Abstract
Background
Increased second-line antiretroviral therapy (ART) failure rate narrows future options for HIV/AIDS treatment. It has critical implications in resource-limited settings; including sub-Saharan Africa (SSA) where the burden of HIV-infection is immense. Hence, pooled estimate for second-line HIV treatment failure is relevant to suggest valid recommendations that optimize ART outcomes in SSA.
Methods
We retrieved literature systematically from PUBMED/MEDLINE, EMBASE, CINAHL, Google Scholar, and AJOL. The retrieved studies were screened and assessed for eligibility. We also assessed the eligible studies for their methodological quality using the Joanna Briggs Institute’s appraisal checklist. The pooled estimates for second-line HIV treatment failure and its associated factors were determined using STATA, version 15.0 and MEDCALC, version 18.11.3, respectively. We assessed publication bias using Comprehensive Meta-analysis software, version 3. Detailed study protocol for this review/meta-analysis is registered and found on PROSPERO (ID: CRD42018118959).
Results
A total of 33 studies with the overall 18,550 participants and 19,988.45 person-years (PYs) of follow-up were included in the review. The pooled second-line HIV treatment failure rate was 15.0 per 100 PYs (95% CI: 13.0–18.0). It was slightly higher at 12–18 months of follow-up (19.0/100 PYs; 95% CI: 15.0–22.0), in children (19.0/100 PYs; 95% CI: 14.0–23.0) and in southern SSA (18.0/100 PYs; 95% CI: 14.0–23.0). Baseline values (high viral load (OR: 5.67; 95% CI: 13.40–9.45); advanced clinical stage (OR: 3.27; 95% CI: 2.07–5.19); and low CD4 counts (OR: 2.80; 95% CI: 1.83–4.29)) and suboptimal adherence to therapy (OR: 1.92; 95% CI: 1.28–2.86) were the factors associated with increased failure rates.
Conclusion
Second-line HIV treatment failure has become highly prevalent in SSA with alarming rates during the 12–18 month period of treatment start; in children; and southern SSA. Therefore, the second-line HIV treatment approach in SSA should critically consider excellent adherence to therapy, aggressive viral load suppression, and rapid immune recovery.
Introduction
In the past decade, rapid scale-up of antiretroviral therapy (ART) in sub-Saharan Africa (SSA) substantially reduced HIV/AIDS-related morbidity and mortality [1, 2]. It has also prolonged the average life expectancy of HIV-infected individuals [1]. However, these benefits are being challenged by the increasing HIV treatment failure rates with first or second-line antiretroviral therapies [3–6]. HIV treatment failure can be defined in terms of clinical, immunological, or virological failures [7]. Clinical failure is the occurrence of a new or recurrent stage III or stage IV clinical event (s). Immunological failure is the decline of CD4 counts either to less than the pre-treatment value or to <50% of a peak value on ART or persistently lower than 100 cells/ml [7]. Virological failure could be a definite failure (i.e., when a single viral load (VL) is greater than 10,000 copies/ml at 12 months of follow-up) or a probable failure (i.e., when either a single VL is >1000 copies/ml at 12 months or a VL at 12 months is ≥ 400 copies/ml confirmed by a second measurement taken 30 days later) [8–10]. Clinical and immunological failure criteria are not sufficient for the definite diagnosis of treatment failure and each of them should be accompanied with VL tests as a confirmation [11]. With this, VL testing is efficient to indicate direct plasma effects of ART on HIV ribonucleic acid (RNA) [12]. It also helps preserve the limited HIV treatment options available by reducing the probability of incorrect switching to the next more expensive and toxic regimens [13].
The HIV-infected patients on ART are recalling earlier fears of death from the infection because of treatment failures [14]. Patients who experienced first-line HIV treatment failure may be switched to second-line regimens [15, 16]. Many countries in resource-limited settings switch a failed first-line ART to second-line regimen after an initial delay mainly related to inadequate VL tests [11]. The inadequacy of VL informed differentiated care for the HIV-infected patients commenced with second-line ART [17] could increase the risks of death and opportunistic infections especially in patients with advanced HIV at the time of first-line HIV treatment failure [18].
The HIV treatment failure involving second-line regimens has very narrow options for further switching, and this is a serious concern in resource-limited settings [19]. The World Health Organization (WHO) recommends few second-line regimens as preferred ART (i.e., ritonavir-boosted atazanavir- or lopinavir-based ART and dolutegravir-based ART) [20, 21]. Despite the limited second-line HIV treatment options, many countries in SSA have financial constraints to adopt third-line regimens [20, 21]. As a result, the optimal use of second-line therapies after the occurrence of first-line HIV treatment failure is alarmingly essential for SSA, the epicenter of HIV/AIDS. However, many countries in SSA have no national strategic guidelines for the optimal use of second-line therapy despite the occurrence of a number of treatment failures related to the therapies [17].
Suboptimal adherence (i.e., missing of any dose in the past 3 days [22] or 7 days [23]; or less than 95% adherence in the past 30 days [24] or less than 90% adherence in the past year [25]) was indicated as a key determinant of second-line HIV treatment failure [26, 27]. Suboptimal adherence could be a result of regimen toxicities [28]. It may require a tailored adherence intervention based on the degree of suboptimal adherence [27, 29]. Baseline characteristics such as delayed initiation of second-line therapy [30] and high VL might result in unfavorable treatment outcomes [25]. To maximize the durability of the second-line regimens, early identification of first-line treatment failure and switching to a second-line regimen at a relatively higher CD4 cell count is very important [31, 32]. Advanced clinical stage at baseline and lack of VL monitoring were also identified to have associations with second-line HIV treatment failures [33]. In addition, the clinical status of patients such as baseline clinical stage IV and CD4 counts below 100 cells/mm3 were significantly linked with increased rates of treatment failure [34]. As a result, pooling the proportion of second-line HIV treatment failures and factors associated with these failures are required to assist the optimization of HIV treatment outcomes in SSA. Therefore, the aim of this systematic review and meta-analysis was to estimate the proportion of second-line HIV treatment failure and its associated factors in SSA.
Materials and methods
Study protocol
The method of this systematic review and meta-analysis was reported as per the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement recommendations [35]. Identification of records, screening by titles and abstracts, and eligibility evaluation of full texts for final inclusion was conducted in accordance with the PRISMA flow diagram [36]. During the execution of this systematic review and meta-analysis, the PRISMA checklist was strictly followed. The protocol is registered on the International Prospective Register of Systematic Reviews (PROSPERO) with a registration number of CRD42018118959 and it is available at https://www.crd.york.ac.uk/prospero/#recordDetails.php?ID=CRD42018118959 [37].
Data sources and searches
We performed a systematic literature search from PubMed, MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCOhost), Cochrane Library, Google Scholar, Health Technology Assessment, African Journals Online (AJOL) and ResearchGate. Websites of organizations and University repositories were also visited to retrieve any remaining relevant record including unpublished (gray) kinds of literature. In our search strategy, search terms we employed were “treatment failure”; “second-line”; “protease inhibitor”; “antiretroviral therapy”; and names of countries in the SSA. During the search, we accomplished a careful selection of keywords and indexing terms that did not limit the year of publication. In the search strategy, Boolean operators and truncations were also employed. The search was conducted from 15 December 2018 to 14 January 2019. Accordingly, all published and unpublished literature identified during the period of searching were retrieved.
Study selection
We set predefined inclusion and exclusion criteria for initial screening by titles or abstracts and evaluation of full texts for their eligibility assessment. We considered articles with at least an outcome of failure to second-line ART for their potential to be included. Next, we assessed the original articles reporting second-line HIV treatment failure after at least 6 month period of follow-up; reported in English language; and conducted in countries of SSA for their eligibility. In addition, we assessed the eligible original articles for quality using the Joanna Briggs Institute’s (JBI) critical appraisal checklist and articles with moderate (50–75%) to high (>75%) quality were considered as per the appraisers’ evaluation results. However, we excluded articles with outcomes not related to second-line therapy failure; with no separate failure data for SSA patients in case of mixed multi-center study settings involving SSA and other countries; and with no separate data of second-line therapy failure in studies involving first and second-line therapies during the screening and eligibility assessments.
Screening and eligibility
We identified and selected records retrieved through a search of the electronic databases and indexing services. Following this, we exported them to ENDNOTE reference software version 8.2 (Thomson Reuters, Stamford, CT, USA). Next, we identified, registered, and removed duplicates by the use of ENDNOTE. Accordingly, two authors, Dumessa Edessa (DE) and Mekonnen Sisay (MS), independently screened titles and abstracts of the retained records based on the predefined inclusion criteria. A third author, Fekede Asefa (FA), was consulted in case of disagreement between the two authors. With this, DE and FA individually collected and evaluated full texts of the retained articles for their quality and final eligibility assessment. In the end, we included articles that fulfilled the quality evaluation criteria.
Quality assessment and data extraction
We accomplished quality assessment for the articles by employing the JBI’s critical appraisal checklist for cohort and analytical cross-sectional studies [38, 39]. Two authors (DE and MS) critically appraised the articles. For the final decision of inclusion, we considered scores of the two authors in consultation with the third author’s score (in case of disagreement between the two authors’ appraisal results). Lastly, we ranked the articles by their methodological qualities based on the total number of appraisers’ score marked as ‘yes’ to questions of the JBI’s critical appraisal checklist. Accordingly, we included all studies with their overall positive responses in ranges of 50% to 75% (moderate quality studies) or higher than 75% (high quality studies) for the review and meta-analysis.
To extract relevant data, we employed a customized data abstraction format that has been prepared in a Microsoft Excel sheet. Two of the authors independently abstracted data pertaining to first author; year of publication; study design (analytical cross-sectional, follow-up); study region/country; study participants (children, adults, mixed-age groups); types of second-line ART (ritonavir-boosted protease inhibitor (PI)-based ART, PI-based ART with no ritonavir-boosting); sample size; median months of follow-up; person/patient-years (PYs) of follow-up; and event of interest (number of second-line therapy failure and factors associated with the failure).
Outcome variables
Proportion of second-line HIV treatment failure that includes clinical, immunological or virological failure, as defined by the WHO [7, 9] was the primary outcome variable we estimated in this systematic review and meta-analysis. The secondary outcome measure we estimated was factors associated with the second-line HIV treatment failures.
Data synthesis and analysis
Proportion of second-line HIV treatment failure we pooled together was accomplished with the help of STATA software, version 15.0. Again, we performed sensitivity and subgroup analyses to minimize the risks of bias. With this, we used forest plots to graphically report the various meta-analysis results. We also applied the Mantel-Haenszel random-effects model to conduct meta-analyses at a 95% confidence level. Likewise, we assessed the heterogeneity status of the included studies and presented it with the use of Cochran’s Q test (chi-squared (I2) statistic). We also accomplished tests for factors associated with second-line therapy failure by using MEDCALC statistical software (MedCalc Software bvba, Ostend, Belgium), version 18.11.3. Besides, we employed Comprehensive Meta-analysis software (Biostat, Englewood, New Jersey, USA), version 3, for publication bias assessment. Similarly, we evaluated the presence of publication bias with the use of Egger's regression and Begg’s correlation tests. Lastly, we considered all statistical tests with p-values less than 0.05 (two-tailed) as significant.
Results
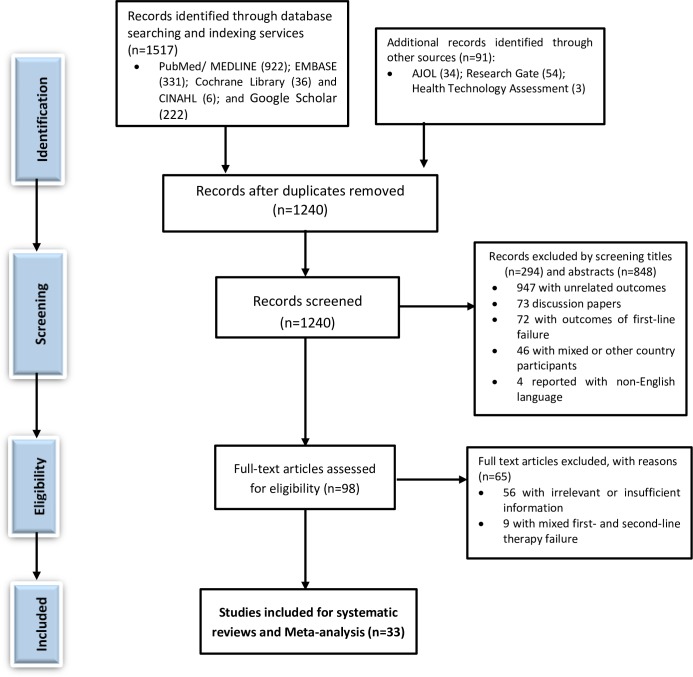
We identified a total of 1,608 records from the search of legitimate databases and indexing services. After the removal of 368 duplicates, we retained 1240 records for screening by titles and abstracts. Again, we excluded a total of 1,142 literature by screening titles (n = 294) and abstracts (n = 848). Of this 1,142 literature, 947 of them had unrelated outcomes of interest; 73 of them were discussion papers; 72 of them had outcomes of first-line therapy failure; 46 of them had mixed and/or other country studies, and 4 of them reported their outcomes with non-English languages. Accordingly, we conducted an eligibility evaluation of 98 full texts as per the predefined eligibility criteria for inclusion. Again, we excluded 65 studies with justifiable reasons (i.e., 56 of them with irrelevant/insufficient outcomes of interest; 9 of them with mixed first and second-line HIV treatment failures and no separate data for second-line therapy failure). PRISMA flow chart depicting the selection, screening, and eligibility assessment process is shown in Fig 1 and S1 Table. We also assessed these records for their methodological quality by employing the JBI’s critical appraisal checklists (Table 1). Finally, we included 33 articles with the primary outcome of interest and with a high or moderate percentage in its score of methodological quality assessment for the systematic reviews and meta-analysis.
Fig 1. PRISMA flow diagram depicting the selection process.
Table 1. Quality assessment for included studies.
| References | JBI’s Critical Appraisal Checklist | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | |
| Adetunji et al, 2013 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | UC | No | Yes |
| Akanmu et al, 2015 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | UC | No | Yes |
| Berhanu et al, 2014 | NA | NA | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Boender et al, 2016 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Boerma et al, 2017 | NA | NA | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Castelnuovo et al, 2009 | NA | NA | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Ciaffi et al, 2015 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Collier et al, 2017 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Court et al, 2014 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| Evans et al, 2018 | NA | NA | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| Evans et al, 2018 | NA | NA | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| Fox et al, 2010 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Fox et al, 2016 | NA | NA | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Garone et al, 2013 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hosseinipour et al, 2010 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Johnston et al, 2012 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Johnston et al, 2014 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Levison et al, 2012 | NA | NA | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Murphy et al, 2012 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Musiime et al, 2013 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Ongubo et al, 2017 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| Onyedum et al, 2013 | NA | NA | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Paton et al, 2014 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Paton et al, 2017 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pujades et al, 2010 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Rawizza et al, 2013 | NA | NA | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Schoffelen et al, 2013 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Shearer et al, 2017 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Sigaloff et al, 2012 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Tsegaye et al, 2016 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Wandeler et al, 2012 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Wandeler et al, 2014 | NA | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Van Zyl et al, 2011 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | |||
Note: NA, not applicable; UN, unclear; Q1-8, JBI’s Critical Appraisal Checklist for Analytical Cross Sectional studies {Q1: Were the criteria for inclusion in the sample clearly defined? Q2: Were the study subjects and the setting described in detail? Q3: Was the exposure measured in a valid and reliable way? Q4: Were objective, standard criteria used for measurement of the condition? Q5: Were confounding factors identified? Q6: Were strategies to deal with confounding factors stated? Q7: Were the outcomes measured in a valid and reliable way? Q8: Was appropriate statistical analysis used?}; Q1-11, JBI’s Critical Appraisal Checklist for Cohort studies {Q1: Were the two groups similar and recruited from the same population? Q2: Were the exposures measured similarly to assign people to both exposed and unexposed groups? Q3: Was the exposure measured in a valid and reliable way? Q4: Were confounding factors identified? Q5: Were strategies to deal with confounding factors stated? Q6: Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? Q7: Were the outcomes measured in a valid and reliable way? Q8: Was the follow up time reported and sufficient to be long enough for outcomes to occur? Q9: Was follow up complete, and if not, were the reasons to loss to follow up described and explored? Q10: Were strategies to address incomplete follow up utilized? Q11: Was appropriate statistical analysis used?}.
Study characteristics
The 33 studies included for the systematic reviews and meta-analysis had a total of 18,550 participants, and 2,473 of them experienced treatment failures to their second-line HIV treatment. The study participants had a total of 19,988.45 PYs of follow-up. All the included studies were published during the year 2009 to 2018. Sample sizes for the included studies range from 40 patients enrolled by a study conducted in Uganda [40] to 6,714 patients enrolled by a study accomplished in Nigeria [41]. The study participants of 26 studies were adults [19, 23–25, 27, 34, 40, 42–60], while that of 2 studies [22, 61] and 5 studies [6, 41, 62–64] were children and mixed age groups, respectively. Sixteen studies (n = 16) were from southern Africa [23, 27, 43, 45–52, 57, 58, 60, 62, 64]; 7 studies were from eastern Africa [22, 34, 40, 53, 55, 61, 63]; 5 studies were from western Africa [25, 41, 42, 44, 54]; and 5 studies were from mixed regions in SSA [6, 19, 24, 55, 59]. The second-line HIV treatment regimens received by the study participants were PI-based, 18 of them with ritonavir-boosted PI-based ART [24, 25, 27, 40, 42, 44, 45, 48, 49, 52–56, 58, 60, 61, 63] and 15 of them with no ritonavir in their PI-based ART regimens [6, 19, 22, 23, 34, 41, 43, 46, 47, 50, 51, 57, 59, 62, 64]. Thirteen studies (n = 13) defined the second-line HIV treatment failure by using the WHO definition of RNA VL more than 400 copies/ml [6, 19, 24, 25, 27, 42, 47, 49, 53, 54, 56–58] while 16 of the studies employed the WHO criteria of HIV RNA VL above 1000 copies/ml [22, 23, 40, 43–46, 48, 50–52, 55, 59–61, 63]. However, 4 of the studies employed mixed definitions of the WHO criteria for ART failure that included clinical, immunological and virological failures and/or death/lost to follow-up [34, 41, 62, 64] (Table 2 and S2 Table).
Table 2. Characteristics of studies describing second-line ART failure among patients on treatment follow-up in sub-Saharan Africa.
| References | Year of publication | Study design | Study setting | Patient groups | Second-line regimen | Sample size | Number with TF | PYs of follow-up |
|---|---|---|---|---|---|---|---|---|
| Adetunji et al [25] | 2013 | RFU | Nigeria | Adults | PI/r-based | 225 | 34 | 225 |
| Akanmu et al [42] | 2015 | RFU | Nigeria | Adults | LPV/r-based | 318 | 25 | 636 |
| Berhanu et al [43] | 2014 | RFU | South Africa | Adults | PI-based | 372 | 129 | 465 |
| Boender et al [19] | 2016 | FU | Zambia, South Africa, Kenya, Uganda, Zimbabwe and Nigeria | Adults | PI-based | 227 | 32 | 227 |
| Boerma et al [22] | 2017 | FU | Uganda | Children | PI-based | 60 | 12 | 120 |
| Castelnuovo et al [40] | 2009 | FU | Uganda | Adults | LPV/r-based | 40 | 7 | 120 |
| Ciaffi et al [44] | 2015 | FU | Cameroon, Senegal and Burkina Faso | Adults | PI/r-based | 451 | 5 | 451 |
| Collier et al [45] | 2017 | FU | South Africa | Adults | LPV/r-based | 101 | 23 | 202 |
| Court et al [46] | 2014 | RFU | South Africa | Adults | PI-based | 228 | 26 | 228 |
| Evans et al [23] | 2018 | RFU | South Africa | Adults | PI-based | 128 | 50 | 192 |
| Evans et al [47] | 2018 | RFU | South Africa | Adults | PI-based | 719 | 36 | 1438 |
| Fox et al [49] | 2010 | FU | South Africa | Adults | PI-based | 262 | 59 | 262 |
| Fox et al [48] | 2016 | FU | South Africa | Adults | LPV/r-based | 388 | 106 | 446.6 |
| Garone et al [62] | 2013 | FU | South Africa | Mixed-age groups | PI-based | 40 | 7 | 30 |
| Hosseinipour et al [63] | 2010 | FU | Malawi | Mixed-age groups | LPV/r-based | 101 | 15 | 101 |
| Johnston et al [50] | 2014 | FU | South Africa | Adults | PI-based | 122 | 39 | 518.75 |
| Johnston et al [51] | 2012 | FU | South Africa | Adults | LPV/r-based | 417 | 43 | 152.5 |
| Levison et al [52] | 2012 | RFU | South Africa | Adults | LPV/r-based | 322 | 43 | 268.3 |
| Murphy et al [27] | 2012 | FU | South Africa | Adults | LPV/r-based | 136 | 26 | 136 |
| Musiime et al [61] | 2013 | FU | Uganda | Children | LPV/r-based | 142 | 55 | 142 |
| Ongubo et al [53] | 2017 | RFU | Malawi | Adults | ATV/r-based | 376 | 35 | 282 |
| Onyedum et al [54] | 2013 | RFU | Nigeria | Adults | LPV/r-based | 68 | 12 | 68 |
| Paton et al [55] | 2014 | FU | Five countries in SSA | Adults | LPV/r-based | 379 | 35 | 758 |
| Paton et al [56] | 2017 | FU | Malawi, Uganda, Zimbabwe and Kenya | Adults | LPV/r-based | 336 | 45 | 1008 |
| Pujades et al [6] | 2010 | FU | Burkina Faso, Democratic Republic of Congo, Kenya, Malawi, Mozambique, Nigeria, Zimbabwe, South Africa, Uganda, Zambia | Mixed-age groups | PI-based | 493 | 91 | 493 |
| Rawizza et al [41] | 2013 | RFU | Nigeria | Mixed-age groups | PI-based | 6714 | 673 | 3357 |
| Schoffelen et al [64] | 2013 | RFU | South Africa | Mixed-age groups | PI-based | 191 | 48 | 318.3 |
| Shearer et al [57] | 2017 | RFU | South Africa | Adults | PI-based | 927 | 233 | 927 |
| Sigaloff et al [24] | 2012 | FU | Uganda, South Africa, Kenya, Nigeria, Zambia and Zimbabwe | Adults | PI/r-based | 232 | 63 | 232 |
| Tsegaye et al [34] | 2016 | RFU | Ethiopia | Adults | PI-based | 356 | 67 | 712 |
| Van Zyl et al [58] | 2011 | CS | South Africa | Adults | LPV/r-based | 93 | 37 | 93 |
| Wandeler et al [59] | 2014 | FU | South Africa, Zambia, Zimbabwe | Adults | PI-based | 1256 | 122 | 3495 |
| Wandeler et al [60] | 2012 | FU | Zambia and South Africa | Adults | LPV/r-based | 2330 | 240 | 1884 |
| Total | 18, 550 | 2, 473 | 19, 988.45 | |||||
Note: CS, cross sectional; FU, follow-up; RFU, retrospective follow-up; ATV/r, ritonavir-boosted atazanavir; PI/r, ritonavir-boosted protease inhibitor; LPV/r, ritonavir-boosted lopinavir; PI, protease inhibitor; PYs, Person-years of follow-up; TF, treatment failure; SSA, sub-Saharan Africa.
Proportion of patients with second-line ART failure
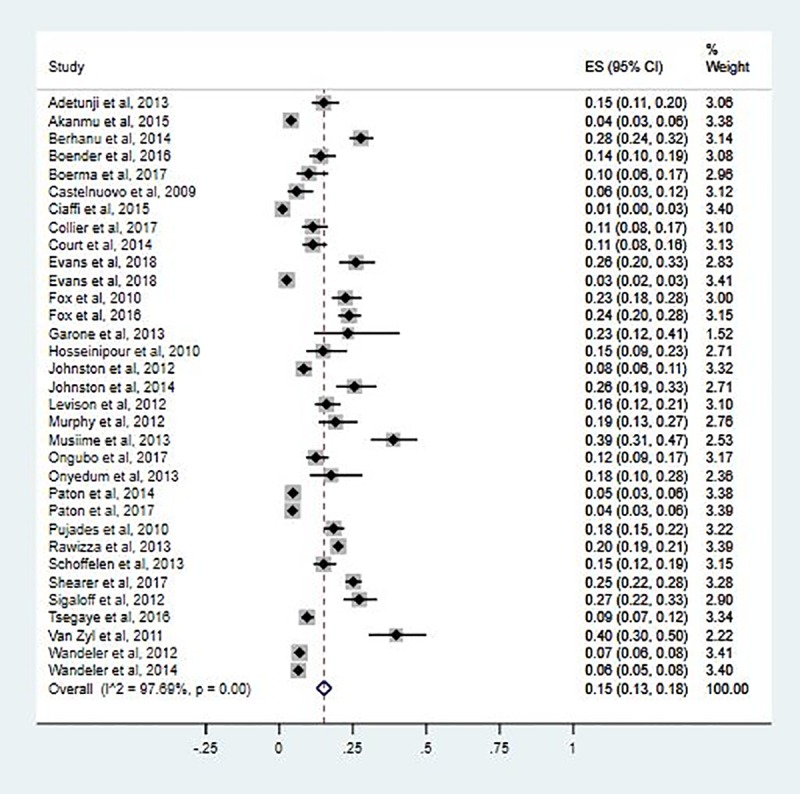
The pooled estimate for rate of second-line HIV treatment failure was 15.0 per 100 PYs of follow-up (95% CI: 13.0–18.0 per 100 PYs; I2 = 97.69%; P<0.001). The second-line treatment failures among the included studies range from 1.0/100 PYs (95% CI: 0.0–3.0 per 100 PYs) to 40.0/100 PYs (95% CI: 30.0–50.0) (Fig 2).
Fig 2. Forest pilot of proportion for second-line HIV treatment failure in SSA.
Sensitivity and subgroup analyses
We performed sensitivity analyses by excluding outliers [44, 58] and one or more studies. They did not have significant changes in the extent of pooled outcome measures. As a result, we included all the studies for the meta-analysis. We performed subgroup analyses on the basis of month period of follow-up after second-line ART initiation (less than 12 months, 12–18 months, above 18 months); patient groups (children, adults, mixed age-groups); regions in SSA (southern Africa, eastern Africa, western Africa, mixed regions of SSA); and type of second-line ART (PI-based ART, ritonavir-boosted PI-based ART). Accordingly, the pooled estimates of second-line ART failure were 19.0/100 PYs (95% CI: 15.0–22.0/100 PYs; I2 = 97.58%; P<0.001) at 12–18 month period of follow-up after second-line therapy initiation; 19.0/100PYs (95% CI: 14.0–23.0/100PYs; I2 = 0.0%) among children; and 18.0/100 PYs (95% CI: 14.0–23.0/100 PYs; I2 = 97.60%; P < 0.001) among patients in the southern SSA (Fig 3A–3D and S3 Table).
Fig 3. Forest pilots of proportion for second-line HIV treatment failure by subgroups.
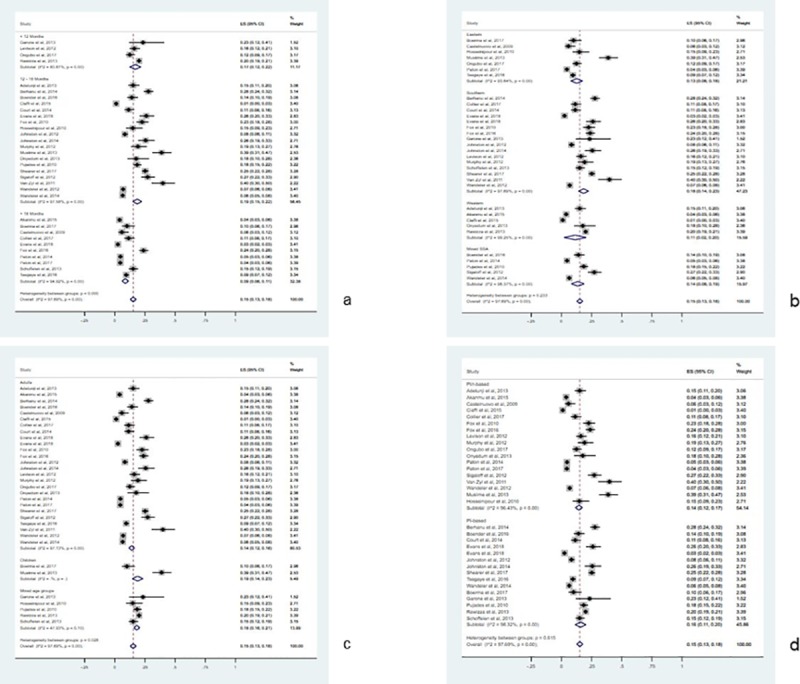
(a) Forest pilot describing failure by months of follow-up. (b) Forest pilot describing failure by regions of SSA. (c) Forest pilot describing failure by age group of participants. (d) Forest pilot describing failure by ritonavir boosting status of PI-based ART.
Factors associated with second-line ART failure
The pooled estimate for factors associated with second-line HIV treatment failure revealed that certain factors were influencing the failure rates. High baseline viral load (OR: 5.67; 95% CI: 13.40–9.45); advanced clinical stage of HIV at baseline (OR: 3.27; 95% CI: 2.07–5.19); low peak CD4 cell counts at baseline (<100 cells/mm3) (OR: 2.80; 95% CI: 1.83–4.29); and suboptimal adherence to second-line therapy (OR: 1.92; 95% CI: 1.28–2.86) were patient factors associated with the significantly increased occurrence of second-line ART failures (Table 3).
Table 3. Pooled estimates of factors associated with second-line HIV treatment failure.
| Factor | OR (95% CI) | Z statistic | P-values |
|---|---|---|---|
| High VL at second-line therapy initiation | 5.67 (3.40–9.45) | 6.67 | <0.0001 |
| Advanced WHO clinical stage at baseline | 3.27 (2.07–5.19) | 5.06 | <0.0001 |
| Low CD4 cell counts (<100 cells/mm3) at baseline | 2.80 (1.83–4.29) | 4.75 | <0.0001 |
| Suboptimal adherence to second-line ART | 1.92 (1.28–2.86) | 3.20 | 0.0013 |
Note: VL, viral load; OR, odds ratio; ART, antiretroviral therapy; WHO, World Health Organization.
Different independent study reports also described depressive symptoms [23]; tuberculosis co-treatment with HIV/AIDS [45]; traditional medicine use [23]; delayed second-line HIV treatment initiation [25]; and younger age [53] as factors that favored second-line therapy failure. On the other hand, a study report indicated obesity [53] and elevated total bilirubin [53] as factors that protected second-line ART failure.
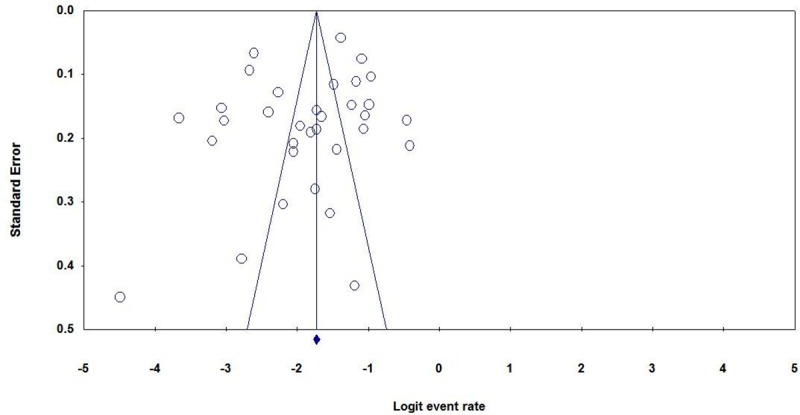
Publication bias
Egger’s regression test did not show any evidence for publication bias among the included studies (P = 0.0992, one-tailed). In addition, Begg’s correlation test did not also show any evidence of publication bias (P = 0.154, one-tailed) (Fig 4).
Fig 4. Funnel plot of standard error by logit event rate for publication bias.
Discussion
In this meta-analysis, the pooled estimate of proportion for second-line HIV treatment failure was 15.0 per 100 PYs of follow-up. This evidence is in line with reports from several studies that revealed a proportion of second-line HIV treatment failure as high as 25% [32, 65–69]. With this, the rate of second-line therapy failure among HIV-infected children was estimated to be 19.0 per 100 PYs. Aligned with this finding, 19% of children treated with PI-based second-line therapy in Thailand encountered treatment failures [70]. Another study conducted in Thailand also reported up to 49% virological failure rates in children treated with second-line therapies [71]. Additionally, an ART audit for pediatric patients in London indicated that 37% of the patients achieved HIV-RNA VL less than 400 copies/ml [72]. Lack of VL monitoring, insufficient early diagnosis of failure, and unstructured and inadequate adherence counseling were the few reasons implicated for the increased treatment failure rates in children [73].
The pooled treatment failure rates before 12 months and 12–18 months of follow-up after second-line therapy initiation were 17.0/100 PYs and 19.0/100 PYs, respectively. The failure rate was 9.0/100 PYs after 18 months of follow-up. This indicated that a relatively sustained virological response is expected after the 18 months of follow-up. Similarly, a multi-centered study in Asia and Africa found that the most frequent experience of second-line therapy failure (i.e., 250.0/1000 PYs) occurred during 6 to 11 months of follow-up compared to the 18 months and more duration of follow-up (i.e., 212.0/1000 PYs) [6].
Subgroup analyses by regions revealed lower second-line HIV treatment failure rates in western (11.0/100 PYs) and eastern (13.0/100 PYs) regions of SSA compared to the rate in southern (18.0/100 PYs) region. These estimates are in line with the 19.6 million people living with HIV in southern and eastern regions compared to the 6.1 million people living with the infection in western and central Africa regions in 2017 [74]. Since a minimal failure rate can naturally occur toward antimicrobial agents, an increased probability of failure might be expected in the southern/eastern regions of Africa with a higher HIV burden. Indeed, in the presence of infection and antimicrobial agent use, there is always a natural phenomenon of drug resistance and failure [75]. This phenomenon can also be accelerated with improper infection control practices and suboptimal adherence to the ART [75]. In addition, two studies indicated consistent findings with the second-line HIV treatment failure rates in southern (19%) [76] and western/eastern regions (11.1%) [19].
High VL (≥ 5000 copies/ml) at second-line HIV treatment initiation increased the odds of treatment failure (odds ratio (OR) 5.67; 95% CI: 3.40–9.45; P<0.0001). Patients who experienced virologic failure with first-line therapy and switched to second-line therapy after 12 months were more likely to experience a further increase in VL as a potential indicator for second-line therapy failure [77]. Several studies indicated that second-line therapy failure was associated with higher baseline VL measurements [26, 78–84]. Patients who had an experience of suboptimal adherence to second-line therapy were more likely to develop treatment failure compared to patients with optimal adherence (OR 1.92; 95% CI: 1.28, 2.86; P = 0.0013). Several published reports explained a relationship between suboptimal adherence to second-line therapy and the increase in failure rates [6, 22, 26, 71, 79, 81, 85–88]. In addition, patients with histories of suboptimal adherence to first-line therapy were also more likely to have suboptimal adherence to second-line therapy [50, 89]. It could increase the odds of second-line HIV treatment failure rate. This increased failure rate may also be linked to poor treatment adherence resulting from the more frequent toxicities associated with second-line ART regimens [65].
An advanced clinical stage (stage III or IV) of HIV at the commencement of second-line therapy increased the odds of treatment failure (OR 3.27; 95% CI: 2.07–5.19; P<0.0001). With this, baseline CD4 cell counts of < 100 cells/ml were linked to increased odds of treatment failure (OR 2.80; 95% CI: 1.83–4.29; P<0.0001). Growing evidence relate the advanced HIV and lower peak CD4 cell counts at baseline to the increased rates of failure with second-line therapy [6, 69, 77, 87, 90].
Some of the included studies also reported that patients with lengthy delays in initiating second-line therapy [25]; who were underweight [22]; who were on tuberculosis co-treatment [45]; who had depressive symptoms [23]; who were with practice of herbal or traditional medicine use [23]; and who were at younger age [53] had increased rates of treatment failure [63, 71, 77, 80, 90]. Although the relationship among depression, adherence and treatment failure is yet to be fully investigated, more than one-third of HIV-infected patients with depressive symptoms were found to have an elevated HIV-RNA VL in South Africa [91]. Patients with higher depression rating scales, with higher HIV-RNA VL and at a younger age were indicated to have increased patterns of ART missed doses [92]. A high probability of suboptimal adherence to ART among alcohol users was also reported [93] which can contribute to the ART failure. Despite widespread concern about concurrent traditional medicine use and ART, yet there is no sufficient evidence of whether traditional medicine use results in adverse effects or interactions that could limit the effectiveness of the ART or not [94]. With regard to HIV-tuberculosis co-treatment, suboptimal adherence to treatments was explained by a study report [95] and this interpretation could be related to the outcome of ART.
Contrary to other studies, one of the included studies reports that obese or overweight patients had a reduced proportion of failure to second-line ART [53]. Obese HIV patients were found to have higher CD4 counts compared to normal-weight patients [96]. A higher plasma concentration of second-line regimen containing darunavir-boosted with ritonavir was revealed in obese patients [97]. The WHO recommended second-line therapies for HIV-infection involve mainly ritonavir-boosted PI-based regimen. The ritonavir inhibits cytochrome P450 enzymes to which many of the medications are substrate [98]. The ritonavir-enzyme interaction can increase the plasma concentration of second-line therapy thereby protects treatment failure experience [99]. Although the relationship between elevated total bilirubin and second-line therapy failure is not fully clear, up to one-third of patients treated with atazanavir had elevated bilirubin as a marker of hepatotoxicity [100, 101]. Aligned with this, a higher discontinuation rate of ritonavir-boosted atazanavir [101] and super-boosting of lopinavir-ritonavir were linked with co-administration of a medication that inhibits hepatic cytochrome P450 enzymes [102].
Although the overall sample size was large enough, there are some limitations to note. First, the majority of data were derived from observational studies which resulted in a high degree of heterogeneity and a range of potential biases. As a result, we have used a random-effects model which is more appropriate in such anticipated heterogeneity. A series of subgroup analyses were also considered to reduce the degree of heterogeneity and presented them in percentages to indicate the extent of differences. Second, other additional potential explanations for second-line therapy failures including medication toxicities and drug-drug interactions might not have been adequately addressed. Third, we have included articles published only in the English language and this could under-or over-estimate the pooled proportion of second-line therapy failure in the SSA. Finally, the reporting of some variables pertaining to clinical and programmatic follow-ups were inconsistent, limiting the conclusiveness of the pooled factors associated with the second-line HIV treatment failure.
Conclusion
The pooled proportion of second-line HIV treatment failure experienced by HIV-infected patients in SSA was found to be high. More common failure rates occurred at a 12–18 month period of follow-up after second-line therapy start, in children, and in the southern region of the SSA. Suboptimal adherence to second-line ART, higher HIV-RNA VL at baseline, lower peak values for CD4 cells, and advanced WHO clinical stages were among the key factors that have accelerated second-line HIV treatment failure in the setting. With this, prolonged delays in switching prior therapy, tuberculosis co-treatment, and other patient factors including younger age, depressive symptoms, underweight, and traditional medicine use were linked with the occurrence of second-line treatment failure. Therefore, optimal second-line HIV treatment approaches should critically consider immediate and aggressive VL suppression, rapid immune recovery, and excellent adherence to the therapy. Together with these approaches, more frequent clinical follow-ups and VL monitoring are recommended for the HIV-infected patients in SSA that help in rapid identification and intervention of failure cases. Finally, countries in the SSA should develop strategies and guidelines related to containment of second-line HIV treatment including intensive adherence support and intervention as routine clinical practice especially for patients with slow response to the therapies.
Supporting information
(DOC)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Haramaya University, College of Health and Medical Sciences’ staff without them this systematic review and meta-analysis would not have been realized.
Abbreviations
- AIDS
Acquired Immunodeficiency Syndrome
- ART
Antiretroviral Therapy
- CI
Confidence Interval
- ES
Effect Size
- HIV
Human Immunodeficiency Virus
- JBI
Joanna Briggs Institute
- OR
Odds Ratio
- PRISMA
Preferred Reporting Items for Systematic review and Meta-analysis
- RNA
Ribonucleic Acid
- SSA
sub-Saharan Africa
- VL
Viral Load
- WHO
World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
We have received no funding for this work.
References
- 1.Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. : Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Annals of internal medicine 2011, 155(4):209–216. 10.7326/0003-4819-155-4-201108160-00358 [DOI] [PubMed] [Google Scholar]
- 2.Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, Munthali F, et al. : Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. The Lancet 2008, 371(9624):1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. : Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. Jama 2006, 296(6):679–690. 10.1001/jama.296.6.679 [DOI] [PubMed] [Google Scholar]
- 4.Long L, Fox M, Sanne I, Rosen S: The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. Aids 2010, 24(6):915–919. 10.1097/QAD.0b013e3283360976 [DOI] [PubMed] [Google Scholar]
- 5.Keiser O, Tweya H, Braitstein P, Dabis F, MacPhail P, Boulle A, et al. : Mortality after failure of antiretroviral therapy in sub‐Saharan Africa. Tropical medicine & international health 2010, 15(2):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pujades-Rodríguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A: Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. Jama 2010, 304(3):303–312. 10.1001/jama.2010.980 [DOI] [PubMed] [Google Scholar]
- 7.Van Oosterhout JJ, Brown L, Weigel R, Kumwenda JJ, Mzinganjira D, Saukila N, et al. : Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Tropical medicine & international health 2009, 14(8):856–861. [DOI] [PubMed] [Google Scholar]
- 8.Murri R, Lepri AC, Cicconi P, Poggio A, Arlotti M, Tositti G, et al. : Is moderate HIV viremia associated with a higher risk of clinical progression in HIV-infected people treated with highly active antiretroviral therapy: evidence from the Italian cohort of antiretroviral-naive patients study. JAIDS Journal of Acquired Immune Deficiency Syndromes 2006, 41(1):23–30. [DOI] [PubMed] [Google Scholar]
- 9.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD: Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. Aids 2008, 22(15):1971–1977. 10.1097/QAD.0b013e32830e4cd8 [DOI] [PubMed] [Google Scholar]
- 10.Labhardt ND, Bader J, Lejone TI, Ringera I, Hobbins MA, Fritz C, et al. : Should viral load thresholds be lowered?: revisiting the WHO definition for virologic failure in patients on antiretroviral therapy in resource-limited settings. Medicine 2016, 95(28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joram SL, Paul G, Moses K, Stanley B, Isaac M, Allan G, et al. : Misdiagnosis of HIV treatment failure based on clinical and immunological criteria in Eastern and Central Kenya. BMC Infect Dis 2017, 17(1):383 10.1186/s12879-017-2487-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpadi SM, Shiau S, De Gusmao EP, Violari A: Routine viral load monitoring in HIV-infected infants and children in low- and middle-income countries: challenges and opportunities. J Int AIDS Soc 2017, 20 Suppl 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandormael AM, Boulware DR, Tanser FC, Barnighausen TW, Stott KE, de Oliveira T: Brief Report: Virologic Monitoring Can Be a Cost-Effective Strategy to Diagnose Treatment Failure on First-Line ART. J Acquir Immune Defic Syndr 2016, 71(4):462–466. 10.1097/QAI.0000000000000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns R, Borges J, Blasco P, Vandenbulcke A, Mukui I, Magalasi D, et al. : 'I saw it as a second chance': A qualitative exploration of experiences of treatment failure and regimen change among people living with HIV on second- and third-line antiretroviral therapy in Kenya, Malawi and Mozambique. Global public health 2019:1–13. [DOI] [PubMed] [Google Scholar]
- 15.Siripassorn K, Manosuthi W, Chottanapund S, Pakdee A, Sabaitae S, Prasithsirikul W, et al. : Effectiveness of boosted protease inhibitor-based regimens in HIV type 1-infected patients who experienced virological failure with NNRTI-based antiretroviral therapy in a resource-limited setting. AIDS research and human retroviruses 2010, 26(2):139–148. 10.1089/aid.2009.0125 [DOI] [PubMed] [Google Scholar]
- 16.Stockdale AJ, Saunders MJ, Boyd MA, Bonnett LJ, Johnston V, Wandeler G, et al. : Effectiveness of Protease Inhibitor/Nucleos (t) ide Reverse Transcriptase Inhibitor–Based Second-line Antiretroviral Therapy for the Treatment of Human Immunodeficiency Virus Type 1 Infection in Sub-Saharan Africa: A Systematic Review and Meta-analysis. Clinical infectious diseases 2017, 66(12):1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, et al. : Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015, 528(7580):S68–76. 10.1038/nature16046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy RA, Court R, Maartens G, Sunpath H: Second-Line Antiretroviral Therapy in Sub-Saharan Africa: It Is Time to Mind the Gaps. AIDS Res Hum Retroviruses 2017, 33(12):1181–1184. 10.1089/AID.2017.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boender TS, Hamers RL, Ondoa P, Wellington M, Chimbetete C, Siwale M, et al. : Protease inhibitor resistance in the first 3 years of second-line antiretroviral therapy for HIV-1 in sub-Saharan Africa. The Journal of infectious diseases 2016, 214(6):873–883. 10.1093/infdis/jiw219 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach: World Health Organization; 2016. [PubMed] [Google Scholar]
- 21.World Health Organization: Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. In.: World Health Organization; 2018. [Google Scholar]
- 22.Boerma RS, Kityo C, Boender TS, Kaudha E, Kayiwa J, Musiime V, et al. : Second-line HIV Treatment in Ugandan Children: Favorable Outcomes and No Protease Inhibitor Resistance. J Trop Pediatr 2017, 63(2):135–143. 10.1093/tropej/fmw062 [DOI] [PubMed] [Google Scholar]
- 23.Evans D, Dahlberg S, Berhanu R, Sineke T, Govathson C, Jonker I, et al. : Social and behavioral factors associated with failing second-line ART—results from a cohort study at the Themba Lethu Clinic, Johannesburg, South Africa. AIDS care 2018, 30(7):863–870. 10.1080/09540121.2017.1417527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. : Second-line antiretroviral treatment successfully resuppresses drug-resistant HIV-1 after first-line failure: prospective cohort in Sub-Saharan Africa. J Infect Dis 2012, 205(11):1739–1744. 10.1093/infdis/jis261 [DOI] [PubMed] [Google Scholar]
- 25.Adetunji AA, Achenbach C, Feinglass J, Darin KM, Scarsi KK, Ekong E, et al. : Optimizing treatment switch for virologic failure during first-line antiretroviral therapy in resource-limited settings. J Int Assoc Provid AIDS Care 2013, 12(4):236–240. 10.1177/1545109712463733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd MA, Moore CL, Molina JM, Wood R, Madero JS, Wolff M, et al. : Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: an exploratory analysis. The lancet HIV 2015, 2(2):e42–51. 10.1016/S2352-3018(14)00061-7 [DOI] [PubMed] [Google Scholar]
- 27.Murphy RA, Sunpath H, Castilla C, Ebrahim S, Court R, Nguyen H, et al. : Second-line antiretroviral therapy: long-term outcomes in South Africa. J Acquir Immune Defic Syndr 2012, 61(2):158–163. 10.1097/QAI.0b013e3182615ad1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wynberg E, Williams E, Tudor-Williams G, Lyall H, Foster C: Discontinuation of Efavirenz in Paediatric Patients: Why do Children Switch? Clinical drug investigation 2018, 38(3):231–238. 10.1007/s40261-017-0605-1 [DOI] [PubMed] [Google Scholar]
- 29.Khan S, Das M, Andries A, Deshpande A, Mansoor H, Saranchuk P, et al. : Second-line failure and first experience with third-line antiretroviral therapy in Mumbai, India. Glob Health Action 2014, 7:24861 10.3402/gha.v7.24861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ssempijja V, Nakigozi G, Chang L, Gray R, Wawer M, Ndyanabo A, et al. : Rates of switching to second-line antiretroviral therapy and impact of delayed switching on immunologic, virologic, and mortality outcomes among HIV-infected adults with virologic failure in Rakai, Uganda. BMC infectious diseases 2017, 17(1):582 10.1186/s12879-017-2680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keiser O, Tweya H, Boulle A, Braitstein P, Schecter M, Brinkhof MW, et al. : Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. Aids 2009, 23(14):1867–1874. 10.1097/QAD.0b013e32832e05b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boettiger DC, Nguyen VK, Durier N, Bui HV, Heng Sim BL, Azwa I, et al. : Efficacy of second-line antiretroviral therapy among people living with HIV/AIDS in Asia: results from the TREAT Asia HIV observational database. J Acquir Immune Defic Syndr 2015, 68(2):186–195. 10.1097/QAI.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy RA, Court R, Maartens G, Sunpath H: Second-line antiretroviral therapy in sub-Saharan Africa: It is time to mind the gaps. AIDS research and human retroviruses 2017, 33(12):1181–1184. 10.1089/AID.2017.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsegaye AT, Wubshet M, Awoke T, Alene KA: Predictors of treatment failure on second-line antiretroviral therapy among adults in northwest Ethiopia: a multicentre retrospective follow-up study. BMJ open 2016, 6(12):e012537 10.1136/bmjopen-2016-012537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. : Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 2015, 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 2009, 6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edessa D, Sisay M, Asefa F: Treatment failure for second-line antiretroviral therapy among HIV-infected patients in sub-Saharan Africa: a systematic review and meta-analysis National Institute for Health Research; 2018, CRD42018118959. [Google Scholar]
- 38.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K et al. : Chapter 7: Systematic reviews of etiology and risk In: Aromataris E, Munn Z (Editors). Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute; Available from https://reviewersmanualjoannabriggsorg/2017. [Google Scholar]
- 39.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. : Chapter 7: Systematic reviews of etiology and risk In: Aromataris E, Munn Z (Editors). Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute; Available from https://reviewersmanualjoannabriggsorg/ 2017. [Google Scholar]
- 40.Castelnuovo B, John L, Lutwama F, Ronald A, Spacek LA, Bates M, et al. : Three-year outcome data of second-line antiretroviral therapy in Ugandan adults: good virological response but high rate of toxicity. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill: 2002) 2009, 8(1):52–59. [DOI] [PubMed] [Google Scholar]
- 41.Rawizza HE, Chaplin B, Meloni ST, Darin KM, Olaitan O, Scarsi KK, et al. : Accumulation of protease mutations among patients failing second-line antiretroviral therapy and response to salvage therapy in Nigeria. PLoS One 2013, 8(9):e73582 10.1371/journal.pone.0073582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akanmu AS, Adeyemo T, Lesi F, Bello FO, Okwuegbuna K, Oloko K, et al. : Immunological and Virological Outcomes of Patients Switched from LPV/r to ATV/r-Containing Second- Line Regimens. Current HIV research 2015, 13(3):176–183. [DOI] [PubMed] [Google Scholar]
- 43.Berhanu RH, Evans D, Ive P, Spencer D, Firnhaber C, Sanne I, et al. : Second-line failure and protease inhibitor resistance in a clinic in Johannesburg, South Africa. Topics in antiviral medicine 2014, 22 (E-1):557–558. [Google Scholar]
- 44.Ciaffi L, Koulla-Shiro S, Sawadogo A, le Moing V, Eymard-Duvernay S, Izard S, et al. : Efficacy and safety of three second-line antiretroviral regimens in HIV-infected patients in Africa. Aids 2015, 29(12):1473–1481. 10.1097/QAD.0000000000000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collier D, Iwuji C, Derache A, de Oliveira T, Okesola N, Calmy A, et al. : Virological Outcomes of Second-line Protease Inhibitor-Based Treatment for Human Immunodeficiency Virus Type 1 in a High-Prevalence Rural South African Setting: A Competing-Risks Prospective Cohort Analysis. Clin Infect Dis 2017, 64(8):1006–1016. 10.1093/cid/cix015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Court R, Leisegang R, Stewart A, Sunpath H, Murphy R, Winternheimer P, et al. : Short term adherence tool predicts failure on second line protease inhibitor-based antiretroviral therapy: an observational cohort study. BMC Infect Dis 2014, 14:664 10.1186/s12879-014-0664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans D, Hirasen K, Berhanu R, Malete G, Ive P, Spencer D, et al. : Predictors of switch to and early outcomes on third-line antiretroviral therapy at a large public-sector clinic in Johannesburg, South Africa. AIDS Res Ther 2018, 15(1):10 10.1186/s12981-018-0196-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox MP, Berhanu R, Steegen K, Firnhaber C, Ive P, Spencer D, et al. : Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa. Trop Med Int Health 2016, 21(9):1131–1137. 10.1111/tmi.12741 [DOI] [PubMed] [Google Scholar]
- 49.Fox MP, Ive P, Long L, Maskew M, Sanne I: High rates of survival, immune reconstitution, and virologic suppression on second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010, 53(4):500–506. 10.1097/QAI.0b013e3181bcdac1 [DOI] [PubMed] [Google Scholar]
- 50.Johnston V, Cohen K, Wiesner L, Morris L, Ledwaba J, Fielding KL, et al. : Viral suppression following switch to second-line antiretroviral therapy: associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch. J Infect Dis 2014, 209(5):711–720. 10.1093/infdis/jit411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnston V, Fielding K, Charalambous S, Mampho M, Churchyard G, Phillips A, et al. : Second-line antiretroviral therapy in a workplace and community-based treatment programme in South Africa: determinants of virological outcome. PLoS One 2012, 7(5):e36997 10.1371/journal.pone.0036997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levison JH, Orrell C, Gallien S, Kuritzkes DR, Fu N, Losina E, et al. : Virologic failure of protease inhibitor-based second-line antiretroviral therapy without resistance in a large HIV treatment program in South Africa. PLoS One 2012, 7(3):e32144 10.1371/journal.pone.0032144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ongubo DM, Lim R, Tweya H, Stanley CC, Tembo P, Broadhurst R, et al. : A cross-sectional study to evaluate second line virological failure and elevated bilirubin as a surrogate for adherence to atazanavir/ritonavir in two urban HIV clinics in Lilongwe, Malawi. BMC Infect Dis 2017, 17(1):461 10.1186/s12879-017-2528-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onyedum CC, Iroezindu MO, Chukwuka CJ, Anyaene CA, Obi FI, Young EE: Profile of HIV-infected patients receiving second-line antiretroviral therapy in a resource-limited setting in Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2013, 107(10):608–614. 10.1093/trstmh/trt071 [DOI] [PubMed] [Google Scholar]
- 55.Paton NI, Kityo C, Hoppe A, Reid A, Kambugu A, Lugemwa A, et al. : Assessment of second-line antiretroviral regimens for HIV therapy in Africa. New England journal of medicine 2014, 371(3):234‐247. 10.1056/NEJMoa1311274 [DOI] [PubMed] [Google Scholar]
- 56.Paton NI, Kityo C, Thompson J, Nankya I, Bagenda L, Hoppe A, et al. : Nucleoside reverse-transcriptase inhibitor cross-resistance and outcomes from second-line antiretroviral therapy in the public health approach: an observational analysis within the randomised, open-label, EARNEST trial. The lancet HIV 2017, 4(8):e341‐e348. 10.1016/S2352-3018(17)30065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shearer K, Evans D, Moyo F, Rohr JK, Berhanu R, Van Den Berg L, et al. : Treatment outcomes of over 1000 patients on second-line, protease inhibitor-based antiretroviral therapy from four public-sector HIV treatment facilities across Johannesburg, South Africa. Trop Med Int Health 2017, 22(2):221–231. 10.1111/tmi.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, et al. : Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011, 56(4):333–339. 10.1097/QAI.0b013e31820dc0cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wandeler G, Gerber F, Rohr J, Chi BH, Orrell C, Chimbetete C, et al. : Tenofovir or zidovudine in second-line antiretroviral therapy after stavudine failure in southern Africa. Antiviral therapy 2014, 19(5):521–525. 10.3851/IMP2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wandeler G, Keiser O, Mulenga L, Hoffmann CJ, Wood R, Chaweza T, et al. : Tenofovir in second-line ART in Zambia and South Africa: collaborative analysis of cohort studies. J Acquir Immune Defic Syndr 2012, 61(1):41–48. 10.1097/QAI.0b013e3182632540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musiime V, Kaudha E, Kayiwa J, Mirembe G, Odera M, Kizito H, et al. : Antiretroviral drug resistance profiles and response to second-line therapy among HIV type 1-infected Ugandan children. AIDS Res Hum Retroviruses 2013, 29(3):449–455. 10.1089/aid.2012.0283 [DOI] [PubMed] [Google Scholar]
- 62.Garone D, Conradie K, Patten G, Cornell M, Goemaere E, Kunene J, et al. : High rate of virological re-suppression among patients failing second-line antiretroviral therapy following enhanced adherence support: A model of care in Khayelitsha, South Africa. Southern African journal of HIV medicine 2013, 14(4):170–175. [Google Scholar]
- 63.Hosseinipour MC, Kumwenda JJ, Weigel R, Brown LB, Mzinganjira D, Mhango B, et al. : Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med 2010, 11(8):510–518. 10.1111/j.1468-1293.2010.00825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoffelen AF, Wensing AM, Tempelman HA, Geelen SP, Hoepelman AI, Barth RE: Sustained virological response on second-line antiretroviral therapy following virological failure in HIV-infected patients in rural South Africa. PLoS One 2013, 8(3):e58526 10.1371/journal.pone.0058526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnett W, Patten G, Kerschberger B, Conradie K, Garone D, Van Cutsem G, et al. : Perceived adherence barriers among patients failing second-line antiretroviral therapy in Khayelitsha, South Africa. Southern African journal of HIV medicine 2013, 14(4):166–169. [Google Scholar]
- 66.Boerma RS, Bunupuradah T, Dow D, Fokam J, Kariminia A, Lehman D, et al. : Multicentre analysis of second‐line antiretroviral treatment in HIV‐infected children: adolescents at high risk of failure. Journal of the International AIDS Society 2017, 20(1):21930 10.7448/IAS.20.1.21930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Beaudrap P, Thiam M, Diouf A, Toure-Kane C, Ngom-Gueye NF, Vidal N, et al. : Risk of virological failure and drug resistance during first and second-line antiretroviral therapy in a 10-year cohort in Senegal: results from the ANRS 1215 cohort. J Acquir Immune Defic Syndr 2013, 62(4):381–387. 10.1097/QAI.0b013e31827a2a7a [DOI] [PubMed] [Google Scholar]
- 68.Gross R, Zheng L, La Rosa A, Sun X, Rosenkranz SL, Cardoso SW, et al. : Partner-based adherence intervention for second-line antiretroviral therapy (ACTG A5234): a multinational randomised trial. The lancet HIV 2015, 2(1):e12–e19. 10.1016/S2352-3018(14)00007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shearer K, Evans D, Moyo F, Rohr JK, Berhanu R, Van Den Berg L, et al. : Treatment outcomes of over 1000 patients on second‐line, protease inhibitor‐based antiretroviral therapy from four public‐sector HIV treatment facilities across Johannesburg, South Africa. Tropical Medicine & International Health 2017, 22(2):221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puthanakit T, Jourdain G, Suntarattiwong P, Chokephaibulkit K, Siangphoe U, Suwanlerk T, et al. : High virologic response rate after second-line boosted protease inhibitor-based antiretroviral therapy regimens in children from a resource limited setting. AIDS Res Ther 2012, 9(1):20 10.1186/1742-6405-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suaysod R, Ngo-Giang-Huong N, Salvadori N, Cressey TR, Kanjanavanit S, Techakunakorn P, et al. : Treatment Failure in HIV-Infected Children on Second-line Protease Inhibitor-Based Antiretroviral Therapy. Clin Infect Dis 2015, 61(1):95–101. 10.1093/cid/civ271 [DOI] [PubMed] [Google Scholar]
- 72.Doerholt K, Sharland M, Ball C, DuMont G: Paediatric antiretroviral therapy audit in South London. HIV Med 2002, 3(1):44–48. [DOI] [PubMed] [Google Scholar]
- 73.Bernheimer JM, Patten G, Makeleni T, Mantangana N, Dumile N, Goemaere E, et al. : Paediatric HIV treatment failure: a silent epidemic. J Int AIDS Soc 2015, 18:20090 10.7448/IAS.18.1.20090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.World Health Organization: FactSheet: People living with HIV, WHO regional officier Africa. 2017.
- 75.USAID/SIAPS: COMBATING ANTIMICROBIAL RESISTANCE WITH STRONGER HEALTH SYSTEMS. TECHNICAL PROGRAM UPDATE 2016, Arlington, VA 22203 USA.
- 76.De Beaudrap P, Thiam M, Diouf A, Toure-Kane C, Ngom-Guèye NF, Vidal N, et al. : Risk of virological failure and drug resistance during first and second-line antiretroviral therapy in a 10-year cohort in Senegal: results from the ANRS 1215 cohort. JAIDS Journal of Acquired Immune Deficiency Syndromes 2013, 62(4):381–387. 10.1097/QAI.0b013e31827a2a7a [DOI] [PubMed] [Google Scholar]
- 77.Ssempijja V, Nakigozi G, Chang L, Gray R, Wawer M, Ndyanabo A, et al. : Rates of switching to second-line antiretroviral therapy and impact of delayed switching on immunologic, virologic, and mortality outcomes among HIV-infected adults with virologic failure in Rakai, Uganda. BMC Infectious Diseases 2017, 17(582). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.BOETTIGER DC, NGUYEN VK, DURIER N, BUI HV, SIM BLH, AZWA I, et al. : Efficacy of second-line antiretroviral therapy among people living with HIV/AIDS in Asia: Results from the TREAT Asia HIV Observational Database. J Acquir Immune Defic Syndr 2015. 68(2):186–195. 10.1097/QAI.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chakravarty J, Sundar S, Chourasia A, Singh PN, Kurle S, Tripathy SP, et al. : Outcome of patients on second line antiretroviral therapy under programmatic condition in India. BMC Infectious Diseases 2015, 15(517). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cardoso SW, Luz PM, Velasque L, Torres TS, Tavares IC, Ribeiro SR, et al. : Outcomes of second-line combination antiretroviral therapy for HIV-infected patients: a cohort study from Rio de Janeiro, Brazil. BMC Infectious Diseases 2014, 14(699). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thao VP, Quang VM, N. J, Day JN, Chinh NT, Shikuma CM, Farrar J, et al. : High prevalence of PI resistance in patients failing second-line ART in Vietnam. Antimicrob Chemother 2016, 71:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prasitsuebsai W, Teeraananchai S, Singtoroj T, Truong KH, Ananworanich J, Do VC, et al. : Treatment outcomes and resistance patterns of children and adolescents on second-line antiretroviral therapy in Asia. J Acquir Immune Defic Syndr 2016. 72(4):380–386. 10.1097/QAI.0000000000000971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haggblom A, Santacatterina M, Neogi U, Gisslen M, Hejdeman B, Flamholc L, et al. : Effect of therapy switch on time to second-line antiretroviral treatment failure in HIV-infected patients. PLoS One 2017, 12(7):e0180140 10.1371/journal.pone.0180140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kyaw NTT, Kumar AMV, Oo MM, Oo HN, Kyaw KWY, Thiha S, et al. : Long-term outcomes of second-line antiretroviral treatment in an adult and adolescent cohort in Myanmar. Glob Health Action 2017, 10(1):1290916 10.1080/16549716.2017.1290916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans D, Berhanu R, Moyo F, Nguweneza A, Long L, Fox MP: Can Short-Term Use of Electronic Patient Adherence Monitoring Devices Improve Adherence in Patients Failing Second-Line Antiretroviral Therapy? Evidence from a Pilot Study in Johannesburg, South Africa. AIDS Behav 2016, 20(11):2717–2728. 10.1007/s10461-016-1417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hawkins C, Hertzmark E, Spiegelman D, Muya A, Ulenga N, Kim S, et al. : Switching to second-line ART in relation to mortality in a large Tanzanian HIV cohort. The Journal of antimicrobial chemotherapy 2017, 72(7):2060–2068. 10.1093/jac/dkx098 [DOI] [PubMed] [Google Scholar]
- 87.Wilhelmson S, Reepalu A, Balcha TT, Jarso G, Bjorkman P: Retention in care among HIV-positive patients initiating second-line antiretroviral therapy: a retrospective study from an Ethiopian public hospital clinic. Glob Health Action 2016, 9:29943 10.3402/gha.v9.29943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J, Zhang M, Shang M, Yang W, Wang Z, Shang H: Research on the treatment effects and drug resistances of long-term second-line antiretroviral therapy among HIV-infected patients from Henan Province in China. BMC Infect Dis 2018, 18(1):571 10.1186/s12879-018-3489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramadhani HO, Bartlett JA, Thielman NM, Pence BW, Kimani SM, Maro VP, et al. : Association of first-line and second-line antiretroviral therapy adherence. Open forum infectious diseases 2014, 1(2):ofu079 10.1093/ofid/ofu079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rohr JK, Ive P, Horsburgh CR, Berhanu R, Shearer K, Maskew M, et al. : Marginal Structural Models to Assess Delays in Second-Line HIV Treatment Initiation in South Africa. PLoS One 2016, 11(8):e0161469 10.1371/journal.pone.0161469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shearer K, Evans D, Xhosa B, Hirasen K, Bracken C, Mahomed K, et al. : Low prevalence of depressive symptoms among stable patients on antiretroviral therapy in Johannesburg, South Africa. PLoS One 2018, 13(9):e0203797 10.1371/journal.pone.0203797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tufano CS, Amaral RA, Cardoso LR, Malbergier A: The influence of depressive symptoms and substance use on adherence to antiretroviral therapy. A cross-sectional prevalence study. Sao Paulo medical journal = Revista paulista de medicina 2015, 133(3):179–186. 10.1590/1516-3180.2013.7450010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lancaster KE, Lungu T, Mmodzi P, Hosseinipour MC, Chadwick K, Powers KA, et al. : The association between substance use and sub-optimal HIV treatment engagement among HIV-infected female sex workers in Lilongwe, Malawi. AIDS care 2017, 29(2):197–203. 10.1080/09540121.2016.1211244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Appelbaum Belisle H, Hennink M, Ordonez CE, John S, Ngubane-Joye E, Hampton J, et al. : Concurrent use of traditional medicine and ART: Perspectives of patients, providers and traditional healers in Durban, South Africa. Global public health 2015, 10(1):71–87. 10.1080/17441692.2014.967709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webb Mazinyo E, Kim L, Masuku S, Lancaster JL, Odendaal R, Uys M, et al. : Adherence to Concurrent Tuberculosis Treatment and Antiretroviral Treatment among Co-Infected Persons in South Africa, 2008–2010. PLoS One 2016, 11(7):e0159317 10.1371/journal.pone.0159317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Madelain V, Le MP, Champenois K, Charpentier C, Landman R, Joly V, et al. : Impact of obesity on antiretroviral pharmacokinetics and immuno-virological response in HIV-infected patients: a case-control study. The Journal of antimicrobial chemotherapy 2017, 72(4):1137–1146. 10.1093/jac/dkw527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lloret-Linares C, Rahmoun Y, Lopes A, Chopin D, Simoneau G, Green A, et al. : Effect of body weight and composition on efavirenz, atazanavir or darunavir concentration. Therapie 2018, 73(3):185–191. 10.1016/j.therap.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 98.Dresser GK, Spence JD, Bailey DG: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clinical pharmacokinetics 2000, 38(1):41–57. 10.2165/00003088-200038010-00003 [DOI] [PubMed] [Google Scholar]
- 99.Cohen K, Stewart A, Kengne AP, Leisegang R, Coetsee M, Maharaj S, et al. : A Clinical Prediction Rule for Protease Inhibitor Resistance in Patients Failing Second-Line Antiretroviral Therapy. J Acquir Immune Defic Syndr 2019, 80(3):325–329. 10.1097/QAI.0000000000001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roy-Chowdhury J, Roy-Chowdhury N, Listowsky I, Wolkoff AW: Drug- and Drug Abuse-Associated Hyperbilirubinemia: Experience With Atazanavir. Clinical pharmacology in drug development 2017, 6(2):140–146. 10.1002/cpdd.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giacomelli A, Oreni L, Franzetti M, Di Cristo V, Colella E, Ridolfo AL, et al. : Factors involved in continuance of atazanavir-based regimens: Results from a cohort of HIV1-positive patients. Antiviral research 2016, 129:52–57. 10.1016/j.antiviral.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 102.Rabie H, Denti P, Lee J, Masango M, Coovadia A, Pillay S, et al. : Lopinavir-ritonavir super-boosting in young HIV-infected children on rifampicin-based tuberculosis therapy compared with lopinavir-ritonavir without rifampicin: a pharmacokinetic modelling and clinical study. The lancet HIV 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.