I metabotropic glutamate receptor activity. PLoS One 7, e32503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin L., Ye Y., Zakeri Z. (2006) p53, Apaf-1, caspase-3, and -9 are dispensable for Cdk5 activation during cell death. Cell Death Differ. 13, 141–150 [DOI] [PubMed] [Google Scholar]
- 45.Samsa M. M., Mondotte J. A., Iglesias N. G., Assunção-Miranda I., Barbosa-Lima G., Da Poian A. T., Bozza P. T., Gamarnik A. V. (2009) Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 5, e1000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanida I., Minematsu-Ikeguchi N., Ueno T., Kominami E. (2005) Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1, 84–91 [DOI] [PubMed] [Google Scholar]
- 47.Mizushima N., Yoshimori T., Levine B. (2010) Methods in mammalian autophagy research. Cell 140, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal D., Schmader K. E., Kossenkov A. V., Doyle S., Kurupati R., Ertl H. C. J. (2018) Immune response to influenza vaccination in the elderly is altered by chronic medication use. Immun. Ageing 15, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu H. T., Shen L. J., Chen Y. C., Lin J. H., Wang C. C. (2018) Effect of statin use on the risk of medically attended acute respiratory illness among influenza vaccinated elderly. Vaccine 36, 6133–6137 [DOI] [PubMed] [Google Scholar]