Abstract
Ageing populations pose one of the main public health crises of our time. Reprogramming gene expression by altering the activities of sequence-specific transcription factors (TFs) can ameliorate deleterious effects of age. Here we explore how a circuit of TFs coordinates pro-longevity transcriptional outcomes, which reveals a multi-tissue and multi-species role for an entire protein family: the E-twenty-six (ETS) TFs. In Drosophila, reduced insulin/IGF signalling (IIS) extends lifespan by coordinating activation of Aop, an ETS transcriptional repressor, and Foxo, a Forkhead transcriptional activator. Aop and Foxo bind the same genomic loci, and we show that, individually, they effect similar transcriptional programmes in vivo. In combination, Aop can both moderate or synergise with Foxo, dependent on promoter context. Moreover, Foxo and Aop oppose the gene-regulatory activity of Pnt, an ETS transcriptional activator. Directly knocking down Pnt recapitulates aspects of the Aop/Foxo transcriptional programme and is sufficient to extend lifespan. The lifespan-limiting role of Pnt appears to be balanced by a requirement for metabolic regulation in young flies, in which the Aop-Pnt-Foxo circuit determines expression of metabolic genes, and Pnt regulates lipolysis and responses to nutrient stress. Molecular functions are often conserved amongst ETS TFs, prompting us to examine whether other Drosophila ETS-coding genes may also affect ageing. We show that five out of eight Drosophila ETS TFs play a role in fly ageing, acting from a range of organs and cells including the intestine, adipose and neurons. We expand the repertoire of lifespan-limiting ETS TFs in C. elegans, confirming their conserved function in ageing and revealing that the roles of ETS TFs in physiology and lifespan are conserved throughout the family, both within and between species.
Author summary
Understanding the basic biology of ageing may help us to reduce the burden of ill-health that old age brings. Ageing is modulated by changes to gene expression, which are orchestrated by the coordinate activity of proteins called transcription factors (TFs). E-twenty six (ETS) TFs are a large family with cellular functions that are conserved across animal taxa. In this study, we examine a longevity-promoting transcriptional circuit composed of two ETS TFs, Pnt and Aop, and Foxo, a forkhead TF with evolutionarily-conserved pro-longevity functions. This leads us to demonstrate that the activity of the majority of ETS TFs in multiple tissues and even different animal taxa regulates lifespan, indicating that roles in ageing are a general feature of this family of transcriptional regulators.
Introduction
Ageing is characterised by a steady systematic decline in biological function, and increased likelihood of disease[1]. Understanding the basic biology of ageing therefore promises to help improve the overall health of older people, who constitute an ever-increasing proportion of our populations. In experimental systems, healthy lifespan can be extended by altered transcriptional regulation, coordinated by sequence-specific TFs[2–6]. Thus, understanding TFs’ functions can reveal how to promote health in late life. Forkhead family TFs, especially Forkhead Box O (Foxo) orthologues, have been studied extensively in this context. This effort has been driven by the association of Foxo3a alleles with human longevity[7]; and the findings that the activation of Foxos is necessary and sufficient to explain the extension of lifespan observed following reduced insulin/IGF signalling (IIS) in model organisms[8–11]. Foxos interact with additional TFs in regulatory circuits, and it is in this context that their function must be understood. For example, in Caenorhabditis elegans, the pro-longevity activity of Daf-16 is orchestrated with further TFs including Hsf, Elt-2, Skn-1, Pqm-1 and Hlh-30/Tfeb [3,12–15]. Examining regions bound by Foxos across animals has highlighted the conserved presence of sites to bind ETS family TFs[16]. In Drosophila, two members of this family, namely Aop (a.k.a. Yan) and Pnt, have been linked to ageing via genetic interactions with Foxo and IIS[4], and similar interactions are evident in C. elegans [17]. These findings raise questions of the overall roles of ETS factors in ageing, and their relationship to the activities of Foxos.
The ETS TFs are conserved across animals, including 28 representatives in humans[18,19]. Their shared, defining feature is a core helix-turn-helix DNA-binding domain, which binds DNA on 5’-GGA(A/T)-3’ ETS-binding motifs (EBMs). They are differentiated by tissue-specific expression, and variation in peripheral amino acid residues which, along with variation in nucleotides flanking the core EBM, confers DNA-binding specificity[20]. ETS TFs generally function as transcriptional activators, but a few repress transcription[21,22]. Aop is one such repressor in Drosophila. Aop and its human orthologue Tel are thought to repress transcription by competing with activators for binding sites[21,23], recruiting co-repressors[22,24,25], and forming homo-oligomers that limit activator access to euchromatin[26–30]. Consequently, Aop's role in physiology must be explored in the context of its interactions with additional TFs, especially activators. Foxo is one such activator[31]. Both Foxo and Aop are required for longevity by IIS inhibition[9], each is individually sufficient to extend lifespan[4], and both are recruited to the same genomic loci in vivo. Whilst activating either in the gut and fat body extends lifespan, the effect of activating both is not additive. Furthermore, if Aop is knocked down, activating Foxo not only ceases to extend lifespan, but even becomes deleterious for lifespan[4]. Overall, these findings suggest that gene expression downstream of IIS is orchestrated by the coordinated activity of Aop and Foxo, and that there is a redundancy in the function of the two TFs, even though Foxo is a transcriptional activator and Aop a transcriptional repressor. We started this study by characterising Aop and its relationship with relevant transcriptional activators, including Foxo. This led us to uncover that roles in ageing are widespread throughout the ETS TF family, extending across multiple fly tissues and diverse animal taxa.
Results
AOP orchestrates an equivalent transcriptional programme to FOXO in vivo
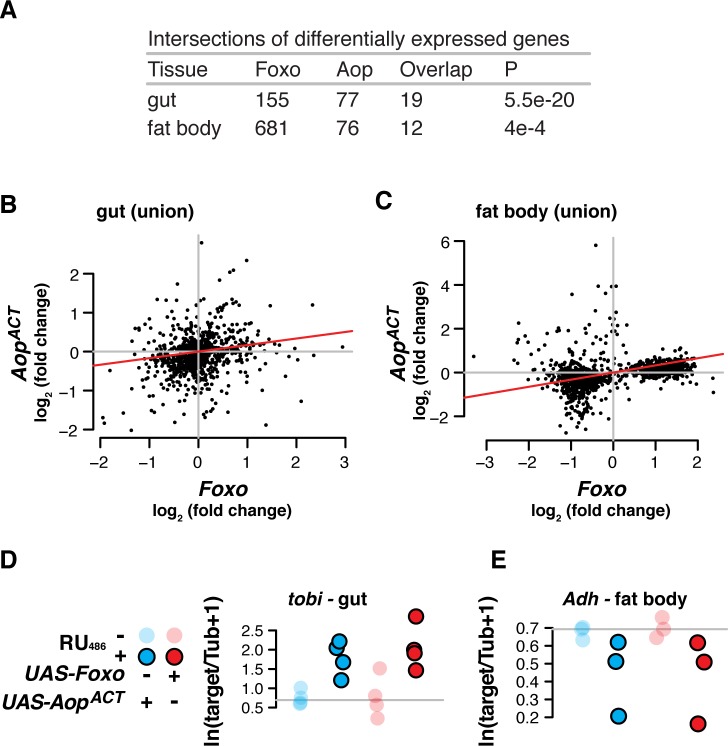
How does the transcriptional programme triggered by Aop relate to that triggered by Foxo? We sought to identify genes that were differentially regulated in response to activation of either TF. We focused on adult female fly guts and fat bodies (equivalent to mammalian liver and adipose), since these are the organs from which Foxo and Aop promote longevity[4]. We induced expression of Foxo, AopACT (encoding a constitutively active form of AOP) or both under the control of the S1106 driver by feeding flies with the RU486 inducer. We profiled genome-wide transcriptional changes in dissected guts and abdominal fat bodies (as associated with the cuticle) with RNA-Seq and identified genes responding to RU486 within each genotype at a False Discovery Rate (FDR) of 10% (these and all subsequently mentioned gene set assignments are given in S1 Supplementary Information, along with full statistics for all genes in all genotypes; the key to the location of each sheet is contained within the S1 Supplementary Information). In both tissues, we found that the sets of genes regulated by either Foxo or AopACT overlapped significantly (gut p<10−19, fat body p<10−4, Fig 1A). To further assess whether Aop’s and Foxo’s transcriptional programmes were similar, we tested for correlated expression changes in response to the two TFs within the union of all 712 genes differentially regulated by either TF in the gut, or the equivalent 727 genes in the fat body. The transcriptional programmes triggered by Foxo or AopACT were significantly correlated within these unions (Fig 1B and 1C, Kendall's Tau rank-correlation test: gut tau = 0.17, p = 1e-14; fat body tau = 0.32, p<2.2e-16). Interestingly, the sets of differentially expressed genes were largely tissue-specific (S1 Fig), suggesting that this correlated response may be a general feature of the Aop and Foxo regulons and independent of the tissue-specificity of target promoters. Gene Ontology (GO) enrichment analysis suggested that, in the gut, this combined set of Aop- and Foxo-regulated genes tended to be involved in translation and energy metabolism, whilst the equivalent analysis in the fat body showed enrichment for regulators of gene expression (details of this GO analysis and all those subsequently mentioned are given in S1 Supplementary Information). We independently confirmed this correlated response to Aop and Foxo using qRT-PCR of two transcripts identified by transcriptomics: a characterised transcriptional target of IIS [32], tobi (Fig 1D, linear model: RU486 F1,13 = 26.04, p = 2e-4; no effect of genotype, full details of this and all subsequent linear models are contained in one sheet of the S1 Supplementary Information), and alcohol dehydrogenase (Adh, Fig 1E—linear model: RU486 F1,9 = 7.83, p = 0.02; no effect of genotype). Hence, Aop and Foxo not only promote longevity, but also individually effect equivalent transcriptional programmes.
Fig 1. Aop recapitulates Foxo’s transcriptional output.
(A) Transcriptomic analysis reveals that overexpression of either Foxo or AopACT under control of S106 induces differential expression of an overlapping set of genes, in both gut and fat body. P values from hypergeometric tests. (B-C) In both gut and fat body, in the unions of sets of differentially expressed genes, transcriptomic effects of AopACT correlate those of Foxo (log2 fold-change expression, calculated by DESeq2). Red lines show correlation coefficients (Kendall’s Tau, p<10e-14 for both tissues). (D-E) qRT-PCR confirms congruent effects of AopACT and Foxo in fat body and gut (linear model: RU486 p<0.05 for either target, no effect of TF).
Aop modulates Foxo's transcriptional outputs
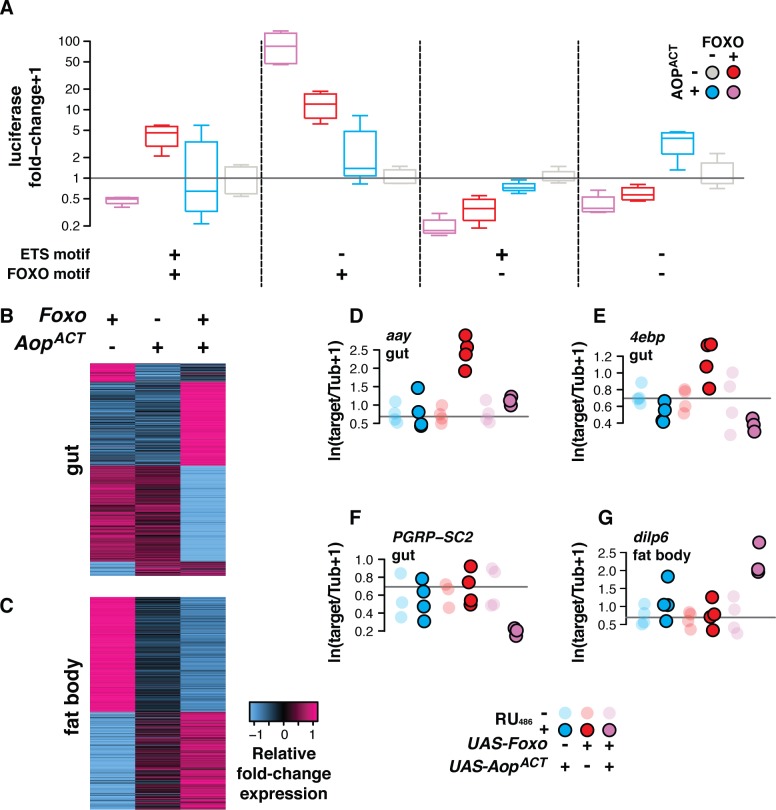
What are the outcomes of combining Aop and Foxo activity? FOXO co-localises extensively with AOP in the genome, with 60% of FOXO-bound loci also bound by AOP in the adult gut and fat body[4]. Since AOP functions by repressive interactions with transcriptional activators, we hypothesised that FOXO activity would be modulated by AOP. We tested this hypothesis in vitro. Transcriptional reporters were constructed by combining the Adh basal promoter with FOXO-responsive elements (FREs: AACA), ETS-binding motifs (EBMs: GGAA) or both, and examined for their response to FOXO and AOPACT in Drosophila S2 cells (Fig 2A, S2 Fig). In the presence of EBMs, AOP prevents activation by ETS activators [e.g. 33]. In the presence of FREs, FOXO is known to activate transcription [31]. We confirmed published observations for individual TFs on the reporters that contained their individual binding elements: FOXO was sufficient to activate transcription from the FREs (t-test t = 6.64, p = 3.7e-5), while, as expected [23,26,28], AOPACT did not impact expression from EBMs (t-test t = -0.66, p = 0.26).
Fig 2. Aop modulates Foxo’s transcriptional outputs.
(A) In vitro (Drosophila S2 cells), AOPACT alters FOXO's transcriptional output. Representative of three replicate experiments shown, see S2 Fig for each separate replicate and their pool. When both AOPACT and FOXO can bind a promoter (ETS and FOXO motifs present), AOPACT directly moderates FOXO activity. When only FOXO can bind a promoter (only FOXO motif present), AOPACT indirectly synergises with FOXO activity. No significant reporter activity is detected in the absence of FOXO motifs. Statistical modelling confirms that consequence of combining TFs depends on combination of promoter motifs (linear model: Foxo:Aop:FRE:EBM F1,48 = 11.41, p = 1e-3). Plot shows luciferase reporter activity from Drosophila S2 cells transfected with expression constructs containing combined ETS-binding motifs (EBMs—GGAA) and FOXO-responsive elements (FREs—AACA), upstream of a basal Adh-Fireflyluciferase reporter. Activity is shown following normalisation to internal Renillaluciferase controls, and calculation of fold-change over the median expression of each reporter in the absence of FOXO and AOPACT. (B-C) In vivo, expressing AopACT moderates and synergises with Foxo to determine transcriptomic output in the gut and fat body (S106). Sets of differentially expressed genes regulated interactively by Aop and Foxo (10% FDR) are shown. Rows represent relative fold-change in expression (Z-scores, log2 fold-change expression from DESeq2 output). (D-G) qRT-PCR validation of patterns indicated in vivo by transcriptomics. In gut, (D—E) AopACT abrogates Foxo's activation of aay and 4ebp (linear models: aay genotype:RU486 F2,17 = 15.43, p = 1e-4; 4ebp genotype:RU486 F2,17 = 8.38, p = 2e-3), while (F) they synergise to repress PGRP-SC2 in the gut (linear model: genotype:RU486 F2,15 = 4.06, p = 0.03); (G) AopACT and Foxo synergise to activate dilp6 in fat body (linear model: genotype:RU486 F2,17 = 6.61, p = 8e-3).
We conducted three replicate experiments to assess the interactive output of AOP and FOXO. Combining the FREs and EBMs allowed AOPACT to attenuate activation by FOXO, revealing that AOP can moderate FOXO’s activity when brought onto the same promoter. By striking contrast, in the absence of EBMs, AOPACT synergised with FOXO to stimulate induction to an order of magnitude greater than FOXO alone, indicating that AOPACT can indirectly accentuate FOXO’s ability to activate transcription (Fig 2A). While the magnitude of these effects varied, it was consistently present across three independent experiments (S2 Fig). To analyse these data we used linear modelling, testing how the complement of TF binding motifs altered the output of combining the TFs, both in each individual experiment, and across the three experiments. This analysis confirmed that the output of combining AOP and FOXO was promoter-dependent (Linear model: FOXO:AOP:FRE:EBM—data from all three replicates, F1,158 = 21.06, p = 9e-6; data from Fig 2A F1,48 = 15.34, p = 2e-4; see also statistical analysis section of S1 Supplementary Information). Since the synergistic interaction occurred in the absence of EBMs, this is most likely an indirect effect, occurring not via a direct interaction on the promoter but rather via AOP-induced transcriptional changes elsewhere in the genome. Note that in the presence of EBMs, any synergistic effect of AOP appears counteracted by the repression occurring from direct AOP binding to the promoter. Synergy may account in part for the similarity of AOP’s and FOXO’s transcriptional programmes in vivo (Fig 1). Hence, AOP is not only able to moderate the activity of other ETS activators, but also the Forkhead TF FOXO, with the presence or absence of EBMs in a promoter determining whether AOP enhances or moderates FOXO activity.
The in vitro analysis suggested that Foxo’s in vivo output should depend on Aop activity. To examine if synergy and antagonism of Foxo by Aop can be observed on native promoters in vivo, we looked at what happens when Aop and Foxo were combined. We used our above-described RNA-Seq experiment and sorted the union of differentially expressed genes by the direction of regulation upon induction of Foxo, AopACT, or both, paying attention to altered regulation when the TFs were co-induced. To visualise the groupings, we compared the fold-change values for each gene between different conditions by calculating per gene Z-score (number of standard deviations away from the mean fold-chage; Fig 2B and 2C). In this way, we could identify sets of genes that may be synergistically or antagonistically regulated by Aop and Foxo. We note that neither Aop nor Foxo were significantly down-regulated by the other in either tissue, indicating that their combined transcriptomic outputs result from interactive effects on promoters (S1 Supplementary Information). We selected specific candidates for validation by qRT-PCR, and used linear models to test for interactive effects of the TFs, indicated by differential effects of RU486 feeding on the study genotypes. Indeed, we found that Aop was able to antagonise Foxo’s induction of aay and 4ebp in the gut (Fig 2D and 2E; aay genotype:RU486 F2,17 = 15.43, p = 1e-4; 4ebp genotype:RU486 F2,17 = 8.38, p = 2e-3; full analysis in S1 Supplementary Information). On the other hand, Aop synergised with Foxo to modulate expression of PGRP-SC2 in the gut and dilp6 in the fat body (Fig 2F and 2G; PGRP-SC2 genotype:RU486 F2,15 = 4.06, p = 0.03; dilp6 genotype:RU486 F2,17 = 6.61, p = 8e-3; full analysis in S1 Supplementary Information). Thus, transcript profiling followed by qRT-PCR validation confirmed that the two modes of AOP:FOXO interaction observed on synthetic reporters can also occur in vivo. This simultaneous synergy and antagonism of AOP and FOXO may explain why, whilst activation of either TF is sufficient to promote longevity, their co-activation does not extend lifespan additively[4].
Aop and Foxo broker transcriptomic outcomes in vivo with Pnt
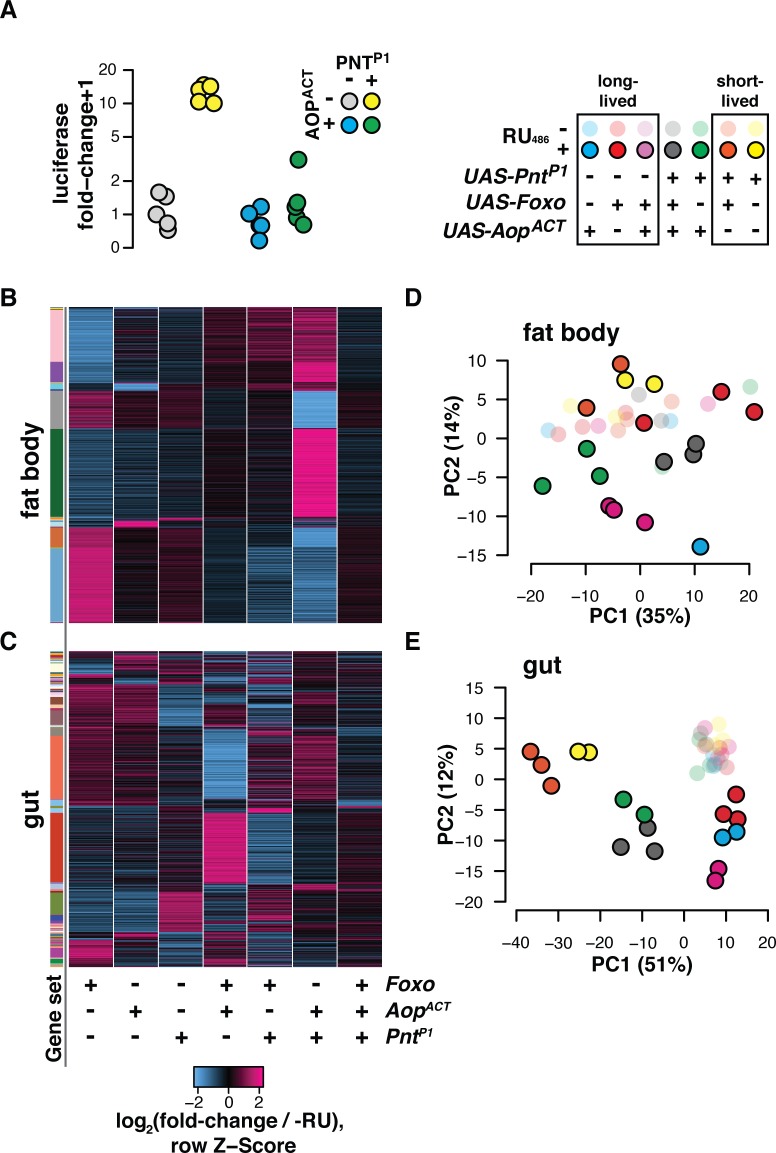
Whilst interactions with FOXO appear to account for some of the transcriptional outputs of AOP, 80% of AOP-bound genomic sites are not bound by FOXO in vivo[4]. Since AOP alone is insufficient to regulate transcription when brought onto a promoter (Fig 2A and references[21,23,26,28]), interactions with other transcriptional activators must account for the full breadth of Aop's physiological and transcriptomic effects. Pnt is one such transcriptional activator. Pnt and Aop have mutually antagonistic roles in development, which is presumed to occur by competition for binding sites since the two recognise the same DNA sequence[23,30,34]. We confirmed this interaction on reporters in S2 cells: Transcriptional induction by PNTP1 (a constitutively active isoform[35]) was completely blocked by AOPACT (Fig 3A, linear model AOP:PNT F1,16 = 41.8, p = 7.9e-6; also see references[23,28,36,37]), suggesting that PNT inhibition may be a key factor in Aop’s pro-longevity effect. Additionally, Pnt over-expression can block the longevity effects of both Foxo and IIS [4,9], suggesting that Pnt may also modify Foxo’s transcriptional output. To evaluate emergent interactions in vivo, the transcriptome-wide effects of co-expressing AopACT, PntP1 and Foxo in the gut and fat body were examined.
Fig 3. Aop and Foxo broker transcriptomic outcomes with Pnt.
(A) In vitro (Drosophila S2 cells) AOPACT counteracts activation by PNTP1 of a synthetic promoter containing EBMs, upstream of an Adh-Fireflyluciferase reporter. Activity is shown following normalisation to internal Renillaluciferase controls, and calculation of fold-change over median expression in the absence of PNTP1 and AOPACT. Promoter activation was subject to a significant AOPACT:PNTP1 interaction (linear model F1,16 = 41.725, p = 7.9e-6) (B-C) Foxo and AopACT coordinate transcription by jointly countering Pnt's transcriptional output. Heatmaps show per-gene Z-score of log2 fold-change expression (DESeq2 output) upon RU486 feeding relative to controls. Rows represent all genes in the union of targets identified in flies bearing S106 transgenes along with UAS-Foxo, UAS-AopACT, UAS-PntP1, or combinations thereof. Gene sets are defined by pattern of response to RU486 amongst genotypes. Set assignments shown by coloured side bar. (D-E) AopACT, Foxo and PntP1 interact to establish transcriptional programs corresponding to lifespan. For gut and fat body, plots show coordinates of samples on the first two dimensions of PCA for the genes whose expression was responsive to the combinatorial, interactive effects of the three TFs. Key shows samples’ groupings by previously-published lifespan outcomes[4] resulting from TF induction in the gut and fat body or the gut alone (noting that lifespan effects of combined AopACT and PntP1 expression are not known).
We assessed the transcriptomic outcomes of induction of PntP1 either alone or in combination with AopACT and Foxo (note that this is an extension of the above-described transcriptomic experiment, which was performed at the same time). For each of the gut and the fat body, we assembled sets of genes that were differentially regulated upon induction of any of the three TFs or their combinations (union of all genes differentially expressed at FDR 10%, set assignments per tissue in S1 Supplementary Information, noting that the preceding Foxo/Aop-regulated genes are a subset). This formed a union of 945 genes in the gut, and 1214 genes in the fat body. We sorted these genes by their pattern of regulation (i.e. set assignment) and visualised the groupings based on per-gene Z-score. This revealed a complex pattern in both tissues where each TF appeared able to influence the outcomes of the other two (Fig 3B and 3C). To distil these interactions, we tested explicitly for genes whose regulation is subject to a statistically significant three-way interaction of Foxo, AopACT and PntP1 induction. In the gut, 511 transcripts were subject to the combinatorial, interactive effects of the three TFs, as were 617 in the fat body (10% FDR, see results in S1 Supplementary Information). To reveal emergent transcriptional programmes in each tissue, principal components analysis (PCA) was performed over these sets of transcripts (Fig 3D and 3E). Remarkably, the first principal component (PC) of differentially expressed genes in the gut distinguished flies by published lifespan outcomes[4], with short-lived flies expressing PntP1 alone or in combination with Foxo at one end of the PC; long-lived flies expressing one or both Foxo and AopACT forming a distinct group at the other end of the PC; and AopACT countering the effect of PntP1 to form an intermediate group (Fig 3E). In the fat body, a similar grouping was apparent on the diagonal of PCs 1 and 2 (Fig 3D), despite more variability in the data, probably resulting from the difficulty of dissecting this organ. To infer functional consequences of these distinct transcriptional programmes, transcripts from the input set corresponding to the PCs were isolated and GO enrichment analysis performed. This revealed a strong enrichment of genes with roles in energy metabolism, whose expression was strongly correlated to the PCs (S3 Fig). Overall, a combined view of the PCA and GO analysis predicted that: (1) inhibiting Pnt may recapitulate the transcriptional programme of Aop and Foxo and promote longevity, and (2) that Pnt, alongside Aop and Foxo, may regulate metabolism in young flies.
Pnt limits lifespan
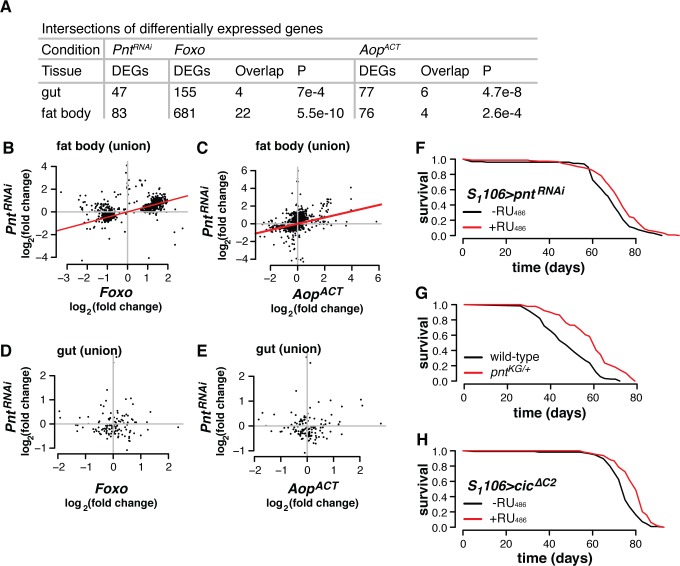
Since Aop and Foxo appeared to drive a transcriptional programme opposed to that of Pnt, we hypothesised that directly limiting physiological levels of Pnt would be sufficient to recapitulate their effect on gene expression. We first assessed the transcriptome-wide changes in the gut and fat body induced by RNAi-mediated knockdown of Pnt. The sets of genes differentially regulated by Pnt knockdown (FDR 10%) significantly overlapped those regulated by AopACT or Foxo in both the gut and the fat body (Fig 4A). Additionally, in the union of the genes regulated by PntRNAi, AopACT or Foxo induction in the fat body, correlated effects of Pnt knockdown and Foxo or Aop activation were evident (Fig 4B and 4C). However, such broad correlations were not evident in the gut (Fig 4D and 4E). Hence, reducing the physiological levels of Pnt can recapitulate some aspects of the Aop/Foxo transcriptional programme. But is this sufficient to extend lifespan?
Fig 4. Physiological Pnt regulates lifespan and genes in the Aop/Foxo regulon.
(A) Transcriptomic analysis reveals that expressing RNAi against Pnt under control of S106 induces differential expression of set of genes that overlap with the Foxo-Aop regulon, in both gut and fat body. P-values from hypergeometric tests. (B-C) In fat body, in the union of transcripts responding to PntRNAi, Foxo or AopACT, impacts of PntRNAi are correlated to those of Foxo or AopACT (log2 fold-change expression from DESeq2 output). Red lines show correlation coefficients (Kendall’s Tau, P≤2.2e-16 for both tissues). (D-E). In gut, in the union of transcripts responding to PntRNAi, Foxo or AopACT, impacts of PntRNAi do not correlate those of Foxo or AopACT (log2 fold-change expression from DESeq2 output). (F) Adult-onset Pnt inhibition in the gut and fat body is sufficient to extend lifespan. Log-rank test p = 7.2e-4. (G) Heterozygous Pnt mutants are long-lived. Log-rank test p = 9.2e-11. (H) Overexpressing Cic (an inhibitor of Pnt), in the gut and fat body extends lifespan. Log-rank test p = 1.5e-7.
Inducing RNAi against Pnt from day three of adulthood in the gut and fat body was indeed sufficient to increase lifespan (Fig 4F, log-rank p = 7.2e-4). To further validate this finding, we backcrossed a loss-of-function p-element insertion in Pnt (PntKG04968, henceforth PntKG), into an outbred, wild-type background for ten generations. The mutation was homozygous lethal. However, heterozygote females exhibited a 20% increase in median lifespan (Fig 4G, log-rank p = 9.2e-11). We also tested whether expressing a transcriptional repressor of the Pnt locus extended lifespan. The HMG-box repressor capicua (cic) represses expression of Pnt[38] and, consistent with the effects of PntRNAi, overexpressing cicΔC2 (a cic mutant lacking a known MAPK phosphorylation site) in the gut and fat body also substantially extended lifespan (Fig 4H, log-rank p = 1.5e-7). These experiments demonstrated that countering Pnt is sufficient to recapitulate aspects of the Aop/Foxo transcriptional programme and extend lifespan, and corroborate the conclusions of transcriptomic analysis that Aop and Foxo act in part by countering Pnt.
Pnt determines metabolic outcomes
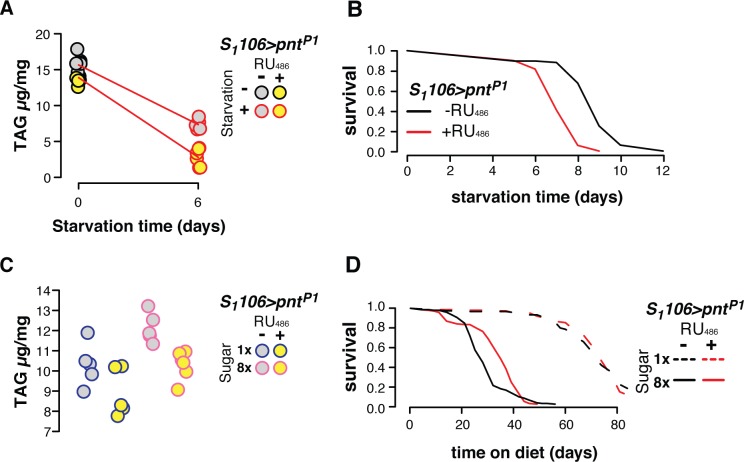
Our data show that each member of the Foxo-Aop-Pnt circuit can be targeted in the gut and fat body to extend lifespan. What is the function of this circuit, and Pnt in particular, in young flies, before ageing occurs? The RNA-Seq data sets suggested metabolic regulation. Since the levels of Pnt appeared to dictate transcriptional and lifespan outcomes (Figs 3 and 4), we evaluated its metabolic role in more detail. The presence of genes including lipases and perilipin (Lsd-2) in the transcriptome data suggested that Pnt modulates lipid metabolism. Therefore, we applied nutritional stresses to alter triacylglyceride (TAG) storage, and assessed how PntP1 altered the response to these stresses. We quantified TAG after a week of PntP1 induction, and then after a subsequent six days of starvation. PntP1 accentuated the loss of TAG per unit weight induced by starvation (Fig 5A; linear model RU486:starvation F1,19 = 7.03, p = 0.02), but not overall weight loss (S4 Fig), suggesting that Pnt sensitises flies specifically to cues for lipolysis. The mobilisation of TAG stores was associated with decreased resistance to starvation, with flies over-expressing PntP1 dying 24% earlier on average (Fig 5B log-rank p = 1.3e-14). This ability of Pnt to promote catabolism of energy stores may be beneficial in the face of over-nutrition, and relevant to the Western human epidemic of metabolic disease associated with energy-rich diets. A Drosophila model of such energy-rich diets is feeding flies a high sugar diet. Flies fed 40% sugar die substantially earlier than controls fed a 5% sugar diet, and accumulate TAG[39,40]. However PntP1 overexpression restored TAG levels in flies on a high-sugar diet to those observed on a low-sugar diet (Fig 5C). Whilst there was no statistically significant interaction of sugar and PntP1 induction in a linear model (RU486:sugar F1,17 = 0.32, p = 0.57), the adipogenic effect of sugar was opposed by Pnt, such that TAG levels on a high-sugar diet with Pnt induction were not different from those on a low-sugar diet without Pnt induction (t-test: t = 0.01, p = 0.99). Moreover, PntP1 induction spared flies from the full extent of the early death induced by dietary sugar, increasing median survival time by 26%, despite having no effect on the low-sugar diet (Fig 5D; cox proportional hazards RU486:sugar p = 6.2e-3). Altogether, these results indicate that while Pnt activity is detrimental during ageing, in youth it predisposes flies to leanness, which correlates survival of nutritional stress. This may suggest that metabolic regulation is an adaptive function of the Foxo-Aop-Pnt circuit in early life, but that the configuration which is optimal for metabolism is deleterious for later survival.
Fig 5. Pnt impacts the response to nutritional stress.
(A) Over-expressing PntP1 in the gut and fat body accelerates loss of TAG under starvation stress (linear model: RU486:starvation F1,19 = 7.03, p = 0.02; see S4 Fig for corresponding plot of body mass). (B) Over-expressing PntP1 in the gut and fat body reduces survival under starvation stress (log-rank test p = 1.3e-14). (C) Over-expressing PntP1 in the gut and fat body reduces accumulation of TAG on a high-sugar diet. PntP1 reduced TAG (linear model F1,17 = 14.4, p = 1.4e-3), and sugar increased TAG (linear model: F1,17 = 15.25, p = 1.1e-3), with PntP1 appearing to counteract the effect of sugar (mean±SE: low sugar-RU486 10.31±0.48, high sugar+RU486 10.30±0.29; t-test t = 0.009, p = 0.99). (D) Over-expressing PntP1 in the gut and fat body enhances survival on a high-sugar diet (Cox Proportional Hazards diet:RU486 p = 6e-3).
The majority of ETS TFs limit fly lifespan
Animal genomes encode multiple ETS factors: In Drosophila the ETS family comprises the repressor Aop and activators: Pnt, Eip74EF, Ets21C, Ets65A, Ets96B, Ets97D and Ets98B, each of which is expressed with its own unique tissue-specific pattern (Fig 6A). Finding lifespan-limiting roles of Pnt in addition to the previously described pro-longevity role of Aop, suggested that other ETS TFs with functions as transcriptional activators may also have the same lifespan-limiting effect. We examined the function of the other ETS TFs in Drosophila lifespan by knocking down their expression levels with RNAi in combination with inducible drivers. The data obtained in >40 lifespan assays are summarised in Fig 6B, including information on Aop, Pnt and Foxo. Summary statistics of each lifespan, along with associated genetic information, are presented in S1 Supplementary Information, while individual lifespan curves are presented in S5–S8 Figs. We identified each of Eip74EF, Ets21C and Ets97D as limiting lifespan in at least one tissue. For Ets21C, we confirmed the result using an available mutant (S5 Fig). Whilst some of the effects we observed were modest, overall the data pointed to roles in ageing for five of the eight Drosophila ETS TFs.
Fig 6. Lifespan regulation is a conserved function of ETS TFs.
(A) The diversity and tissue-specific expression of ETS TFs in Drosophila. A variance-stabilising transformation was applied to published expression data from the same population of flies and same media as used in Figs 1–5 [5], and medians were calculated. (B) Tissue-specific lifespan regulation is a conserved function of ETS TFs in Drosophila. Plot summarises 44 lifespan experiments. Bubbles are colour-coded for percentage change in median lifespan, either in mutants relative to controls, or upon feeding RU486 to flies bearing the indicated geneswitch drivers along with either RNAi or overexpression constructs. Bubble size represents p-values from log-rank tests. Full genotype information and p-values are given in S1 Supplementary Information. Note that lifespans of S106 flies overexpressing dominant-negative InR were significantly extended due to delayed mortality in early or late portions of survival curves, despite absence of differential median survival (See S8 Fig). (C) ETS TFs have evolutionarily-conserved functions in lifespan: Feeding lin-1RNAi to the nematode C. elegans from egg promotes longevity (log-rank test p = 3.3e-2).
The effects were in general tissue specific. RNAi against Pnt, Ets21C and Ets97D restricted to the gut and the fat body with the S1106 driver extended lifespan (Fig 6B, S5 Fig), the same tissues where Foxo and Aop act [4]. Knockdown of Pnt but not of Ets21C in enterocytes (ECs), using the GS5966 driver, was sufficient to extend lifespan, as was activating either AopACT or Foxo (Fig 6B, S6 Fig). Pnt and Ets21C have been characterised as regulators of intestinal stem cell (ISC) proliferation[38], but activating cognate RNAi constructs with drivers that are active in ISCs (GS5961 and TIGS) did not consistently or substantially extend lifespan (Fig 6B, S6 Fig). Pnt functions in neurogenesis[41], and its continued expression in adult neurons suggested an ongoing, physiologically relevant role. However, neuronal PntRNAi induction, achieved with the ElavGS driver, did not affect lifespan, while over-expressing either AopACT or Foxo was deleterious and in contrast to their benefits in gut and fat body (Fig 6B, S7 Fig). Eip74EF is more highly expressed in the brain than other tissues (Fig 6A), indicating that neurons may mediate the beneficial effect of its ubiquitous knockdown. Indeed, expressing Eip74EFRNAi in neurons using the inducible, neuron-specific driver Elav-GS extended lifespan (Fig 6B, S7 Fig). Overall, these data show that members of the Drosophila ETS family, along with Foxo, have distinct effects on lifespan in distinct tissues.
The ETS TFs act downstream of receptor tyrosine kinase (RTK) pathways. The insulin receptor InR is an established regulator of Aop and Foxo[8], and reducing its activity promotes lifespan[9]. Whilst expressing InRDN (a dominant-negative form) in the gut and fat body enhanced lifespan, expressing the same construct in ECs did not (Fig 6B, S8 Fig), indicating that another RTK may function upstream of ETS TFs and Foxo in the ECs. The epidermal growth factor receptor EGFR can signal to Pnt and Ets21C via cic[38], suggesting EGFR in ECs may regulate lifespan. Indeed, inducing the dominant-negative form EGFRDN in the gut and fat body or ECs extended lifespan (Fig 6B, S8 Fig). Hence, different ETS factors may limit lifespan downstream of different RTK pathways in different tissues.
The evidence suggested that a role in ageing is shared amongst multiple ETS factors in Drosophila. ETS TFs are conserved throughout multicellular animals, and the extensive conservation of roles in lifespan amongst the ETS family in the fly suggested that this lifespan modulation may be a fundamental property of these TFs, that extends to other species. The genome of the nematode C. elegans encodes 11 ETS TFs in total. At least one of these, Ets-4, has been reported to limit lifespan in the worm intestine[17]. We screened the majority of the other C. elegans ETS TFs for roles in lifespan by feeding worms RNAi from egg or L4 onwards (S1 Supplementary Information). Expanding the repertoire of proteins that limit worm lifespan, we found that knockdown of Lin-1 (an orthologue of human ELK1, ELK3 and ELK4) consistently extended C. elegans lifespan, in multiple independent trials from L4 stage or egg (e.g. Fig 6C). Thus, multiple ETS factors limit lifespan in species separated by hundreds of millions of years of evolutionary divergence, hinting at a general role for this family of TFs in animal longevity.
Discussion
Promoting healthy ageing by transcriptional control is an attractive prospect, because targeting one specific protein can restructure global gene expression to provide broad-scale benefits. This study suggests key roles for ETS TFs in such optimisation. The results show dual roles for Aop: balancing Foxo’s outputs, and opposing Pnt’s outputs. These functions coordinate transcriptional changes that correspond to lifespan. Repressing transcription from the ETS site appears to be the key longevity-promoting step, and indeed lifespan was extended by limiting multiple ETS TFs, in multiple fly tissues, and in multiple taxa. Altogether, these results show that inhibiting lifespan is a general feature of ETS transcriptional activators. Presumably the expression of these TFs is maintained, despite costs in late life, because of benefits in other contexts. For example, Pnt is important during development[23,34–36], and expression may simply run-on into adulthood. We have now shown that Pnt is also important for adults facing nutritional variation or stress, and genomic evidence suggests equivalent functions for Ets-4 in C. elegans[17]. In addition, Ets21C is required to mount an effective immune response[42], and both Ets21C and Pnt control gut homeostasis[38]. Tissue environment appears to be another important contextual factor that determines the lifespan effects of specific ETS TFs. Differences between tissues in chromatin architecture are likely to alter the capacity of a given TF to bind a given site, and our results show that a given TF, and also upstream RTKs, do not necessarily lead to the same lifespan effect across all tissues. The tissue-specific functions that we show for ETS TFs, Foxo and RTKs, suggests that transcription is locally coordinated by distinct receptors and TFs in distinct tissues, but that lifespan-regulatory signalling nevertheless converges on the ETS site. This differentiation makes it all the more remarkable that roles in lifespan appear to be conserved amongst ETS family TFs, even in diverse tissue contexts.
The structure of molecular networks and their integration amongst tissues underpins phenotype, including into old age. Unravelling the basics of these networks is a critical step in identifying precise anti-ageing molecular targets. Identifying the least disruptive perturbation of these networks, by targeting the “correct” effector, is a key goal in order to achieve desirable outcomes without undesirable trade-offs that may ensue from broader-scale perturbation. This targeting can be at the level of specific proteins, cell types, points in the life-course, or a combination of all three. The tissue-specific expression pattern of ETS TFs, and the apparent conservation of their roles in longevity, highlights them as important regulators of tissue-specific programs that may be useful in precise medical targeting of specific senescent pathologies.
Methods
D. melanogaster culture
All experiments were carried out in outbred, Wolbachia-free Dahomey flies, bearing the w1118 mutation and maintained at large population size since domestication in 1970. All transgenes (S1 Supplementary Information) were backcrossed into this background at least 6 times prior to experimentation, and stocks were maintained without bottlenecking. Cultures were maintained on 10% yeast (MP Biomedicals, OH, USA), 5% sucrose (Tate & Lyle, UK), 1.5% agar (Sigma-Aldrich, Dorset, UK), 3% nipagin (Chemlink Specialities, Dorset, UK), and 0.3% propionic acid (Sigma-Aldrich, Dorset, UK), at a constant 25°C and 60% humidity, on a 12:12 light cycle. Experimental flies were collected as embryos following 18h egg laying on grape juice agar, cultured at standardised density until adulthood, and allowed to mate for 48h before males were discarded and females assigned to experimental treatments at a density of 15 females/vial. To induce transgene expression using the GeneSwitch system, the inducer RU486 (Sigma M8046) was dissolved in absolute ethanol and added to the base medium to a final concentration of 200 μM. Ethanol was added as a vehicle control in RU-negative food. For lifespan experiments, flies were transferred to fresh food and survival was scored thrice weekly. Feeding RU486 to driver-only controls did not affect lifespan (S1 Supplementary Information). For starvation stress experiments, flies were fed RU486 or EtOH-supplemented media for one week, before switching to 1% agarose with the equivalent addition of RU486 or EtOH, with death scored daily until the end. For sugar stress experiments, sugar content was increased to 40% w/v sucrose[39,40].
C. elegans culture
Worms were maintained by Brenner’s protocol[43], at 20°C on NGM plates seeded with Escherichia coli OP50. For lifespan experiments, N2 (wildtype N2 male stock, N2 CGCM) were used at 20°C on NGM plates supplemented with 15μM FUDR to block progeny production. RNAi treatment was started from egg or late larval stages (details in Supplementary Materials). Animals that died from internal hatching were censored.
Molecular cloning
The pGL3Basic-4xFRE-pADH-Luc construct (called pGL4xFRE, reference [31]) was used as template to generate PCR products containing 6xETS-4xFRE-pADH, 4xFRE-pADH, 6xETS-pADH- or pADH (primers in S1 Supplementary Information, ETS sequence described in [44]), flanked by XhoI and HindIII sites, cloned into the corresponding sites in pGL3-Basic and confirmed by sequencing. PntP1 was amplified from UAS-PntP1 genomic DNA with Q5 High-Fidelity Polymerase (NEB M0491S - primers in S1 Supplementary Information), and AopACT was cloned from genomic DNA of UAS-AopACT flies[4]. PntP1 and AopACT sequences were then cloned into the pENTR-D-TOPO gateway vector (Thermo 450218) before recombination into the pAW expression vector.
S2 cell culture
Drosophila S2 cells were cultured in Schneider’s medium (Gibco/Thermo Scientific 21720024), supplemented with 10% FBS (Gibco/Thermo Scientific A3160801) and Penicillin/Streptomycin (Thermo 15070063). Cells were split into fresh media 24h before transfection, then resuspended to a density of 106 ml-1 and transfected using Effectene reagent (Qiagen 301425) in 96-well plates, according to the manufacturer’s instructions. Reporters and TF expression plasmids were co-transfected with pAFW-eGFP to visually confirm transfection, and pRL-TK-Renillaluc as an internal control for normalisation of reporter-produced Firefly luciferase. Reporters and pRL-TK-Renillaluc were transfected 1:1. When multiple TF expression plasmids were transfected, it was done 1:1. Each TF expression plasmid was transfected 4:1 relative to reporters or pRL-TK-Renillaluc (i.e. for every ng TF expression plasmid, 0.25 ng reporter and 0.25 ng pRL-TK-Renillaluc were transfected). The total amount of DNA transfected was then topped up to a standard quantity across all experimental conditions with pAFW-eGFP, in equal volumes of TE buffer. Reporter activity was measured 18h after transfection using Dual-Luciferase reagents (Promega E1960). pAHW-Foxo and/or pAW-AopACT were co-transfected with promoters bearing combinations of FREs and EBMs. pAW-AopACT and pAW-PntP1 were co-transfected with a promoter bearing EBMs.
Transcriptomics
Flies bearing combinations of UAS-Foxo, UAS-AopACT and UAS-PntP1, or UAS-PntRNAi in an S106-GS background were dissected after six days adult feeding on RU486. Tissues were dissected in ice-cold PBS. Guts were dissected by cutting off the head and last abdominal segment, pulling on the crop through an incision at the abdomenal-thoracic junction, then removing tubules. Reproductive anatomy was then removed from the abdomen and the remainder of the abdomen taken as fat body. Dissected tissues were placed directly into ice-cold Trizol (Ambion 15596026). In the Foxo-AopACT-PntP1 epistasis RNA-Seq experiment, four experimental replicates were sampled per condition, each comprising a pool of 12 fat bodies or guts. In the PntRNAi experiment three replicates were sampled per condition, also each comprising organs from 12 flies. RNA was extracted by Trizol-chloroform extraction, quantified on a NanoDrop, and integrity was assessed on an Agilent Bioanalyzer. Poly(A) RNA was pulled down using NextFlex Poly(A) beads (PerkinElmer NOVA-512981) and integrity was re-assessed. In the Foxo-AopACT-PntP1 epistasis RNA-Seq experiment, only samples with the highest RNA yields and integrity were included in library preparation, leaving 2–3 samples per experimental condition. All three replicates were prepped and sequenced in the PntRNAi RNA-Seq experiment. RNA fragments were given unique molecular identifiers and libraries were prepared for sequencing using NextFlex qRNAseq v2 reagents, (barcode sets C and D, PerkinElmer NOVA-5130-14 and NOVA-5130-15) and 16 cycles of PCR. Individual and pooled library quality was assessed on an Agilent Bioanalyzer and quantified with a Qubit spectrophotometer. Sequencing was performed by the UCL Cancer Institute, using an Illumina HiSeq 2500 instrument (paired-end 50bp) for the Foxo-AopACT-PntP1 epistasis experiment, and a NextSeq 500 (paired-end 75bp) for the PntRNAi experiment.
cDNA and qRT-PCR
cDNAs were made from the polyA RNA preps that were prepared for sequencing, using SuperScript II Reverse Transcriptase (Thermo 18064014) and OligoDT. qRT-PCR was performed on an Applied Biosystems QuantStudio 6 Flex real-time PCR instrument with Fast SYBR Green PCR Master Mix (Thermo Fisher), with primers supplied by EuroFins Genomics (all oligo sequences in S1 Supplementary Information), relative to a standard curve comprising a pool of all samples and the instrument's standard PCR cycle.
Metabolic assays
TAG was measured as in [45] in whole adult S106; UAS-PntP1 flies following one week of RU486 feeding. Briefly, flies were CO2-anaesthetised, weighed on a microbalance, and immediately flash-frozen in liquid N2. Flies were thawed in ice-cold TEt buffer (10 mM Tris, 1 mM EDTA, 0.1% v/v Triton-X-100) and homogenised by shaking with glass beads (Sigma G8772) for 30s in a ribolyser at 6500 Hz. Aliquots of homogenates were heated to 72°C for 15m to neutralise enzymatic activity, then spun 1m at 4500g and 4°C to pellet debris. Triglyceride was measured by treating 5 μl sample with 200 μl Glycerol Reagent (Sigma F6428) for 15m at 37°C and measuring absorbance at 540 nm, then incubating with 50 μl Triglyceride Reagent (Sigma F2449) for 15m at 37°C and re-measuring absorbance at 540 nm, calculating glycerol content in each reading, then quantifying triglyceride content as the difference between the first and second measurement.
Data analysis
Sequence libraries were quality-checked by FastQC 0.11.3, duplicate reads were removed using Je 1.2, and reads were aligned to D. melanogaster genome 6.19 with HiSat2 2.1. Alignments were enumerated with featureCounts 1.6. All downstream analyses were performed in R 3.3.1. The gut and fat body were analysed in parallel. In the RNA-Seq experiment analysing Foxo-AopACT-PntP1 epistasis, genes with no counted transcripts were excluded (S1 Supplementary Information). In the subsequent PntRNAi experiment, genes were filtered by the same criteria and any genes that were not analysed in the first experiment were also excluded. Read counts are given in S1 Supplementary Information. The transcriptomic effect of RU486 feeding was established for each individual genotype in the experiment, using DESeq2 at a false discovery rate (IHW) of 10%. To identify correlated effects amongst genotypes, sets of shared targets were formed as unions of DE gene sets from individual genotypes. Log2 fold-change values (Figs 1–4) were plotted from the DESeq2 output. Three-way epistatic interaction amongst TFs were identified by fitting models of the form
where block represented experimental replicate. The tripartite interaction of Foxo, AopACT and PntP1 was identified by applying the model to all genes across all experimental conditions, and isolating genes with a significant genotype:RU486 term.
GO analysis was performed using the TopGO package, applying Fisher’s test with the weight01 algorithm. Principal Components Analysis was performed on read counts of these genes following a variance-stabilizing transformation. To characterise gene-expression correlates of principal components, loadings onto principal components were extracted using the dimdesc function from the FactoMineR library, and GO analysis performed as previously. Transcripts of genes annotated with enriched GO terms were then plotted per term by centring variance-stabilised reads to a mean of zero and plotting against PC values per sample. Heatmaps were plotted using the heatmap.2 function from the gplots library, ordering rows by hierarchical clustering by Ward’s method on Euclidian distance, and scaling to row.
Fly lifespan data were analysed using log-rank tests in Microsoft Excel or Cox Proportional Hazards in R for the interaction of sugar and PntP1 expression. Worm lifespan data were analysed by log-rank tests in JMP.
Luciferase reporter data were normalised by taking the ratio of firefly luciferase to renilla luciferase signal and, for each promoter, taking the median reporter signal in the absence of FOXO and AOPACT as the start value, then calculating fold-change (i.e. difference in start and end values, divided by start value) for each sample. To assess the interaction of FOXO and AOP with promoters’ complements of TF-binding motifs, these normalised data were analysed by fitting a linear model of the form
in which y was the natural log of fold-change+1, FRE and EBM represented the TF-binding complement, and FOXO and AOPACT represented co-transfection with pAHW-Foxo or pAW-AopACT. By the same approach, the interactive effect of PNTP1 and AOPACT were assessed by fitting a linear model of the form
in which y represented the natural log of fold-change+1.
The effect of PntP1 overexpression on TAG and lifespan responses to nutrient stress (starvation or high-sugar diet) were analysed by a model of the form
where y represented TAG normalised to unit weight in a linear model, or survival in a Cox Proportional Hazards model (survival library).
Supporting information
Numbers represent set and intersection size.
(EPS)
Results were qualitatively consistent in each of the three replicates: AOPACT both moderates and synergises with transcriptional activation by FOXO on synthetic promoters containing combined ETS-binding motifs (EBMs) and FOXO-responsive elements (FREs), upstream of a basal Adh-Fireflyluciferase reporter. Activity is shown following normalisation to internal Renillaluciferase controls, and calculation of fold-change over the median expression of each reporter in the absence of FOXO and AOPACT. A significant four-way interaction of promoter and TF combination (i.e. FRE:EBM:FOXO:AOP) was detected in each experiment and in the pool. Full statistical analysis for each replicate experiment and their pool in S1 Supplementary Information.
(EPS)
Expression values were derived by applying DESeq2's variance-stabilising transformation to read counts, taking medians per transcript, and mean-sweeping values. Principal component values are shown in Fig 3D and 3E.
(EPS)
Linear model: RU486, F1,19 = 4.9, p = 0.04; starvation F1,19 = 465.55, p = 8e-15; RU486:starvation F1,19 = 2.45, p = 0.13).
(EPS)
Corresponding percentage changes in lifespan and associated p-values are given in S1 Supplementary Information and Fig 6B. (A-F) Expressing RNAi against each ETS TFs other than Aop and Pnt. Ets21C or Ets97D knockdown extended lifespan. (G-L) Expressing RNAi against the same set of ETS with the ubiquitous DaGS driver. Ets21C or Eip74EF knockdown extended lifespan. (M-N) Heterozygous and homozygous mutants of Ets21C were long-lived. Note that results from one experiment, with the same control population, are plotted in the two separate panels. (O-P) Knocking down Pnt with the ubiquitous DaGS or ActGS drivers limits lifespan, noting that an equivalent result is observed in S6I Fig, indicating that strong knockdown in the wrong adult tissues is costly. (Q-R) Confirmation with ActGS that neither Ets65A nor Ets98B limit lifespan.
(EPS)
Expressing Foxo (A-C) or limiting ETS activation (AopACT (D-F), PntRNAi (G-I), Ets21CRNAi (J-L)) has cell type-specific effects in the gut. TFs were chosen for experimentation based on strength of lifespan extension with S106, excluding Ets97DRNAi due to its relatively mild effect therein.
(EPS)
(A) Neuronal Eip74EFRNAi extends lifespan. (B) Neuronal PntRNAi does not impact lifespan. (C-D) Neuronal AopACT or Foxo are toxic.
(EPS)
(A-B) Expressing dominant-negative forms of the insulin receptor InR or epidermal growth factor receptor EGFR in gut and fat body with S106, extended lifespan. (C-D) Expressing the same constructs using GS5966 reveals differentiation between impacts of insulin and EGF signalling in the gut and fat body. (E-F) Neither RTK construct extended lifespan when expressed using the ISC-specific driver GS5961. (G-H) Dominant-negative InR, but not EGFR, mildly extends lifespan when expressed under control of TiGS.
(EPS)
(XLSX)
Acknowledgments
We thank Danny Filer, Kami Shalfrooshan, Seda Sakaci and Caesar Chi for technical assistance; Oscar Puig, Rachel Hunt, Joeseph Bateman, Cathy Slack and Ekin Bolukbasi for sharing plasmids and cells; Bruce Edgar for UAS-Cic flies; Steven Parratt for statistical advice; Nathan Woodling for tobi and dilp6 primers; Cristina Cotobal-Martin for assistance in planning sequencing experiments; Jennifer Lohr for conducting pilot experiments in C. elegans; and Pawan Dhami and Alex McLatchie of the CRUK-UCL Genomics and Genome Engineering Core Facility at the UCL Cancer Institute, for running sequencing instruments.
Data Availability
RNAseq data are available on the NCBI GEO repository (accession GSE130533). All other data are available on the Dryad repository (doi:10.5061/dryad.5qv9750), https://datadryad.org/review?doi=doi:10.5061/dryad.5qv9750.
Funding Statement
This work was funded by BBSRC grants BB/M029093/1 and BB/R014507/1 (https://bbsrc.ukri.org/) and Royal Society grant RG140694 (royalsociety.org) to NA. A. Gregoriou was a recipient of Wellcome Trust Biomedical Vacation Scholarship 206777/Z/17/Z (wellcome.ac.uk). The funders did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Niccoli T. & Partridge L. Ageing as a Risk Factor for Disease. Curr. Biol. 22, R741–R752 (2012). 10.1016/j.cub.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 2.Murphy C. T. et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–284 (2003). 10.1038/nature01789 [DOI] [PubMed] [Google Scholar]
- 3.Hsu A., Murphy C. & Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145 (2003). 10.1126/science.1083701 [DOI] [PubMed] [Google Scholar]
- 4.Alic N. et al. Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in Drosophila melanogaster. PLoS Genetics 10, (2014). 10.1371/journal.pgen.1004619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobson A. J. et al. Tissue-specific transcriptome profiling of Drosophila reveals roles for GATA transcription factors in longevity by dietary restriction. Npj Aging Mech Dis 4, 5 (2018). 10.1038/s41514-018-0024-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budovskaya Y. V. et al. An elt-3/elt-5/elt-6 GATA Transcription Circuit Guides Aging in C. elegans. Cell 134, 1–13 (2007). 10.1016/j.cell.20m08.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris B. J., Willcox D., Donlon T. A. & Willcox B. J. FOXO3: a major gene for human longevity-a mini-review. Gerontology 61, 515–525 (2015). 10.1159/000375235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slack C. et al. The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity. Cell 162, 72–83 (2015). 10.1016/j.cell.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slack C., Giannakou M. E., Foley A., Goss M. & Partridge L. dFOXO‐independent effects of reduced insulin‐like signaling in Drosophila. Aging Cell 10, 735–748 (2011). 10.1111/j.1474-9726.2011.00707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolukbasi E. et al. Intestinal Fork Head Regulates Nutrient Absorption and Promotes Longevity. Cell Reports 21, 641–653 (2017). 10.1016/j.celrep.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon C., Chang J., Gensch E., Rudner A. & Tabtiang R. A. C. elegans mutant that lives twice as long as wild type. Nature 366, 461 (1993). 10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- 12.Zhang P., Judy M., Lee S.-J. & Kenyon C. Direct and Indirect Gene Regulation by a Life-Extending FOXO Protein in C. elegans: Roles for GATA Factors and Lipid Gene Regulators. Cell Metab. 17, 85–100 (2013). 10.1016/j.cmet.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tullet J. et al. Direct Inhibition of the Longevity-Promoting Factor SKN-1 by Insulin-like Signaling in C. elegans. Cell 132, 1025–1038 (2008). 10.1016/j.cell.2008.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tepper R.J., et al. PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 154, 676–690 (2013). 10.1016/j.cell.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin h X.X., et al. DAF-16/FOXO and HLH-30/TFEB function as combinatorial transcription factors to promote stress resistance and longevity. Nat Comms 9, 4400 (2018). 10.1038/s41467-018-06624-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb A., Kundaje A., Brunet A. Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 15, 673–685 (2016). 10.1111/acel.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thyagarajan B. et al. ETS-4 Is a Transcriptional Regulator of Life Span in Caenorhabditis elegans. PLoS Genetics 6, e1001125 (2010). 10.1371/journal.pgen.1001125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollenhorst P. C., McIntosh L. P. & Graves B. J. Genomic and Biochemical Insights into the Specificity of ETS Transcription Factors. Annu Rev Biochem 80, 437–471 (2011). 10.1146/annurev.biochem.79.081507.103945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharrocks A. The ETS-domain transcription factor family. Nature Reviews Molecular Cell Biology 2, 827–837 (2001). 10.1038/35099076 [DOI] [PubMed] [Google Scholar]
- 20.Wei G.-H. H. et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. The EMBO journal 29, 2147–60 (2010). 10.1038/emboj.2010.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavrothalassitis G. & Ghysdael J. Proteins of the ETS family with transcriptional repressor activity. Oncogene 19, 6524–6532 (2000). 10.1038/sj.onc.1204045 [DOI] [PubMed] [Google Scholar]
- 22.Wang L. & Hiebert S. TEL contacts multiple co-repressors and specifically associates with histone deacetylase-3. Oncogene 20, 3716–25 (2001). 10.1038/sj.onc.1204479 [DOI] [PubMed] [Google Scholar]
- 23.O’Neill E. M., Rebay I., Tjian R. & Rubin G. M. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78, 137–147 (1994). 10.1016/0092-8674(94)90580-0 [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti S. R. & Nucifora G. The Leukemia-Associated Gene TEL Encodes a Transcription Repressor Which Associates with SMRT and mSin3A. Biochemical and Biophysical Research Communications 264, 871–877 (1999). 10.1006/bbrc.1999.1605 [DOI] [PubMed] [Google Scholar]
- 25.Guidez F. et al. Recruitment of the nuclear receptor corepressor N-CoR by the TEL moiety of the childhood leukemia-associated TEL-AML1 oncoprotein. Blood 96, 2557–61 (2000). [PubMed] [Google Scholar]
- 26.Hope C., Webber J. L., Tokamov S. A. & Rebay I. Tuned polymerization of the transcription factor Yan limits off-DNA sequestration to confer context-specific repression. eLife 7, (2018). 10.7554/eLife.37545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hope M. C., Rebay I. & Reinitz J. DNA Occupancy of Polymerizing Transcription Factors: A Chemical Model of the ETS Family Factor Yan. Biophysical Journal 112, 180–192 (2017). 10.1016/j.bpj.2016.11.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao F. et al. Derepression by depolymerization; structural insights into the regulation of Yan by Mae. Cell 118, 163–73 (2004). 10.1016/j.cell.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 29.Webber J. L. et al. The relationship between long-range chromatin occupancy and polymerization of the Drosophila ETS family transcriptional repressor Yan. Genetics 193, 633–49 (2012). 10.1534/genetics.112.146647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez R. et al. TEL is a sequence-specific transcriptional repressor. The Journal of biological chemistry 274, 30132–8 (1999). 10.1074/jbc.274.42.30132 [DOI] [PubMed] [Google Scholar]
- 31.Puig O., Marr M. T., Ruhf L. M. & Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes & Development 17, 2006–2020 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buch S., Melcher C., Bauer M., Katzenberger J., Pankratz M.J. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab 7, 312–332 (2008). 10.1016/j.cmet.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 33.Baker D. A., Mille-Baker B., Wainwright M. S., Ish-Horowicz D. & Dibb N. J. Mae mediates MAP kinase phosphorylation of ETS transcription factors in Drosophila. Nature 411, 330–334 (2001). 10.1038/35077122 [DOI] [PubMed] [Google Scholar]
- 34.Rebay I. & Rubin G. Yan functions as a general inhibitor of differentiation and is negatively regulated by activation of the Ras1/MAPK pathway. Cell 81, 857–66 (1995). 10.1016/0092-8674(95)90006-3 [DOI] [PubMed] [Google Scholar]
- 35.Klämbt C. The Drosophila gene pointed encodes two ETS-like proteins which are involved in the development of the midline glial cells. Development (Cambridge, England) 117, 163–76 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Vivekanand P., Tootle T. L. & Rebay I. MAE, a dual regulator of the EGFR signaling pathway, is a target of the Ets transcription factors PNT and YAN. Mechanisms of development 121, 1469–79 (2004). 10.1016/j.mod.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 37.Tootle T. L., Lee P. S. & Rebay I. CRM1-mediated nuclear export and regulated activity of the Receptor Tyrosine Kinase antagonist YAN require specific interactions with MAE. Development 130, 845–857 (2003). 10.1242/dev.00312 [DOI] [PubMed] [Google Scholar]
- 38.Jin Y. et al. EGFR/Ras Signaling Controls Drosophila Intestinal Stem Cell Proliferation via Capicua-Regulated Genes. PLoS genetics 11, e1005634 (2015). 10.1371/journal.pgen.1005634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saud S., Summerfield A. C. & Alic N. Ablation of insulin-producing cells prevents obesity but not premature mortality caused by a high-sugar diet in Drosophila. Proceedings of the Royal Society of London B: Biological Sciences 282, 20141720 (2015). 10.1098/rspb.2014.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mair W., Goymer P., Pletcher S. D. & Partridge L. Demography of dietary restriction and death in Drosophila. Science 301, 1731–1733 (2003). 10.1126/science.1086016 [DOI] [PubMed] [Google Scholar]
- 41.Zhu S., Barshow S., Wildonger J., Jan L. & Jan Y.-N. Ets transcription factor Pointed promotes the generation of intermediate neural progenitors in Drosophila larval brains. Proceedings of the National Academy of Sciences 108, 20615–20620 (2011). 10.1073/pnas.1118595109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayres J., Freitag N. & Schneider D. Identification of Drosophila mutants altering defense and endurance of Listeria monocytogenes infection. Genetics 178, 1807–1815 (2008). 10.1534/genetics.107.083782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers E.M., et al. Pointed regulates an eye-specific transcriptional enhancer in the Drosophila hedgehog gene, which is required for the movement of the morphogenetic furrow. Development 132, 4833–4843 (2005). 10.1242/dev.02061 [DOI] [PubMed] [Google Scholar]
- 45.Dobson A. J. et al. Host genetic determinants of microbiota-dependent nutrition revealed by genome-wide analysis of Drosophila melanogaster. Nature communications 6, 6312 (2015). 10.1038/ncomms7312 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Numbers represent set and intersection size.
(EPS)
Results were qualitatively consistent in each of the three replicates: AOPACT both moderates and synergises with transcriptional activation by FOXO on synthetic promoters containing combined ETS-binding motifs (EBMs) and FOXO-responsive elements (FREs), upstream of a basal Adh-Fireflyluciferase reporter. Activity is shown following normalisation to internal Renillaluciferase controls, and calculation of fold-change over the median expression of each reporter in the absence of FOXO and AOPACT. A significant four-way interaction of promoter and TF combination (i.e. FRE:EBM:FOXO:AOP) was detected in each experiment and in the pool. Full statistical analysis for each replicate experiment and their pool in S1 Supplementary Information.
(EPS)
Expression values were derived by applying DESeq2's variance-stabilising transformation to read counts, taking medians per transcript, and mean-sweeping values. Principal component values are shown in Fig 3D and 3E.
(EPS)
Linear model: RU486, F1,19 = 4.9, p = 0.04; starvation F1,19 = 465.55, p = 8e-15; RU486:starvation F1,19 = 2.45, p = 0.13).
(EPS)
Corresponding percentage changes in lifespan and associated p-values are given in S1 Supplementary Information and Fig 6B. (A-F) Expressing RNAi against each ETS TFs other than Aop and Pnt. Ets21C or Ets97D knockdown extended lifespan. (G-L) Expressing RNAi against the same set of ETS with the ubiquitous DaGS driver. Ets21C or Eip74EF knockdown extended lifespan. (M-N) Heterozygous and homozygous mutants of Ets21C were long-lived. Note that results from one experiment, with the same control population, are plotted in the two separate panels. (O-P) Knocking down Pnt with the ubiquitous DaGS or ActGS drivers limits lifespan, noting that an equivalent result is observed in S6I Fig, indicating that strong knockdown in the wrong adult tissues is costly. (Q-R) Confirmation with ActGS that neither Ets65A nor Ets98B limit lifespan.
(EPS)
Expressing Foxo (A-C) or limiting ETS activation (AopACT (D-F), PntRNAi (G-I), Ets21CRNAi (J-L)) has cell type-specific effects in the gut. TFs were chosen for experimentation based on strength of lifespan extension with S106, excluding Ets97DRNAi due to its relatively mild effect therein.
(EPS)
(A) Neuronal Eip74EFRNAi extends lifespan. (B) Neuronal PntRNAi does not impact lifespan. (C-D) Neuronal AopACT or Foxo are toxic.
(EPS)
(A-B) Expressing dominant-negative forms of the insulin receptor InR or epidermal growth factor receptor EGFR in gut and fat body with S106, extended lifespan. (C-D) Expressing the same constructs using GS5966 reveals differentiation between impacts of insulin and EGF signalling in the gut and fat body. (E-F) Neither RTK construct extended lifespan when expressed using the ISC-specific driver GS5961. (G-H) Dominant-negative InR, but not EGFR, mildly extends lifespan when expressed under control of TiGS.
(EPS)
(XLSX)
Data Availability Statement
RNAseq data are available on the NCBI GEO repository (accession GSE130533). All other data are available on the Dryad repository (doi:10.5061/dryad.5qv9750), https://datadryad.org/review?doi=doi:10.5061/dryad.5qv9750.